Abstract
Thermotolerant yeasts are widely considered to be alternative strains to traditional yeasts for bioethanol production at high temperatures. In this study, thirty-two yeasts isolated from lychees were screened for thermotolerance, and seven selected isolates were identified as Candida tropicalis (isolates H8, H19, and H23), Meyerozyma guilliermondii (isolates H1 and H12) and Saccharomyces cerevisiae (isolates H10 and H18). They tolerated up to 45 °C, 12% (v/v) ethanol concentration, 10 g/L acetic acid, and 5 g/L furfural, respectively, and produced 47.96 to 70.18 g/L of ethanol from 160 g/L glucose at 40 °C during 48 h of fermentation. Among the evaluated yeasts, M. guilliermondii H1 showed great potential for second-generation bioethanol fermentation with its ability to ferment xylose and arabinose. Under the optimal conditions resulting from a Plackett Burman design and a Box Behnken design, the highest ethanol concentration of 11.12 g/L was produced from 40 g/L substrate-based sugarcane bagasse hydrolysate (non-detoxified hydrolysate) at 40 °C by M. guilliermondii H1. These findings suggested that the newly isolated thermotolerant yeast M. guilliermondii H1 is a good candidate for ethanol production from agricultural wastes.
1. Introduction
Nowadays, fossil energy sources such as oil and coal are increasingly being depleted causing petroleum prices to escalate and affecting energy security and economic development. Therefore, it is necessary to search for new energy source that are eco-friendly, renewable, sustainable, and low-cost are required to replace non-renewable fossil fuels [1]. Recently, some different biofuel energy resources have been developed. Among them, bioethanol is gaining attention as a clean energy alternative to fossil energy [2]. Undoubtedly, it is a clean-burning fuel and environmentally safe energy since it is an oxygenated product with low toxicity and biodegradability. Moreover, it is easy to mix efficiently with fossil-derived liquid fuels, such as gasoline or petrol, to directly serve as energy for private vehicles or transportation [3,4].
At an the early stage of bioethanol production, sugar or starch, originating from wheat, sugar cane, cassava, or corn it required as a substrate for fermentation [5]. However, the use of these materials for bioethanol production can directly or indirectly affect food security through competition and price escalation since food crops are also important for humans and livestock [2,5]. Lignocellulosic biomass from agricultural waste, retaining a high sugar content, has become an attractive renewable resource for the sustainable production of ethanol (second-generation bioethanol production) [6,7]. Sugarcane bagasse (SCB) is generated from sugar production after sugarcane juice extractions. It is one of the most important lignocellulosic biomass, produced in massive amounts and available in Vietnam.SCB can be easily broken down in diluted acid to make simple sugars for bioethanol production [1]. The main advantage of bioethanol production from SCB hydrolysate pre-treated with diluted acid is the reducion in air pollution since it is less carbon-intensive than fossil fuel [8,9]. However, some inhibitors are formed after the pre-treatment of SCB, such as furfural, hydroxymethylfurfural (HMF), and acetic acid, which may have negative effects on fermenting microorganisms, including yeasts [10]. Therefore, the use of yeasts that can tolerate these inhibitors is also a critical characteristic.
The production of bioethanol at high temperatures has attracted much interest since it helps us to save energy during the cooling process and reduce contamination [11]. Consequently, plenty of yeast characteristics must be considered the yeast’s ability to grow and produce ethanol in restricted conditions in high temperatures or in the presence of inhibitory chemicals. Moreover, to efficiently produce bioethanol from lignocellulosic biomass, the most desired yeast characteristic is the ability to use different sugar types besides glucose, such as D-xylose and arabinose (pentose), which are released from the hydrolysis of agricultural waste [12]. To date, relatively few yeasts that can ferment pentose sugars have been isolated from nature, including Candida shehatae, Pichia stipitis [13] and C. Tropicalis [14]. Simultaneously, by using genome editing, researchers have tried to generate industrial yeast strains that can ferment the xylose. However, the generated strains were not suitable for application in industrial settings since the fermentation efficiency, yield, and stress tolerances of the strain were too low [15].
Thermotolerant yeasts isolated from nature have received considerable attention, more than laboratory strains, since they not only have many advantageous characteristics in fermentation, particularly in producing ethanol at high temperatures and metabolizing carbon sources, xylose, arabinose, and cellobiose, but they also have unique properties that support their growth in adverse conditions (acid, heat, heavy metal) [16]. This is especially relevant for central Vietnam, a hot tropical area, which has a diversity of bioresources, especially microorganisms yeast, which may have favorable properties for ethanol production from agricultural wastes [17,18,19]. Thus, it would be exciting to research novel yeasts and to uncover their benefits for waste-based bioethanol production.
The aim of this study, therefore, focuses on isolating and screening indigenous thermotolerant yeasts in Hue of Vietnam during the summer season for fermenting agricultural waste to produce ethanol at high temperatures.
2. Materials and Methods
2.1. Materials
Sugarcane bagasse (SCB) was collected at Lam Son factory in Thanh Hoa, Vietnam. SCB was air-dried until the moisture content was about 6.5% (w/w), it then was ground with a cutting mill and sieved through a 40 mesh (size particle 900 µm). Pentose-rich fractions were extracted from dried biomass by pretreating it at a ratio of 1:10 with sulfuric acid 6% (w/w) for 60 min, 121 °C, and 4 bar pressure in autoclave [20]. After cooling, the mixed biomass was separated by filtration. The chemical composition of hydrolysate was then applied for HPLC analysis as previously described [8] and as shown in Supplementary Table S1. The pH of SCB hydrolysate was adjusted by NaOH to the levels of the experimental design (pH 4 and 6), then supplemented with 2 g/L yeast extract.The total sugar of the mixture (non-detoxified SCB hydrolysate and yeast extract) was adjusted to obtain the range of 20–40 g/L by supplementation with xylose and glucose to achieve a working concentration of 15–40 g/L and 5 g/L, respectively. This mixture was used as substrate for bioethanol production.
2.2. Sample Collection, Yeast Isolation and Thermotolerant Yeasts Screening
Lychees were collected from Thua Thien Hue (16°27′59.6″ N 107°33′48.2″ E) a central highland of Vietnam, where the range of average temperatures in summer time (April to June, 2020), is usually 35–37 °C. Thirty samples were collected from each garden and five gardens were sampled. Lychees were carefully harvested and placed in a sterile bag before being brought to the laboratory for yeast isolation.
Yeasts were isolated as previously reported [21] with some modifications. In brief, lychees were cut into small pieces with a size of 0.5 cm and placed in 250 mL Erlenmeyer flasks with 50 mL sterilized YM broth (1% malt extract, 1% yeast extract, 1% peptone, and 2% glucose) on a shaking incubator (H2010, Benchmark Scientific) at 150 rpm at 35 °C for 48 h.
The solutions were centrifuged (5417R, Eppendorf) for 1 min at 3000 rpm. The pellet was collected and resuspended in 0.5 mL of YM broth, and serially diluted before spreading 0.1 mL of each dilution on YMA plates (YM and 2% agar) with 100 mg/L of chloramphenicol. The plates were incubated at 35 °C for 72 h, and different colonies were picked and streaked onto fresh YMA plates.
To isolate thermotolerant yeasts, an equal volume of overnight shaking culture at 35 °C of all isolates were freshly streaked on YMA plates and incubated at 37, 40 and 45 °C until yeast colonies appeared. After identification, purified yeast isolates were stored at −80 °C in 10% glycerol for further study.
