Non-goal-Driven Eye Movement After Visual Search Task
Abstract
:Introduction
Methods
Participants
Apparatus
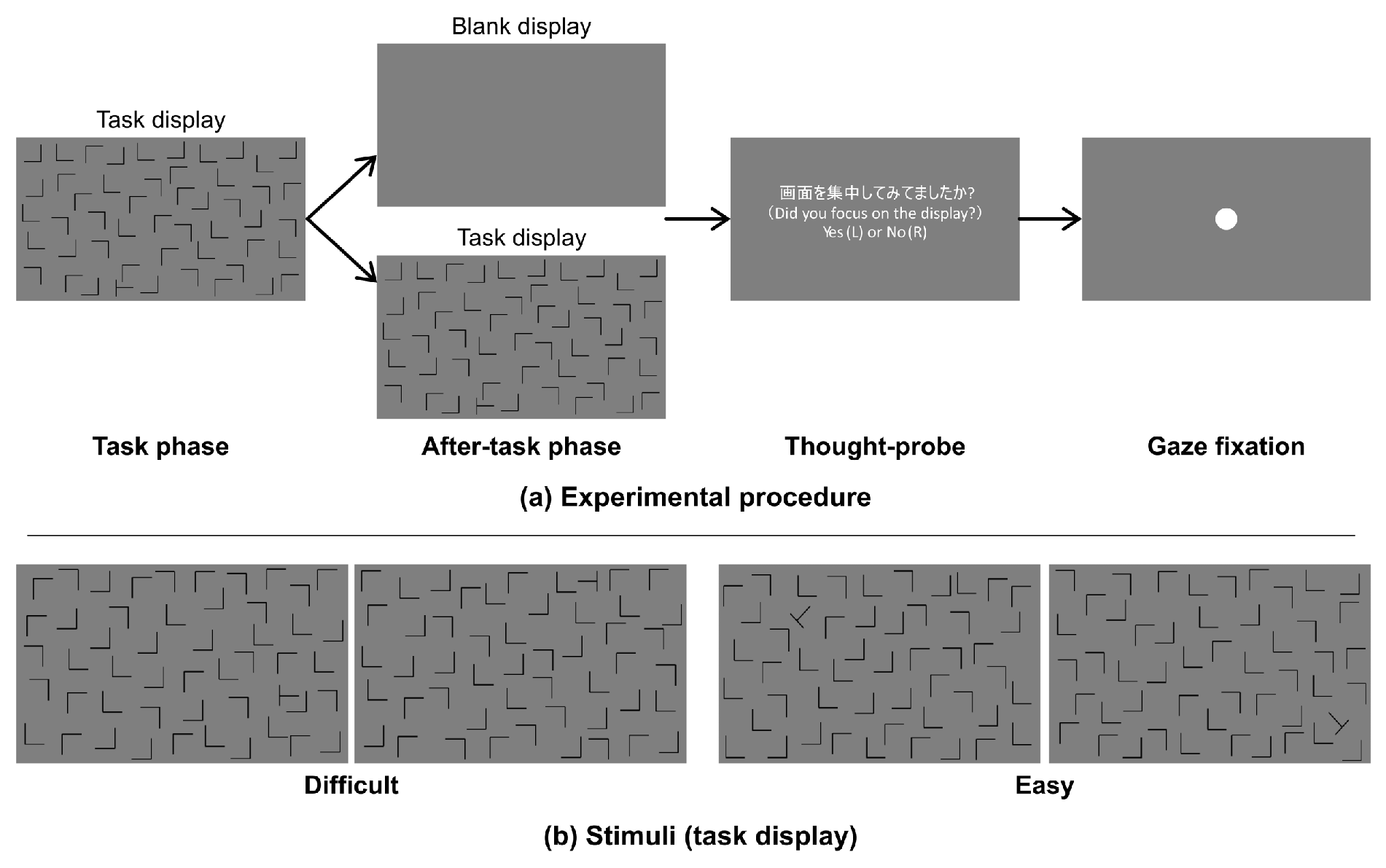
Procedure
Materials
Analysis
- Eye movement analysis
- 2.
- Saccade frequency
- 3.
- Gaze-shift pattern
- 4.
