Abstract
Although Severe Acute Respiratory Syndrome Coronavirus 2 infection (SARS-CoV-2) is primarily recognized as a respiratory disease, mounting evidence suggests that it may lead to neurological and cognitive impairments. The current study used three eye-tracking tasks (free-viewing, fixation, and smooth pursuit) to assess the oculomotor functions of mild infected cases over six months with symptomatic SARS-CoV-2 infected volunteers. Fifty symptomatic SARS-CoV-2 infected, and 24 self-reported healthy controls completed the eye-tracking tasks in an initial assessment. Then, 45, and 40 symptomatic SARS-CoV-2 infected completed the tasks at 2- and 6-months post-infection, respectively. In the initial assessment, symptomatic SARS-CoV-2 infected exhibited impairments in diverse eye movement metrics. Over the six months following infection, the infected reported overall improvement in health condition, except for self-perceived mental health. The eye movement patterns in the free-viewing task shifted toward a more focal processing mode and there was no significant improvement in fixation stability among the infected. A linear discriminant analysis shows that eye movement metrics could differentiate the infected from healthy controls with an accuracy of approximately 62%, even 6 months post-infection. These findings suggest that symptomatic SARS-CoV-2 infection may result in persistent impairments in oculomotor functions, and the employment of eye-tracking technology can offer valuable insights into both the immediate and long-term effects of SARS-CoV-2 infections. Future studies should employ a more balanced research design and leverage advanced machine-learning methods to comprehensively investigate the impact of SARS-CoV-2 infection on oculomotor functions.
1. Introduction
The COVID-19 pandemic has caused unprecedented disruptions to the global economy and inflicted over 770 million individuals worldwide (World Health Organization, n.d.). While SARS-CoV-2 is predominantly recognized as respiratory disease, mounting evidence also suggests nervous system disruption (Andalib et al., 2021; Desforges et al., 2019; Mao et al., 2020; Meinhardt et al., 2021; Miners et al., 2020; Nagu et al., 2021), with notable clinical symptoms like sensorineural hearing loss (Degen et al., 2020), dysautonomia (Eldokla et al., 2022), anosmia (Vaira et al., 2020), headache (Nazari et al., 2021), etc. Autopsies on patients who died with COVID-19 also revealed that the SARS-CoV-2 RNA persisted throughout the brain, as late as 230 days following symptom onset (Stein et al., 2022). Similarly, recent studies have shown that SARS-CoV-2 infections may affect cognitive functions (Becker et al., 2021; Ceban et al., 2022; He et al., 2023; Miners et al., 2020; Zhao et al., 2023). For instance, mild SARS-CoV-2 infected cases show cognitive deficits that may relate to neuroinflammation (De Paula et al., 2023).
Standardized neuropsychological tests are commonly used in clinical settings to assess cognitive functions (Kanchan et al., 2018). However, these tests require highly qualified individuals to administer and may fail to detect mild cognitive or neurological impairments that do not reveal themselves in observable symptoms or magnetic resonance imaging (MRI) scans, such as mild traumatic brain injuries (Hunt et al., 2016). While researchers and medical practitioners continue to search for alternative diagnostic methods, there has been a recent surge in the utilization of eye-tracking technologies for detecting neurological impairments (Levantini et al., 2020; McDonald et al., 2022; Tao et al., 2020), assessing cognitive deficits (Caldani et al., 2020; Panagiotidi et al., 2017), and evaluating treatment responses (Fletcher-Watson & Hampton, 2018; Mihara et al., 2023; Stafford et al., 2019; Vassallo & Douglas, 2021). Eye-tracking allows quick acquisition of objective and real-time analytics (Benson et al., 2012), and various eye-tracking tasks have been designed to capture eye movement metrics that help to reveal the neurological or psychological profile of an individual. For example, in a recent study, Zhang et al. (2022) tested depressed patients with fixation stability, antisaccade, and free-viewing tasks, and revealed noticeable differences in eye movement patterns between depressed participants and healthy controls. Hunfalvay et al. (2021) also discovered that smooth pursuit tasks hold promise for detecting deficits in oculomotor functions stemming from concussions.
As noted, SARS-CoV-2 infection has been shown to burden the nervous system and impair cognitive functions (e.g., Zhao et al., 2023). However, in cases with mild symptoms, the impact of SARS-CoV-2 infection may be subtle and therefore may not be detected using traditional neuropsychological tests. The oculomotor system is closely linked to higher cognitive functions such as attention and executive control (Van Ede et al., 2019), and oculomotor function abnormalities have been studied as potential markers or proxies for cognitive impairments in various neurological and neuropsychiatric conditions. The current study examined whether impairments in oculomotor functions exist in recovered symptomatic SARS-CoV-2 infected participants immediately after recovery and at two- and six-month follow-ups. While oculomotor function tasks cannot yet replace cognitive assessments, the results of the present study should provide insights into the short- and long-term neural and cognitive impact of SARS-CoV-2.
2. Methods
The research protocol reported in this paper was approved by a local ethics committee at the Center for Psychological Sciences, Zhejiang University. The experiments were carried out following the guidelines outlined in the Declaration of Helsinki, and all participants gave written informed consent before the assessments.