2.3. Glucose-Based Bioethanol Production by Selected Thermotolerant Yeasts
Each selected isolate was cultured overnight in YM broth with shaking incubation (150 rpm) at 35 °C. The next day, 1 mL of diluted overnight cultured cells (with a density of approximately 107 cells/mL) was added to 200 mL YM broth containing 160 g/L glucose [22]. The flask was sealed with a rubber stopper to keep favorable conditions for ethanol fermentation and also equipped with a sampling port and a one-way gas valve to remove the CO2 released. The fermentation flasks were statically incubated at 37, 40, and 45 °C for 48 h. Ethanol levels were examined every 12 h: briefly, approximately 600 µL broths were collected via sampling port and centrifuged at 13,000 rpm for 5 min. The supernatant was applied to gas chromatography with a simultaneous flame-ionization (GC-FID) detector for the estimation of the ethanol concentration (g/L).
2.4. DNA Sequencing and Yeast Identification
The total DNA of each isolated yeast colony was extracted using a Favorgene kit (Favorgen, Ping-Tung, Taiwan) according to the manufacturer’s manual. Briefly, a single yeast colony was selected and inoculated overnight in YM broth with a shaking incubator (H2010, Benchmark Scientific) at 150 rpm at 35 °C. Cells were collected from cultures by centrifugation (5417R, Eppendorf) and then lysed in lysis buffer. DNA solutions were purified via application to purification columns. Extracted DNA concentration was checked by nanodrop (Thermal Scientific). The appropriate amount of genomic DNA was used as template to amplify the 26S rDNA D1/D2 region using forward primer NL1 (5′-GCATATCAATAAGCGGGGAAAAG-3′) and reverse primer NL4 (5′-GGTCCGTGTTTCAAGACGG-3′) as previously described [23]. OneTaq Master Mix (England Biolabs) was applied with the following thermal program for both of the PCRs: initial denaturation at 95 °C for 5 min, following 35 cycles of denaturation at 95 °C for 30 sec, annealing at 52 °C for 30 sec, and extension at 68 °C for 1 min. Expected PCR products with a size of 560 bp were purified by FavorPrep GEL/PCR Purification Kit (Favorgen, Ping-Tung, Taiwan) according to the manufacturer’s instruction. Sequencing was performed by Genlab (Hanoi, Vietnam) using Sanger sequencing. Yeasts were identified by aligning their sequences with published sequences deposited in the National Centre for Biotechnology Information (NCBI) database using the Basic Logarithmic Alignment Search Tool (BLAST).
2.5. Screening of the Thermotolerant Yeasts for Second-Generation Bioethanol Production
2.5.1. Tolerance of Thermotolerant Yeasts to Individual Stress Factors
The growth ability of selected thermotolerant yeasts under the different stresses of ethanol, acetic acid, and furfural was examined by a spot dilution growth assay according to Li et al. (2015) [24]. Briefly, 24 h cultures of yeast cells in YM broth at 35 °C were harvested, washed, and adjusted to an OD600 of 1.0 (equal 107 CFU/mL). Then, a serial 10-fold dilution was made to obtain 10−1, 10−2, and 10−3 before spotting 4 μL of each dilution onto the YM plates with different stressors: ethanol (5, 7, 10, and 12% (v/v)), acetic acid (3, 5, 7, and 10 g/L), and furfural (1, 2, 3, and 5 g/L). Plates were incubated at 35 °C for 48 h and the growth performance of yeast cells was checked.
2.5.2. Fermentation of Sugars
The ability of selected yeast isolates to carry out fermentation of different carbon sources (glucose, galactose, fructose, saccharose, lactose, xylose, maltose, and arabinose) was examined according to a previous description by Chamnipa et al. (2018), with some modifications. Each selected yeast was grown on a Wikerham medium (1% peptone, 0.5% yeast extract, and 2% (w/v) of different sugars) supplemented with phenol red in Durham tubes, and incubated for three weeks at 40 °C. The tests were carried out in duplicate.
2.6. Optimization for Bioethanol Production from SCB Hydrolysate by Statistical Experimental Design
2.6.1. Plackett–Burman (PB) Design
Different fermentation factors that may affect bioethanol production were evaluated by Plackett–Burman (PB) design. Based on a literature review and our preliminary experiments, 8 fermentation factors as the independent variables were chosen for PB design including substrate concentration (SCB hydrolysate), pH, ammonium sulfate ((NH4)2SO4), calcium chloride (CaCl2·2H2O), zinc sulfate heptahydrate (ZnSO4·7H2O), magnesium sulfate (MgSO4·7H2O), manganese sulfate (MnSO4·H2O), and potassium phosphate monobasic (KH2PO4). The fermentation of twelve random runs which were generated from PB design by different combinations with two values of each investigated factor was performed at 40 °C for 48 h in 500 mL flasks with 20% working volume and shaking at 150 rpm (H2010, Benchmark Scientific).
Each experiment was carried out in triplicate. Statistical analysis was carried out by Design-Expert 12 (Stat-Ease Inc, Minneapolis, MN, USA). Pareto charts were plotted to stress the most significant factor responsible for bioethanol production. Each independent variable had two values and was investigated for the screening design as shown in Supplementary Table S2.
2.6.2. Response Surface Methodology
Based on the PB experiment results, three variables (substrate concentration, pH, ammonium sulfate (NH4)2SO4) that affect bioethanol production were further optimized using the Box-Behnken design (BBD). BBD was carried out with three significant variables each having 3 levels (low, middle, and high values) with 17 experimental designs as shown in Supplementary Table S3. Bioethanol production was represented as a second-order model in the form of a quadratic polynomial in the following equation:
where Y is the measured response; a, bi, bij, bii are constant coefficients and Xi, Xj represent coded values of the independent variables.
Y = a + ∑biXi + ∑bijXiXj + ∑biiX2i
Fermentation experiments were carried out in triplicate, with the same conditions mentioned above. Design Expert® Software (Version 12, Stat-Ease Inc, Minneapolis, MN, USA) was applied for designing the optimization experiments and data analysis. An analysis of variance (ANOVA) was used to evaluate the significance of fermentation factors. Statistically significant differences were designated as p < 0.05.
Validation experiments were carried out based on the optimized conditions determined from the response surface plots. Experiments were carried out as mentioned above. During fermentation, the fermentation broth was withdrawn periodically, and ethanol concentration and total residual sugars were analyzed following previously described protocols.
2.7. Analytical Methods
The bioethanol production was determined using aas chromatography (GC-2014, Shimadzu, Kyoto, Japan) equipped with a flame ionization detector and a polyethylene glycol (PEG-20M) packed column.
