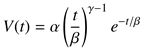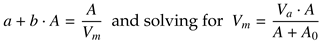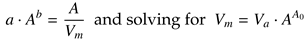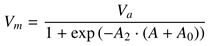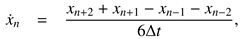Introduction
Several models characterizing the relationship be-tween saccadic peak velocity and amplitude have been proposed, including the power law, an exponential curve, and an inverse-linear model. Each of these can be made to express the interdependence of the main sequence parameters of amplitude, duration, and peak velocity. However, no model appears to adequately span a wide range of amplitudes. Some models per-form better at the small-amplitude range others do bet-ter at the mid-amplitude range while others are better suited to the large-amplitude range.
To derive a robust model of the main sequence, we propose fitting the saccadic peak velocity-amplitude relation with an S curve such that peak velocity dis-plays a fairly flat slope over very small and very large amplitudes. Meanwhile, the relation of saccadic peak velocity to duration and amplitude suggests that the model should also conform to the linearity of the duration-amplitude relation. Finally, parameters of the model should be easy to interpret. In this paper, we derive the S curve model from the logistic function and show how this model satisfies all of the aforemen-tioned requirements. To satisfy the criterion of interde-pendence between non-linear peak velocity and linear duration relations to amplitude, the model requires an inverse-linear component, producing an inverse-linear logistic model, suitable for expressing both relations.
We demonstrate the utility and robustness of the model when fit to
aggregate data collected from three experiments at three different laboratories, utilizing two different eye trackers. The first two experiments required maintenance of steady gaze while the third did not. Using saccade and microsaccade detection algorithms based on the work of Engbert and col-leagues (Engbert, Rothkegel, Backhaus, & Trukenbrod, 2016; Engbert, 2006; Engbert & Kliegl, 2003), we show how our model provides superior fits to peak velocity-amplitude relations of microsaccades, saccades, and to the superposition of both. Our model is simultaneously capable of providing a linear fit to duration-amplitude indistinguishable from a fit provided by the established linear main sequence (for an example of a linear fit to aggregate microsaccade data, see
Siegenthaler et al. (
2014).
Our work is similar to that of
Diaz-Piedra et al. (
2016), who also model the saccade peak velocity-amplitude relation nonlinearly and test different fits to individuals’ data (using a first-order polynomial and power-law fits). We model and empirically test our inverse-linear logistic
S curve fit against three other fits (power-law, exponential, and inverse-linear) and find that ours provides the best statistical fit. Unlike Diaz-Piedra et al., we fit our data to the aggregate collection microsaccade and saccade data from many participants instead of fitting the function per individual. Diaz-Piedra et al. also do not appear to consider the recip-rocal of their velocity-amplitude fits, i.e., they do not show whether the nonlinear fits they use for velocity-amplitude can also simultaneously be used to model the
linear duration-amplitude relation. Due to the in-terdependence of main sequence parameters pointed out by Lebedev, Van Gelder, and Tsui (1996), any non-linear function chosen to model the velocity-amplitude relation should
also be suitable as a model of the linear duration-amplitude relation. Below we first go through the mathematical derivation of our inverse-linear logis-tic model, showing how it can serve to model both re-lations, then we describe the three experiments whose data we test our four function fits on.
Saccadic Characteristics
Human saccades are stereotyped (
Fuchs, 1967) and presumed to be ballistic (
Carpenter, 1977), meaning programmed motor movements whose trajectory is un-changeable once in flight. Saccades follow the
main sequence describing the relationship between saccadic peak velocity and amplitude (Bahill, Clark, & Stark, 1975; Baloh et al., 1975; Knox, 2001). The main sequence
also relates saccade duration to amplitude. Treating saccade duration as movement time MT, the main sequence can be expressed as a linear relation
or a power law
where amplitude
A is given in degrees visual angle in both instances (
Becker, 1989). Example fits are given for the power law (1b) by
Yarbus (
1967) with
a = 0.021 and
b = 0.4 and for the linear relation (1a) by
Baloh et al. (
1975) with
a = 37 and
b = 2.7, in milliseconds and milliseconds/degree, respectively, see
Figure 1a.(Other variations of the linear main sequence include
a = 23,
b = 2.7 from
Collewijn et al. (
1988) and
a ∈ [20, 30] ms.,
b ∈ [2, 2.7] ms/deg from Lee, Badler, and Badler (2002) and Gu, Lee, Badler, and Badler (2008) in their graphical simula-tion of saccades known as
Eyes Alive) The lower limit (
a) of saccade duration is due to finite rise-time of muscle fibre twitches (for microsaccades of am-plitude less than 0.5
◦, duration of 14 ms have been re-ported (
Becker, 1989)). The relationship between dura-tion and saccade amplitude is normally linear for sac-cades of up to about 80
◦ (although most naturally oc-curring saccades range up to about 15
◦-20
◦ (
Bahill et al., 1975) and up to 30
◦ without head movement (
Lebedev et al., 1996)).
Unlike the linear relation of duration-amplitude, the peak velocity of saccadic eye movements is related in a nonlinear manner to their amplitude over a thousand-fold range (from 3
j minutes of arc to 50
◦), with data scatter noted as “extremely small” (
Bahill et al., 1975). Peak velocity is related in a quasi-linear manner to sac-cadic amplitude up to about 15
◦ or 20
◦, when a soft sat-uration limit is reached.
Lebedev et al. (
1996) provide an inverse-linear model (the Michaelis-Menten equa-tion) where peak velocity
Vp is modeled by
where
A denotes saccade amplitude,
Va is an asymp-totic maximum of peak velocity (the saturation value, in degrees per second), and
A0 is the half-maximum amplitude (in degrees), i.e., the amplitude at which 50% of the peak velocity is reached.
