Microsaccade Activity During Visuospatial Working Memory in Early-Stage Parkinson’s Disease
Highlights
- We examined the potential modulatory role of dopamine in fixational eye movements (i.e., microsaccades) during spatial working memory tasks, as other studies have not comprehensively examined microsaccades in this context.
- We revealed that dopamine deficiencies affect some characteristics of microsaccades, and levodopa medication does not restore them in patients with early-stage Parkinson’s disease to the level observed in healthy control subjects.
- We found that regardless of dopamine status or task demand, microsaccade direction remained the same and microsaccades exhibited a characteristic rate change, consistent with previous research.
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Apparatus and Visual Stimuli
2.3. Visuospatial Working Memory Paradigm
2.4. Microsaccade Identification and Analysis
2.5. Statistical Analysis
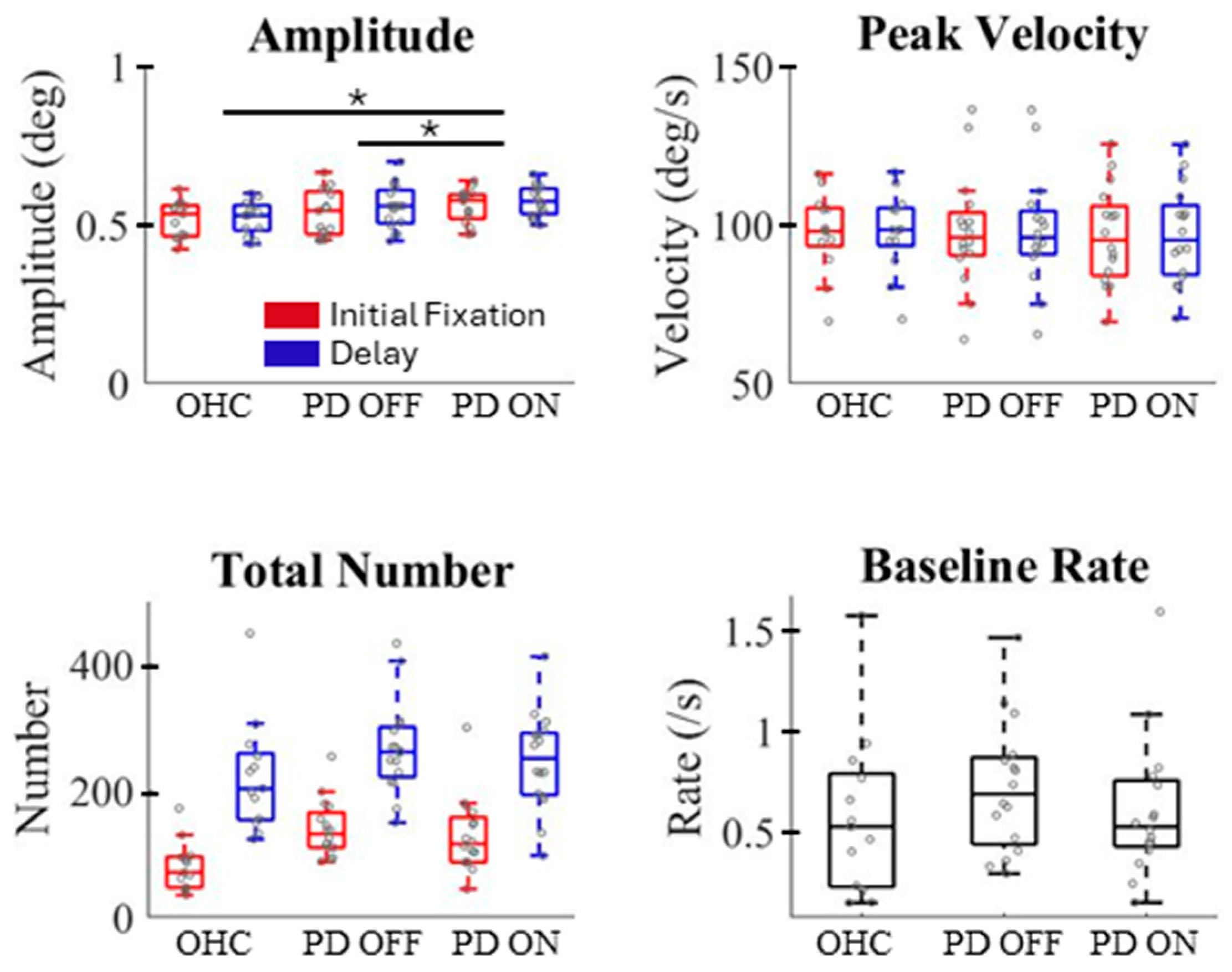
3. Results
4. Discussion
4.1. Dopamine Deficiency Alters Microsaccade Characteristics and Activity
4.2. Microsaccade Rate During Working Memory
4.3. Lack of Directionally Biased Microsaccades
4.4. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PD | Parkinson’s disease |
| OHC | Older healthy controls |
| SWJ | Square wave jerk |
References
- Baddeley, A.D.; Hitch, G.J. Working Memory. In Recent Advances in Learning and Motivation; Bower, G.A., Ed.; Academic Press: New York, NY, USA, 1974; Volume 8, pp. 47–89. [Google Scholar] [CrossRef]
- Awh, E.; Vogel, E.K.; Oh, S.H. Interactions between attention and working memory. Neuroscience 2006, 139, 201–208. [Google Scholar] [CrossRef]
- Smyth, M.M.; Scholey, K.A. Interference in immediate spatial memory. Mem. Cogn. 1994, 22, 1–13. [Google Scholar] [CrossRef]
- Heuer, A.; Ohl, S.; Rolfs, M. Memory for action: A functional view of selection in visual working memory. Vis. Cogn. 2020, 28, 388–400. [Google Scholar] [CrossRef]
- Olivers, C.N.L.; Roelfsema, P.R. Attention for action in visual working memory. Cortex A J. Devoted Study Nerv. Syst. Behav. 2020, 131, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Ditchburn, R.W.; Ginsborg, B.L. Involuntary eye movements during fixation. J. Physiol. 1953, 119, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Gill, C.; Poletti, M.; Rucci, M. Monocular microsaccades: Do they really occur? J. Vis. 2018, 18, 18. [Google Scholar] [CrossRef]
- Watanabe, M.; Matsuo, Y.; Zha, L.; Munoz, D.P.; Kobayashi, Y. Fixational saccades reflect volitional action preparation. J. Neurophysiol. 2013, 110, 522–535. [Google Scholar] [CrossRef]
- Zuber, B.L.; Stark, L.; Cook, G. Microsaccades and the velocity-amplitude relationship for saccadic eye movements. Science 1965, 150, 1459–1460. [Google Scholar] [CrossRef]