Samples from SCB hydrolysate and fermentation were filtered through a 0.22 µm membrane filter and analyzed using a high-performance liquid chromatography (HPLC) (Shimadzu, Kyoto, Japan) equipped with a Bio-Rad Aminex HPX-87H (300 × 7.8 mm) column. HPLC-RI method (refractive index detector) was used to detect sugars (glucose, fructose, xylose, arabinose), while other target compounds (acetic acid, formic acid, and furfural) were detected at the wavelength of 210 nm using the UV-VIS detector. In the mobile phase using (H2SO4) (5 mM), there was a flow rate of 0.6 mL/min at 40 °C. Ethanol productivity (Qp, g/L∙h) was calculated as the ratio between the greatest ethanol concentration (P, g/L) and the corresponding fermentation time (t, h) as the following equation:
Qp = P/t
3. Results and Discussion
3.1. Yeast Isolation and Thermotolerant Screening
Seventy-five yeast isolates were isolated from 15 samples of lychee at Thua Thien Hue. They were preliminarily screened on YMA supplemented with 4% (v/v) ethanol at 35 °C according to Limtong et al. (2007) [25]. Thereafter, all of them were examined for their ability to grow on YMA at 37, 40, and 45 °C. The results indicated that 32 isolates (H1–H32) of the yeasts grew well at 37 °C (Figure 1A), of which seven isolates could grow at 40 °C (Figure 1B). Four isolates could grow at 45 °C, and 12 exhibited poor growth, whereas three isolates (H1, H19, and H23) could grow well at this high temperature (Figure 1C). Our results are in line with those previous studies, in which thermotolerant yeasts could grow from 37 °C to 45 °C [18,26,27] and even up to 50 °C [28]. As a result, the seven isolates that grew at 40 °C were chosen to further examine bioethanol production.
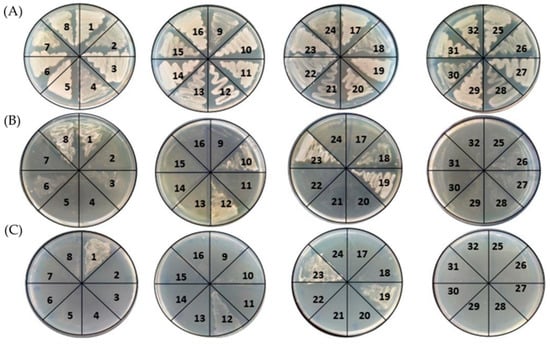
Figure 1.
Growth of 32 yeast isolates under various growth temperatures at 37 °C (A), 40 °C (B), and 45 °C (C), respectively, on YM medium agar for 48 h. H1-H32 isolates were indicated as 1–32.
3.2. Ethanol Fermentation Using Thermotolerant Yeasts
Seven strains (H1, H8, H10, H12, H18, H19, and H23) were selected to estimate their ability to produce bioethanol at high temperatures using YM broth with 160 g/L glucose for 48 h, and the results are given in Table 1.

Table 1.
Efficiency of bioethanol production at different temperatures by selected yeasts using glucose as sole carbon source.
At 37 °C, the yeast isolates that produced the maximum ethanol concentrations and ethanol volumetric productivities were in the range of 51.58–72.19 g/L and 1.07–1.5 g/L∙h, respectively. The thanol concentration produced by all isolates was slightly decreased and varied from 47.96 to 70.18 g/L with productivities from 1 to 1.46 at 40 °C. Notably, the ethanol concentrations as well as the volumetric ethanol productivities were significantly decreased at 45 °C, which confired the negative effect of high temperatures on the fermentation of the yeasts.
A comparison of the findings here with those of previous studies clearly illustrates that all isolates are highly efficient bioethanol producers. Chamnipa et al. (2018) [22] reported that 40 thermotolerant yeasts were able to produce ethanol in a range from 22.78 to 70.42 g/L at 40 °C from the same amount of glucose as we used. A slightly higher yield was reported by Phong et al. (2019) [27], who found that P. kudriavzevii CM4.2 produces 71.98 g/L at 40 °C for 48 h. However, our results were greater than those from S. cerevisiae VS3 (60 g/L) [17], S. cerevisiae DBKKU Y-53 (58.14 g/L) [29], and 26 isolated yeasts that could produce 16.52 to 53.89 g/L of ethanol from 160 g/L glucose at 40 °C in the study by Techaparin et al. (2017) [18].
3.3. Identification of Selected Thermotolerant Yeasts
The identification of yeast isolates was performed by a sequencing analysis of the 26S rDNA D1/D2 region as described in the methods sections. This technique has been widely used for the identification of hundreds of yeast strains, S. cerevisiae, K. marxianus, and P. kudriavzevii [27,30]. In accordance with the sequencing analysis of yeast isolates, seven selected thermotolerant isolates in this study were identified: isolates H1 and H12 (Genbank accession number: OP010064 and OP010065, respectively) belong to the M. guilliermondii species with 99% sequence homology. Isolates H10 and H18 (Genbank accession number: OP010083, ON775518) are 99% identical to S. cerevisiae while isolates 8, 19, and 23 (Genbank accession number: OP010088, OP010089, and OP010090) belong to C. tropicalis with 99% sequence similarity. Along with S. cerevisiae, it has been reported that C. tropicalis and M. guilliermondii yeasts are promising strains for bioethanol fermentation from different carbon sources, such as xylose [31] and sugarcane bagasse [32]. Therefore, identified strains may be good candidates for bioethanol production and hence be chosen for further experiments.
3.4. Selection of Superior Yeast Strains for Second-Generation Bioethanol Production
3.4.1. Tolerance of Thermotolerant Yeasts to Individual Stress Factors
In the second-generation ethanol production process, yeast cells may encounter various stresses, hyperosmolarity, high temperatures, weak acids, furan derivatives, and high ethanol concentration [33]. Therefore, seven selected strains were examined for their growth ability under different concentrations of stress factors, and the results are shown in Figure 2.

Figure 2.
Yeast growth under different stresses. Serial 10-fold dilutions of different isolates were spotted onto YMA plates supplemented with different stress concentrations of ethanol (A), acetic acid (B) and furfural (C). All tests were incubated at 37 °C for 48 h.
As results show in Figure 2A, all isolates were able to grow well in a medium supplemented with 5% ethanol. However, the growth of yeast cells was slightly decreased when they were grown on plates containing 7% ethanol, and significantly decreased in the presence of 10% ethanol, especially isolates H18 and H19. The egative effects of ethanol on yeast cell growth were clearly observed in the presence of 12% ethanol compared with other conditions. Among the seven selected strains, four isolates (H1, H8, H12, and H23) were able to grow in media supplemented with 12% (v/v) of ethanol, while three isolates (H10, H18, and H19) could not grow at all at this concentration. Nevertheless, the range of ethanol tolerance of isolated yeasts (H1, H8, H12, and H23) was 5–12%, compatible with the previous studies on thermotolerant yeasts isolated from some sources (soil, fruit, flowers) in the Mekong region [18,27].
In regards to acetic acid stress, all the isolates grew well in a medium supplemented with 3 g/L acetic acid and were slightly reduced in the presence of 5 g/L acetic acid. However, the growth performance of all isolates was negatively affected as the acetic acid concentration increased from 7 to 10 g/L: isolates H18 and H19 especially exhibited poor growth in the presence of 7 g/L acetic acid. Similar to our results, Favaro et al. (2013) [34] and Chamnipa et al. (2018) [22] reported that thermotolerant S. cerevisiae Fm17 and kudriavzevii RZ8-1 strains grow poorly in media containing 7.2 and 7.5 g/L acetic acid, respectively. On the other hand, two isolates, H1 and H23, were able to grow in the presence of 10 g/L acetic acid, while others could not (Figure 2B). A similar result was reported by Phong et al. (2019) [27], in which P. kudriavzevii CM4.2 was tolerant to 10 g/L acetic acid.
