The Effect of Germanium-Loaded Hydroxyapatite Biomaterials on Bone Marrow Mesenchymal Stem Cells Growth
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials and Composition
2.2. Hydroxyapatite Synthesis
2.3. Fabrication of HA-Ge Composite
2.4. Composite Characterization
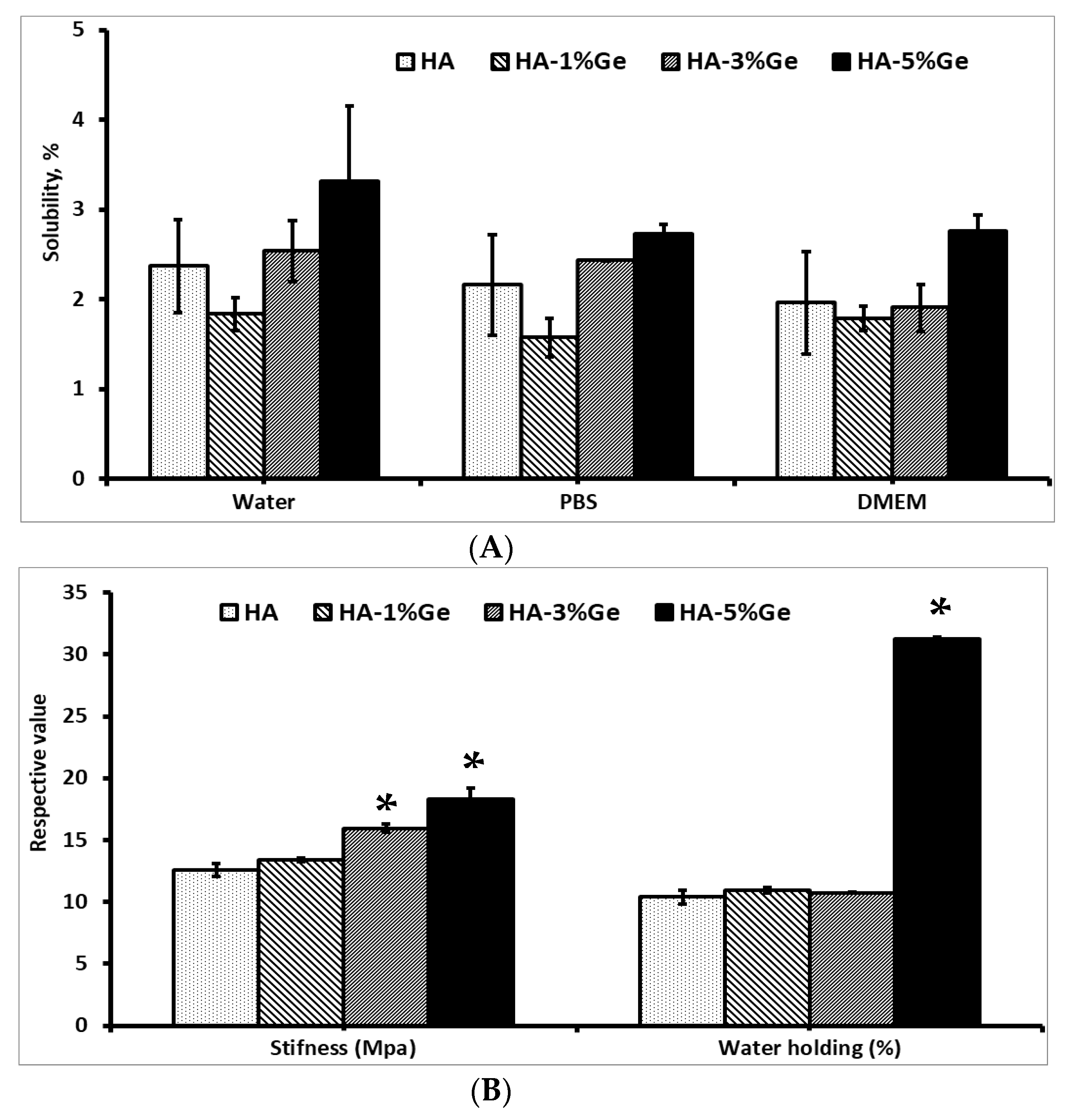
2.5. Mechanical Properties
2.6. Solubility
2.7. Water Absorption Rate
2.8. Swelling Rate
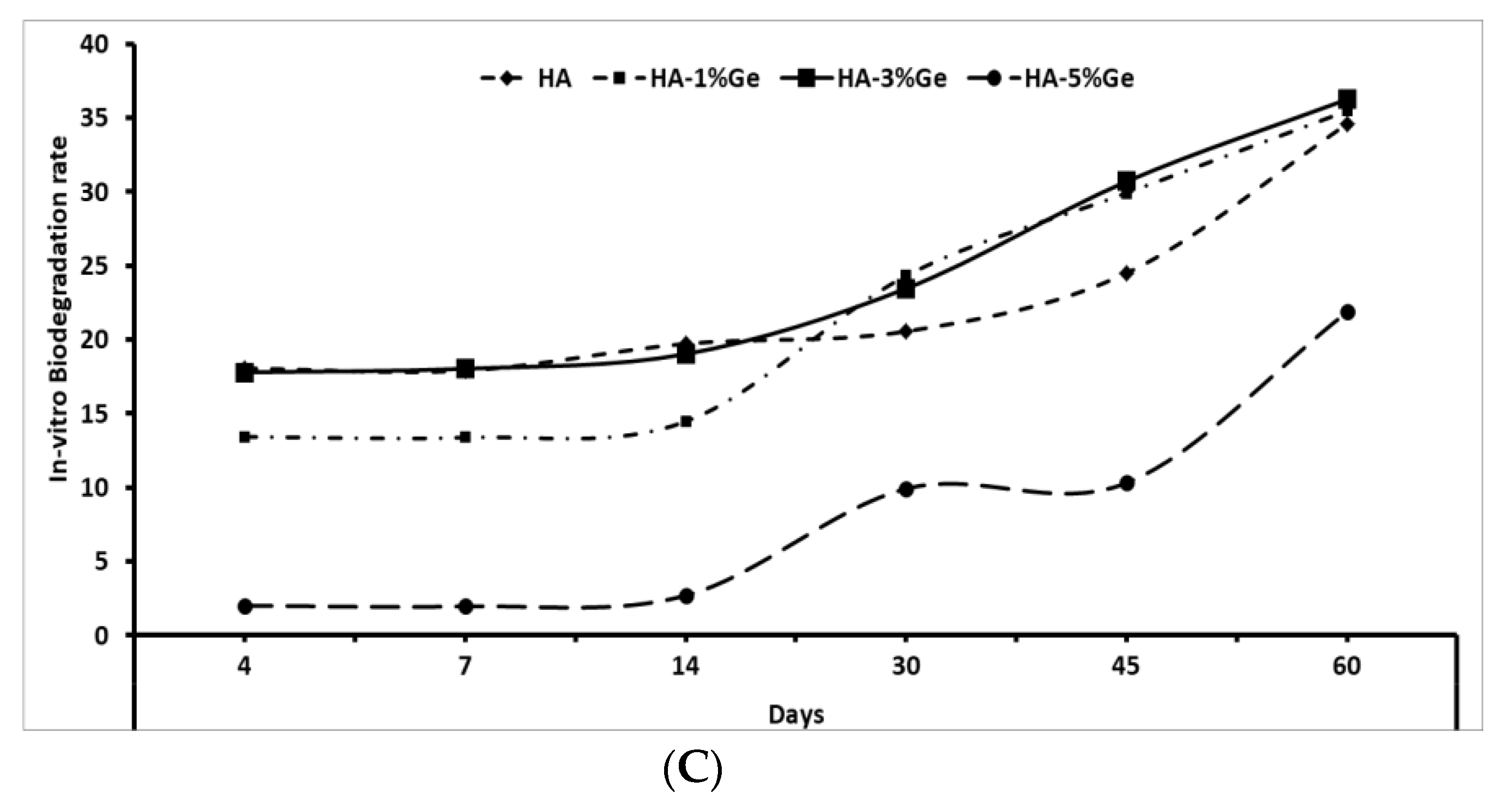
2.9. In-Vitro Degradation
2.10. Water Angle Contact
2.11. Protein Adsorption Ability
2.12. Bioactivity
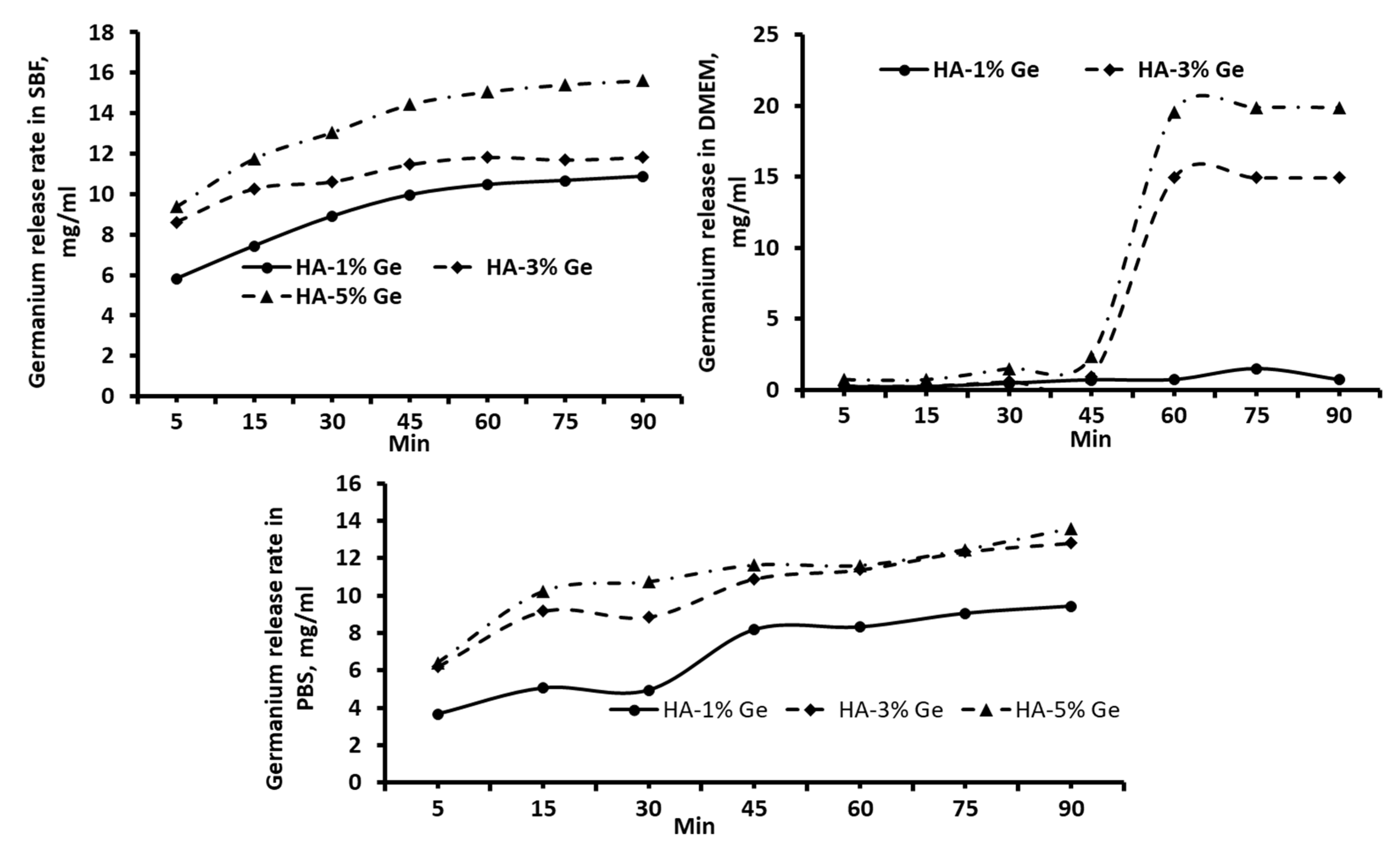
2.13. Drug Release Behavior
2.14. In-Vitro Cell Culture
2.15. Cell Loading Density
2.16. Statistical Analysis
3. Results
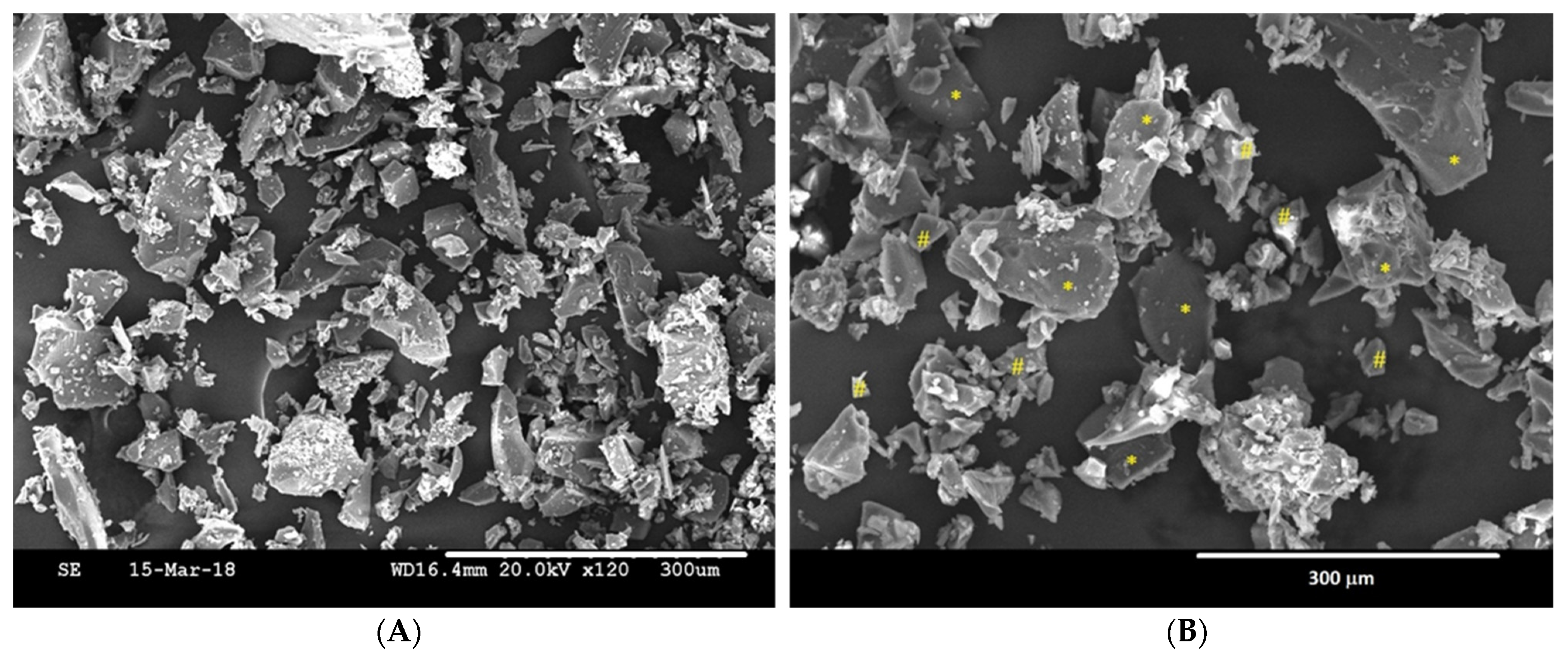
3.1. Characterization
3.2. Drug-Release
3.3. Contact Angle Test
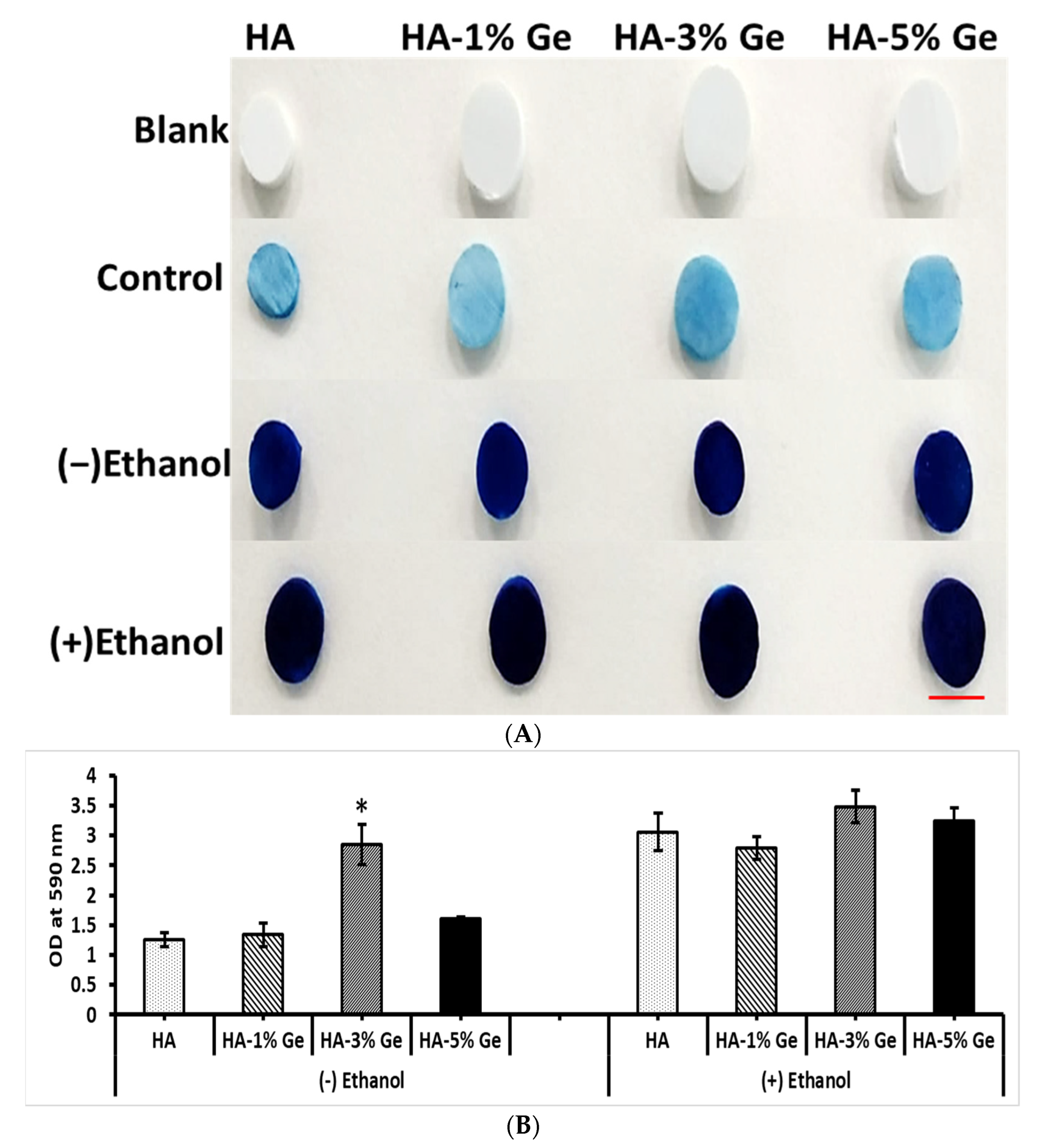
3.4. Protein Adsorption Ability
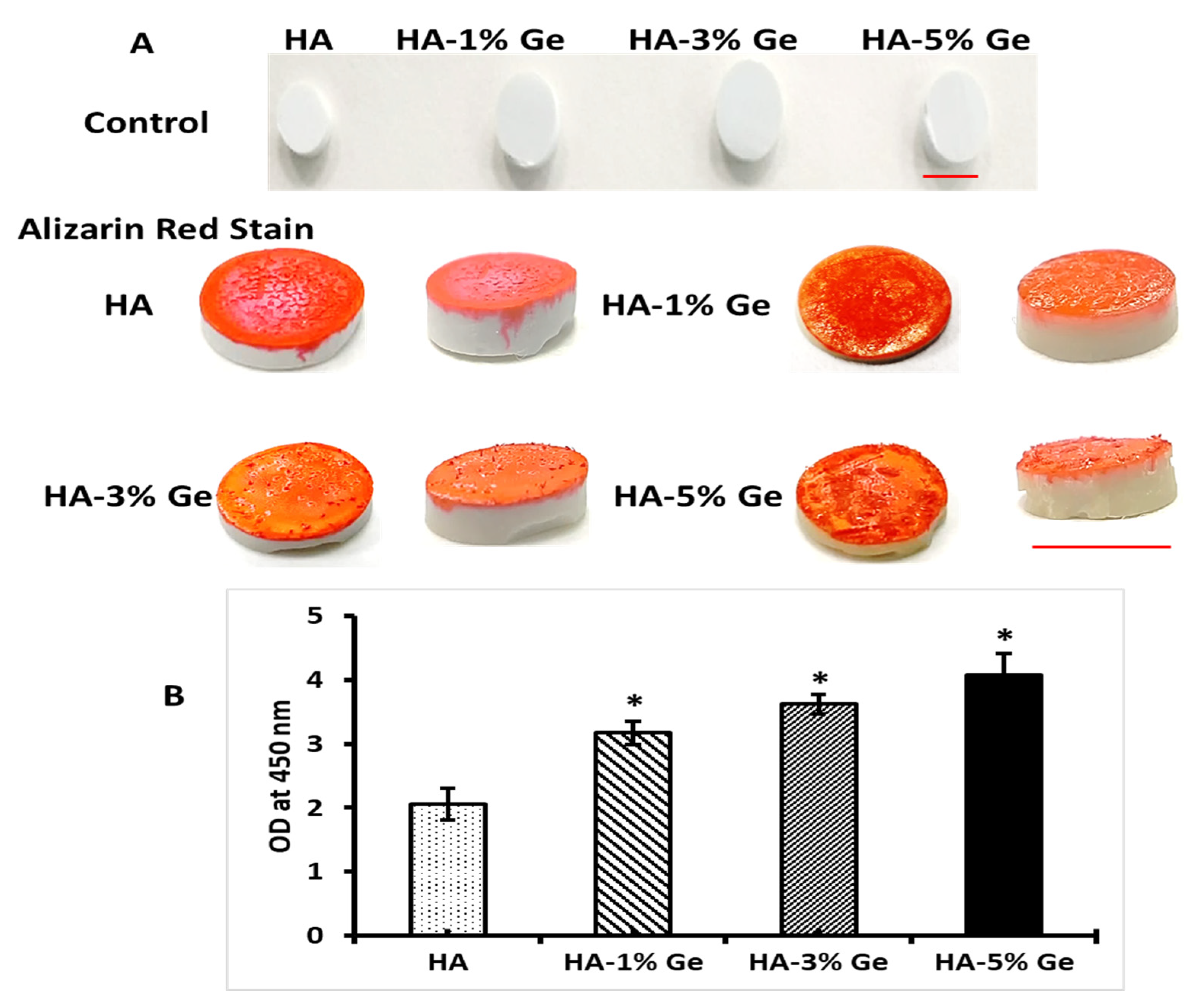
3.5. In-Vitro Bioactivity
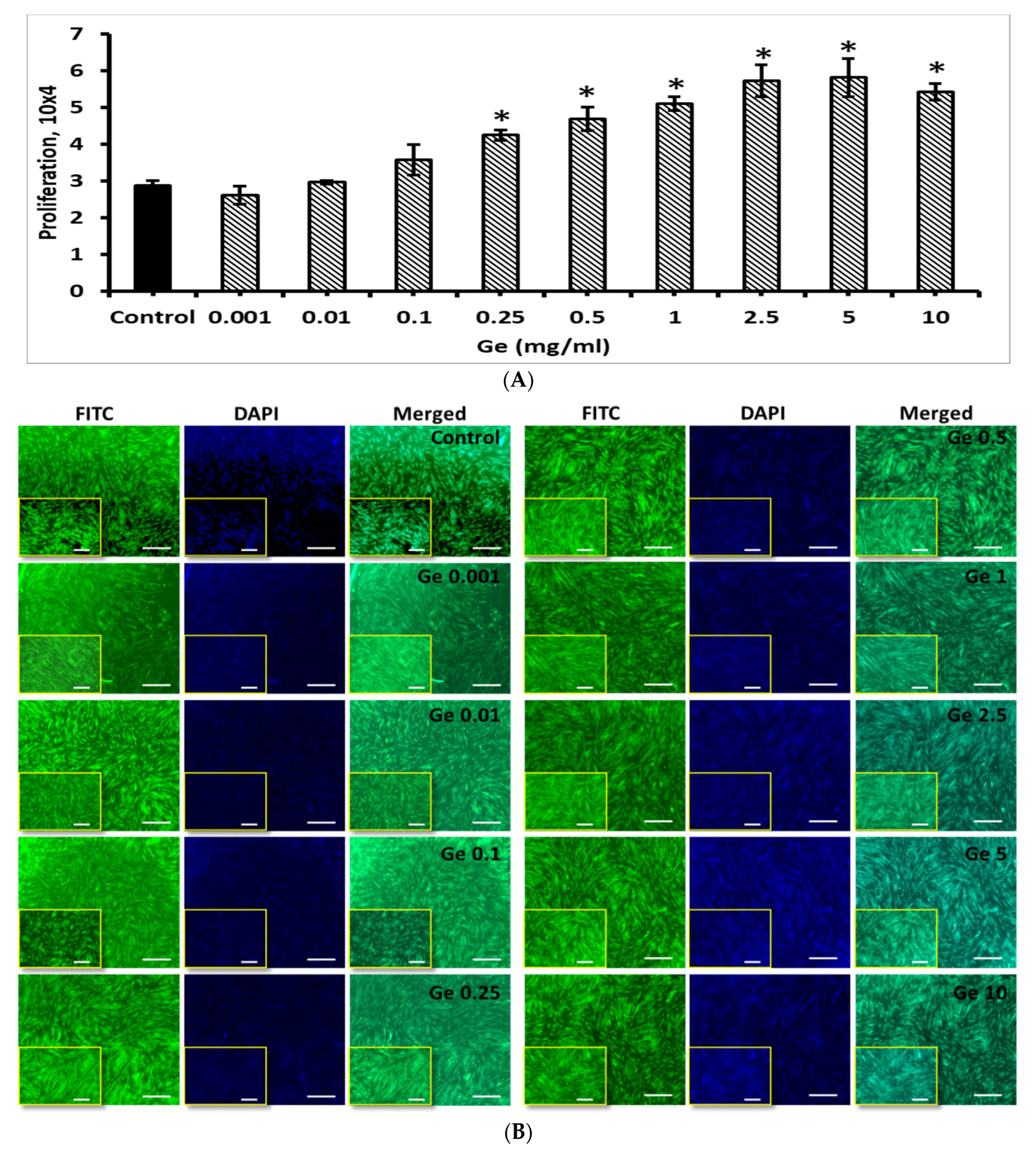
3.6. Effect of Ge on BM-MSCs
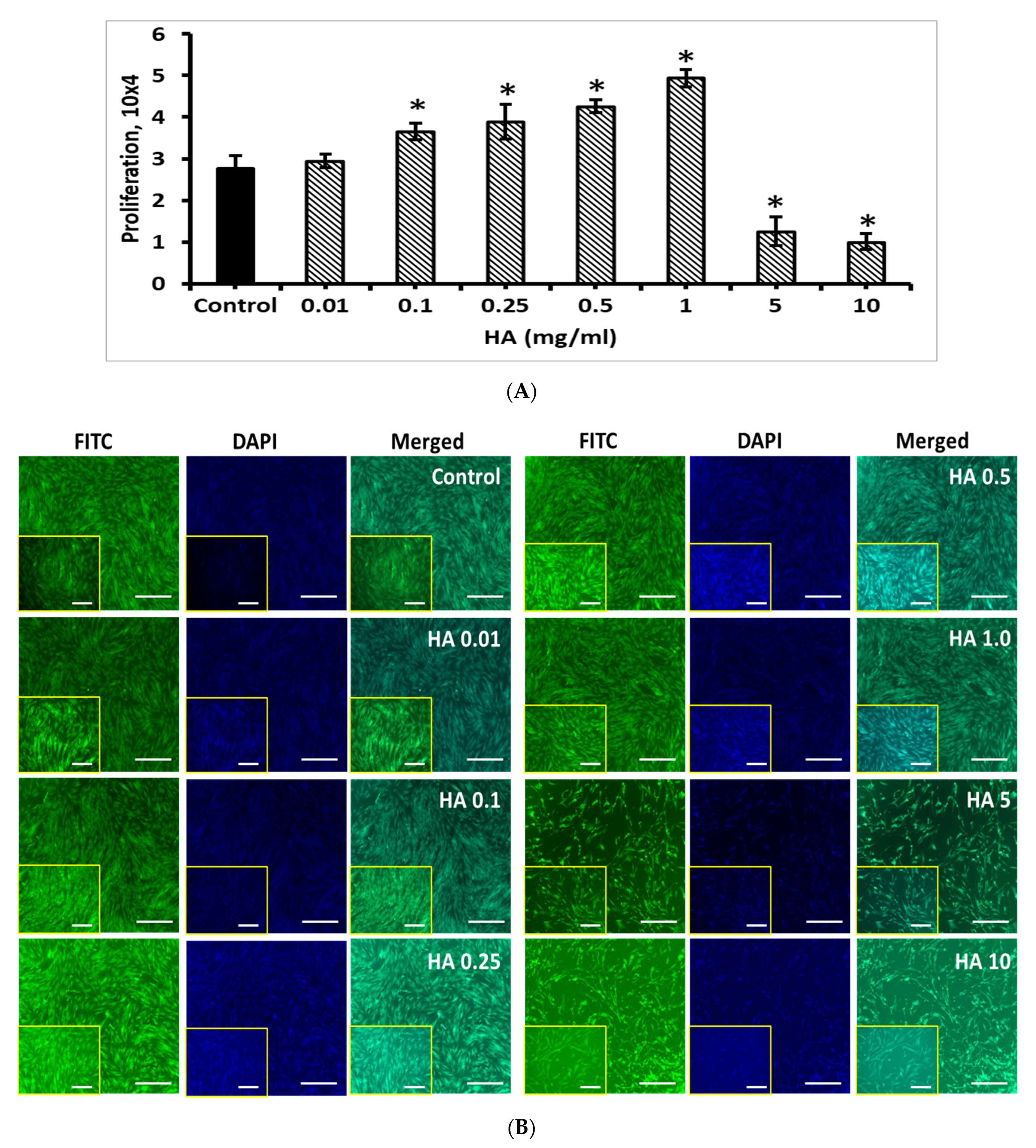
3.7. Effect of HA on BM-MSCs
3.8. Effect of HA-Ge Coating on BM-MSCs
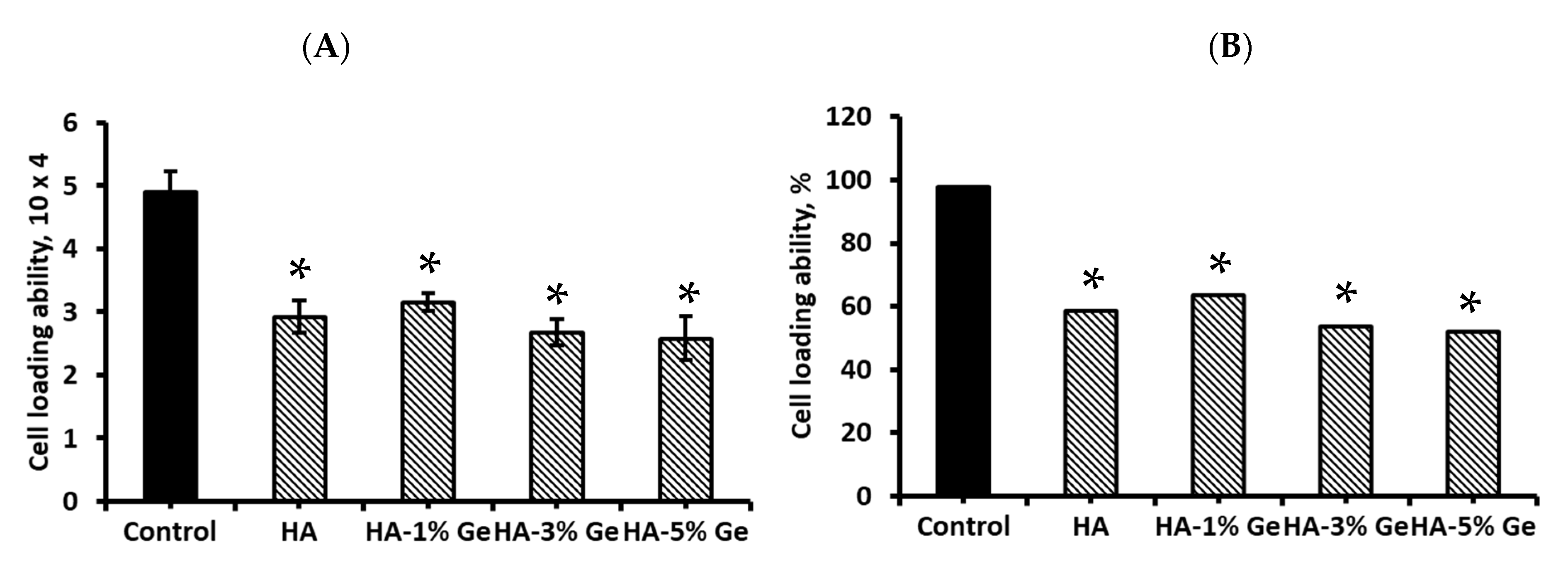
3.9. Cell Loading Density
