No Waste from Waste: Membrane-Based Fractionation of Second Cheese Whey for Potential Nutraceutical and Cosmeceutical Applications, and as Renewable Substrate for Fermentation Processes Development
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Downstream Process
2.3. Analytical Methods
2.4. Protein Assay
2.5. Protein Molecular Weight Analyses
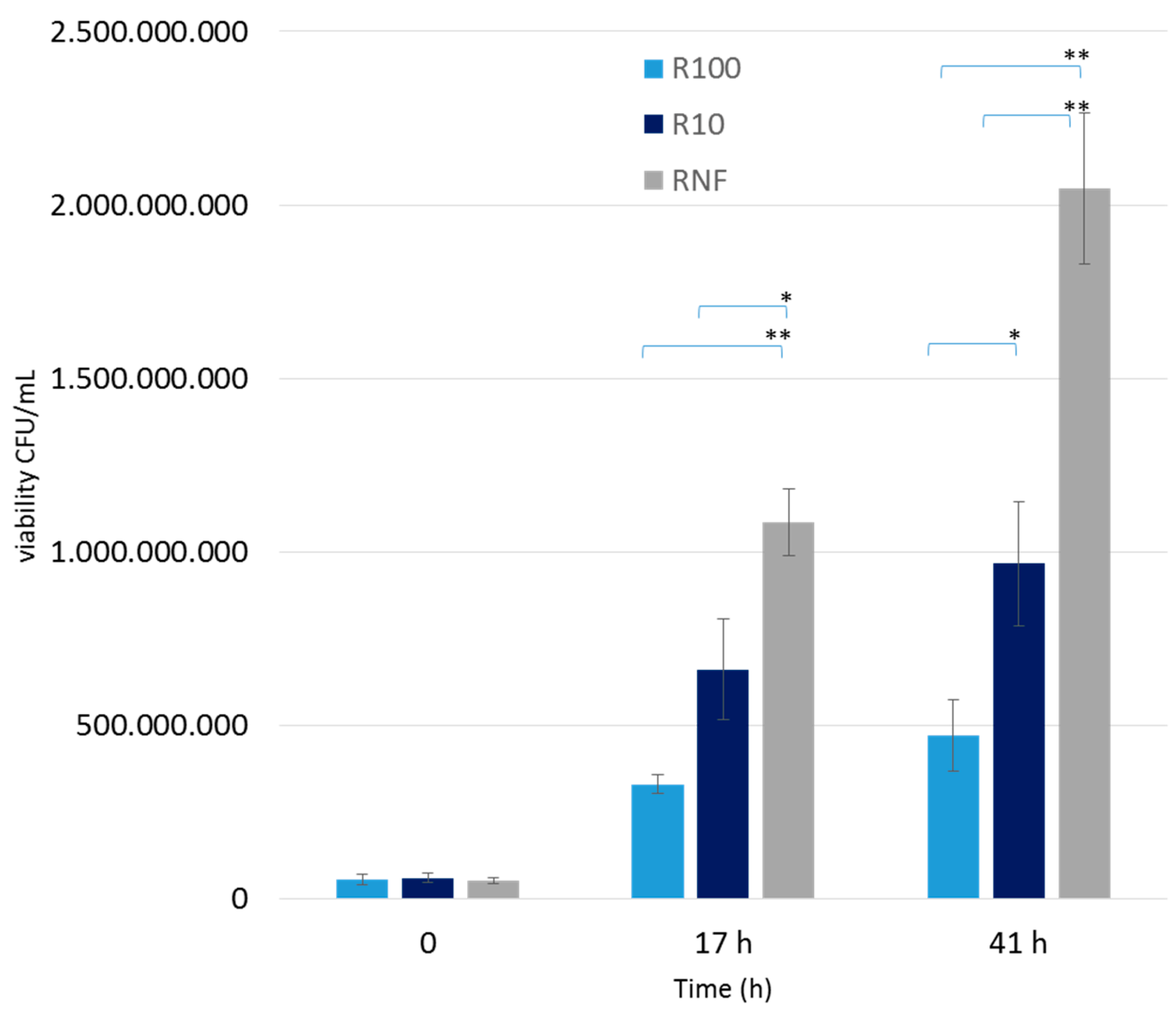
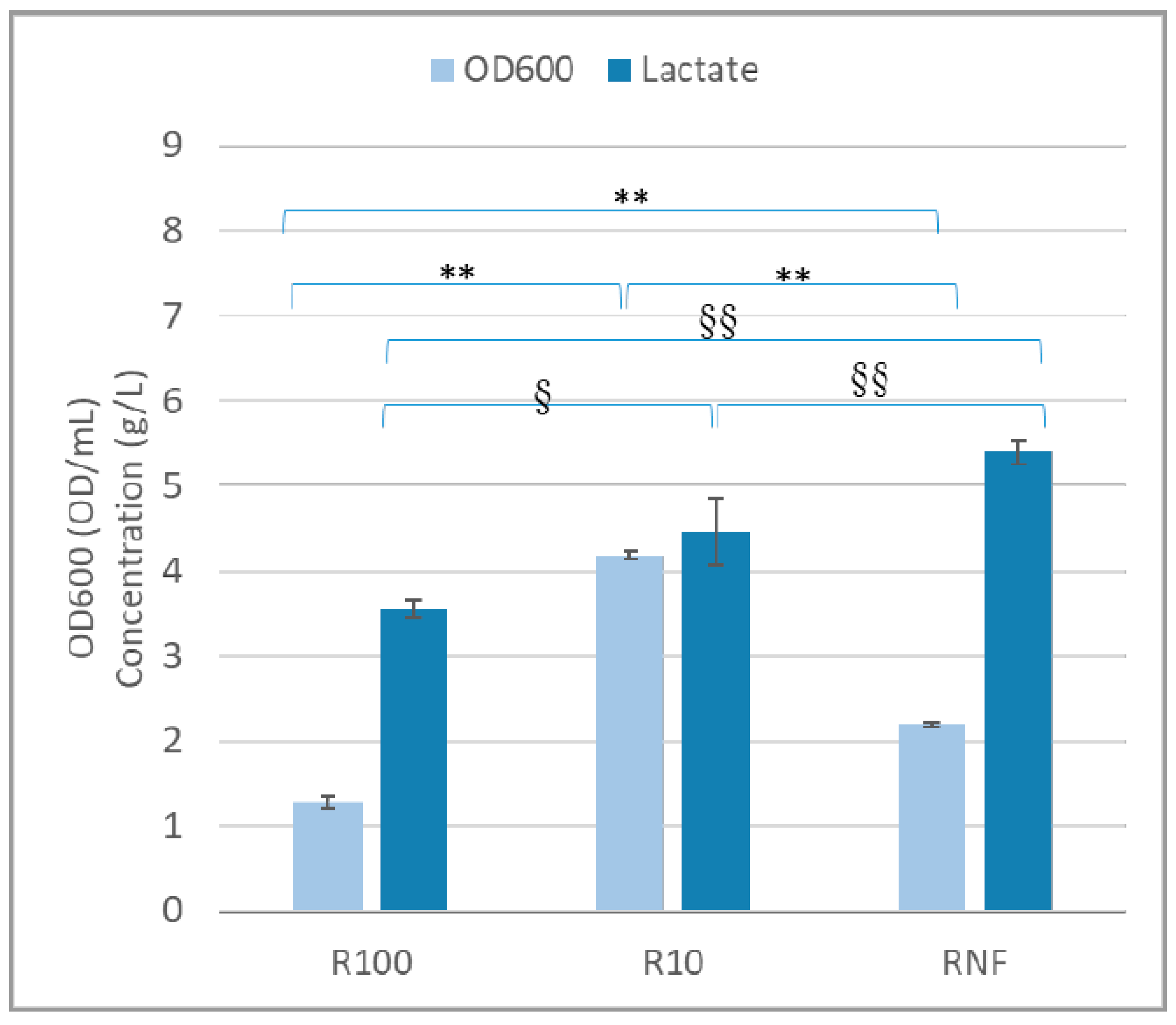
2.6. Growth of Lactic Acid Bacteria in Small-Scale Bottle Experiments
2.7. Whey Protein Films Preparation
2.8. Dehydration Test
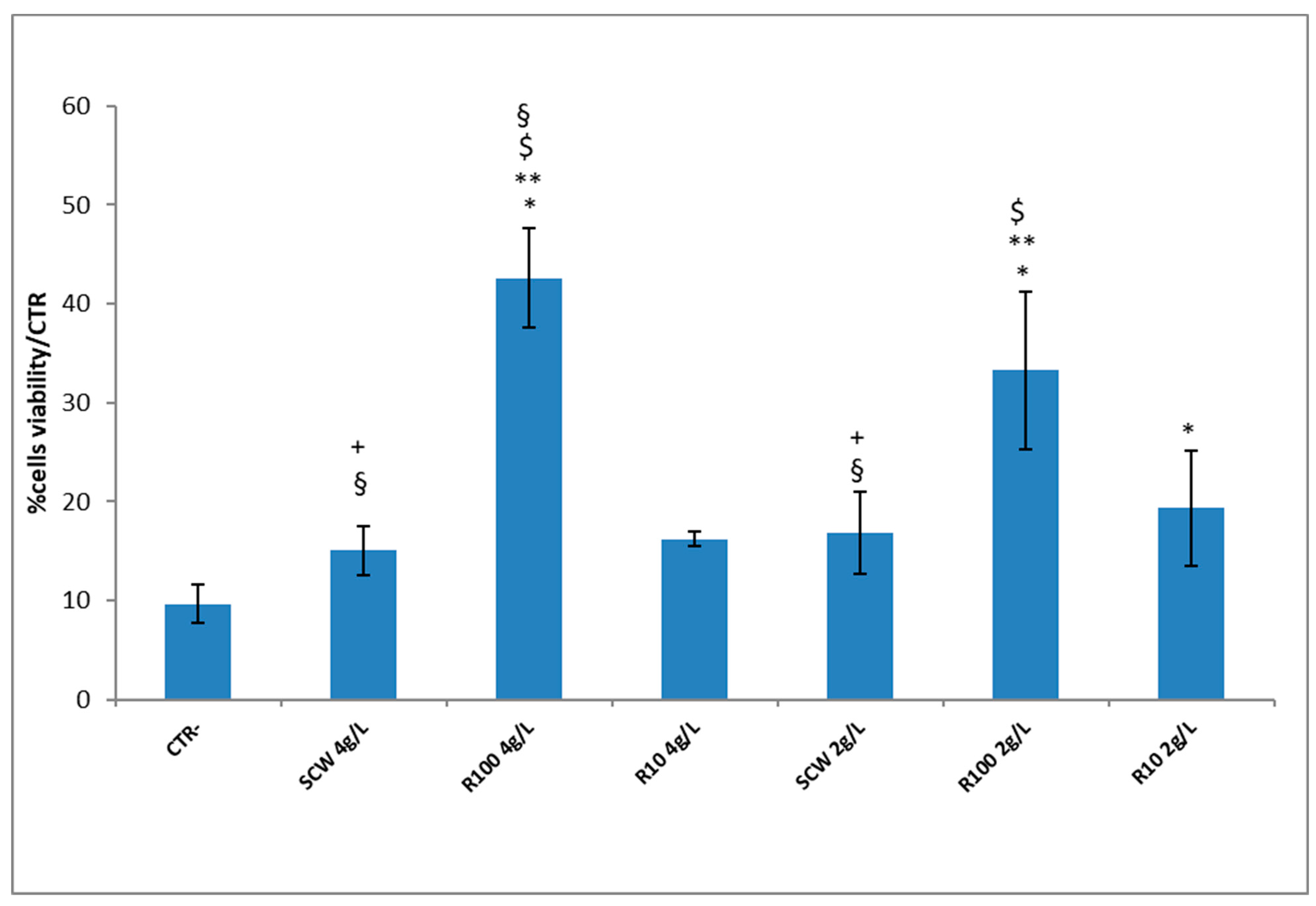
3. Results
3.1. Downstream Process
3.2. Small-Scale Bottle Experiments
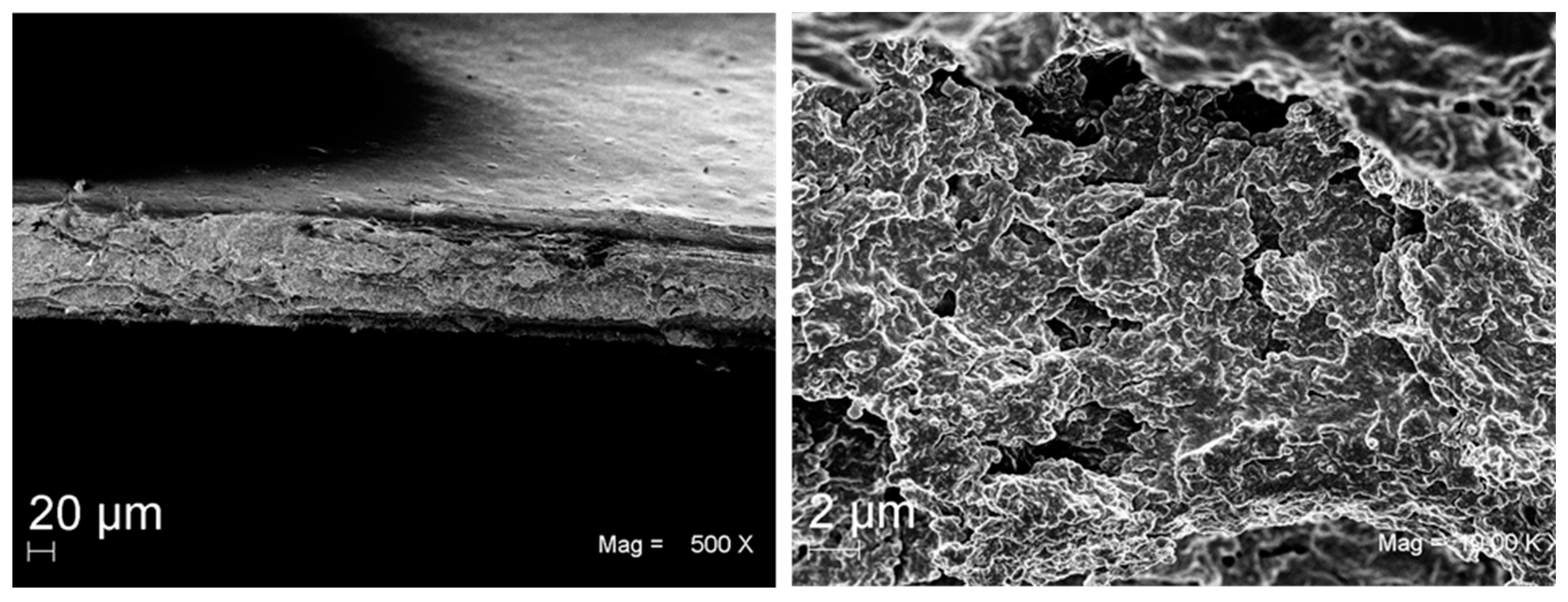
3.3. Evaluation of Casting Conditions of Whey Proteins
3.4. Dehydration Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lievore, P.; Simões, D.R.S.; Silva, K.M.; Drunkler, N.L.; Barana, A.C.; Nogueira, A.; Demiate, I.M. Chemical characterisation and application of acid whey in fermented milk. J. Food Sci. Technol. 2015, 52, 2083–2092. [Google Scholar] [CrossRef] [PubMed]
- Zandona, E.; Blažić, M.; Jambrak, A.R. Whey Utilization: Sustainable Uses and Environmental Approach. Food Technol. Biotechnol. 2021, 59, 147–161. [Google Scholar] [CrossRef]