Concerning furfural stress, the growth of all selected isolates in the presence of 1 g/L furfural was remarkably similar, except for isolate H18 (Figure 2C). The growth performance of the isolates (H18, and H19) was significantly decreased when the plates contained 2 g/L furfural; others were not affected much at this dose of furfural. Increasing the furfural concentration in the medium to 3 g/L resulted in severely affected the growth of four isolates (H8, H10, H18, and H19). On the other hand, three isolates (H1, H12, and H23) could grow even at 5 g/L. Our results indicated that the selected isolates are more tolerant to furfural than some industrial S. cerevisiae [24]. In general, Figure 1 indicated that isolates H1 and H23 showed better growth than other isolates in ethanol, acetic acid, and furfural stress conditions.
3.4.2. Selected Yeast Fermenting Different Sugars
The ability of seven isolates to ferment different sugars at 40 °C was determined by their ability to produce gas in Durham test tubes. As the results show in Table 2, glucose, fructose, sucrose, galactose, and maltose were fermented by all isolates. While xylose and arabinose were only fermented by isolate H1, none of these isolates could ferment the lactose (Table 2).

Table 2.
Carbon fermentation assays of the isolated yeasts using the Durham test.
Generally, hexose sugars, maltose, sucrose, glucose, and mannose are the main carbon sources that yeasts prefer to use, but a few yeasts have the ability to ferment pentose, particularly xylose and arabinose [32]. According to Bai et al. (2008) [1], an important characteristic of industrial strains is their capability to ferment many different carbon sources in addition to glucose. The ability of yeast to convert both hexoses and pentoses to bioethanol is an extremely important factor to reduce the cost of bioethanol [30].
Our results indicated that the H1 isolate as M. guilliermondii H1 can produce bioethanol by using xylose and arabinose. These sugars are abundant in agricultural waste, especially in sugar cane bagasse. Therefore, the H1 yeast strain is the potential candidate for alcohol production from agricultural wastes.
3.5. Screening Factors Affecting Ethanol Production from SCB Hydrolysate Using M. guilliermondii H1 Strain by Plackett–Burman Design
Xylose is the main pentose sugar, making up approximately 70% of the overall total sugars obtained from the hemicellulosic acidic hydrolysate. Therefore, to obtain high yield bioethanol production from SCB, we have to choose a suitable strain. Among the selected isolates, the H19 isolate produced an ethanol concentration at the highest level compared to others at 40 °C, but it was less tolerant of the tested stressors (ethanol, acetic acid and furfural). H23 is highly tolerant of the tested stressors and produced bioethanol efficiently compared to H1. However, H1 is more suitable for second-generation bioethanol production since this strain can grow at 45 °C, in the presence of 12% (v/v) ethanol, 10 (g/L) acetic acid, and 5 (g/L) furfural (Figure 1 and Figure 2), and more importantly it can utilize xylose and arabinose, which are the main components of SCB.
We aim to find out the key factors that have significant effects on bioethanol production from SCB hydrolysate by M. guilliermondii H1 at 40 °C. Eight variables, substrate concentration (non-detoxified SCB hydrolysate supplementation with yeast extract, xylose and glucose), pH, (NH4)2SO4, MgSO4·7H2O, MnSO4·H2O, ZnSO4·7H2O, KH2PO4, and CaCl2·2H2O were investigated using the Plackett–Burman design. The experimental matrices and the variables from the 12 experimental runs were evaluated and the results are shown in Supplementary Table S2. The results showed that experimental run no 11 achieved the highest ethanol concentration (11.08 g/L), followed by experimental run no 7 and run no 3 (10.15 and 9.48 g/L, respectively), while the lowest ethanol concentration (5.17 g/L) was produced from experimental run no.9. The results of the statistical analysis given in Table 3 pointed out that the model is significant since p = 0.008 (< 0.05) and revealed three variables: (NH4)2SO4, substrate concentration, and pH, which are considered to significantly affect bioethanol production (p-value <0.05) from SCB hydrolysate using M. guilliermondii H1, while the rest of the variables had no significant effect on bioethanol production. In agreement with the statistical analysis, the results from the Pareto chart (Figure 3) indicated that the t-values of three variables, (NH4)2SO4, substrate concentration, and pH exceeded the t-value limit of 3.182, which suggests these factors were highly significant factors affecting the bioethanol production from SCB hydrolysate using M. guilliermondii H1. Based on the PB design, a variable has a significant effect if the studied effect is greater than zero, whereas if the studied effect is less than zero, the effect is insignificant. Consequently, substrate concentration, pH, (NH4)2SO4, MgSO4·7H2O, CaCl2·2H2O, and ZnSO4·7H2O had positive effects on bioethanol production, whereas MnSO4·H2O and KH2PO4 had negative effects on bioethanol production.

Table 3.
Analysis of variance of PB design for ethanol production by M. guilliermondii H1.
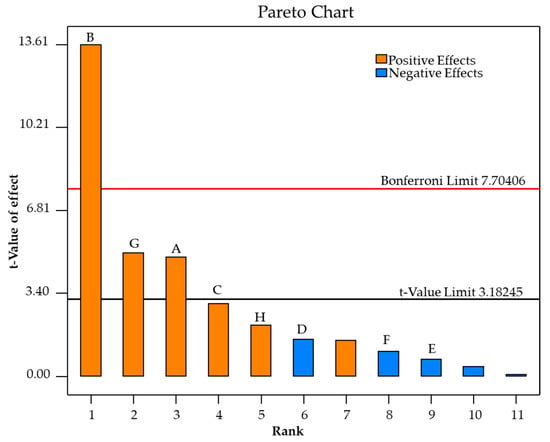
Figure 3.
Pareto chart exhibiting effects of (NH4)2SO4 (A); Substrate concentration (B); ZnSO4.7H2O concentration (C); MgSO4·7H2O concentration (D); MnSO4·H2O concentration (E), KH2PO4 concentration (F); initial pH (G) and CaCl2. 2H2O (H) on bioethanol production from substrate-based SCB hydrolysate.
3.6. Optimization of Ethanol Production from SCB Hydrolysate by M. guilliermondii H1 Strain Using Box–Behnken Design
The interaction of three significant factors, generated from the PB design, was investigated by the Box–Behnken design to identify the best combination of these factors for the highest bioethanol production. The random combination of three values of each significant factor which generated seventeen experimental runs is shown in Supplementary Table S3. The variation in the ethanol concentration from different combinations ranged from 6.35 to 11.12 g/L. The relationship between the concentration of (NH4)2SO4 (A), substrate concentration (B), and the initial pH (C) was estimated in a model of quadratic multiple regression and used to develop a second-order polynomial equation (1) for predicting the final ethanol concentration (P, g/L). The prediction model for the three variables made the following equation:
P(g/L) = 10.3 + 0.76A + 1.48B + 0.94C + 0.1AB + 0.13AC + 0.19BC − 0.84A2 − 0.9B2 − 0.83C2
Table 4 shows that this model (p < 0.01) based on a statistical analysis of variance (ANOVA) with a 95% confidence interval. Moreover, the quadratic of the individual investigated factors (A2, B2, and C2) were super significant (p < 0.001), and the interactions between A and C as well as B and C were more significant than that between A and B. The coefficient of determination (R2) of the regression model in this study was 0.9993, indicating our model was accurate to explain 99.93% of the data Myers et al. (2016) [36] suggested that R2 values (≥ 0.75) indicate that the model is accurate, hence the model in this study is reliable.

Table 4.