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lett, J.A.; Sagadevan, S.; Fatimah, I.; Hoque, E.; Lokanathan, Y.; Léonard, E.; Alshahateet, S.F.; Schirhagl, R.; Oh, W.C. Recent advances in natural polymer-based hydroxyapatite scaffolds: Properties and applications. Eur. Polym. J. 2021, 148, 110360. [Google Scholar] [CrossRef]
- Senra, M.R.; de Lima, R.B.; Souza, D.D.H.S.; Marques, M.D.F.V.; Monteiro, S.N. Thermal characterization of hydroxyapatite or carbonated hydroxyapatite hybrid composites with distinguished collagens for bone graft. J. Mater. Res. Technol. 2020, 9, 7190–7200. [Google Scholar] [CrossRef]
- Fiume, E.; Magnaterra, G.; Rahdar, A.; Verné, E.; Baino, F. Hydroxyapatite for Biomedical Applications: A Short Overview. Ceramics 2021, 4, 542–563. [Google Scholar] [CrossRef]
- Saleem, M.; Rasheed, S.; Yougen, C. Silk fibroin/hydroxyapatite scaffold: A highly compatible material for bone regeneration. Sci. Technol. Adv. Mater. 2020, 21, 242–266. [Google Scholar] [CrossRef] [PubMed]
- Farokhi, M.; Mottaghitalab, F.; Shokrgozar, M.A.; Ai, J.; Hadjati, J.; Azami, M. Bio-hybrid silk fibroin/calcium phosphate/PLGA nanocomposite scaffold to control the delivery of vascular endothelial growth factor. Mater. Sci. Eng. C 2014, 35, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Daugela, P.; Pranskunas, M.; Juodzbalys, G.; Liesiene, J.; Baniukaitiene, O.; Afonso, A.; Sousa Gomes, P. Novel cellulose/hydroxyapatite scaffolds for bone tissue regeneration: In-vitro and in vivo study. J. Tissue Eng. Regen. Med. 2018, 12, 1195–1208. [Google Scholar] [CrossRef] [PubMed]