- Yadav, J.; Yan, S.; Pilli, S.; Kumar, L.; Tyagi, R.D.; Surampalli, R.D. Cheese whey: A potential resource to transform into bioprotein, functional/nutritional proteins and bioactive peptides. Biotechnol. Adv. 2015, 33, 756–774. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, K.; Nakagawa, S.; Kanawaku, R.; Kawamura, S. Ethanol production from Cheese Whey and Expired Milk by the Brown Rot Fungus Neolentinus lepideus. Fermentation 2019, 5, 49. [Google Scholar] [CrossRef]
- Baldasso, C.; Barros, T.C.; Tessaro, I.C. Concentration and purification of whey proteins by ultrafiltration. Desalination 2011, 278, 381–386. [Google Scholar] [CrossRef]
- Maragkoudakis, P.; Vendramin, V.; Bovo, B.; Treu, L.; Corich, V.; Giacomini, A. Potential use of scotta, the by-product of the ricotta cheese manufacturing process, for the produc-tion of fermented drinks. J. Dairy Resour. 2016, 83, 104. [Google Scholar] [CrossRef]
- Zotta, T.; Solieri, L.; Iacumin, L.; Picozzi, C.; Gullo, M. Valorization of cheese whey using microbial fermentations. Appl. Microbiol. Biotechnol. 2020, 104, 2749–2764. [Google Scholar] [CrossRef]
- Madureira, A.R.; Pereira, I.C.; Gomes, M.P.; Pintado, M.E.; Malcata, F.X. Bovine whey proteins—Overview on their main biological properties. Food Res. Int. 2007, 40, 1197–1211. [Google Scholar] [CrossRef]