2.1. Participants
The present study recruited 53 volunteers with self-reported prior SARS-CoV-2 infection and 25 volunteers self-reported having no prior SARS-CoV-2 infection from Zhejiang University. Those with prior SARS-CoV-2 infection all reported SARS-CoV-2 symptoms (see Table 1). For convenience, we will refer to these two groups of participants as SARS-CoV-2 infected and controls, respectively. Three SARS-CoV-2 infected participants were excluded from the analysis: two had been infected several months ago, one due to equipment failure. One participant in the control group was excluded because he reported being on medication for depressive disorder. So, in the initial assessment, there were 50 SARS-CoV-2 infected (27 females; M = 24.98 years, SD = 3.86) and 24 controls (20 females; M = 23.92 years, SD = 2.73). Statistical analysis showed that the ages of these two groups did not differ, t(72) = 1.56, p = 0.125, Hedges’ g = 0.30. Among the SARS-CoV-2 infected, 45 (25 females/20 males, M = 25.07 years, SD = 3.89) returned to complete the 2-month follow-up assessment, and 40 (20 females/20 males; M = 24.90 years, SD = 4.07) returned to complete the 6-month follow-up assessment.

Table 1.
Clinical symptoms experienced by the SARS-CoV-2 infected.
All participants reported having normal or corrected-to-normal visual acuity and having no other vision conditions (e.g., color blindness, amblyopia, high astigmatism, strabismus, cataracts, glaucoma, floaters, etc.). They were also required to report psychological conditions (e.g., depression, mania, schizophrenia, etc.) to the experimenter if there were any. No ophthalmologic evaluation was performed on the participants.
2.2. Materials and Equipment
The participants completed three eye movement tasks in a quiet laboratory. The visual stimuli were presented against a gray background on a 21-inch ViewSonic LCD monitor. The refresh rate of this monitor was 144 Hz and its visible screen area measured 50° × 29.5° at a viewing distance of 57 cm (maintained with a chinrest). Stimulus presentation and eye-movement data recording were controlled by Python scripts running on a Windows 10 gaming laptop. Eye movement data were recorded at 1000 Hz with an EyeLink Portable Duo eye tracker (SR Research, Ottawa, Canada). The tracker was set to record binocular gaze data, but in rare occasions where only one eye could be reliably tracked, monocular gaze data was recorded. The typical tracking accuracy of this tracker was reported to be around 0.25–0.5° (SR Research Ltd., 2022).
2.3. Procedure and Design
The initial assessment was administered shortly after the Chinese government lifted COVID-19 restrictions in late 2022. All participants completed a fixation task, a smooth pursuit task, and a free-viewing task. Details of the three eye movement tasks (i.e., fixation, smooth pursuit, and free viewing) are presented below.
Fixation task. Each participant completed four trials in the fixation task, two with and two without visual distractors. Visual distractors were presented to elicit intrusive saccades, which help assess the ability to maintain sustained attention (e.g., Benson et al., 2012; Zhang et al., 2022). A driftcheck was performed at the beginning of each trial. During the drift-check, a target was presented at the center of the screen and the participant was asked to press an assigned button when looking at the target to validate the accuracy of the calibration model (SR Research Ltd., 2022). Following the drift-check, a fixation target (a white cross “+”) appeared at the center of the screen. In trials with distractors, a salient distractor (red disk; diameter = 0.44°) would appear at a random location 1.5° away from the fixation target. Participants were instructed to maintain their gaze on the fixation target and ignore the distractor. The visual distractor would show up on the screen at random intervals throughout a trial. Each trial lasted for 30 s, and the distractor would show up a total of 10 times. In trials without distractors, only the fixation target was presented at the screen center for 30 s.
Smooth pursuit task. Smooth pursuit tasks are commonly used for clinical assessment of neurological deficits (Kullmann et al., 2021). At the start of each trial, a drift-check target appeared 10° left or right of the screen center. Following a successful drift-check, a target stimulus (white disk; diameter = 0.69°) appeared at the same positions and began to move in a Lissajous pattern (frequency ratio = 3/4, amplitude = 10°). The participants were instructed to follow the target movement with their gaze. Each trial lasted for 48 s, and four trials were tested on each participant.
Free-viewing task. In the free-viewing task, the participants viewed 30 color pictures (31.38° × 18.11°) selected from publicly available datasets, HKU-IS (G. Li & Yu, 2015), DUST (Wang et al., 2017), and PASCAL-S (Y. Li et al., 2014). The task consisted of thirty trials. Each trial started with a drift-check, followed by a picture that was presented for 5 s. The participant was instructed to freely view the picture.
The overall quality of life (QoL) of the participants was also assessed with the 36-item Short Form (SF-36; McHorney et al., 1993; Ware & Sherbourne, 1992). The same cohort of SARS-CoV-2 infected participants were re-assessed, using the same testing protocol, two and six months after the initial assessment.
2.4. Data analysis
In the analysis, we first examined the immediate impact of SARS-CoV-2 infection by comparing the SARS-CoV-2 infected against the controls on various eye movement metrics. We then shifted focus to the SARS-CoV-2 infected and examined the evolving effect of SARS-CoV-2 infection on eye movement metrics over time. Lastly, linear discriminant analyses were performed to examine whether eye movement metrics can effectively differentiate individuals with a history of SARS-CoV-2 infection from controls.