- Sancilio, S.; Gallorini, M.; Di Nisio, C.; Marsich, E.; Di Pietro, R.; Schweikl, H.; Cataldi, A. Alginate/Hydroxyapatite-Based Nanocomposite Scaffolds for Bone Tissue Engineering Improve Dental Pulp Biomineralization and Differentiation. Stem Cells Int. 2018, 2018, 9643721. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, M.P.; da Silva, B.C.R.; Hamouda, A.E.I.; de Toledo, M.A.S.; Schalla, C.; Rütten, S.; Goetzke, R.; Mattoso, L.H.C.; Zenke, M.; Sechi, A. PLA/Hydroxyapatite scaffolds exhibit in-vitro immunological inertness and promote robust osteogenic differentiation of human mesenchymal stem cells without osteogenic stimuli. Sci. Rep. 2022, 12, 2333. [Google Scholar] [CrossRef]
- Biscaia, S.; Branquinho, M.V.; Alvites, R.D.; Fonseca, R.; Sousa, A.C.; Pedrosa, S.S.; Caseiro, A.R.; Guedes, F.; Patrício, T.; Viana, T.; et al. 3D Printed Poly(ε-caprolactone)/Hydroxyapatite Scaffolds for Bone Tissue Engineering: A Comparative Study on a Composite Preparation by Melt Blending or Solvent Casting Techniques and the Influence of Bioceramic Content on Scaffold Properties. Int. J. Mol. Sci. 2022, 23, 2318. [Google Scholar] [CrossRef]