Analysis of variance of the Box–Behnken model for ethanol production by M. guilliermondii H1.
The three-dimensional (3D) response surface plots and their related contour plots were depicted based on the equation models to explore the interactive effect of the (NH4)2SO4 concentration (A), substrate concentration (B), and initial pH (C) on bioethanol production, and also to determine the optimum level of each factor for bioethanol production from SCB hydrolysate using M. guilliermondii H1. The effects of varying concentrations of (NH4)2SO4 (A) and one of the other factors are displayed in Figure 4. The shape of the contour plot represents the interaction between the variables. Circular plots represent weak interactions, whereas elliptical plots represent significant interactions (Prakash et al., 2008). The shape of contour plots was found to be elliptical (Figure 4D–F) indicating the increasing level of each variable contributes to the yield of ethanol production. The 3D response surface plots describing the interactive effects of (NH4)2SO4 concentration (A) and the substrate concentration (B) are presented in Figure 4A. A simultaneous increase in the (NH4)2SO4 concentration (above 1.5) and the substrate concentration (B) (above 20 g/L), resulted in increases in ethanol production when the initial pH was fixed at the center point (pH = 5). In agreement with this, the ethanol concentration increased when the (NH4)2SO4 concentration increased from 1.5 to3 g/L and the pH value was in the range of 5–6 (Figure 4B). Figure 4C describes the effects of substrate concentration (B) and initial pH (C) on ethanol production. The higher level of the two variables enhances bioethanol production from 6.35 g/L to a maximum concentration of 11.12 g/L when the (NH4)2SO4 concentration is kept at the center point value (1.55 g/L).
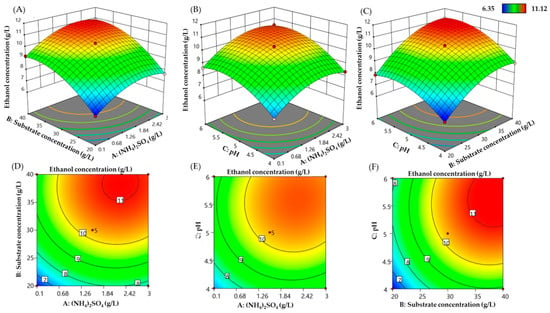
Figure 4.
Response surface plot and related contour plot displaying the simultaneous effects of (NH4)2SO4 andsubstrate concentration (A,D); (NH4)2SO4 and initial pH (B,E); substrate concentration and initial pH (C,F) on ethanol production.
Therefore, the bioethanol production was significantly impacted by selected factors: (NH4)2SO4, substrate concentration, and pH, in which more effects were produced by the substrate concentration.
The carbon and nitrogen sources are important factors, influencing yeast cell growth and bioethanol production [37]. The effect of (NH4)2SO4 on bioethanol production from SCB using M. guilliermondii H1 was determined, with a concentration ranging from 0.1 to 3 g/L. When two variables, substrate concentration 40 g/L and pH 5, were kept constant, ethanol production was increased from 9.22 to 10.85 g/L when the medium was supplemented with 0.1 g/L and with 3 g/L (NH4)2SO4, respectively. A positive relationship between (NH4)2SO4 concentration and ethanol was also found in previous studies; for example, Li et al. (2017) [38] revealed that a small portion of ethanol was produced by S. cerevisiae using (NH4)2SO4 as the sole nitrogen source. Moreover, the ethanol concentration was increased from 6.85 to 7.8% when the medium was supplemented with 0.07% (NH4)2SO4 [25]. On the other hand, the effect of (NH4)2SO4 on ethanol production was not determined in another study [39] or it had an insignificant effect [40].
The substrate concentration promoted bioethanol production as described in both the Plackett–Burman screening test and also in the response surface Box–Behnken experiments. The significantly positive effects of a substrate are possibly because of its sugar capacity, which induces yeast cell growth and bioethanol production. The maximum ethanol concentration, 11.12 g/L, was achieved when the substrate concentration increased to 40 g/L (and two factors (NH4)2SO4 and initial pH were 1.55 g/L and 6, respectively). These findings are optimistic and comparable with previous studies using SCB as substrate. For example, Dasgupta et al. (2013) [41] obtained an 11.51 g/L ethanol concentration from 40 g/L bagasse pith hydrolysate using the Kluyveromyces sp. IIPE45 strain. S. shehatae CG8-8BY S. shehatae BR6-2AY produced 10.96 and 11.49 g/L ethanol concentrations from SCB supplemented with 0.3% yeast extract [42]. The ethanol concentration obtained in this study is significantly higher than in several previous ones. For example, Ferreira et al. (2011) [43] obtained a 6.4 g/L ethanol concentration from SCB supplemented with 30 g/L xylose using the yeast S. stipitis UFMG-IMH 43.2. Moreover, Canilha et al. (2010) [44] reported that using an SB-based substrate supplemented with 0.3% yeast extract, 0.3 % malt extract, and 0.5 % peptone (w/v) could produce a 6.1 g/L ethanol concentration.
The substrate is one of the critical factors in the production of ethanol, not only in regards to type but also to the concentration, because too much substrate assembly could induce an inhibitory effect on fermenting ethanol [41]. Moreover, the production of ethanol also dependeds on working volume, inoculum type and size, and terms of interactions between these factors with the substrate and others [45].
The pH is known to be vital, particularly in influencing yeast growth and metabolism. A pH in the range from 4 to 6, has been determined as optimal for fermenting ethanol; however, these values also depend other factors, such as fermentation temperature, yeast species, and others [45,46]. Our data indicated that the minimal values of ethanol concentration were achieved from fermentation at pH 4 as a result of runs no (1, 7, 11, and 16), and significantly increased when fermentation was performed at higher pH, in which the optimal pH for ethanol production from SCB using M. guilliermondii H1 was 6. Similar results were observed in previous studies: ethanol production from pineapple waste hydrolysate by P. kudriavzevii CM4.2 had an optimal pH of 5.8, while Pichia stipitis NRRL-124 produced the highest ethanol concentration at a pH 5.89 [47]. A pH 5.82 was optimal in the studies by Nuanpeng et al. (2016) [29] and Techaparin et al. (2017) [18]. Limtong et al. (2007) [25] found that sugar cane juice fermentation at pH 4–4.5 yielded lower ethanol concentrations than those obtained from fermentation at pH 5–5.5 by Kluyveromyces marxianus.
Under optimal conditions, M. guilliermondii H1 could convert sugars to ethanol with its highest productivity at 0.23 g/L.h (equal to 11.12/48 h). Comparable results were also observed in the study of Cadete et al. (2012) [48], in which S. stipitis UFMG-XMD-15.2 could produce 0.2 g/L ethanol per hour from SCB. The obtained result was lower than that of the study by Sasikumar and Viruthagiri (2008) [49], in which 32.6 g/L ethanol was produced from 180 g/L pretreated sugarcane bagasse using Kluyveromyces fragilis at the optimal fermentation for 72 h. However, our results are significantly higher than previous reports, in which thermotolerant C. tropicalis HNMA-1 converted 35 g/L of total sugar from SCB to ethanol with a productivity of 0.150 g/L·h [14] and ethanol productivities of 0.12 g/L·h by S. stipitis NRRL Y-7124 [20] and 0.041 g/L.h by Pichia strain BY2 from SCB acid hydrolysate [50]. The differences may be that M. guilliermondii H1 can tolerate highly inhibitory compounds derived from SCB pretreatment, furfural, and hydroxymethyl furfural, which inhibit yeast cell growth by prolonging the lag phase of cell growth [51]. With its ability to grow in non-detoxified hydrolysates, the M. guilliermondii H1 strain has a great potential for the use in bioethanol production due to saving sugar, energy, and time in the detoxification process. This is a fascinating result since most of the yeasts are unable to survive and metabolize in the culture containing inhibitors originating from acidic hydrolysis hemicellulose.