- Bassan, J.C.; Goulart, A.J.; Nasser, A.L.M.; Bezerra, T.M.S.; Garrido, S.; Rustiguel, C.B.; Guimarães, L.; Monti, R. Buffalo Cheese Whey Proteins, Identification of a 24 KDa Protein and Characterization of Their Hydrolysates: In Vitro Gastrointestinal Digestion. PLoS ONE 2015, 10, e0139550. [Google Scholar] [CrossRef]
- Lappa, I.K.; Papadaki, A.; Kachrimanidou, V.; Terpou, A.; Koulougliotis, D.; Eriotou, E.; Kopsahelis, N. Cheese Whey Processing: Integrated Biorefinery Concepts and Emerging Food Applications. Foods 2019, 8, 347. [Google Scholar] [CrossRef] [PubMed]
- Pires, A.F.; Marnotes, G.; Rubio, O.; Garcia, A.; Pereira, C. Dairy By-Products: A Review on the Valorization of Whey and Second Cheese Whey. Foods 2021, 10, 1067. [Google Scholar] [CrossRef] [PubMed]
- Sommella, E.; Pepe, G.; Ventre, G.; Pagano, F.; Conte, G.M.; Ostacolo, C.; Manfra, M.; Tenore, G.C.; Russo, M.; Novellino, E. Detailed peptide profiling of “Scotta”: From a dairy waste to a source of potential health-promoting compounds. Dairy Sci. Technol. 2016, 96, 763–771. [Google Scholar] [CrossRef]
- Zoppellari, F.; Bardi, L. Production of bioethanol from effluents of the dairy industry by Kluyveromyces marxianus. New Biotechnol. 2013, 30, 607–613. [Google Scholar] [CrossRef]