Baloh et al. (
1975) compare alternative peak velocity models using a power-law equation
and an exponential curve
were
A0 in this latter form represents the amplitude for which peak velocity reaches 63% of its saturation (
Lebedev et al., 1996), see
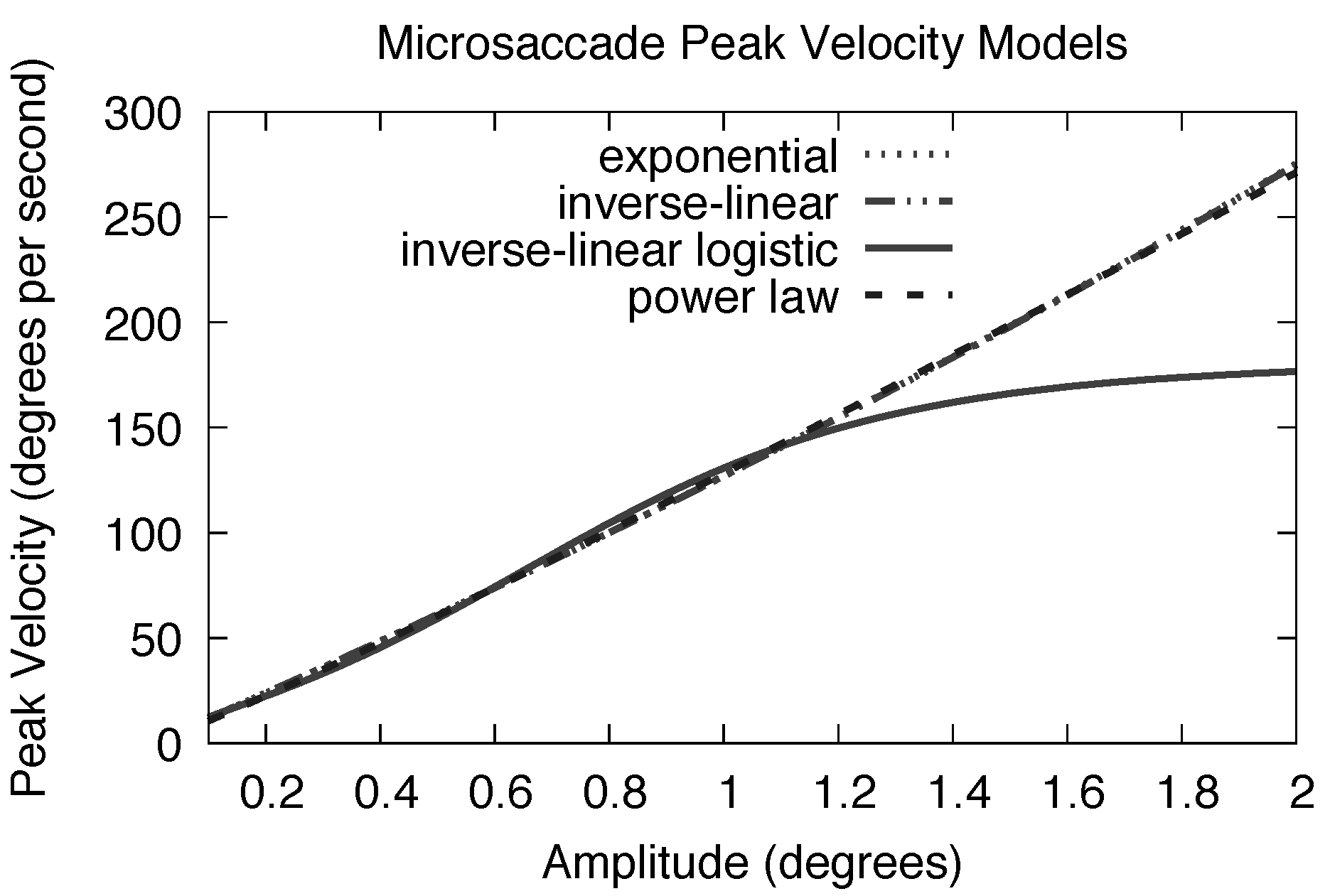
Figure 1b where the expo-nential model is plotted for
Va = 551 degrees/second and
A0 = 14 degrees as fit by
Baloh et al. (
1975) to their observations, corresponding to their MT = 37 + 2.7
A duration-amplitude regression shown in
Figure 1a.
The interdependence between the dynamic proper-ties of saccadic amplitude, duration, and peak veloc-ity might not be immediately obvious. By itself, the main sequence does not provide a complete descrip-tion of the saccadic system, which as a whole, is non-linear (Van Opstal & Van Gisbergen, 1987). Saccades of different amplitudes have differently shaped velocity profiles. The velocity profile of small saccades is sym-metrical while it is skewed for large saccades, and can be modeled by the expression
where time
t ≥ 0, α, β > 0 are scaling constants for ve-locity and duration, respectively, and 2 < γ < 15 is the shape parameter that determines the degree of asym-metry. Small values of γ yield asymmetrical velocity profiles and as γ tends to infinity, the function assumes a symmetrical (Gaussian) shape see Van Opstal and Van Gisbergen (1987) as well as
Baloh et al. (
1975) or
Collewijn et al. (
1988) for illustrations.
Interdependence of the main saccadic parameters of amplitude, duration, and peak velocity is explained by a strong linear relationship (
r ≥ 0.98) between mean ve-locity
Vm and peak velocity
Vp (
Lebedev et al., 1996). By definition, the mean velocity of saccades is computed directly from their duration and amplitude,
where solving for MT =
A/
Vm and equating with (1a) leads to the inverse-linear dependence of mean veloc-ity on amplitude,
produces the Michaelis-Menten equation (2) for
Vm with
A0 = (
a/
b) and
Va = 1/
b (
Becker, 1989). Similarly, equating (6) with (1b)
leads to the power-law equation (3) for Vm with A0 = (1−b) and Va = 1/b
Peak velocity initially rises in proportion to saccade amplitude and then saturates as the amplitude be-comes larger. Plotted as a function of saccade ampli-tude,
Vp resembles a scaled-up version of
Vm,
where
K is constant (
Becker, 1989;
Lebedev et al., 1996).