Data analyses were performed in R 4.2.1 (R Core Team, 2022), and the effect size measure (Hedges’ g) reported in this paper was calculated with the esc package in R (Lüdecke, 2019). Repeated measure ANOVAs, when needed, were performed using the bruceR package in R (Bao, 2023). Significant main effects observed in the ANOVA were further examined with Bonferroni-corrected post-hoc comparisons. The t-tests reported in this study were corrected using a method recommended by Ruxton (2006). This approach involved an initial examination of the distribution of the dependent measure, followed by the performance of an unequal variance t-test on either ranked or unranked data, depending on whether the dependent measure exhibited a normal distribution or not. The scikit-learn library in python was applied to the linear discriminant analysis.
The present study recorded binocular gaze data, with a few exceptions where good binocular calibration could not be obtained (5, 5, and 8 instances in the smooth pursuit task, free-viewing task, and fixation task, respectively). Sample eye movement recordings from the three oculomotor tasks are presented in Figure 1. Various eye movement metrics that have been previously used in clinical studies were compared between the two groups (Benson et al., 2012). The eye movement metrics examined in all three tasks include fixation number, fixation duration (s), saccade number, saccade duration (ms), saccade amplitude (°), saccade average velocity (°/s), and saccade peak velocity (°/s). In the smooth pursuit task, we also examined velocity gain and root mean square error (RMSE), which reflect how closely the gaze followed the pursuit target. In the fixation task, we also examined two fixation stability metrics, i.e., bivariate contour ellipse area (BCEA) and RMSE. These eye movement metrics were extracted from the raw recordings using SR Data Viewer (version 4.3.1).
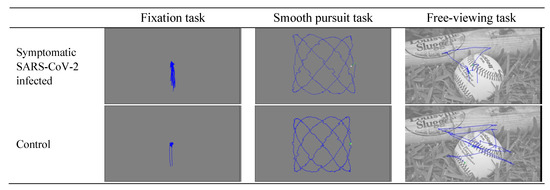
Figure 1.
Sample eye movement recordings from the three oculomotor tasks.
Saccades in the present study were identified with a velocity threshold of 30°/s and an acceleration threshold of 8000°/s2. It is important to note that blink saccades were excluded from the analysis. In the free viewing task, nearby fixations were merged based on an amplitude threshold of 1.0° and a duration threshold of 50 ms. Fixations flanking blinks were treated as separate ones, as the gaze position usually changes following blinks. The EyeLink event detection algorithm categorized smooth pursuit movements as “fixations”. In the smooth pursuit task, velocity gain was calculated individually for all fixations by dividing the average gaze angular velocity by the average target velocity and then aggregated for each trial.
3. Results
3.1. The Immediate and Evolving Impact of SARS-CoV-2 Infection
Quality of life ratings (SF-36). Both the SARS-CoV-2 infected and controls completed the SF-36 survey in the initial and follow-up assessments. The SF-36 domain scores of the three assessments are summarized in Table 2. In the initial assessment, the SARS-CoV-2 infected reported deterioration in several domains, including role limitation due to physical health, social functioning, bodily pain, role limitation due to emotional problems, and general health perceptions. Over the six months following the infection, the quality of life of the SARS-CoV-2 infected showed improvement across all SF-36 domains, except for self-perceived mental health, F = 0.08, p = 0.921.

Table 2.
SF-36 domain scores of the SARS-CoV-2 infected and controls.
Eye movement metrics. At the beginning of the recording session, participants were instructed to look at 9 fixation targets on the monitor sequentially to calibrate the tracker. No significant difference in the calibration result was observed between the controls (M = 0.37°, SD = 0.11) and the SARS-CoV-2 infected (M = 0.40°, SD = 0.17), p = 0.129. The eye movement metrics extracted from the tasks are summarized in Table 3.

Table 3.
Eye movement metrics extracted from three eye movement tasks.
In the initial assessment, a significant elevation in saccade amplitude, saccade average velocity, saccade peak velocity, and a fixation stability (RMSE), were observed among the SARS-CoV-2 infected in the fixation task, regardless of the presence of visual distractors. There was a decrease in fixation duration in the SARS-CoV-2 infected when no distractor was present. In the free-viewing task, the SARS-CoV-2 infected had fewer fixations than controls. The smooth pursuit task revealed no reliable difference between the two groups, all |t’s| ≤ 1.19, all p’s ≥ 0.243.
In the second-month follow-up (T1), the SARS-CoV-2 infected continued to exhibit slightly poorer fixation stability, as evidenced by marginally higher RMSE (with distractor) and log10(BCEA) (without distractor). Furthermore, there was a decrease in fixation duration (without distractor), coupled with an increase in saccade amplitude and average velocity (with and without distractor) among the SARS-CoV-2 infected in the fixation task. When compared to the control group, the SARS-CoV-2 infected also had a decrease in fixation number in the free-viewing task and an increase in velocity gain in the smooth pursuit task.
In the sixth-month follow-up (T2), there was a significant rise in saccade amplitude and average velocity, and a marginal increase in saccade peak velocity and RMSE among the SARS-CoV-2 infected in the fixation task (with distractor). There was a decrease in fixation and saccade number and an increase in fixation duration in the free-viewing task.
Over the six months following the infection ([T0 T1 T2] in Table 3), a significant increase in fixation duration, and decreases in fixation number, saccade amplitude, saccade average velocity, saccade peak velocity, and saccade number were observed among the SARS-CoV-2 infected in the free-viewing task. The smooth pursuit task, on the other hand, revealed an increase in velocity gain and a decrease in saccade peak velocity.