- Lopes, A.C.A.; Eda, S.H.; Andrade, R.P.; Amorim, J.C.; Duarte, W.F. New Alcoholic Fermented Beverages—Potentials and Challenges. Fermented Beverages 2019, 2019, 577–603. [Google Scholar]
- Secchi, N.; Giunta, D.; Pretti, L.; García, M.; Roggio, T.; Mannazzu, I.; Catzeddu, P. Bioconversion of ovine scotta into lactic acid with pure and mixed cultures of lactic acid bacteria. J. Ind. Microbiol. Biotechnol. 2012, 39, 175–181. [Google Scholar] [CrossRef]
- Sarkar, B.; Chakrabarti, P.P.; Vijaykumar, A.; Kale, V. Wastewater treatment in dairy industries possibility of reuse. Desalination 2006, 195, 141–152. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Ko, S.; Xiao, L. Use of whey proteins for encapsulation and controlled delivery applications. J. Food Eng. 2007, 83, 31–40. [Google Scholar] [CrossRef]
- Khanzadi, M.; Jafari, S.M.; Mirzaei, H.; Chegini, F.K.; Maghsoudlou, Y.; Dehnad, D. Physical and mechanical properties in biodegradable films of whey protein concentrate-pullulan by application of beeswax. Carbohydr. Polym. 2015, 118, 24–29. [Google Scholar] [CrossRef]
- Gilbert, V.; Rouabhi, M.; Wang, H.; Arnould, A.; Remondetto, G.; Subirade, M. Characterization and evaluation of whey protein-based biofilms as substrates for in vitro cell cultures. Biomaterials 2005, 26, 7471–7480. [Google Scholar] [CrossRef]