As a numerical example, solving the main sequence provided by
Baloh et al. (
1975) MT = 37 + 2.7
A =
A/
Vm for
Vm produces the Michaelis-Menten inverse-linear dependence of peak velocity on amplitude (2) with
A0 = 13.70 and
Va = 0.37 · 551 where 0.37 is 1/
b from (1a) and 551 is the asymptotic maximum peak velocity used in (4). Using (7), we found a good approximation to (4) by setting
K = 3.11, see
Figure 1(b). For a numerical example of the power law, see
Lebedev et al. (
1996).
In their comparison of various models, including the inverse-linear, exponential, and power-law models of saccadic peak velocity,
Lebedev et al. (
1996) note that the inverse-linear and exponential models are of no use outside the range of estimation (i.e., < 1.5
◦ or > 30
◦) and that their parameters do not allow any reasonable in-terpretation. They give an approximation of (horizon-tal) saccadic eye movements’ peak velocity in the form of a square-root model, effectively Yarbus’ power-law model given in (6) with
A0 fixed to 1/2. They claim that the square-root model is adequate for a range of ampli-tudes from 1.5
◦ to 30
◦, i.e., the mid-amplitude range. Furthermore, they show that all models except the square-root model are unstable with respect to a small shift of the amplitude ranges from which they were drawn. Meanwhile, within the 7
◦-15
◦ range of ampli-tudes, they found the power-law model served better than the square-root model with respect to goodness of fit, with the inverse-linear and exponential models unacceptably unstable.
From the model comparison of
Lebedev et al. (
1996), it appears no model provides a good fit to the data across a wide range of saccadic amplitudes. They note that the interdependence of the main sequence param-eters, specifically relations (6) and (7), allows catego-rization of saccades into three ranges of saccade ampli-tudes: the small-amplitude range (
A < 1.5
◦) in which duration remains fairly constant and increase in ampli-tude is caused by an increase in peak velocity; the mid-amplitude range (1.5
◦ ≤
A ≤ 35
◦) in which the increase in the amplitude is caused by an increase in saccade dura-tion and peak velocity; and the large-amplitude range (35
◦ <
A) in which peak velocity saturates such that an increase in amplitude is caused primarily by dura-tion.(
Carpenter (
1988) states,
“The situation is rather like that of a man falling off a cliff: at first, acceleration dominates, and his peak velocity depends on how far he falls. But if his drop is a long one, most of the way he will be falling at his terminal velocity, so the duration of his fall will be in proportion to the height of the cliff.”) The majority of naturally occurring saccadic eye movements fall within the mid-amplitude range and are made without head movements.
The above description by
Lebedev et al. (
1996) of the main sequence suggests a model resembling an
S curve with peak velocity displaying a fairly flat slope over very small and very large amplitudes. Meanwhile, the definition of saccadic mean velocity in relation to their duration and amplitude given in (6) suggests that such a model conform to the observed near-linearity of the duration-amplitude relation e.g., as given by the linear expression for MT in (1a). Finally, parameters of the model should be easy to interpret. A model derived from the logistic function satisfies all of these require-ments.
A logistic function model for saccadic velocity can be expressed as
where
A2 is the sigmoid curve’s steepness (slope),
A0 is the sigmoid’s midpoint,
Va is the curve’s asymp-totic maximum (see
Figure 2). Unfortunately, while the logistic function can be made to fit peak velocity data, it does not satisfy the remaining requirement of near-linearity when using it to express the duration-amplitude relation. The problem rests in the resultant expression for MT being fixed at 0 for the
y-intercept. To achieve the desired flexibility in the model’s expres-sivity, the logistic function is augmented with what re-sembles an inverse-linear component
which we term the inverse-linear logistic model of sac-cadic peak velocity (save for the constant scalar
K as per (7)). The additional parameter
A1 produces a shift of the function, which is made clear in the function’s expression for the duration-amplitude relation.
Using (6) and solving for MT =
A/
Vm yields
with
a = 1/
Va,
b =
k0 –
A0,
c =
k2 ·
A2, and
d =
k1 ·
A1. Choos-ing parameters
Va = 551,
A0 = –0.71,
A1 = 4.0,
A2 = 3.70,
k0 = 14.99,
k1 = 3,
k2 = 0.48, and
K = 2.5 ·
Va as per (7) to produce MT =
K ·
Vm, yields the functions plotted in
Figure 1a,b that visually match the functions fit by
Baloh et al. (
1975) with
Va = 551 and
A0 = 14 (see above) in the large-amplitude range.
The goodness of fit problem of the exponential and inverse-linear functions may lie in the small-amplitude (e.g., microsaccadic) range. Examining this range, it is clear that these two functions and the inverse-linear lo-gistic functions diverge, see
Figure 3. In this instance it may appear that the inverse-linear logistic function reaches asymptote too quickly. Because none of the fits were based on actual microsaccade data, it is diffi-cult to tell which curves are growing at an appropriate rate. Inspecting
Figure 3a and focusing on the expo-nential and inverse-linear fits would suggest that mi-crosaccades reach peak velocities of less than 100
◦ per second. This is not very likely. Reaching asymptote at about 150
◦ per second is probably more realistic. More-over, neither of the exponential nor the inverse-linear functions possess an initial inflection seen at very low amplitudes (the bottom part of the
S curve).