- Elango, J.; Saravanakumar, K.; Rahman, S.U.; Henrotin, Y.; Regenstein, J.M.; Wu, W.; Bao, B. Chitosan-collagen 3D matrix mimics trabecular bone and regulates RANKL-mediated paracrine cues of differentiated osteoblast and mesenchymal stem cells for bone marrow macrophage-derived osteoclastogenesis. Biomolecules 2019, 9, 173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elango, J.; Zhang, J.; Bao, B.; Palaniyandi, K.; Wang, S.; Wenhui, W.; Robinson, J.S. Rheological, biocompatibility and osteogenesis assessment of fish collagen scaffold for bone tissue engineering. Int. J. Biol. Macromol. 2016, 91, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Elango, J.; Selvaganapathy, P.R.; Lazzari, G.; Bao, B.; Wenhui, W. Biomimetic collagen-sodium alginate-titanium oxide (TiO2) 3D matrix supports differentiated periodontal ligament fibroblasts growth for periodontal tissue regeneration. Int. J. Biol. Macromol. 2020, 163, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Li, S.H.; De Wijn, J.R.; Layrolle, P.; De Groot, K. Synthesis of macroporous hydroxyapatite scaffolds for bone tissue engineering. J. Biomed. Mater. Res. 2002, 61, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Pengfei, M.; Wenjing, W.; Yu, W.; Le, R.; Shuxian, L.; Junhua, W. Biomimetic gelatin/chitosan/polyvinyl alcohol/nano-hydroxyapatite scaffolds for bone tissue engineering. Mater. Des. 2021, 207, 109865. [Google Scholar] [CrossRef]