- La Gatta, A.; Corsuto, L.; Salzillo, R.; D’Agostino, A.; De Rosa, M.; Bracco, A.; Schiraldi, C. In vitro evaluation of hybrid cooperative complexes of hyalouronic acid as a potential new ophthalmic treatment. J. Ocul. Pharmacol. Ther. 2018, 34, 677–684. [Google Scholar] [CrossRef]
- Alfano, A.; D’ambrosio, S.; D’Agostino, A.; Finamore, R.; Schiraldi, C.; Cimini, D. Concentrated Buffalo Whey as Substrate for Probiotic Cultures and as Source of Bioactive Ingredients: A Local Circular Economy Approach towards Reuse of Wastewaters. Fermentation 2021, 7, 281. [Google Scholar] [CrossRef]
- Cuartas-Uribe, B.; Alcaina-Miranda, M.I.; Soriano-Costa, E.; Mendoza-Roca, J.A.; Iborra-Clar, M.I.; Lora-García, J. A study of the separation of lactose from whey ultrafiltration permeate using nanofiltration. Desalination 2009, 241, 244–255. [Google Scholar] [CrossRef]
- Pires, A.F.; Marnotes, N.G.; Bella, A.; Viegas, J.; Gomes, D.M.; Henriques, M.H.; Pereira, C.J. Use of ultrafiltrated cow’s whey for the production of whey cheese with Kefir or probiotics. J. Sci. Food Agric. 2020, 101, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Yorgun, M.; Balcioglu, I.A.; Saygin, O. Performance comparison of ultrafiltration, nanofiltration and reverse osmosis on whey treatment. Desalination 2008, 229, 204–216. [Google Scholar] [CrossRef]