Do microsaccades exhibit an inflection at very low amplitudes? Examining fits to microsaccade data (see
Figure 4) suggests that there is an inflection point at very low amplitudes (about about 0.5
◦ amplitude). At about 1.5
◦ a microsaccadic asymptote is apparent. The exponential and inverse-linear models, when fit to mi-crosaccadic peak velocities, “miss the turn” at both lo-cations. The
S inherent in the logistic function affords a better fit. Below we compare statistical fits to microsac-cade data from three experiments conducted that were in part designed to capture microsaccades.
Empirical Methodology
To compare and contrast saccadic main sequence model fits, we examine data captured from three eye tracking experiments.The first two were designed to replicate the experiment of
Siegenthaler et al. (
2014) but using eye trackers sampling at two different rates (500 Hz and 300 Hz). The study was originally de-signed to test microsaccadic response to task difficulty and mental fatigue, necessitating exclusion of saccades (controlled within the experimental procedure; see be-low). The third experiment considers microsaccades and saccades from an experiment conducted to eval-uate affective response to images of faces gradually changing shape (morphing) to express one of several emotions. Below we provide detailed description of the study methodologies, including a brief review of exper-imental design with independent and dependent mea-sures, procedure, participants, and equipment. Our focus here is not so much on the analyses of results pertaining to the study hypotheses, rather we are con-centrating on characteristics of saccades and microsac-cades observed in each of the three experiments.
Experiments 1 and 2: Microsaccades
The first two experiments closely followed the ex-perimental design of
Siegenthaler et al. (
2014). In each of our two experiments we used a 3 × 6 within-subjects design where the first fixed factor was task type (Dif-ficult vs. Easy vs. Control) and the second fixed fac-tor was Time-on-Task where six blocks of trials within the experimental procedure constituted the six levels of this fixed factor. In the Difficult and Easy tasks, partic-ipants were asked to perform difficult and easy mental calculations, while in the Control task, they were not asked to perform any mental calculations at all (see Ex-perimental Procedure below).
Following
Siegenthaler et al. (
2014), we focused on microsaccade magnitude and rate, and following Di Stasi et al. (2013), we analyzed fits of the relationship between microsaccadic amplitude and peak velocity.
Experimental Procedure. Following signing of a consent form and completion of an online demographic questionnaire, participants sat at the eye-tracking com-puter with their head stabilized by a chin rest. After making sure participants were comfortable, a 5-point eye tracker calibration was performed. Experimental tasks started when the average calibration error was lower than 0.5◦ visual angle.
The experimental procedure followed that of
Siegenthaler et al. (
2014), described here for complete-ness. Three types of number counting trials, Difficult, Easy, and Control, were grouped into 6 blocks, giving 18 trials total. Each block started with the Control trial, followed by the Easy and Difficult trials in counterbal-anced order. Between each block, participants were asked to take a short break lasting 2–5 minutes; they were not allowed to start the next block until at least 2 minutes had elapsed.
Each trial started with an instruction screen and in-cluded a break at the end of each of the six blocks. In the Difficult trials, participants were asked to mentally count backwards, as fast and accurately as possible, in steps of 17 starting at one of the following 4-digit num-bers drawn randomly from this set: {1375, 8489, 5901, 5321, 4819, 1817}.
The Easy and Control trials were constructed simi-larly to Difficult trials, but differed in task performance and initial instructions. In the Easy tasks, participants were instructed to mentally count forward, as fast and accurately as possible, in steps of 2 starting at one of the following 3-digit numbers drawn randomly from this set: {363, 385, 143, 657, 935, 141}. In the Control tri-als, participants were asked just to gaze at the fixation point with no mental task assigned.
When doing the experimental task, participants were asked to gaze at the fixation point appearing at screen center. Whenever their gaze shifted 3◦ visual angle away from the fixation point a warning beep sounded.
During each trial, participants were prompted four times to enter in their current number in a text box shown on the screen. A limit of 9 seconds was given for providing the entry. Three prompts appeared at random times during each trial, and the fourth at the very end of the trial. The gap between prompts was a minimum of 15 seconds and a maximum of 80 seconds. Since we do not focus specifically on the impact of mental calculations on the microsaccade-peak velocity relationship, only data from the Control tasks were se-lected for analysis.
Participants. Participants (N= 17) volunteered for Experiment 1, recruited verbally and via social media. Due to problems with eye tracker calibration or misun-derstanding of the task by participants (i.e., in at least one case the participant stopped counting after one it-eration, see Experimental Procedure above), data from four subjects were discarded resulting in a final sample of N = 13. Data from 7 males and 6 females aged be-tween 20 and 40 years old (M = 29.77; SD = 7.15) was used in the analysis. All participants reported normal, uncorrected vision.
Participants (N = 10) volunteered for Experiment 2, recruited verbally. All participants reported normal, corrected or uncorrected vision.
Experimental Setting and Apparatus. In Experi-ment 1, an SR Research EyeLink 1000 eye tracker was used for eye tracking data acquisition. Eye movements were recorded binocularly at a sampling rate of 500 Hz. Each participant’s head was stabilized with a chin rest during the entire experimental procedure, see
Figure 5. The accuracy of the EyeLink 1000 tracker is reported by the manufacturer as 0.25
◦–0.5
◦ visual angle on average, with microsaccade resolution of 0.05
◦.
The experimental procedure was controlled by a per-sonal computer connected to the eye-tracking com-puter. Visual stimuli were displayed on a 24 inch computer screen with 1920×1080 resolution at a view-ing distance of 57 cm. Responses made by partici-pants were performed on a standard numerical key-board connected to the stimuli presentation computer and placed by the participant’s dominant hand.
In Experiment 2, conditions were similar, except that a 300 Hz eye tracker from Tobii was used. As in Ex-periment 1, each participant’s head was stabilized with a chin rest during the entire experimental procedure, see
Figure 6. The accuracy of the Tobii TX300 tracker is reported by the manufacturer as 0.3
◦–0.6
◦ visual angle on average.