The saccade peak velocity to amplitude ratio (main sequence) was also examined. When comparing to the health controls, there was a decrease among the SARS-CoV-2 infected in the fixation task (with distractor) at T0 (p = 0.004), T1 (p = 0.033), and T2 (p = 0.029). Over the six months following infection, there was a change in peak velocity to amplitude ratio in the free-viewing task (p = 0.005). No other significant effect was observed in other tasks (all ps ≥ 0.176).
3.2. Linear discriminant analysis
We also investigated whether the eye movement metrics could effectively differentiate between the SARS-CoV-2 infected and controls with linear discriminant analyses (LDA). The participants included in this analysis include the controls who completed all tasks in the initial assessment (n = 23; 20 females/3 males; M = 24.00 years, SD = 2.76), the SARS-CoV-2 infected who completed all tasks in the initial assessments (n = 49; 26 females/23 males; M = 25.12 years, SD = 3.77), and the SARS-CoV-2 infected that completed all tasks in the 2-month (n = 44; 24 females/20 male; M = 25.14 years, SD = 3.91) and 6-month (n = 40; 20 females/20 males; M = 24.90 years, SD = 4.07) follow-up assessments.
LDA was performed on the eye movement metrics exacted from individual experimental tasks and all experimental tasks combined. When considering data from all three experimental tasks, the linear discriminant analysis demonstrated the ability to differentiate between SARS-CoV-2 infected and controls with an accuracy of 59.72%, 59.70%, and 61.90% at the initial assessment (T0), 2-month (T1), and 6-month (T2) follow-up assessments, respectively. When examining individual tasks, the fixation task with distractors yielded discriminative accuracies of 65.28%, 53.73%, and 50.79% at T0, T1, and T2, respectively. In the fixation task without distractors, the discriminant accuracies reached 63.89%, 64.18%, and 60.32% at T0, T1, and T2, respectively. With data from the free-viewing task, the discriminative accuracies were 56.94%, 61.19%, and 58.73% at T0, T1, and T2, respectively. Meanwhile, with data from the smooth pursuit task, the discriminative accuracies were 58.33%, 53.73%, and 53.97%.
4. Discussion
Previous studies have shown that the rich features derived from eye-tracking data can effectively distinguish clinical groups from healthy controls with exceptional accuracy (e.g., Benson et al., 2012; Zhang et al., 2022). In the current study, we investigated both the immediate and long-term impact of SARS-CoV-2 infection on oculomotor functions with three eye-tracking tasks.
4.1. The Immediate Impact of SARS-CoV-2 Infection
During the initial assessment, the SF-36 survey, and three eye movement tasks were administered to the SARS-CoV-2 infected and controls. We observed significant group differences in multiple eye movement metrics in the free-viewing and fixation tasks.
In the free-viewing task, the SARS-CoV-2 infected exhibited a marginal decrease in the number of fixations. The number of fixations during free viewing has been associated with several mental disorders (schizophrenia, depression, etc.; e.g., Benson et al., 2012; Holmqvist et al., 2011). Previous studies have also linked this metric with exploratory behaviors (e.g., Ohya et al., 2014). Few volunteers in the current study reported experiencing mental problems; the observed decline in fixation number among the SARS-CoV-2 infected is likely associated with impairments in exploratory eye movements.
In the fixation task, the SARS-CoV-2 infected exhibited an increase in saccade amplitude, saccade average velocity, saccade peak velocity, and RMSE, regardless of the presence of visual distractors. Furthermore, there was a marginal increase in the number of saccades. Saccades that occur during a prolonged fixation are also known as intrusive saccades and have been associated with attention-deficit/hyperactivity disorder (e.g., ADHD; Falck-Ytter et al., 2020). These observations suggest that the SARS-CoV-2 infected may suffer from impairments in attention and executive function to some extent.
Besides, the SARS-CoV-2 infected participants exhibited deteriorated health conditions in several SF-36 domains when compared to the healthy controls. These findings were not surprising, considering that the SARS-CoV-2 infected were surveyed soon after their recovery, and most of them had reported experiencing one or more physical symptoms associated with the infection (see Table 1 for details).
4.2. Long-Lasting Oculomotor Impairments Due to SARS-CoV-2 Infection
Following the initial assessment, most of the SARS-CoV-2 infected returned to the lab to complete the 2- and 6-month follow-up assessments. Over the six months following the SARS-CoV-2 infection, the SARS-CoV-2 infected showed overall improvements in health conditions across all SF-36 domains, except for general mental health. In the eye movement tasks, we observed an increase in fixation duration, coupled with a decrease in fixation number, saccade amplitude, saccade average/peak velocity, and saccade number among the SARS-CoV-2 infected in the free-viewing task. These observations suggest that the SARS-CoV-2 infected participants may have adopted a relatively focal processing mode (Ito et al., 2017), as previously observed in participants with mental disorders, potentially indicating cognitive dysfunctions (Zhang et al., 2022). In the smooth pursuit task, there was an increase in velocity gain and a decrease in saccade peak velocity. The linear discriminant analysis further revealed that eye movement metrics extracted from all three tasks could differentiate between the SARS-CoV-2 infected and controls with accuracies of 59.72%, 59.70%, and 61.90% at the initial, 2-month, and 6-month assessments, respectively.