- De Souza, R.R.; Bergamasco, R.; da Costa, S.C.; Feng, X.; Faria, S.H.B.; Gimenes, M.L. Recovery and purification of lactose fromwhey. Chem. Engeneering Process 2010, 49, 1137–1143. [Google Scholar] [CrossRef]
- Tong, M.; Sasaki, S.; McClements, D.J.; Decke, A. Mechanisms of the Antioxidant Activity of a High Molecular Weigh Fraction of Whey. J. Agric. Food Chem. 2000, 48, 1473–1478. [Google Scholar] [CrossRef]
- Aguirre-Ezkauriatza, E.J.; Aguilar-Yáñez, J.M.; Ramírez-Medrano, A.; Alvarez, M.M. Production of probiotic biomass (Lactobacillus casei) in goat milk whey: Comparison of batch, continuous and fed-batch cultures. Bioresour. Technol. 2010, 101, 837–2844. [Google Scholar] [CrossRef]
- Kumar, M.; Jain, A.K.; Ghosh, M.; Ganguli, A. Industrial whey utilization as a medium supplement for biphasic growth and bacteriocin production by probiotic Lactobacillus casei LA-1. Probiotics Antimicrob. Proteins 2012, 4, 198–207. [Google Scholar] [CrossRef]
- Alfano, A.; Donnarumma, G.; Cimini, D.; Fusco, A.; Marzaioli, I.; De Rosa, M.; Schiraldi, C. Lactobacillus plantarum: Microfiltration experiments for the production of probiotic biomass to be used in food and nutraceutical preparations. Biotechnol. Prog. 2015, 31, 325–333. [Google Scholar] [CrossRef]