- Wu, C.-S.; Wang, S.-S.; Wu, D.-Y.; Shih, W.-L. Novel composite 3D-printed filament made from fish scale-derived hydroxyapatite, eggshell and polylactic acid via a fused fabrication approach. Addit. Manuf. 2021, 46, 102169. [Google Scholar] [CrossRef]
- Jahangir, M.U.; Islam, F.; Wong, S.Y.; Jahan, R.A.; Matin, M.A.; Li, X.; Arafat, M.T. Comparative analysis and antibacterial properties of thermally sintered apatites with varied processing conditions. J. Am. Ceram. Soc. 2021, 104, 1023–1039. [Google Scholar] [CrossRef]
- Tram, N.X.T.; Ishikawa, K.; Minh, T.H.; Benson, D.; Tsuru, K. Characterization of carbonate apatite derived from chicken bone and its in-vitro evaluation using MC3T3-E1 cells. Mater. Res. Express 2021, 8, 025401. [Google Scholar] [CrossRef]
- Cestari, F.; Agostinacchio, F.; Galotta, A.; Chemello, G.; Motta, A.; Sglavo, V.M. Nano-hydroxyapatite derived from biogenic and bioinspired calcium carbonates: Synthesis and in-vitro bioactivity. Nanomaterials 2021, 11, 264. [Google Scholar] [CrossRef] [PubMed]
- Muthu, D.; Kumar, G.S.; Kattimani, V.; Viswabaskaran, V.; Girija, E. Optimization of a lab scale and pilot scale conversion of eggshell biowaste into hydroxyapatite using microwave reactor. Ceram. Int. 2020, 46, 25024–25034. [Google Scholar] [CrossRef]