- Otero-Millan, J.; Troncoso, X.G.; Macknik, S.L.; Serrano-Pedraza, I.; Martinez-Conde, S. Saccades and microsaccades during visual fixation, exploration, and search: Foundations for a common saccadic generator. J. Vis. 2008, 8, 21. [Google Scholar] [CrossRef]
- Hafed, Z.M.; Goffart, L.; Krauzlis, R.J. A neural mechanism for microsaccade generation in the primate superior colliculus. Science 2009, 323, 940–943. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.; Walker, D.G.; Husain, M.; Kennard, C. Control of voluntary and reflexive saccades. Exp. Brain Res. 2000, 130, 540–544. [Google Scholar] [CrossRef]
- Rolfs, M.; Kliegl, R.; Engbert, R. Toward a model of microsaccade generation: The case of microsaccadic inhibition. J. Vis. 2008, 8, 5. [Google Scholar] [CrossRef]
- Gu, Q.; Zhang, Q.; Han, Y.; Li, P.; Gao, Z.; Shen, M. Microsaccades reflect attention shifts: A mini review of 20 years of microsaccade research. Front. Psychol. 2024, 15, 1364939. [Google Scholar] [CrossRef]
- Ramat, S.; Leigh, R.J.; Zee, D.S.; Shaikh, A.G.; Optican, L.M. Applying saccade models to account for oscillations. Prog. Brain Res. 2008, 171, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Munoz, D.P. Probing basal ganglia functions by saccade eye movements. Eur. J. Neurosci. 2011, 33, 2070–2090. [Google Scholar] [CrossRef] [PubMed]
- Basso, M.A.; Liu, P. Context-dependent effects of substantia nigra stimulation on eye movements. J. Neurophysiol. 2007, 97, 4129–4142. [Google Scholar] [CrossRef] [PubMed]
- Bayer, H.M.; Glimcher, P.W. Midbrain dopamine neurons encode a quantitative reward prediction error signal. Neuron 2005, 47, 129–141. [Google Scholar] [CrossRef]
- Hikosaka, O.; Takikawa, Y.; Kawagoe, R. Role of the basal ganglia in the control of purposive saccadic eye movements. Physiol. Rev. 2000, 80, 953–978. [Google Scholar] [CrossRef]
- Otero-Millan, J.; Optican, L.M.; Macknik, S.L.; Martinez-Conde, S. Modeling the Triggering of Saccades, Microsaccades, and Saccadic Intrusions. Front. Neurol. 2018, 9, 346. [Google Scholar] [CrossRef]
- Hafed, Z.M.; Yoshida, M.; Tian, X.; Buonocore, A.; Malevich, T. Dissociable Cortical and Subcortical Mechanisms for Mediating the Influences of Visual Cues on Microsaccadic Eye Movements. Front. Neural Circuits 2021, 15, 638429. [Google Scholar] [CrossRef]
- Munoz, D.P.; Wurtz, R.H. Fixation cells in monkey superior colliculus. I. Characteristics of cell discharge. J. Neurophysiol. 1993, 70, 559–575. [Google Scholar] [CrossRef] [PubMed]
- Klaus, K.; Pennington, K. Dopamine and Working Memory: Genetic Variation, Stress and Implications for Mental Health. Curr. Top. Behav. Neurosci. 2019, 41, 369–391. [Google Scholar] [CrossRef]
- Surmeier, D.J. Calcium, ageing, and neuronal vulnerability in Parkinson’s disease. Lancet Neurol. 2007, 6, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Kori, A.; Miyashita, N.; Kato, M.; Hikosaka, O.; Usui, S.; Matsumura, M. Eye movements in monkeys with local dopamine depletion in the caudate nucleus. II. Deficits in voluntary saccades. J. Neurosci. Off. J. Soc. Neurosci. 1995, 15 Pt 2, 928–941. [Google Scholar] [CrossRef]