The above observations are consistent with recent studies revealing neurological and cognitive impairments following SARS-CoV-2 infection (e.g., Zhao et al., 2023), suggesting persistent alterations in oculomotor functions among the SARS-CoV-2 infected. The mechanisms that associate SARS-CoV-2 infection and cognition and brain health are unclear, but possible candidates may include cerebrovascular factors, dysregulated autoimmunity and neuroinflammation, direct viral invasion of the central nervous system, and psychological factors (Zhao et al., 2023).
The reversibility of oculomotor impairments as a sequela of SARS-CoV-2 infection remains to be substantiated by further long-term research evidence.
4.3. Limitations of the Current Study
It should be noted that previous researchers have found a series of ocular anomalies potentially arising from SARS-CoV-2 infection, including, retinal changes (Invernizzi et al., 2020), ocular motor nerve palsy (Jeong et al., 2022; Wei et al., 2020), and visuo-constructive deficit (De Paula et al., 2023). The current study did not assess these ocular anomalies due to a lack of resources. However, ocular anomalies due to SARS-CoV-2 infection are rare and last for a relatively short period (Al-Sharif et al., 2021); they are unlikely to confound the present findings.
The dysfunctions and complications resulting from SARS-CoV-2 infection could endure for at least six months among certain discharged patients (The Lancet, 2020). Several studies have also suggested that individuals may even develop new-onset long COVID-19 symptoms several months after their initial recovery (Soriano et al., 2022). In the context of the present finding, previous research has highlighted the prevalence of cognitive dysfunctions and the prolonged recovery process among participants infected with or suspected of being infected with SARS-CoV-2 (Davis et al., 2021). We cannot rule out the possibility of reinfection with SARS-CoV-2 during the present study, as antibody levels tend to decrease over time, increasing the likelihood of reinfection (Ye, 2023). However, the current observation of persistent oculomotor dysfunctions is more likely attributable to long COVID-19, a phenomenon where individuals may experience persistent symptoms following the initial SARS-CoV-2 infection (Spudich & Nath, 2022).
The relatively small and unbalanced sample may have contributed to the relatively low accuracy of the linear discriminant analyses. It is also possible that the eye movement metrics examined in the present study might not be sufficiently sensitive to discern the effects of SARS-CoV-2 infection. As such, caution is warranted when interpreting the results of the linear discriminant analysis. To address these limitations in future investigations, it is advisable to explore more advanced machine learning techniques, such as support vector machines.
Preliminary findings indicate that integrating a support vector machine (SVM) with principal component analysis (PCA) yields classification accuracies of 81.94%, 73.13%, and 74.19% at T0, T1, and T2, respectively.
One methodological limitation of this study is the lack of follow-up on the control group, as most of those in the control group were later infected during the surge of infections following the lift of COVID-19 restrictions. The present study also does not have a balanced design because there was a scarcity of uninfected volunteers who were willing to participate in the initial assessment.
5. Conclusions
The current research found diverse deficits in eye movement metrics immediately following SARS-CoV-2 infection, persisting at 2 and 6 months later. A linear discriminant analysis shows that eye movement metrics could differentiate individuals with a history of SARS-CoV-2 infection from healthy controls with an accuracy of approximately 62%, even 6 months post-infection. The present findings suggest that symptomatic SARS-CoV-2 infection may result in impairments in oculomotor functions, and the employment of eye-tracking technology can offer valuable insights into both the immediate and long-term effects of SARS-CoV-2 infection.
Ethics and declaration of conflict
All authors declare that the contents of the article are in agreement with the ethics described in http://biblio.unibe.ch/portale/elibrary/BOP/jemr/ethics.html and that there is no conflict of interest regarding the publication of this paper.
Acknowledgments
We are grateful to the anonymous reviewers for their constructive comments on an early version of this study. Preliminary results of the initial assessment were presented as a poster at the 2023 Annual Meeting of the General and Experimental Psychology Division of the Chinese Psychological Society.
References
- Al-Sharif, E., D. Strianese, N. H. AlMadhi, A. D’Aponte, R. dell’Omo, R. Di Benedetto, and C. Costagliola. 2021. Ocular tropism of coronavirus (CoVs): A comparison of the interaction between the animal-to-human transmitted coronaviruses (SARS-CoV-1, SARS-CoV-2, MERS-CoV, CoV-229E, NL63, OC43, HKU1) and the eye. International Ophthalmology 41, 1: 349–362. [Google Scholar] [CrossRef] [PubMed]
- Andalib, S., J. Biller, M. Di Napoli, N. Moghimi, L. D. McCullough, C. A. Rubinos, C. O’Hana Nobleza, M. R. Azarpazhooh, L. Catanese, I. Elicer, M. Jafari, F. Liberati, C. Camejo, M. Torbey, and A. A. Divani. 2021. Peripheral nervous system manifestations associated with COVID-19. Current Neurology and Neuroscience Reports 21, 3: 9. [Google Scholar] [CrossRef]
- Bao, H.-W.-S. 2023. bruceR: Broadly Useful Convenient and Efficient R Functions (2023.9) [Computer software. Available online: https://cran.r-project.org/web/packages/bruceR/index.html.