- Ge, M.; Zong, M.; Xu, D.; Chen, Z.; Yang, J.; Yao, H.; Wei, C.; Chen, Y.; Lin, H.; Shi, J. Freestanding germanene nanosheets for rapid degradation and photothermal conversion. Mater. Today Nano 2021, 15, 100119. [Google Scholar] [CrossRef]
- Singh, M.; Kumar, D.; Singh, G. Natural Minerals and Cancer. J. Appl. Pharm. Sci. 2012, 2, 158–165. [Google Scholar] [CrossRef]
- Geng, H.; Dai, J.; Li, J.; Di, Z.; Liu, X. Antibacterial ability and hemocompatibility of graphene functionalized germanium. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef]
- Yoshinari, O.; Shiojima, Y.; Igarashi, K. Hepatoprotective effect of germanium-containing Spirulina in rats with D-galactosamine-and lipopolysaccharide-induced hepatitis. Br. J. Nutr. 2014, 111, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ruan, T.; Lyu, Y.; Wu, B. Advances in effect of germanium or germanium compounds on animals—A review. J. Biosci. Med. 2017, 5, 56–73. [Google Scholar] [CrossRef]
- Aso, H.; Suzuki, F.; YAMAGUCHT, T.; Hayashi, Y.; Ebina, T.; Ishida, N. Induction of interferon and activation of NK cells and macrophages in mice by oral administration of Ge-132, an organic germanium compound. Microbiol. Immunol. 1985, 29, 65–74. [Google Scholar] [CrossRef]