- Ogasawara, T.; Nejime, M.; Takada, M.; Matsumoto, M. Primate Nigrostriatal Dopamine System Regulates Saccadic Response Inhibition. Neuron 2018, 100, 1513–1526.e4. [Google Scholar] [CrossRef]
- Albin, R.L.; Young, A.B.; Penney, J.B. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989, 12, 366–375. [Google Scholar] [CrossRef]
- Marsden, C.D. Parkinson’s disease. Postgrad. Med. J. 1992, 68, 538–543. [Google Scholar] [CrossRef]
- Antoniades, C.A.; Spering, M. Eye movements in Parkinson’s disease: From neurophysiological mechanisms to diagnostic tools. Trends Neurosci. 2024, 47, 71–83. [Google Scholar] [CrossRef]
- Bayram, E.; Litvan, I.; Wright, B.A.; Grembowski, C.; Shen, Q.; Harrington, D.L. Dopamine effects on memory load and distraction during visuospatial working memory in cognitively normal Parkinson’s disease. Neuropsychol. Dev. Cogn. Sect. B Aging Neuropsychol. Cogn. 2020, 28, 812–828. [Google Scholar] [CrossRef]
- Bradley, V.A.; Welch, J.L.; Dick, D.J. Visuospatial working memory in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 1989, 52, 1228–1235. [Google Scholar] [CrossRef] [PubMed]
- Beylergil, S.B.; Kilbane, C.; Shaikh, A.G.; Ghasia, F.F. Eye movements in Parkinson’s disease during visual search. J. Neurol. Sci. 2022, 440, 120299. [Google Scholar] [CrossRef] [PubMed]
- Fooken, J.; Patel, P.; Jones, C.B.; McKeown, M.J.; Spering, M. Preservation of Eye Movements in Parkinson’s Disease Is Stimulus- and Task-Specific. J. Neurosci. Off. J. Soc. Neurosci. 2022, 42, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Terao, Y.; Fukuda, H.; Hikosaka, O. What do eye movements tell us about patients with neurological disorders?—An introduction to saccade recording in the clinical setting. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2017, 93, 772–801. [Google Scholar] [CrossRef]
- Alexander, R.G.; Macknik, S.L.; Martinez-Conde, S. Microsaccade Characteristics in Neurological and Ophthalmic Disease. Front. Neurol. 2018, 9, 144. [Google Scholar] [CrossRef] [PubMed]
- McInnis, H. Microsaccades in Parkinson’s Disease. Master’s Thesis, Queen’s University at Kingston, Kingston, ON, Canada, 2014. [Google Scholar]
- Cameron, I.G.; Brien, D.C.; Links, K.; Robichaud, S.; Ryan, J.D.; Munoz, D.P.; Chow, T.W. Changes to saccade behaviors in Parkinson’s disease following dancing and observation of dancing. Front. Neurol. 2013, 4, 22. [Google Scholar] [CrossRef]
- Chan, F.; Armstrong, I.T.; Pari, G.; Riopelle, R.J.; Munoz, D.P. Deficits in saccadic eye-movement control in Parkinson’s disease. Neuropsychologia 2005, 43, 784–796. [Google Scholar] [CrossRef]