- Gaze distribution
Results
Accuracy and mean reaction time
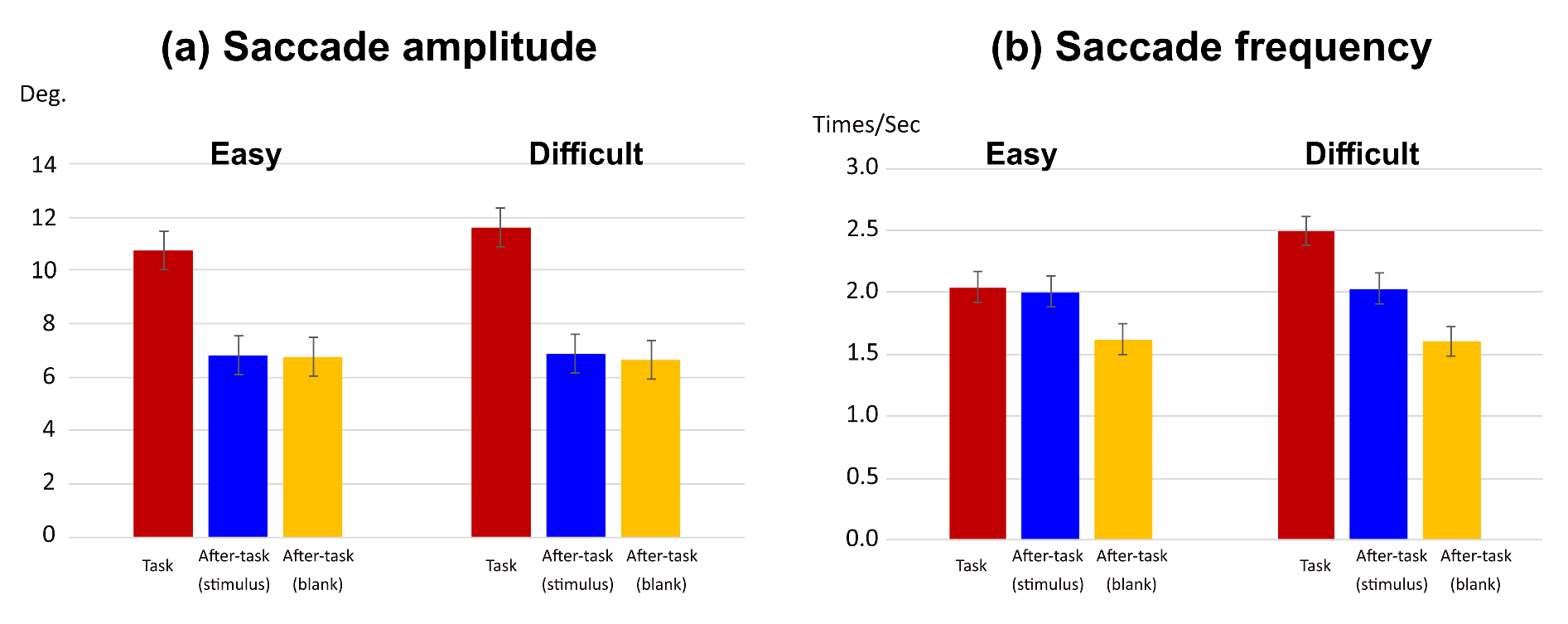
Gaze features as function of phase and task difficulty
Gaze shift angle
Spatial distribution of fixation position
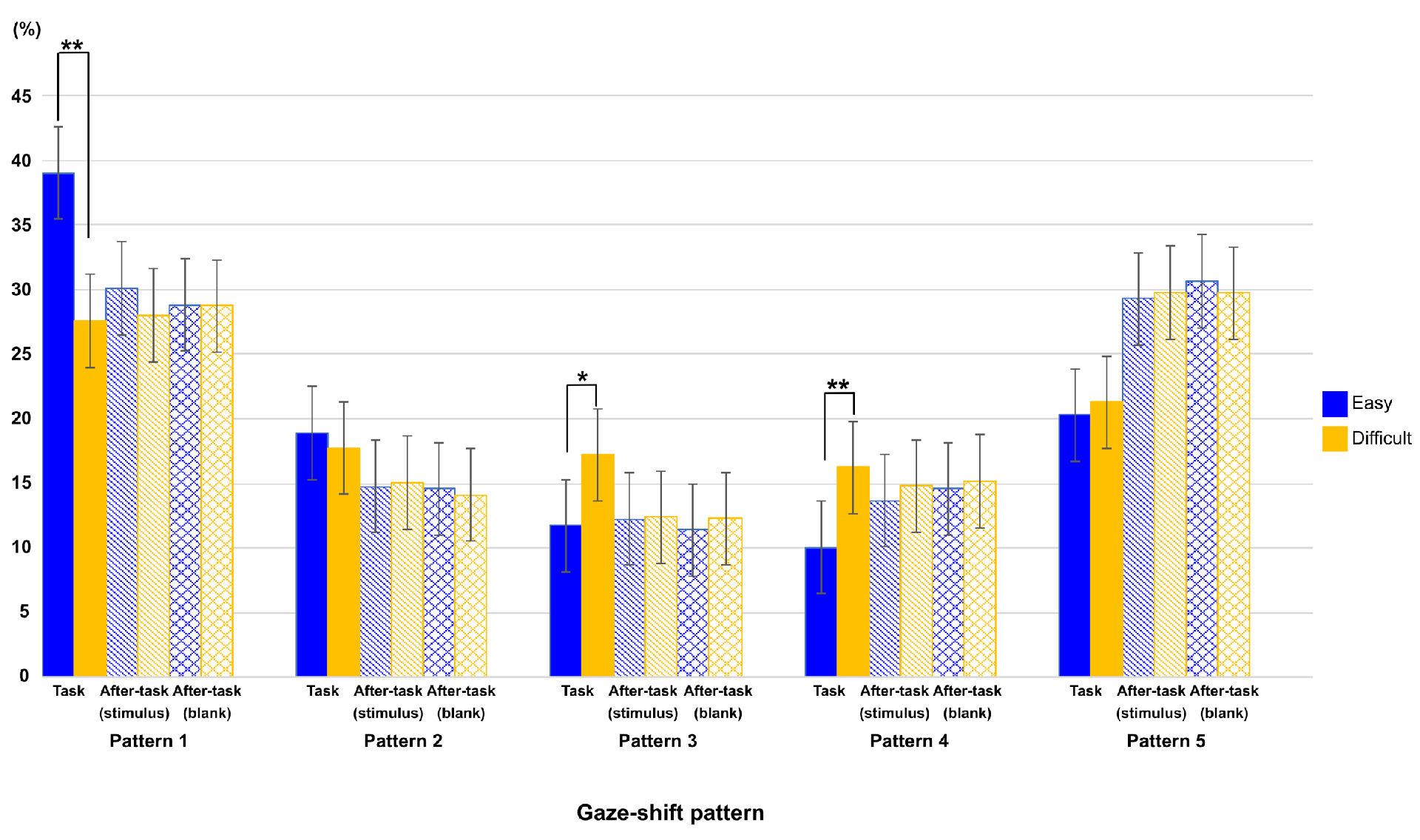
Gaze-shift patterns regarding phase and task difficulty
Discussion
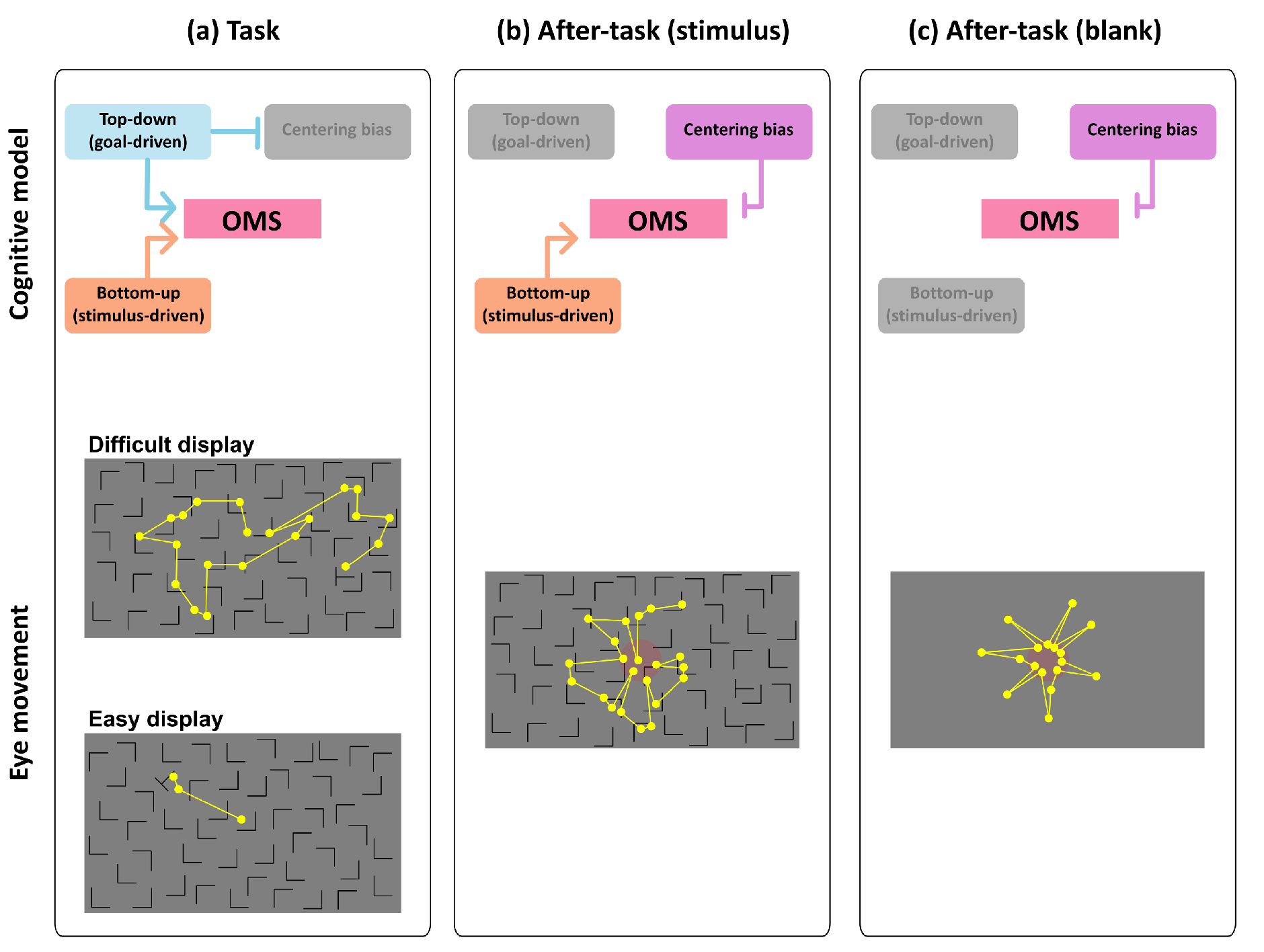
Differences between goal-driven and non-goal-driven eye movements
Aftereffects of task difficulty of preceding task on non-goal-driven eye movements
Gaze-shift-pattern model among phases and difficulties
Limitation
Ethics and Conflict of Interest
References
- Bahill, A. T., M. R. Clark, and L. Stark. 1975. Dynamic overshoot in saccadic eye movements is caused by neurological control signal reversals. Experimental neurology 48, 1: 107–122. [Google Scholar] [CrossRef]
- Bahill, A. T., and B. T. Troost. 1979. Types of saccadic eye movements. Neurology 29, 8: 1150–1152. [Google Scholar] [CrossRef] [PubMed]
- Beattie, G. W., and P. J. Barnard. 1979. The temporal structure of natural telephone conversations (directory enquiry calls). [Google Scholar] [CrossRef]
- Becker, W., and A. F. Fuchs. 1969. Further properties of the human saccadic system: eye movements and correction saccades with and without visual fixation points. Vision research 9, 10: 1247–1258. [Google Scholar] [CrossRef]
- Brien, D. C., B. D. Corneil, J. H. Fecteau, A. H. Bell, and D. P. Munoz. 2010. The behavioural and neurophysiological modulation of microsaccades in monkeys. Journal of Eye Movement Research 3, 2. [Google Scholar] [CrossRef]
- Bulling, A., J. A. Ward, H. Gellersen, and G. Tröster. 2010. Eye movement analysis for activity recognition using electrooculography. IEEE transactions on pattern analysis and machine intelligence 33, 4: 741–753. [Google Scholar] [CrossRef]