Experiment 3: Microsaccades & Saccades
Experiment 3 differed from the first two consider-ably. Its objective was to study facial affect recog-nition and followed procedures similar to those of Schönenberg, Mayer, Christian, Louis, and Jusyte (2015). For the purposes of this analysis, what is most important is that participants viewed a computer mon-itor and maintained their gaze in a fairly central loca-tion. Unlike the first two experiments, Experiment 3 did not restrict eye movement and allowed freedom to visually inspect the stimulus. Recall that Experiments 1 and 2 restricted gaze to a central point on the screen lo-cation with an audible reprimand sounding whenever gaze strayed too far away (3◦) from center.
Although there are considerable differences in tasks and experimental objectives, here we are mainly con-cerned with finding suitable functions to fit microsac-cadic and saccadic peak velocity profiles.
Experimental Procedure. The experimental proce-dure consisted of participants watching a parametri-cally varied image sequence (morphed animation) of a human face. The animated morph task depicted a video sequence of a neutral face slowly developing into one of six basic emotions (fear, sadness, anger, happi-ness, disgust, surprise). Faces were presented in the center of the computer screen and subtended 16.8◦ vi-sual angle in width and 21.1◦ visual angle in height.
Participants were instructed to press a button as soon as they were able to identify the emerging emo-tion. The sequence was then immediately stopped, the face disappeared, and participants were presented with a mask instructing them to indicate which emo-tion they had identified by selecting one of the six ver-bal categories via a button press. The morph intensity level at the time of the button press, as well as the par-ticipant’s judgment of the emotional expression, was recorded.
For the purposes of the analysis of eye movements in the present article, we ignore dependent variables per-taining to affect recognition, e.g., the intensity of emo-tional expression at the time of the button press, cor-rectness of response, etc.