- Lu, Z.; Buchanan, T.; Kennard, C.; FitzGerald, J.J.; Antoniades, C.A. The effect of levodopa on saccades—Oxford Quantification in Parkinsonism study. Park. Relat. Disord. 2019, 68, 49–56. [Google Scholar] [CrossRef]
- Cools, R.; D’Esposito, M. Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol. Psychiatry 2011, 69, e113–e125. [Google Scholar] [CrossRef]
- Hunt, A.R.; Reuther, J.; Hilchey, M.D.; Klein, R.M. The Relationship Between Spatial Attention and Eye Movements. Curr. Top. Behav. Neurosci. 2019, 41, 255–278. [Google Scholar] [CrossRef]
- Brandolani, R.; Galletti, C.; Fattori, P.; Breveglieri, R.; Poletti, M. Distinct modulation of microsaccades in motor planning and covert attention. Sci. Rep. 2025, 15, 19580. [Google Scholar] [CrossRef]
- Van der Stigchel, S.; Hollingworth, A. Visuospatial Working Memory as a Fundamental Component of the Eye Movement System. Curr. Dir. Psychol. Sci. 2018, 27, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Rondina, R., 2nd; Curtiss, K.; Meltzer, J.A.; Barense, M.D.; Ryan, J.D. The organisation of spatial and temporal relations in memory. Memory 2017, 25, 436–449. [Google Scholar] [CrossRef]
- Hodgson, T.L.; Ezard, G.; Hermens, F. Eye Movements in Neuropsychological Tasks. Curr. Top. Behav. Neurosci. 2019, 41, 393–418. [Google Scholar] [CrossRef]
- Spagnolo, C.; Casalvieri, C.; Gambini, A. Different Processes for Graphical Recognition of Derivative of a Function: An Eye-Tracker Analysis. In Proceedings of the Ninth International Congress on Information and Communication Technology, ICICT 2024, London, UK, 19–22 February 2024; Yang, X.S., Sherratt, S., Dey, N., Joshi, A., Eds.; Lecture Notes in Networks and Systems. Springer: Singapore, 2024; Volume 1000. [Google Scholar] [CrossRef]
- Anselm, S.; Kelsey, M.; Andreas, O.; Kristina, R. Eye-tracking methodology in mathematics education research: A systematic literature review. Educ. Stud. Math. 2020, 104, 147–200. [Google Scholar] [CrossRef]
- Hafed, Z.; Clark, J. Microsaccades as an overt measure of covert attention shifts. Vis. Res. 2002, 42, 2533–2545. [Google Scholar] [CrossRef]
- Engbert, R.; Kliegl, R. Microsaccades uncover the orientation of covert attention. Vis. Res. 2003, 43, 1035–1045. [Google Scholar] [CrossRef]
- Meyberg, S.; Sinn, P.; Engbert, R.; Sommer, W. Revising the link between microsaccades and the spatial cueing of voluntary attention. Vis. Res. 2017, 133, 47–60. [Google Scholar] [CrossRef]
- de Vries, E.; van Ede, F. Microsaccades Track Location-Based Object Rehearsal in Visual Working Memory. eNeuro 2024, 11, ENEURO.0276-23.2023. [Google Scholar] [CrossRef]
- de Vries, E.; van Ede, F. Microsaccades reveal preserved spatial organisation in visual working memory despite decay in location-based rehearsal. Cognition 2025, 259, 106111. [Google Scholar] [CrossRef] [PubMed]