- Bernardo, M.P.; Coelho, L.F.; Sass, D.C.; Contiero, J. l-(+)-Lactic acid production by Lactobacillus rhamnosus B103 from dairy industry waste. Braz. J. Microbiol. 2016, 47, 640–646. [Google Scholar] [CrossRef]
- Wang, T.; Lu, Y.; Yan, H.; Li, X.; Wang, X.; Shan, Y.; Yi, Y.; Liu, B.; Zhou, Y.; Lu, X. Fermentation optimization and kinetic model for high cell density culture of a probiotic microorganism: Lactobacillus rhamnosus LS-8. Bioprocess Biosyst. Eng. 2020, 43, 515–528. [Google Scholar] [CrossRef] [PubMed]
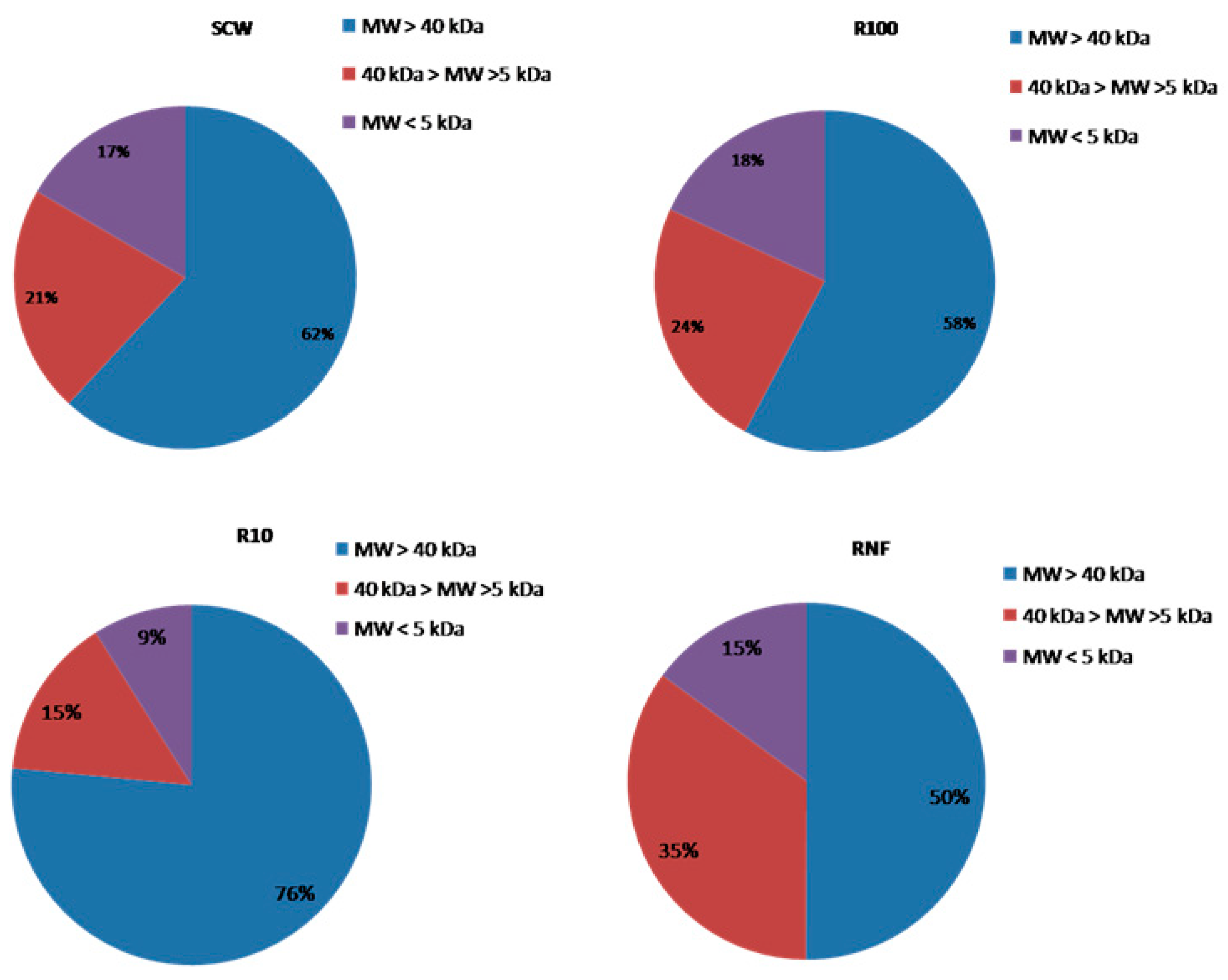


| Process | Concentration Factor | Diafiltration Factor | Initial Transmembrane Pressure (Bar) | Final Transmembrane Pressure (Bar) |
|---|---|---|---|---|
| UF 100 | 9.4 | 4 | 3.1 | 3.3 |
| UF 10 | 5.5 | 8 | 4.0 | 5.2 |
| NF | 4.8 | / | 11.0 | 13.3 |
| Sample | Lactose (g/L) | Glucose (g/L) | Galactose (g/L) | Lactic Acid (g/L) | Proteins (g/L) |
|---|---|---|---|---|---|
| Whey | 34.0 | n.a. | n.a. | 4.6 | 8.0 |
| SCW | 33.1 | 5.3 | 6.7 | 4.5 | 0.84 |
| R100 | 7.9 | 1.2 | 1.6 | 1.1 | 0.22 |
| R10 | 28.2 | 3.5 | 4.4 | 3.2 | 1.53 |
| RNF | 41.9 | 3.8 | 5.1 | 5.1 | 0.67 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfano, A.; D’ambrosio, S.; Cimini, D.; Falco, L.; D’Agostino, M.; Finamore, R.; Schiraldi, C. No Waste from Waste: Membrane-Based Fractionation of Second Cheese Whey for Potential Nutraceutical and Cosmeceutical Applications, and as Renewable Substrate for Fermentation Processes Development. Fermentation 2022, 8, 514. https://doi.org/10.3390/fermentation8100514