Participants. Data were collected from three groups of participants: two clinical groups and a Con-trol group of people who reported no mental disorders (N = 20). Note that for the sake of internal coherence of the present analysis, we focus only on data from the Control group since we are not interested in the differ-ences between research samples.
Experimental Setting and Apparatus. In Experi-ment 3, the same type of eye tracker was used as in Experiment 1, model EyeLink 1000 from SR Research. Eye movements were recorded binocularly at a sam-pling rate of 500 Hz.
Visual stimuli were displayed on a 19 inch computer screen with 1024×768 resolution at a viewing distance of 60 cm.
Results
Analysis of results aims to answer two questions:
how well does the inverse-linear logistic function (9) fit peak velocity-amplitude data for microsac-cades and saccades; and
can the inverse-linear logistic function, in its ex-pression for duration (10), adequately describe the linear duration-amplitude relationship of mi-crosaccades and saccades?
Before providing empirical evidence for these queries, in
Table 1 we first present descriptive statistics of microsaccades from our three experiments (saccades captured in the third experiment are discussed later). Microsaccadic amplitudes and durations are similar across all three experiments, and are in line with what is found in the literature, e.g., see Otero-Millan, Tron-coso, Macknik, Serrano-Pedraza, and Martinez-Conde (2008).
However, one should also note in
Table 1 a greater microsaccade mean peak velocity in Experiment 3 where participants were allowed to move their eyes over the given stimuli (pictures of faces). This is also consistent with the literature. For example, Martinez-Conde (2006) previously showed that a steady fixation leads to a decrease in microsaccade amplitude (result-ing in visual fading).
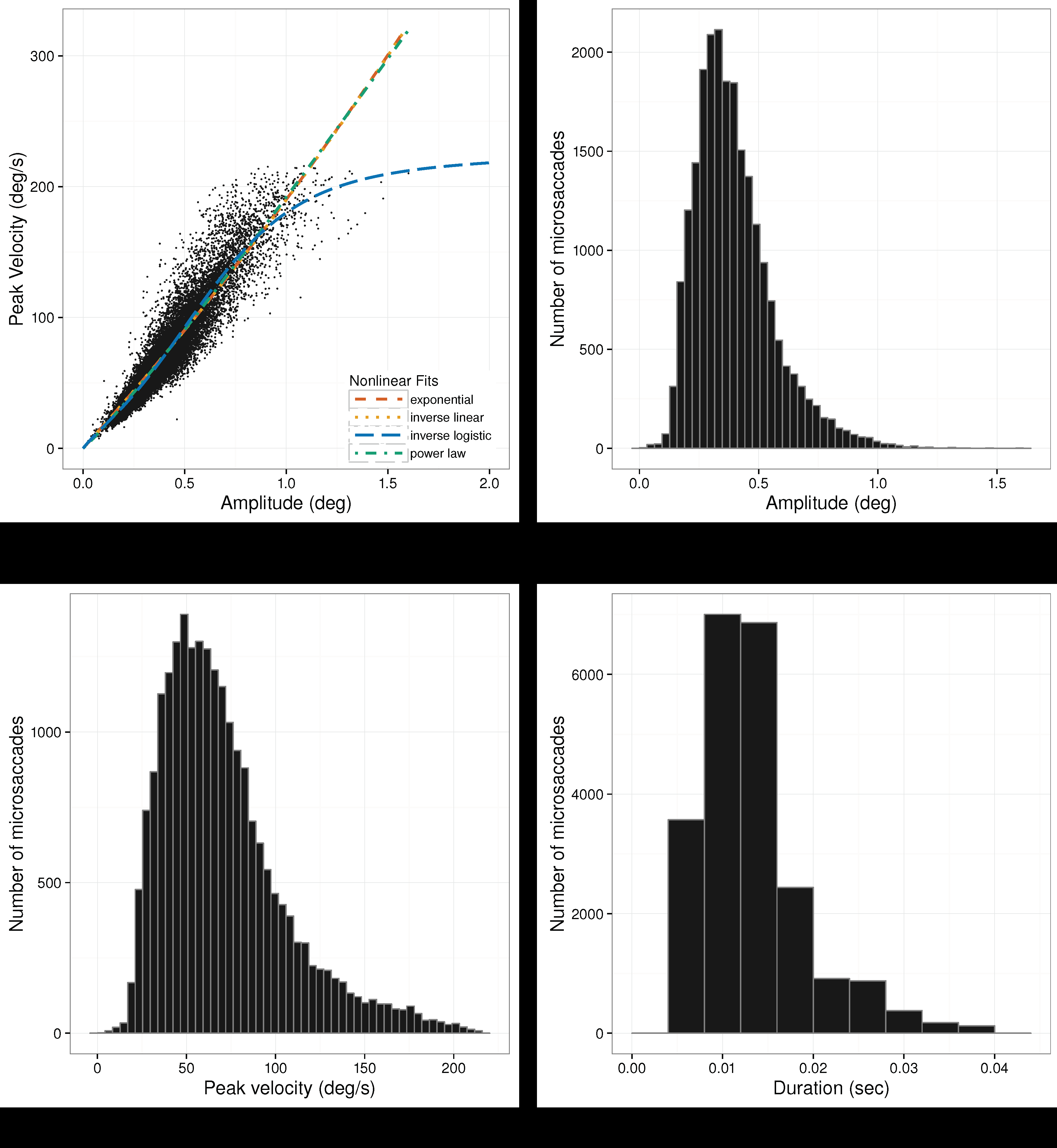
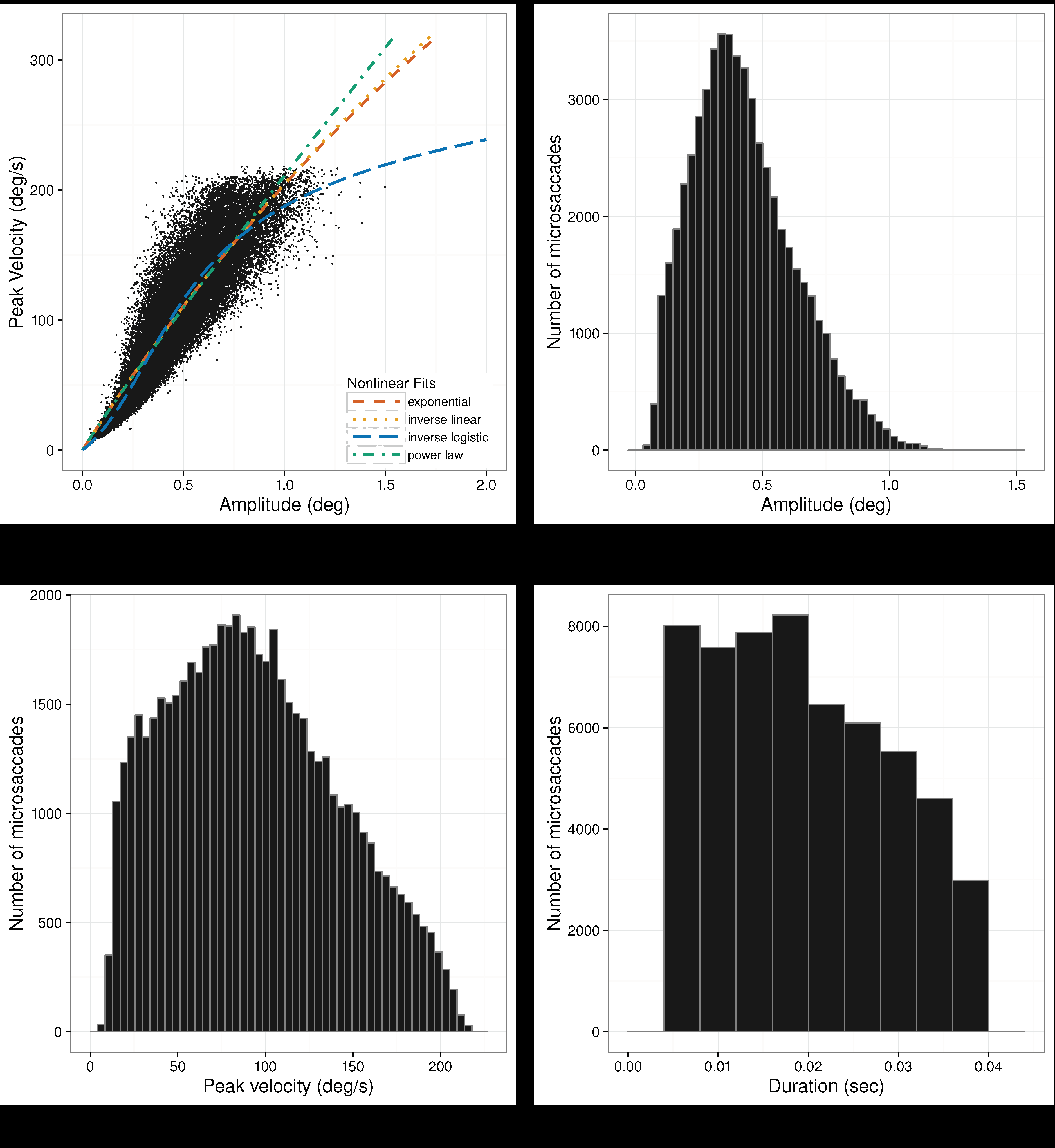
Microsaccadic distributions and the microsaccadic peak velocity vs. amplitude scatterplots are given in
Figure 7,
Figure 8 and
Figure 9 for Experiments 1–3, respectively. Plots of the microsaccadic and saccadic peak velocity-amplitude relation include different functions fit to the data, for comparison with the inverse-linear logistic function fit. These function fits are discussed below.