- Schauss, A.G. Nephrotoxicity and neurotoxicity in humans from organogermanium compounds and germanium dioxide. Biol. Trace Elem. Res. 1991, 29, 267–280. [Google Scholar] [CrossRef]
- Li, J.; Wang, G.; Zhang, W.; Jin, G.; Zhang, M.; Jiang, X.; Di, Z.; Liu, X.; Wang, X. Graphene film-functionalized germanium as a chemically stable, electrically conductive, and biologically active substrate. J. Mater. Chem. B 2015, 3, 1544–1555. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Heo, J.H.; Kim, D.J.; Namkung, S.M.; Lee, T.B.; Lee, M.W.; Kim, S.W. Anti-cancer effect of hot water extract from mycelium in germanium-enriched Cordyceps militaris. Korean J. Clin. Lab. Sci. 2017, 49, 69–78. [Google Scholar] [CrossRef]
- Mo, R.; Lei, Z.; Rooney, D.; Sun, K. Three-dimensional double-walled ultrathin graphite tube conductive scaffold with encapsulated germanium nanoparticles as a high-areal-capacity and cycle-stable anode for lithium-ion batteries. ACS Nano 2019, 13, 7536–7544. [Google Scholar] [CrossRef] [PubMed]
- Scheschkewitz, D.; Poitiers, N.E.; Huch, V.; Morgenstern, B.; Zimmer, M. Siliconoid Expansion by a Single Germanium Atom through Isolated Intermediates. Angew. Chem. 2022, 61, e202205399. [Google Scholar]