Alfano A, D’ambrosio S, Cimini D, Falco L, D’Agostino M, Finamore R, Schiraldi C. No Waste from Waste: Membrane-Based Fractionation of Second Cheese Whey for Potential Nutraceutical and Cosmeceutical Applications, and as Renewable Substrate for Fermentation Processes Development. Fermentation. 2022; 8(10):514. https://doi.org/10.3390/fermentation8100514
Chicago/Turabian StyleAlfano, Alberto, Sergio D’ambrosio, Donatella Cimini, Luca Falco, Maria D’Agostino, Rosario Finamore, and Chiara Schiraldi. 2022. "No Waste from Waste: Membrane-Based Fractionation of Second Cheese Whey for Potential Nutraceutical and Cosmeceutical Applications, and as Renewable Substrate for Fermentation Processes Development" Fermentation 8, no. 10: 514. https://doi.org/10.3390/fermentation8100514
APA StyleAlfano, A., D’ambrosio, S., Cimini, D., Falco, L., D’Agostino, M., Finamore, R., & Schiraldi, C. (2022). No Waste from Waste: Membrane-Based Fractionation of Second Cheese Whey for Potential Nutraceutical and Cosmeceutical Applications, and as Renewable Substrate for Fermentation Processes Development. Fermentation, 8(10), 514. https://doi.org/10.3390/fermentation8100514