A traditional approach to testing linear model (func-tion) goodness of fit is through linear regression anal-ysis, i.e., estimation of
R2. The computation of
R2, however, relies on estimation of the distance between the observations and the model (residuals), i.e., the line that was determined through least squares mini-mization (i.e., minimization of the distance), see Boggs, Byrd, and Schnabel (1987). For
R2 to be meaningful, the distances computed assume orthogonality between the observations and the line fit to them. For a nonlin-ear fit, the assumption of orthogonality might not hold (
Wolter & Fuller, 1982;
Stefanski, 1985).
Instead of
R2, we examine the relative quality of our nonlinear models through Akaike’s Information-theoretic Criterion (AIC) (
Akaike, 1974). What matters is not AIC itself but the difference in AIC between mod-els (∆AIC). Under this criterion, the model with the smallest AIC exhibits the best fit. Supplementing AIC, we also report results from Vuong’s test, a likelihood-ratio based statistic, for non-nested, nonlinear model comparison (
Vuong, 1989). Vuong’s also tests for statis-tical significance between the fits of the models under consideration.
Microsaccade Peak Velocity-Amplitude. For mi-crosaccade data from all experiments, the inverse-linear logistic function provides the best fit, yielding the smallest AIC. Moreover, as alluded by
Lebedev et al. (
1996), the other nonlinear models show inconsis-tency in their goodness of fits, see
Table 2.
We should point out that for the prolonged fixation of Experiments 1 and 2, the rank ordering of the AIC is the same. In these experiments, the inverse-linear model gave the worst fit. In Experiment 3, where the eyes were free to move, the power-law function gives the worst fit. Similar differences in microsaccades be-tween fixating and free-viewing were noticed by Otero-Millan et al. (2008), although they did not fit different functions to the data.
Pairwise comparison of the inverse-linear logistic function with all others with Vuong’s test (with AIC correction) revealed a statistically significant difference in fits, with the inverse-linear logistic function giving better fits in all cases, see
Table 2.
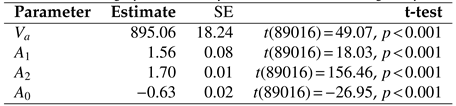
Table 3 gives the parameter estimates for the inverse-linear logistic func-tion in each of the three experiments. Significance tests indicate that all parameters differed significantly from zero.
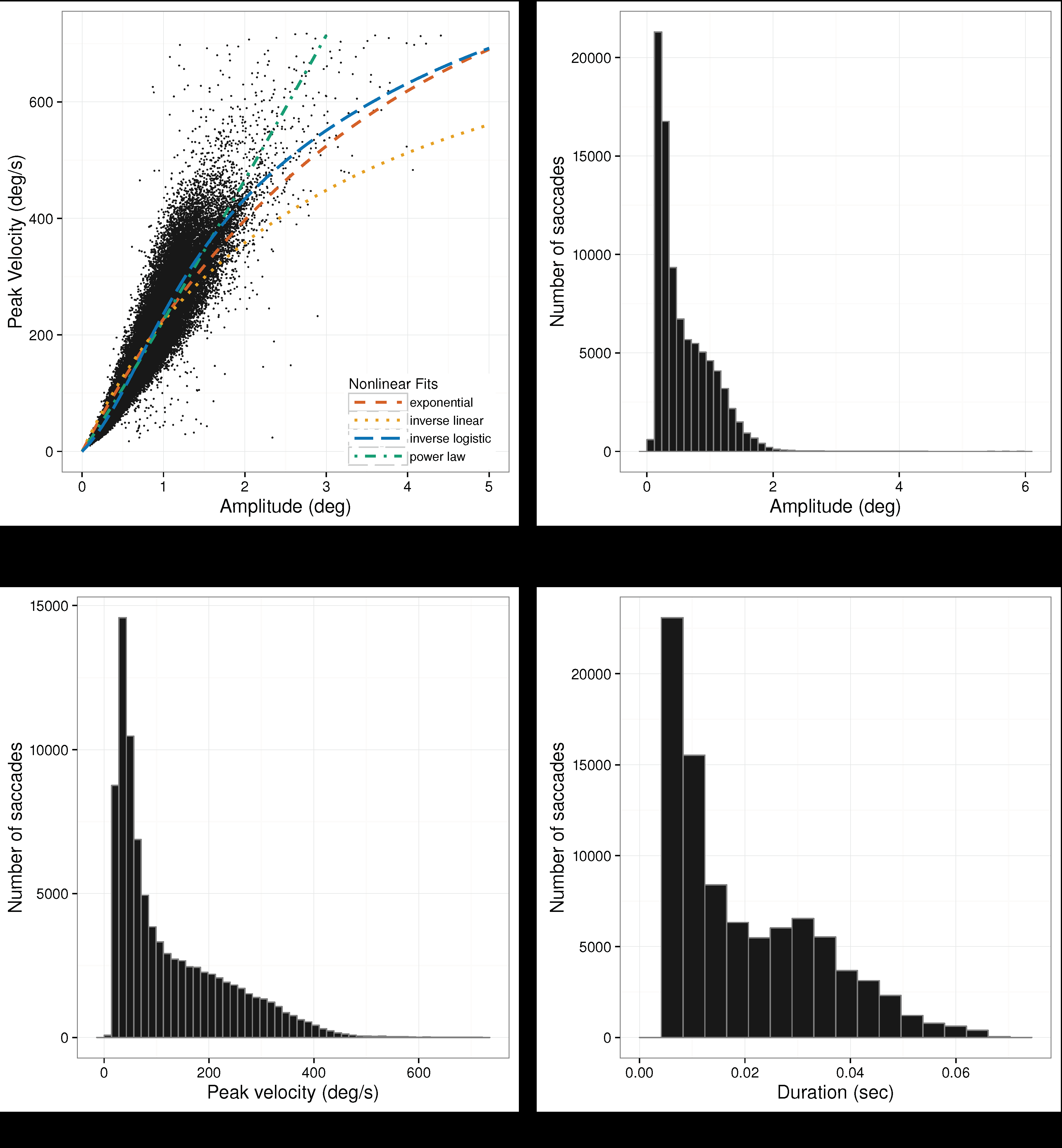
Saccade Peak Velocity-Amplitude. Saccades were captured only in Experiment 3 because only in this re-ported study participants could freely move their eyes. There were
N = 89020 saccades detected in total. De-tailed descriptive statistics for saccades are given in
Table 4. Notice that the maximum amplitude observed was over 6 degrees visual angle, placing these saccades in the small-to mid-amplitude range. Saccade distribu-tions and the saccade peak velocity-amplitude scatter-plot from Experiment 3 is given in
Figure 10, along with depictions of functions fit to the data in
Figure 10(a).