- Couronné, T., A. Guérin-Dugué, M. Dubois, P. Faye, and C. Marendaz. 2010. A statistical mixture method to reveal bottom-up and top-down factors guiding the eye-movements. Journal of Eye Movement Research 3, 2. [Google Scholar] [CrossRef]
- Duncan, J., and G. W. Humphreys. 1989. Visual search and stimulus similarity. Psychological review 96, 3: 433. [Google Scholar] [CrossRef] [PubMed]
- Ehrlichman, H., and J. Barrett. 1983. ‘Random’saccadic eye movements during verbal-linguistic and visualimaginal tasks. Acta psychologica 53, 1: 9–26. [Google Scholar] [CrossRef]
- Findlay, J. M. 1997. Saccade target selection during visual search. Vision research 37, 5: 617–631. [Google Scholar] [CrossRef]
- Greene, H. H., and J. M. Brown. 2017. Where did I come from? Where am I going? Functional differences in visual search fixation duration. Journal of Eye Movement Research 10, 1. [Google Scholar] [CrossRef]
- Groner, R., and M. T. Groner. 1989. Attention and eye movement control: an overview. European archives of psychiatry and neurological sciences 239, 1: 9–16. [Google Scholar] [CrossRef]
- Jang, D., I. Yang, and S. Kim. 2020. Detecting mindwandering from eye movement and oculomotor data during learning video lecture. Education Sciences 10, 3: 51. [Google Scholar] [CrossRef]
- Jaschinski, W. 2016. Pupil size measures of eye position in video eye tracking: implications for recording vergence accuracy. Journal of Eye Movement Research 9, 4. [Google Scholar] [CrossRef]
- König, P., N. Wilming, T. C. Kietzmann, J. P. Ossandón, S. Onat, B. V. Ehinger, R. R. Gameiro, and K. Kaspar. 2016. Eye movements as a window to cognitive processes. Journal of Eye Movement Research 9, 5. [Google Scholar] [CrossRef]