Previous studies also indicated that M. guilliermondii can grow in SCB hydrolysate (non-detoxified) and produce high ethanol concentrations [32,52]. More recently, Trichez et al. (2019) [53] reported that some yeast strains, M. guilliermondii, could produce 8.57 g/L of ethanol concentration from non-detoxified acidic hydrolysis sugarcane biomass consisting of xylose (40 g/L) and glucose (8 g/L) with the presence of 6 (g/L) acetic acid. These findings support our results and suggest that M. guilliermondii H1 is a promising strain for fermenting ethanol from the pretreatment of hemicellulose wastes which commonly contain a high concentration of acetic acid and furfural.
3.7. Validation of the Model
By virtue of the specific characteristics of the BBD, a point prediction and contour plot producing the optimal value of the combination of the three fermentation factors were used to obtain the the highest level of ethanol production. To validate the model, experiments were performed in 500 mL shake flasks under predicted conditions. There was a good agreement between the predicted and experimental values, indicating the validity of the BBD. Based on the validated model and the experimental data, the optimal pH value is 5.95, the substrate concentration is 40 g/L, and (NH4)2SO4 is 1.55 g/L in the second-order polynomial equation, while keeping other factors at their central levels. As per the result shown in Table 5, the highest ethanol concentration (11.13 ± 0.062 g/L), was obtained within 48 h of fermentation from substrate-based SCB hydrolysate by M. guilliermondii H1 at 40 °C under optimal conditions, which was close to the predicted values (11.15), as stated by Levin et al. (2008) [54]; the difference between the predicted and experimental values differ was less than 10%, supporting the validity of the model. Therefore, the model established in this study is reliable and reproducible.

Table 5.
Concentration of individual sugars in ethanol production using M. guilliermondii H1 at pH 5.95, 40 °C and 1.55 g/L (NH4)2SO4 for 48 h.
4. Conclusions
In this study, seven thermotolerant yeast strains belonging to S. cerevisiae, C. tropicalis, and M. guilliermondii were successfully isolated. They could tolerate different levels of stressors, high ethanol, acetic acid, and furfural concentrations, and produce relatively high ethanol concentrations at 40 °C ranging from 47.96 to 70.18 g/L using 160 g/L glucose as the substrate. Among the seven thermotolerant yeasts, M. guilliermondii H1 was found to be a good ethanol producer from SCB hydrolysate since this strain could ferment xylose and arabinose and was also highly tolerant of all tested stressors. Plackett–Burman and Box–Behnken designs were used to optimize the conditions for bioethanol production by M. guilliermondii H1 from substrate-based SCB hydrolysate. A substrate concentration of 40 g/L, an initial pH of 6, and 1.55 g/L of (NH4)2SO4 were used. The highest ethanol concentration was 11.12 g/L when compared to that of the unoptimized condition (6.35 g/L). Our results indicate that this candidate yeast shows potential as a second-generation bioethanol producer and represents a promising starting point to enhance bioethanol production processes from SCB in the near future.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fermentation8100515/s1, Table S1: Concentration of sugars and inhibitors in sugarcane bagasse hydrolysate; Table S2: Matrix design for experimental and predicted values of efficiency of ethanol production (after Plackett–Burman) from SCB using M. guilliermondii H1 at 40 °C for 48 h; Table S3: Matrix design for experimental and predicted values of efficiency of ethanol production (after Box–Behnken) from SCB using M. guilliermondii H1 at 40 °C for 48 h.
Author Contributions
Conceptualization, P.V.N. and K.C.T.N.; original draft preparation, X.T.T.H. and K.H.V.N.; methodology and validation, N.L.N., P.V.N., K.C.T.N.; X.T.T.H. and K.H.V.N.; writing, reviewing and editing, P.V.N., P.H.T., K.H.V.N. and K.C.T.N. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by Hue University under the Core Research Program, Grant No. NCM.DHH2020.13.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
The authors thank Derek Wilkinson for proofreading of the manuscript.
Conflicts of Interest
No potential conflict of interest was reported by the authors.
References
- Bai, F.W.; Anderson, W.A.; Moo-Young, M. Ethanol Fermentation Technologies from Sugar and Starch Feedstocks. Biotechnol. Adv. 2008, 26, 89–105. [Google Scholar] [CrossRef] [PubMed]
- Farrell, A.E.; Plevin, R.J.; Turner, B.T.; Jones, A.D.; O’hare, M.; Kammen, D.M. Ethanol Can Contribute to Energy and Environmental Goals. Science 2006, 311, 506–508. [Google Scholar] [CrossRef] [PubMed]
- Harun, R.; Danquah, M.K.; Forde, G.M. Microalgal Biomass as a Fermentation Feedstock for Bioethanol Production. J. Chem. Technol. Biotechnol. 2010, 85, 199–203. [Google Scholar] [CrossRef]
- Christensen, E.; Yanowitz, J.; Ratcliff, M.; McCormick, R.L. Renewable Oxygenate Blending Effects on Gasoline Properties. Energy Fuels 2011, 25, 4723–4733. [Google Scholar] [CrossRef]
- Månsson, A.; Johansson, B.; Nilsson, L.J. Assessing Energy Security: An Overview of Commonly Used Methodologies. Energy 2014, 73, 1–14. [Google Scholar] [CrossRef]
- Carrillo-Nieves, D.; Alanís, M.J.R.; de la Cruz Quiroz, R.; Ruiz, H.A.; Iqbal, H.M.N.; Parra-Saldívar, R. Current Status and Future Trends of Bioethanol Production from Agro-Industrial Wastes in Mexico. Renew. Sustain. Energy Rev. 2019, 102, 63–74. [Google Scholar] [CrossRef]
- Ho, D.P.; Ngo, H.H.; Guo, W. A Mini Review on Renewable Sources for Biofuel. Bioresour. Technol. 2014, 169, 742–749. [Google Scholar] [CrossRef]
- Canilha, L.; Chandel, A.K.; Suzane dos Santos Milessi, T.; Antunes, F.A.F.; Luiz da Costa Freitas, W.; das Graças Almeida Felipe, M.; da Silva, S.S. Bioconversion of Sugarcane Biomass into Ethanol: An Overview about Composition, Pretreatment Methods, Detoxification of Hydrolysates, Enzymatic Saccharification, and Ethanol Fermentation. J. Biomed. Biotechnol. 2012, 2012, 989572. [Google Scholar] [CrossRef]