- van Ede, F.; Chekroud, S.R.; Nobre, A.C. Human gaze tracks attentional focusing in memorized visual space. Nat. Hum. Behav. 2019, 3, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Tal-Perry, N.; Yuval-Greenberg, S. Pre-target oculomotor inhibition reflects temporal orienting rather than certainty. Sci. Rep. 2020, 10, 21478. [Google Scholar] [CrossRef]
- Abeles, D.; Amit, R.; Tal-Perry, N.; Carrasco, M.; Yuval-Greenberg, S. Oculomotor inhibition precedes temporally expected auditory targets. Nat. Commun. 2020, 11, 3524. [Google Scholar] [CrossRef]
- Engbert, R. Computational modeling of collicular integration of perceptual responses and attention in microsaccades. J. Neurosci. Off. J. Soc. Neurosci. 2012, 32, 8035–8039. [Google Scholar] [CrossRef]
- Contadini-Wright, C.; Magami, K.; Mehta, N.; Chait, M. Pupil Dilation and Microsaccades Provide Complementary Insights into the Dynamics of Arousal and Instantaneous Attention during Effortful Listening. J. Neurosci. Off. J. Soc. Neurosci. 2023, 43, 4856–4866. [Google Scholar] [CrossRef]
- Dalmaso, M.; Castelli, L.; Scatturin, P.; Galfano, G. Working memory load modulates microsaccadic rate. J. Vis. 2017, 17, 6. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.; Sonderegger, A.; Krueger, E.; Meteier, Q.; Luethold, P.; Chavaillaz, A. The interplay between task difficulty and microsaccade rate: Evidence for the critical role of visual load. J. Eye Mov. Res. 2020, 13, 1–12. [Google Scholar] [CrossRef]
- Liu, B.; Alexopoulou, Z.S.; van Ede, F. Attentional shifts bias microsaccade direction but do not cause new microsaccades. Commun. Psychol. 2024, 2, 97. [Google Scholar] [CrossRef] [PubMed]
- Gaunt, J.T.; Bridgeman, B. Microsaccades and Visual-Spatial Working Memory. J. Eye Mov. Res. 2012, 5, 1–16. [Google Scholar] [CrossRef]
- Carter, B.T.; Luke, S.G. Best practices in eye tracking research. Int. J. Psychophysiol. Off. J. Int. Organ. Psychophysiol. 2020, 155, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Casalvieri, C.; Gambini, A.; Spagnolo, C.; Viola, G. The Concept of Derivatives Through Eye-Tracker Analysis. In Proceedings of the 15th International Conference on Computer Supported Education—CSEDU, Prague, Czech Republic, 21–23 April 2023; SciTePress: Setúbal, Portugal, 2023; Volume 2, pp. 378–385, ISBN 978-989-758-641-5. [Google Scholar] [CrossRef]
- Larsen, T.A.; Calne, S.; Calne, D.B. Assessment of Parkinson’s disease. Clin. Neuropharmacol. 1984, 7, 165–169. [Google Scholar] [CrossRef]
- Goetz, C.G.; Tilley, B.C.; Shaftman, S.R.; Stebbins, G.T.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stern, M.B.; Dodel, R.; et al. Movement Disorder Society UPDRS Revision Task Force Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov. Disord. Off. J. Mov. Disord. Soc. 2008, 23, 2129–2170. [Google Scholar] [CrossRef] [PubMed]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Otero-Millan, J.; Castro, J.L.A.; Macknik, S.L.; Martinez-Conde, S. Unsupervised clustering method to detect microsaccades. J. Vis. 2014, 14, 18. [Google Scholar] [CrossRef]