- Krassanakis, V., V. Filippakopoulou, and B. Nakos. 2014. EyeMMV toolbox: An eye movement postanalysis tool based on a two-step spatial dispersion threshold for fixation identification. Journal of Eye Movement Research 7, 1. [Google Scholar] [CrossRef]
- Loftus, G. R., and M. E. Masson. 1994. Using confidence intervals in within-subject designs. Psychonomic bulletin & review 1, 4: 476–490. [Google Scholar] [CrossRef]
- Micic, D., H. Ehrlichman, and R. Chen. 2010. Why do we move our eyes while trying to remember? The relationship between non-visual gaze patterns and memory. Brain and Cognition 74, 3: 210–224. [Google Scholar] [CrossRef]
- Motter, B. C., and E. J. Belky. 1998. The guidance of eye movements during active visual search. Vision research 38, 12: 1805–1815. [Google Scholar] [CrossRef]
- Olma, M. C., T. H. Donner, and S. A. Brandt. 2007. Control of Visual Selection during Visual Search in the Human Brain. Journal of Eye Movement Research 1, 1. [Google Scholar] [CrossRef]
- Pannasch, S., J. R. Helmert, K. Roth, A. K. Herbold, and H. Walter. 2008. Visual fixation durations and saccade amplitudes: Shifting relationship in a variety of conditions. Journal of Eye Movement Research 2, 2. [Google Scholar] [CrossRef]
- Peirce, J. W. 2007. PsychoPy—psychophysics software in Python. Journal of neuroscience methods 162, 1-2: 8–13. [Google Scholar] [CrossRef]
- Rayner, K. 2009. The 35th Sir Frederick Bartlett Lecture: Eye movements and attention in reading, scene perception, and visual search. Quarterly journal of experimental psychology 62, 8: 1457–1506. [Google Scholar] [CrossRef] [PubMed]
- Rigas, I., L. Friedman, and O. Komogortsev. 2018. Study of an extensive set of eye movement features: Extraction methods and statistical analysis. Journal of Eye Movement Research 11, 1. [Google Scholar] [CrossRef]