- Fonseca, B.G.; Moutta, R.d.O.; Ferraz, F.d.O.; Vieira, E.R.; Nogueira, A.S.; Baratella, B.F.; Rodrigues, L.C.; Hou-Rui, Z.; Da Silva, S.S. Biological Detoxification of Different Hemicellulosic Hydrolysates Using Issatchenkia Occidentalis CCTCC M 206097 Yeast. J. Ind. Microbiol. Biotechnol. 2011, 38, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qin, Y.; Xu, J.; Zeng, W.; Zhang, Y.; Wang, W.; Wang, P. Crack Resistance Investigation of Mixtures with Reclaimed SBS Modified Asphalt Pavement Using the SCB and DSCT Tests. Constr. Build. Mater. 2020, 265, 120365. [Google Scholar] [CrossRef]
- Abdel-Banat, B.; Hoshida, H.; Ano, A.; Nonklang, S.; Akada, R. High-Temperature Fermentation: How Can Processes for Ethanol Production at High Temperatures Become Superior to the Traditional Process Using Mesophilic Yeast? Appl. Microbiol. Biotechnol. 2010, 85, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Ryabova, O.B.; Chmil, O.M.; Sibirny, A.A. Xylose and Cellobiose Fermentation to Ethanol by the Thermotolerant Methylotrophic Yeast Hansenula Polymorpha. FEMS Yeast Res. 2003, 4, 157–164. [Google Scholar] [CrossRef]
- Urbina, H.; Blackwell, M. Multilocus Phylogenetic Study of the Scheffersomyces Yeast Clade and Characterization of the N-Terminal Region of Xylose Reductase Gene. PLoS ONE 2012, 7, e39128. [Google Scholar] [CrossRef] [PubMed]
- Nouri, H.; Azin, M.; Mousavi, M.L. Xylan-Hydrolyzing Thermotolerant Candida tropicalis HNMA-1 for Bioethanol Production from Sugarcane Bagasse Hydrolysate. Ann. Microbiol. 2017, 67, 633–641. [Google Scholar] [CrossRef]
- Steensels, J.; Snoek, T.; Meersman, E.; Nicolino, M.P.; Voordeckers, K.; Verstrepen, K.J. Improving Industrial Yeast Strains: Exploiting Natural and Artificial Diversity. FEMS Microbiol. Rev. 2014, 38, 947–995. [Google Scholar] [CrossRef]
- Tanimura, A.; Kikukawa, M.; Yamaguchi, S.; Kishino, S.; Ogawa, J.; Shima, J. Direct Ethanol Production from Starch Using a Natural Isolate, Scheffersomyces Shehatae: Toward Consolidated Bioprocessing. Sci. Rep. 2015, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sree, N.K.; Sridhar, M.; Suresh, K.; Banat, I.M.; Rao, L.V. Isolation of Thermotolerant, Osmotolerant, Flocculating Saccharomyces cerevisiae for Ethanol Production. Bioresour. Technol. 2000, 72, 43–46. [Google Scholar] [CrossRef]
- Techaparin, A.; Thanonkeo, P.; Klanrit, P. High-Temperature Ethanol Production Using Thermotolerant Yeast Newly Isolated from Greater Mekong Subregion. Braz. J. Microbiol. 2017, 48, 461–475. [Google Scholar] [CrossRef]
- Pongcharoen, P.; Chawneua, J.; Tawong, W. High Temperature Alcoholic Fermentation by New Thermotolerant Yeast Strains Pichia Kudriavzevii Isolated from Sugarcane Field Soil. Agric. Nat. Resour. 2018, 52, 511–518. [Google Scholar] [CrossRef]
- Milessi, T.S.S.; Antunes, F.A.F.; Chandel, A.K.; Silva, S.S. Rice Bran Extract: An Inexpensive Nitrogen Source for the Production of 2G Ethanol from Sugarcane Bagasse Hydrolysate. 3 Biotech 2013, 3, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.C.; Van Nguyen, P.; Truong, H. Heavy Metal Tolerance of Novel Papiliotrema Yeast Isolated from Vietnamese Mangosteen. Mycobiology 2020, 48, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Chamnipa, N.; Thanonkeo, S.; Klanrit, P.; Thanonkeo, P. The Potential of the Newly Isolated Thermotolerant Yeast Pichia Kudriavzevii RZ8-1 for High-Temperature Ethanol Production. Braz. J. Microbiol. 2018, 49, 378–391. [Google Scholar] [CrossRef] [PubMed]
- Kurtzman, C.P.; Robnett, C.J. Identification and Phylogeny of Ascomycetous Yeasts from Analysis of Nuclear Large Subunit (26S) Ribosomal DNA Partial Sequences. Antonie Van Leeuwenhoek 1998, 73, 331–371. [Google Scholar] [CrossRef]
- Li, H.; Wu, M.; Xu, L.; Hou, J.; Guo, T.; Bao, X.; Shen, Y. Evaluation of Industrial Saccharomyces cerevisiae Strains as the Chassis Cell for Second-generation Bioethanol Production. Microb. Biotechnol. 2015, 8, 266–274. [Google Scholar] [CrossRef]
- Limtong, S.; Sringiew, C.; Yongmanitchai, W. Production of Fuel Ethanol at High Temperature from Sugar Cane Juice by a Newly Isolated Kluyveromyces Marxianus. Bioresour. Technol. 2007, 98, 3367–3374. [Google Scholar] [CrossRef] [PubMed]
- Pongcharoen, P. The Ability of Pichia Kudriavzevii to Tolerate Multiple Stresses Makes It Promising for Developing Improved Bioethanol Production Processes. Lett. Appl. Microbiol. 2022, 75, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Phong, H.X.; Klanrit, P.; Dung, N.T.P.; Yamada, M.; Thanonkeo, P. Isolation and Characterization of Thermotolerant Yeasts for the Production of Second-Generation Bioethanol. Ann. Microbiol. 2019, 69, 765–776. [Google Scholar] [CrossRef]
- Talukder, A.A.; Easmin, F.; Mahmud, S.A.; Yamada, M. Thermotolerant Yeasts Capable of Producing Bioethanol: Isolation from Natural Fermented Sources, Identification and Characterization. Biotechnol. Biotechnol. Equip. 2016, 30, 1106–1114. [Google Scholar] [CrossRef]
- Nuanpeng, S.; Thanonkeo, S.; Yamada, M.; Thanonkeo, P. Ethanol Production from Sweet Sorghum Juice at High Temperatures Using a Newly Isolated Thermotolerant Yeast Saccharomyces cerevisiae DBKKU Y-53. Energies 2016, 9, 253. [Google Scholar] [CrossRef]
- Koutinas, M.; Patsalou, M.; Stavrinou, S.; Vyrides, I. High Temperature Alcoholic Fermentation of Orange Peel by the Newly Isolated Thermotolerant Pichia Kudriavzevii KVMP 10. Lett. Appl. Microbiol. 2016, 62, 75–83. [Google Scholar] [CrossRef]
- Matos, I.T.S.R.; Cassa-Barbosa, L.A.; Galvão, R.; Nunes-Silva, C.G.; Astolfi Filho, S. Isolation, Taxonomic Identification and Investigation of the Biotechnological Potential of Wild-Type Meyerozyma guilliermondii Associated with Amazonian Termites Able to Ferment D-Xylose. Biosci. J. 2014, 30, 260–266. [Google Scholar]