- Becker, J. H., J. J. Lin, M. Doernberg, K. Stone, A. Navis, J. R. Festa, and J. P. Wisnivesky. 2021. Assessment of cognitive function in patients after COVID-19 infection. JAMA Network Open 4, 10: e2130645. [Google Scholar] [CrossRef] [PubMed]
- Benson, P. J., S. A. Beedie, E. Shephard, I. Giegling, D. Rujescu, and D. St. Clair. 2012. Simple viewing tests can detect eye movement abnormalities that distinguish schizophrenia cases from controls with exceptional accuracy. Biological Psychiatry 72, 9: 716–724. [Google Scholar] [CrossRef] [PubMed]
- Caldani, S., C. L. Gerard, H. Peyre, and M. P. Bucci. 2020. Pursuit eye movements in dyslexic children: Evidence for an immaturity of brain oculomotor structures? Journal of Eye Movement Research 13, 1. [Google Scholar] [CrossRef]
- Ceban, F., S. Ling, L. M. W. Lui, Y. Lee, H. Gill, K. M. Teopiz, N. B. Rodrigues, M. Subramaniapillai, J. D. Di Vincenzo, B. Cao, K. Lin, R. B. Mansur, R. C. Ho, J. D. Rosenblat, K. W. Miskowiak, M. Vinberg, V. Maletic, and R. S. McIntyre. 2022. Fatigue and cognitive impairment in post-COVID-19 syndrome: A systematic review and meta-analysis. Brain, Behavior, and Immunity 101: 93–135. [Google Scholar] [CrossRef]
- Davis, H. E., G. S. Assaf, L. McCorkell, H. Wei, R. J. Low, Y. Re’em, S. Redfield, J. P. Austin, and A. Akrami. 2021. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. eClinicalMedicine 38: 101019. [Google Scholar] [CrossRef] [PubMed]
- De Paula, J. J., R. E. R. P. Paiva, N. G. Souza-Silva, D. V. Rosa, F. L. D. S. Duran, R. S. Coimbra, D. D. S. Costa, P. R. Dutenhefner, H. S. D. Oliveira, S. T. Camargos, H. M. M. Vasconcelos, N. De Oliveira Carvalho, J. B. Da Silva, M. B. Silveira, C. Malamut, D. M. Oliveira, L. C. Molinari, D. B. De Oliveira, J. N. Januário, and M. A. Romano-Silva. 2023. Selective visuoconstructional impairment following mild COVID-19 with inflammatory and neuroimaging correlation findings. Molecular Psychiatry 28, 2: 553–563. [Google Scholar] [CrossRef]
- Degen, C., T. Lenarz, and K. Willenborg. 2020. Acute profound sensorineural hearing loss after COVID-19 pneumonia. Mayo Clinic Proceedings 95, 8: 1801–1803. [Google Scholar] [CrossRef]
- Desforges, M., A. Le Coupanec, P. Dubeau, A. Bourgouin, L. Lajoie, M. Dubé, and P. J. Talbot. 2019. Human coronaviruses and other respiratory viruses: Underestimated opportunistic pathogens of the central nervous system? Viruses 12, 1: 14. [Google Scholar] [CrossRef]
- Eldokla, A. M., A. A. Mohamed-Hussein, A. M. Fouad, M. G. Abdelnaser, S. T. Ali, N. A. Makhlouf, I. G. Sayed, H. A. Makhlouf, J. Shah, and H. Aiash. 2022. Prevalence and patterns of symptoms of dysautonomia in patients with long COVID syndrome: A cross-sectional study. Annals of Clinical and Translational Neurology 9, 6: 778–785. [Google Scholar] [CrossRef] [PubMed]
- Falck-Ytter, T., E. Pettersson, S. Bölte, B. D’Onofrio, P. Lichtenstein, and D. P. Kennedy. 2020. Difficulties maintaining prolonged fixation and attention-deficit/hyperactivity symptoms share genetic influences in childhood. Psychiatry Research 293: 113384. [Google Scholar] [CrossRef]
- Fletcher-Watson, S., and S. Hampton. 2018. The potential of eye-tracking as a sensitive measure of behavioural change in response to intervention. Scientific Reports 8, 1: 14715. [Google Scholar] [CrossRef] [PubMed]
- He, D., M. Yuan, W. Dang, L. Bai, R. Yang, J. Wang, Y. Ma, B. Liu, S. Liu, S. Zhang, X. Liao, and W. Zhang. 2023. Long term neuropsychiatric consequences in COVID-19 survivors: Cognitive impairment and inflammatory underpinnings fifteen months after discharge. Asian Journal of Psychiatry 80: 103409. [Google Scholar] [CrossRef]
- Holmqvist, K., M. Nyström, R. Andersson, R. Dewhurst, H. Jarodzka, and J. van de Weijer. 2011. Eye tracking: A comprehensive guide to methods and measures, 1st ed. Oxford University Press. [Google Scholar]
- Hunfalvay, M., N. P. Murray, R. Mani, and F. R. Carrick. 2021. Smooth pursuit eye movements as a biomarker for mild concussion within 7-days of injury. Brain Injury 35, 14: 1682–1689. [Google Scholar] [CrossRef]
- Hunt, A. W., K. Mah, N. Reed, L. Engel, and M. Keightley. 2016. Oculomotor-based vision assessment in mild traumatic brain injury: A systematic review. Journal of Head Trauma Rehabilitation 31, 4: 252–261. [Google Scholar] [CrossRef]
- Invernizzi, A., A. Torre, S. Parrulli, F. Zicarelli, M. Schiuma, V. Colombo, A. Giacomelli, M. Cigada, L. Milazzo, A. Ridolfo, I. Faggion, L. Cordier, M. Oldani, S. Marini, P. Villa, G. Rizzardini, M. Galli, S. Antinori, G. Staurenghi, and L. Meroni. 2020. Retinal findings in patients with COVID-19: Results from the SERPICO-19 study. EClinicalMedicine 27: 100550. [Google Scholar] [CrossRef]