- Schumann, F., W. Einhäuser, J. Vockeroth, K. Bartl, E. Schneider, and P. König. 2008. Salient features in gaze-aligned recordings of human visual input during free exploration of natural environments. Journal of Vision 8, 14: 12–12. [Google Scholar] [CrossRef] [PubMed]
- Tatler, B. W. 2007. The central fixation bias in scene viewing: Selecting an optimal viewing position independently of motor biases and image feature distributions. Journal of vision 7, 14: 4–4. [Google Scholar] [CrossRef] [PubMed]
- Tatler, B. W., R. J. Baddeley, and B. T. Vincent. 2006. The long and the short of it: Spatial statistics at fixation vary with saccade amplitude and task. Vision research 46, 12: 1857–1862. [Google Scholar] [CrossRef]
- Theeuwes, J., A. F. Kramer, S. Hahn, D. E. Irwin, and G. J. Zelinsky. 1999. Influence of attentional capture on oculomotor control. Journal of experimental psychology: human perception and performance 25, 6: 1595. [Google Scholar] [CrossRef]
- Toivanen, M., K. Pettersson, and K. Lukander. 2015. A probabilistic real-time algorithm for detecting blinks, saccades, and fixations from EOG data. Journal of Eye Movement Research 8, 2. [Google Scholar] [CrossRef]
- Tseng, P. H., R. Carmi, I. G. Cameron, D. P. Munoz, and L. Itti. 2009. Quantifying center bias of observers in free viewing of dynamic natural scenes. Journal of vision 9, 7: 4–4. [Google Scholar] [CrossRef]
- Uzzaman, S., and S. Joordens. 2011. The eyes know what you are thinking: eye movements as an objective measure of mind wandering. Consciousness and cognition 20, 4: 1882–1886. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, Y. 2018. Mind-wandering, how do I measure thee with probes? Let me count the ways. Behavior Research Methods 50, 2: 642–661. [Google Scholar] [CrossRef] [PubMed]