- Martini, C.; Tauk-Tornisielo, S.M.; Codato, C.B.; Bastos, R.G.; Ceccato-Antonini, S.R. A Strain of Meyerozyma guilliermondii Isolated from Sugarcane Juice Is Able to Grow and Ferment Pentoses in Synthetic and Bagasse Hydrolysate Media. World J. Microbiol. Biotechnol. 2016, 32, 1–9. [Google Scholar] [CrossRef]
- Gibson, B.R.; Lawrence, S.J.; Leclaire, J.P.R.; Powell, C.D.; Smart, K.A. Yeast Responses to Stresses Associated with Industrial Brewery Handling. FEMS Microbiol. Rev. 2007, 31, 535–569. [Google Scholar] [CrossRef]
- Favaro, L.; Basaglia, M.; Trento, A.; Van Rensburg, E.; García-Aparicio, M.; Van Zyl, W.H.; Casella, S. Exploring Grape Marc as Trove for New Thermotolerant and Inhibitor-Tolerant Saccharomyces cerevisiae Strains for Second-Generation Bioethanol Production. Biotechnol. Biofuels 2013, 6, 168. [Google Scholar] [CrossRef]
- Kurtzman, C.P.; Fell, J.W.; Boekhout, T.; Robert, V. Methods for Isolation, Phenotypic Characterization and Maintenance of Yeasts. In The Yeasts; Elsevier: Amsterdam, The Netherlands, 2011; pp. 87–110. [Google Scholar]
- Myers, R.H.; Montgomery, D.C.; Anderson-Cook, C.M. Response Surface Methodology: Process and Product Optimization Using Designed Experiments; John Wiley & Sons: New York, NY, USA, 2016; ISBN 1118916034. [Google Scholar]
- Nissen, T.L.; Kielland-Brandt, M.C.; Nielsen, J.; Villadsen, J. Optimization of Ethanol Production in Saccharomyces cerevisiae by Metabolic Engineering of the Ammonium Assimilation. Metab. Eng. 2000, 2, 69–77. [Google Scholar] [CrossRef]
- Li, Z.; Wang, D.; Shi, Y.-C. Effects of Nitrogen Source on Ethanol Production in Very High Gravity Fermentation of Corn Starch. J. Taiwan Inst. Chem. Eng. 2017, 70, 229–235. [Google Scholar] [CrossRef]
- Yue, G.; Yu, J.; Zhang, X.; Tan, T. The Influence of Nitrogen Sources on Ethanol Production by Yeast from Concentrated Sweet Sorghum Juice. Biomass Bioenergy 2012, 39, 48–52. [Google Scholar] [CrossRef]
- Izmirlioglu, G.; Demirci, A. Enhanced Bio-Ethanol Production from Industrial Potato Waste by Statistical Medium Optimization. Int. J. Mol. Sci. 2015, 16, 24490–24505. [Google Scholar] [CrossRef]
- Dasgupta, D.; Suman, S.K.; Pandey, D.; Ghosh, D.; Khan, R.; Agrawal, D.; Jain, R.K.; Vadde, V.T.; Adhikari, D.K. Design and Optimization of Ethanol Production from Bagasse Pith Hydrolysate by a Thermotolerant Yeast Kluyveromyces Sp. IIPE453 Using Response Surface Methodology. SpringerPlus 2013, 2, 159. [Google Scholar] [CrossRef]
- Martiniano, S.E.; Chandel, A.K.; Soares, L.C.S.R.; Pagnocca, F.C.; da Silva, S.S. Evaluation of Novel Xylose-Fermenting Yeast Strains from Brazilian Forests for Hemicellulosic Ethanol Production from Sugarcane Bagasse. 3 Biotech 2013, 3, 345–352. [Google Scholar] [CrossRef]
- Ferreira, A.D.; Mussatto, S.I.; Cadete, R.M.; Rosa, C.A.; Silva, S.S. Ethanol Production by a New Pentose-fermenting Yeast Strain, Scheffersomyces Stipitis UFMG-IMH 43.2, Isolated from the Brazilian Forest. Yeast 2011, 28, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Canilha, L.; Carvalho, W.; de Almeida Felipe, M.d.G.; de Almeida e Silva, J.B.; Giulietti, M. Ethanol Production from Sugarcane Bagasse Hydrolysate Using Pichia Stipitis. Appl. Biochem. Biotechnol. 2010, 161, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Moodley, P.; Kana, E.B.G. Bioethanol Production from Sugarcane Leaf Waste: Effect of Various Optimized Pretreatments and Fermentation Conditions on Process Kinetics. Biotechnol. Rep. 2019, 22, e00329. [Google Scholar] [CrossRef] [PubMed]
- Narendranath, N.V.; Power, R. Relationship between PH and Medium Dissolved Solids in Terms of Growth and Metabolism of Lactobacilli and Saccharomyces cerevisiae during Ethanol Production. Appl. Environ. Microbiol. 2005, 71, 2239–2243. [Google Scholar] [CrossRef] [PubMed]
- Jargalsaikhan, O.; Saraçoğlu, N. Application of Experimental Design Method for Ethanol Production by Fermentation of Sunflower Seed Hull Hydrolysate Using Pichia Stipitis NRRL-124. Chem. Eng. Commun. 2008, 196, 93–103. [Google Scholar] [CrossRef]
- Cadete, R.M.; Melo, M.A.; Dussan, K.J.; Rodrigues, R.C.L.B.; Silva, S.S.; Zilli, J.E.; Vital, M.J.S.; Gomes, F.C.O.; Lachance, M.-A.; Rosa, C.A. Diversity and Physiological Characterization of D-Xylose-Fermenting Yeasts Isolated from the Brazilian Amazonian Forest. PLoS ONE 2012, 7, e43135. [Google Scholar] [CrossRef]
- Sasikumar, E.; Viruthagiri, T. Optimization of Process Conditions Using Response Surface Methodology (RSM) for Ethanol Production from Pretreated Sugarcane Bagasse: Kinetics and Modeling. BioEnergy Res. 2008, 1, 239–247. [Google Scholar] [CrossRef]
- Hande, A.; Mahajan, S.; Prabhune, A. Evaluation of Ethanol Production by a New Isolate of Yeast during Fermentation in Synthetic Medium and Sugarcane Bagasse Hemicellulosic Hydrolysate. Ann. Microbiol. 2013, 63, 63–70. [Google Scholar] [CrossRef]
- Kwon, Y.-J.; Ma, A.-Z.; Li, Q.; Wang, F.; Zhuang, G.-Q.; Liu, C.-Z. Effect of Lignocellulosic Inhibitory Compounds on Growth and Ethanol Fermentation of Newly-Isolated Thermotolerant Issatchenkia Orientalis. Bioresour. Technol. 2011, 102, 8099–8104. [Google Scholar] [CrossRef]
- Perna, M.d.S.C.; Bastos, R.G.; Ceccato-Antonini, S.R. Single and Combined Effects of Acetic Acid, Furfural, and Sugars on the Growth of the Pentose-Fermenting Yeast Meyerozyma Guilliermondii. 3 Biotech 2018, 8, 119. [Google Scholar] [CrossRef]
- Trichez, D.; Steindorff, A.S.; Soares, C.E.V.F.; Formighieri, E.F.; Almeida, J.R.M. Physiological and Comparative Genomic Analysis of New Isolated Yeasts Spathaspora Sp. JA1 and Meyerozyma Caribbica JA9 Reveal Insights into Xylitol Production. FEMS Yeast Res. 2019, 19, foz034. [Google Scholar] [CrossRef] [PubMed]
- Levin, L.; Herrmann, C.; Papinutti, V.L. Optimization of Lignocellulolytic Enzyme Production by the White-Rot Fungus Trametes Trogii in Solid-State Fermentation Using Response Surface Methodology. Biochem. Eng. J. 2008, 39, 207–214. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).