The same nonlinear goodness of fit analysis was car-ried out for the saccadic peak velocity-amplitude rela-tion as for microsaccades, above. Similar to microsac-cades, the AIC shows that the function providing the best fit is the inverse-linear logistic function, see
Table 5. The inverse-linear function gives the worst fit.
Voung’s tests, also in
Table 5, suggest that the inverse-linear logistic function fits the data significantly better than any of the other functions.
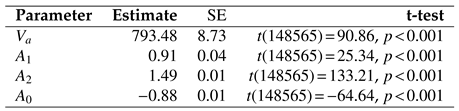
Table 6 lists the param-eter estimates for the inverse-linear logistic function.
Superpositioning Microsaccades and Saccades. To test whether the inverse-linear logistic function pro-vides a good fit across microsaccade and saccade am-plitude ranges, we superpositioned both types of eye movements into a single data set and followed the same analytical procedure as above for the separate mi-crosaccade and saccade analysis.
Results of AIC analysis suggests the inverse-linear logistic function goodness of fit is maintained across the small and mid-amplitude ranges, see
Table 7. Ta-ble 7 also shows results of Voung’s tests which again in-dicate a statistically significant better fit of linear logistic function compared to the others.
Table 8 lists the parameter estimates for the inverse-linear lo-gistic function.
Figure 8.
Experiment 2. Distribution of microsaccade amplitude (b), peak velocity (c), and duration (d). Figure (a) depicts the nonlinear relationship between peak velocity and amplitude with different function fits. Data were captured at 300 Hz.
Figure 8.
Experiment 2. Distribution of microsaccade amplitude (b), peak velocity (c), and duration (d). Figure (a) depicts the nonlinear relationship between peak velocity and amplitude with different function fits. Data were captured at 300 Hz.
Fitting the Duration-Amplitude Main Sequence
While empirical evidence thus far shows that the inverse-linear logistic function produces the best fit to the relation between peak velocity and amplitude, it may not be immediately obvious whether the function can also serve to express the classic linear relation be-tween duration and amplitude, i.e., the main sequence (
Bahill et al., 1975).
We have shown mathematically that the inverse-linear logistic expression (10; restated below) can be used to describe the duration-amplitude relation. Here, we test this assertion empirically by comparing the ex-pression’s fit to that of the linear function given by (1a). To do so, we use the data from the above three exper-iments, this time plotting duration vs. amplitude and compare the linear fit of (1a)
to that of (10).
For the analysis, the inverse-linear logistic function is manually fit to the data, while the linear fit is ob-tained automatically through linear model fitting in software (R). Linear curve fitting for the inverse-linear logistic function proceeds by first automatically fitting expression (9) for peak velocity-amplitude. This was performed automatically in software (using R’s nls() function, see
Fox and Weisberg (
2010). Recall expres-sion (9)
is converted to expression (10)
by using (6) and solving for MT =
A/
Vm with
a = 1/
Va,
b =
k0 –
A0,
c =
k2 ·
A2, and
d =
k1 ·
A1, using constant
e such that
K =
e ·
Va as per (7) to produce MT =
K ·
Vm. Coef-ficients
k0–
k2 and
K, given in
Table 9, were obtained by manually fitting the inverse-linear logistic function to each of the data sets used for fitting the peak velocity-amplitude relation, but replotted using duration vs. amplitude. In each instance, a linear fit of (1a) was ob-tained automatically via minimization of least squares (in R) for comparison to (10).
In line with expectations, for microsaccadic data in Experiments 1, 2, and 3 the linear and inverse-linear lo-gistic functions fit the data similarly. Comparison of the AIC for linear (AIC = –170475.2) and inverse-linear lo-gistic (AIC = –170475.2) functions for microsaccades in Experiment 1 showed that both models fit similarly to the data. Analyses of Experiment 2 also showed similar fits for both linear (AIC = –103460.1) and the inverse-linear logistic model (AIC = –103460.1). Analyses of Experiment 3 also yielded similar results for the lin-ear (AIC=–388684.6) and inverse-linear logistic models (AIC=–388684.7).
Similar AIC results were found for saccades fit by the linear (AIC = –576451.0) and inverse-linear logistic (AIC = –576450.7) models in Experiment 3. Combined saccade and microsaccade data also yielded similar fits by the linear (AIC=–956121.2) and inverse-linear logis-tic (AIC=–956120.7) models.
AIC statistics for each of the pairs of the linear and inverse-linear logistic fits are nearly identical, suggest-ing that both models are indistinguishable. One may argue, as we do above, that AIC statistics are more suit-able to nonlinear goodness of fit estimates and that in this instance of testing linear models, the traditional ap-proach to testing goodness of fit is through linear re-gression analysis, i.e., estimation of R2. Linear regres-sion statistics of each pair of models is provided in Table 10, which shows identical R2 for the model pairs in each of the five data sets.
In sum, when applied to the amplitude-duration re-lation, the inverse-linear logistic function fits the data as well as the classically assumed linear expression. The inverse-linear logistic function fit extends the main sequence from the microsaccadic small to the saccadic mid-amplitude range.