- Ito, J., Y. Yamane, M. Suzuki, P. Maldonado, I. Fujita, H. Tamura, and S. Grün. 2017. Switch from ambient to focal processing mode explains the dynamics of free viewing eye movements. Scientific Reports 7, 1: 1082. [Google Scholar] [CrossRef]
- Jeong, G. U., H.-J. Kwon, W. H. Ng, X. Liu, H. W. Moon, G. Y. Yoon, H. J. Shin, I.-C. Lee, Z. L. Ling, A. G. Spiteri, N. J. C. King, A. Taylor, J. S. Chae, C. Kim, D.-G. Ahn, K.-D. Kim, Y. B. Ryu, S.-J. Kim, S. Mahalingam, and Y.-C. Kwon. 2022. Ocular tropism of SARS-CoV-2 in animal models with retinal inflammation via neuronal invasion following intranasal inoculation. Nature Communications 13, 1: 7675. [Google Scholar] [CrossRef]
- Kanchan, A., A. Singh, N. Khan, M. Jahan, R. Raman, and T. Sathyanarayana Rao. 2018. Impact of neuropsychological rehabilitation on activities of daily living and community reintegration of patients with traumatic brain injury. Indian Journal of Psychiatry 60, 1: 38. [Google Scholar] [CrossRef]
- Kullmann, A., R. C. Ashmore, A. Braverman, C. Mazur, H. Snapp, E. Williams, M. Szczupak, S. Murphy, K. Marshall, J. Crawford, C. D. Balaban, M. Hoffer, and A. Kiderman. 2021. Portable eye-tracking as a reliable assessment of oculomotor, cognitive and reaction time function: Normative data for 18–45 year old. PLoS ONE 16, 11: e0260351. [Google Scholar] [CrossRef]
- Levantini, V., P. Muratori, E. Inguaggiato, G. Masi, A. Milone, E. Valente, A. Tonacci, and L. Billeci. 2020. Eyes are the window to the mind: Eye-tracking technology as a novel approach to study clinical characteristics of ADHD. Psychiatry Research 290: 113135. [Google Scholar] [CrossRef]
- Li, G., and Y. Yu. 2015. Visual saliency based on multiscale deep features. 2015 IEEE Conference on Computer Vision and Pattern Recognition (CVPR); pp. 5455–5463. [Google Scholar] [CrossRef]
- Li, Y., X. Hou, C. Koch, J. M. Rehg, and A. L. Yuille. 2014. The secrets of salient object segmentation. 2014 IEEE Conference on Computer Vision and Pattern Recognition; pp. 280–287. [Google Scholar] [CrossRef]
- Lüdecke, D. 2019. esc: Effect Size Computation for Meta Analysis (0.5.1) [Computer software. Available online: https://cran.r-project.org/web/packages/esc/index.html.
- Mao, L., H. Jin, M. Wang, Y. Hu, S. Chen, Q. He, J. Chang, C. Hong, Y. Zhou, D. Wang, X. Miao, Y. Li, and B. Hu. 2020. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurology 77, 6: 683. [Google Scholar] [CrossRef]
- McDonald, M. A., S. J. Holdsworth, and H. V. Danesh-Meyer. 2022. Eye movements in mild traumatic brain injury: Ocular biomarkers. Journal of Eye Movement Research 15, 2. [Google Scholar] [CrossRef]
- McHorney, C. A., J. E. Ware, and A. E. Raczek. 1993. The MOS 36-item short-form health survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Medical Care 31, 3: 247–263. [Google Scholar] [CrossRef]
- Meinhardt, J., J. Radke, C. Dittmayer, J. Franz, C. Thomas, R. Mothes, M. Laue, J. Schneider, S. Brünink, S. Greuel, M. Lehmann, O. Hassan, T. Aschman, E. Schumann, R. L. Chua, C. Conrad, R. Eils, W. Stenzel, M. Windgassen, and F. L. Heppner. 2021. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nature Neuroscience 24, 2: 168–175. [Google Scholar] [CrossRef] [PubMed]
- Mihara, M., A. Hayashi, K. Kakeue, and R. Tamura. 2023. Changes in saccadic eye movement and smooth pursuit gain in patients with acquired comitant esotropia after strabismus surgery. Journal of Eye Movement Research 16, 4. [Google Scholar] [CrossRef]
- Miners, S., P. G. Kehoe, and S. Love. 2020. Cognitive impact of COVID-19: Looking beyond the short term. Alzheimer’s Research & Therapy 12, 1: 170. [Google Scholar] [CrossRef]
- Nagu, P., A. Parashar, T. Behl, and V. Mehta. 2021. CNS implications of COVID-19: A comprehensive review. Reviews in the Neurosciences 32, 2: 219–234. [Google Scholar] [CrossRef]
- Nazari, S., A. Azari Jafari, S. Mirmoeeni, S. Sadeghian, M. E. Heidari, S. Sadeghian, F. Assarzadegan, S. M. Puormand, H. Ebadi, D. Fathi, and S. Dalvand. 2021. Central nervous system manifestations in COVID-19 patients: A systematic review and meta-analysis. Brain and Behavior 11, 5: e02025. [Google Scholar] [CrossRef]
- Ohya, T., K. Morita, Y. Yamashita, C. Egami, Y. Ishii, S. Nagamitsu, and T. Matsuishi. 2014. Impaired exploratory eye movements in children with Asperger’s syndrome. Brain and Development 36, 3: 241–247. [Google Scholar] [CrossRef] [PubMed]
- Panagiotidi, M., P. Overton, and T. Stafford. 2017. Increased microsaccade rate in individuals with ADHD traits. Journal of Eye Movement Research 10, 1. [Google Scholar] [CrossRef]
- R Core Team. 2022. R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria: URL: https://www.R-project.org/.