- Wienrich, C., U. Heße, and G. Müller-Plath. 2009. Eye movements and attention in visual feature search with graded target-distractor-similarity. Journal of Eye Movement Research 3, 1. [Google Scholar] [CrossRef]
- Yarbus, A. L. 2013. Eye movements and vision. Springer. [Google Scholar] [CrossRef]
- Young, A. H., and J. Hulleman. 2013. Eye movements reveal how task difficulty moulds visual search. Journal of Experimental Psychology: Human Perception and Performance 39, 1: 168. [Google Scholar] [CrossRef]
- Zelinsky, G. J., and D. L. Sheinberg. 1997. Eye movements during parallel–serial visual search. Journal of Experimental Psychology: Human Perception and Performance 23, 1: 244. [Google Scholar] [CrossRef]




Copyright © 2022. This article is licensed under a Creative Commons Attribution 4.0 International License.
Share and Cite
Takemoto, A.; Nakazawa, A.; Kumada, T. Non-goal-Driven Eye Movement After Visual Search Task. J. Eye Mov. Res. 2022, 15, 1-13. https://doi.org/10.16910/jemr.15.2.2
Takemoto A, Nakazawa A, Kumada T. Non-goal-Driven Eye Movement After Visual Search Task. Journal of Eye Movement Research. 2022; 15(2):1-13. https://doi.org/10.16910/jemr.15.2.2
Chicago/Turabian StyleTakemoto, Ayumi, Atsushi Nakazawa, and Takatsune Kumada. 2022. "Non-goal-Driven Eye Movement After Visual Search Task" Journal of Eye Movement Research 15, no. 2: 1-13. https://doi.org/10.16910/jemr.15.2.2
APA StyleTakemoto, A., Nakazawa, A., & Kumada, T. (2022). Non-goal-Driven Eye Movement After Visual Search Task. Journal of Eye Movement Research, 15(2), 1-13. https://doi.org/10.16910/jemr.15.2.2