- Elidan, J.; Gay, I.; Lev, S. Square wave jerks--incidence, characteristic, and significance. J. Otolaryngol. 1984, 13, 375–381. [Google Scholar]
- Otero-Millan, J.; Schneider, R.; Leigh, R.J.; Macknik, S.L.; Martinez-Conde, S. Saccades during attempted fixation in parkinsonian disorders and recessive ataxia: From microsaccades to square-wave jerks. PLoS ONE 2013, 8, e58535. [Google Scholar] [CrossRef]
- Otero-Millan, J.; Serra, A.; Leigh, R.J.; Troncoso, X.G.; Macknik, S.L.; Martinez-Conde, S. Distinctive features of saccadic intrusions and microsaccades in progressive supranuclear palsy. J. Neurosci. Off. J. Soc. Neurosci. 2011, 31, 4379–4387. [Google Scholar] [CrossRef]
- Pinnock, R.A.; McGivern, R.C.; Forbes, R.; Gibson, J.M. An exploration of ocular fixation in Parkinson’s disease, multiple system atrophy and progressive supranuclear palsy. J. Neurol. 2010, 257, 533–539. [Google Scholar] [CrossRef]
- Beylergil, S.B.; Murray, J.; Noecker, A.M.; Gupta, P.; Kilbane, C.; McIntyre, C.C.; Shaikh, A.G.; Ghasia, F.F. Effects of subthalamic deep brain stimulation on fixational eye movements in Parkinson’s disease. J. Comput. Neurosci. 2021, 49, 345–356. [Google Scholar] [CrossRef]
- Hood, A.J.; Amador, S.C.; Cain, A.E.; Briand, K.A.; Al-Refai, A.H.; Schiess, M.C.; Sereno, A.B. Levodopa slows prosaccades and improves antisaccades: An eye movement study in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2007, 78, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, J.A.; Fletcher, W.A.; Lang, A.E.; Zackon, D.H. Smooth pursuit during dose-related on-off fluctuations in Parkinson’s disease. Neurology 1987, 37, 1389–1392. [Google Scholar] [CrossRef]
- Gitchel, G.T.; Wetzel, P.A.; Baron, M.S. Pervasive ocular tremor in patients with Parkinson disease. Arch. Neurol. 2012, 69, 1011–1017. [Google Scholar] [CrossRef]
- Dec-Ćwiek, M.; Tutaj, M.; Gracies, J.M.; Volkmann, J.; Rudzińska, M.; Słowik, A.; Szczudlik, A. Opposite effects of l-dopa and DBS-STN on saccadic eye movements in advanced Parkinson’s disease. Neurol. I Neurochir. Pol. 2017, 51, 354–360. [Google Scholar] [CrossRef]
- Rivaud-Péchoux, S.; Vermersch, A.I.; Gaymard, B.; Ploner, C.J.; Bejjani, B.P.; Damier, P.; Demeret, S.; Agid, Y.; Pierrot-Deseilligny, C. Improvement of memory guided saccades in parkinsonian patients by high frequency subthalamic nucleus stimulation. J. Neurol. Neurosurg. Psychiatry 2000, 68, 381–384. [Google Scholar] [CrossRef]
- Gaunt, J.T.; Bridgeman, B. Visual vs. Spatial Contributions to Microsaccades and Visual-Spatial Working Memory. J. Eye Mov. Res. 2014, 7, 1–14. [Google Scholar] [CrossRef]
- Tse, P.U.; Sheinberg, D.S.; Logothetis, N.K. The Distribution of Microsaccade Directions Need Not Reveal the Location of Attention: Reply to Rolfs, Engbert, and Kliegl. Psychol. Sci. 2004, 15, 708–710. [Google Scholar] [CrossRef]