- Ruxton, G. D. 2006. The unequal variance t-test is an underused alternative to Student’s t-test and the Mann–Whitney U test. Behavioral Ecology 17, 4: 688–690. [Google Scholar] [CrossRef]
- Soriano, J. B., S. Murthy, J. C. Marshall, P. Relan, and J. V. Diaz. 2022. A clinical case definition of post-COVID-19 condition by a Delphi consensus. The Lancet Infectious Diseases 22, 4: e102–e107. [Google Scholar] [CrossRef]
- Spudich, S., and A. Nath. 2022. Nervous system consequences of COVID-19. Science 375, 6578: 267–269. [Google Scholar] [CrossRef]
- SR Research Ltd. 2022. EyeLink® Portable Duo User Manual (version 1.0.9). Oakville, Ontario: SR Research Ltd. [Google Scholar]
- Stafford, T., P. G. Overton, and E. Hampsey. 2019. Can microsaccade rate predict drug response? Journal of Eye Movement Research 12, 6. [Google Scholar] [CrossRef]
- Stein, S. R., S. C. Ramelli, A. Grazioli, J.-Y. Chung, M. Singh, C. K. Yinda, C. W. Winkler, J. Sun, J. M. Dickey, K. Ylaya, S. H. Ko, A. P. Platt, P. D. Burbelo, M. Quezado, S. Pittaluga, M. Purcell, V. J. Munster, F. Belinky, M. J. Ramos-Benitez, and D. S. Chertow. 2022. SARS-CoV-2 infection and persistence in the human body and brain at autopsy. Nature 612, 7941: 758–763. [Google Scholar] [CrossRef]
- Tao, L., Q. Wang, D. Liu, J. Wang, Z. Zhu, and L. Feng. 2020. Eye tracking metrics to screen and assess cognitive impairment in patients with neurological disorders. Neurological Sciences 41, 7: 1697–1704. [Google Scholar] [CrossRef] [PubMed]
- The Lancet. 2020. Facing up to long COVID. The Lancet 396, 10266: 1861. [Google Scholar] [CrossRef]
- Vaira, L. A., G. Salzano, G. Deiana, and G. De Riu. 2020. Anosmia and ageusia: Common findings in covid-19 patients. The Laryngoscope 130, 7: 1787. [Google Scholar] [CrossRef]
- Van Ede, F., S. R. Chekroud, and A. C. Nobre. 2019. Human gaze tracks attentional focusing in memorized visual space. Nature Human Behaviour 3, 5: 462–470. [Google Scholar] [CrossRef]
- Vassallo, S., and J. Douglas. 2021. Visual scanpath training to emotional faces following severe traumatic brain injury: A single case design. Journal of Eye Movement Research 14, 4. [Google Scholar] [CrossRef]
- Wang, L., H. Lu, Y. Wang, M. Feng, D. Wang, B. Yin, and X. Ruan. 2017. Learning to detect salient objects with image-level supervision. 2017 IEEE Conference on Computer Vision and Pattern Recognition (CVPR); pp. 3796–3805. [Google Scholar] [CrossRef]
- Ware, J. E., Jr., and C. D. Sherbourne. 1992. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical Care 30, 6: 473–483. [Google Scholar] [PubMed]
- Wei, H., H. Yin, M. Huang, and Z. Guo. 2020. The 2019 novel coronavirus pneumonia with onset of oculomotor nerve palsy: A case study. Journal of Neurology 267, 5: 1550–1553. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. n.d.WHO Coronavirus (COVID-19) Dashboard. Retrieved October 12, 2023, from https://covid19.who.int.
- Ye, Y. 2023. China’s rolling covid waves could hit every six months. Nature 618, 7965: 442–443. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D., X. Liu, L. Xu, Y. Li, Y. Xu, M. Xia, Z. Qian, Y. Tang, Z. Liu, T. Chen, H. Liu, T. Zhang, and J. Wang. 2022. Effective differentiation between depressed patients and controls using discriminative eye movement features. Journal of Affective Disorders 307: 237–243. [Google Scholar] [CrossRef]
- Zhao, S., S. Toniolo, A. Hampshire, and M. Husain. 2023. Effects of COVID-19 on cognition and brain health. Trends in cognitive sciences 27, 11: 1053–1067. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
This article is licensed under a Creative Commons Attribution 4.0 International license (https://creativecommons.org/licenses/by/4.0/).