- Hafed, Z.M.; Ignashchenkova, A. On the dissociation between microsaccade rate and direction after peripheral cues: Microsaccadic inhibition revisited. J. Neurosci. Off. J. Soc. Neurosci. 2013, 33, 16220–16235. [Google Scholar] [CrossRef] [PubMed]
- Rolfs, M.; Engbert, R.; Kliegl, R. Microsaccade orientation supports attentional enhancement opposite a peripheral cue: Commentary on Tse, Sheinberg, and Logothetis (2003). Psychol. Sci. 2004, 15, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Willeke, K.F.; Tian, X.; Buonocore, A.; Bellet, J.; Ramirez-Cardenas, A.; Hafed, Z.M. Memory-guided microsaccades. Nat. Commun. 2019, 10, 3710. [Google Scholar] [CrossRef] [PubMed]
- Pinkhardt, E.H.; Jürgens, R.; Lulé, D.; Heimrath, J.; Ludolph, A.C.; Becker, W.; Kassubek, J. Eye movement impairments in Parkinson’s disease: Possible role of extradopaminergic mechanisms. BMC Neurol. 2012, 12, 5. [Google Scholar] [CrossRef]




| Title 1 | OHC (n = 13) Mean (SD) | PD (n = 16) Mean (SD) | p (OHC/PD) |
|---|---|---|---|
| Age | 69.15 (9.09) | 61.06 (9.77) | 0.03 |
| Sex (f/m) | 7/6 | 7/9 | 0.22 |
| Education | 15.15 (3.13) | 16.9 (2.92) a | 0.18 |
| MoCA | 26.92 (1.38) | (OFF) 26.5 (2.12) a | 0.87 |
| (ON) 26.82 (2.14) b | 0.57 | ||
| HY | 1.73 (0.47) b | ||
| LEDD | 632.25 (455.78) c | ||
| UPDRS | (OFF) 28.5 (5.83) | ||
| (ON) 22.2 (6.46) d | |||
| Absolute error | 1.56 (0.30) | (OFF) 1.82 (0.52) | 0.04 |
| (ON) 1.83 (0.43) | 0.02 | ||
| Trials included * | 114 (9.68) | (OFF) 120.31 (7.02) | 0.05 |
| (ON) 117.31 (12.91) | 0.45 | ||
| SWJ (%) | 41.15 (10.74) | (OFF) 38.08 (12.52) | 0.50 |
| Broken fixation (%) | 14 (5.5) | (ON) 36.4 (14.92) | 0.34 |
| (OFF) 18.2 (10.34) | 0.20 | ||
| (ON) 21 (11.93) | 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farber, K.; Jiang, L.; Michiels, M.; Obeso, I.; Leung, H.-C. Microsaccade Activity During Visuospatial Working Memory in Early-Stage Parkinson’s Disease. J. Eye Mov. Res. 2025, 18, 46. https://doi.org/10.3390/jemr18050046
Farber K, Jiang L, Michiels M, Obeso I, Leung H-C. Microsaccade Activity During Visuospatial Working Memory in Early-Stage Parkinson’s Disease. Journal of Eye Movement Research. 2025; 18(5):46. https://doi.org/10.3390/jemr18050046
Chicago/Turabian StyleFarber, Katherine, Linjing Jiang, Mario Michiels, Ignacio Obeso, and Hoi-Chung Leung. 2025. "Microsaccade Activity During Visuospatial Working Memory in Early-Stage Parkinson’s Disease" Journal of Eye Movement Research 18, no. 5: 46. https://doi.org/10.3390/jemr18050046
APA StyleFarber, K., Jiang, L., Michiels, M., Obeso, I., & Leung, H.-C. (2025). Microsaccade Activity During Visuospatial Working Memory in Early-Stage Parkinson’s Disease. Journal of Eye Movement Research, 18(5), 46. https://doi.org/10.3390/jemr18050046