- Evtugin, V.; Rogov, A.; Valeeva, L.; Khabipova, N.; Nuzhdin, V.; Valeev, V.; Stepanov, A. Biological cell scaffolds based on nanoporous germanium layers formed by ion implantation. Vacuum 2020, 177, 109403. [Google Scholar] [CrossRef]
- Li, D.; Feng, C.; Liu, H.K.; Guo, Z. Hollow carbon spheres with encapsulated germanium as an anode material for lithium ion batteries. J. Mater. Chem. A 2015, 3, 978–981. [Google Scholar] [CrossRef]
- Dell’Anna, R.; Masciullo, C.; Iacob, E.; Barozzi, M.; Giubertoni, D.; Böttger, R.; Cecchini, M.; Pepponi, G. Multiscale structured germanium nanoripples as templates for bioactive surfaces. RSC Adv. 2017, 7, 9024–9030. [Google Scholar] [CrossRef]
- Xu, C.; Chang, Y.; Wu, P.; Liu, K.; Dong, X.; Nie, A.; Mu, C.; Liu, Z.; Dai, H.; Luo, Z. Two-Dimensional-Germanium Phosphide-Reinforced Conductive and Biodegradable Hydrogel Scaffolds Enhance Spinal Cord Injury Repair. Adv. Funct. Mater. 2021, 31, 2104440. [Google Scholar] [CrossRef]
- Fu, S.; Wang, X.; Guo, G.; Shi, S.; Liang, H.; Luo, F.; Wei, Y.; Qian, Z. Preparation and characterization of nano-hydroxyapatite/poly (ε-caprolactone)− poly (ethylene glycol)− poly (ε-caprolactone) composite fibers for tissue engineering. J. Phys. Chem. C 2010, 114, 18372–18378. [Google Scholar] [CrossRef]
- Love, W.; Millay, D.; Huston, J.S. Properties of disulfide-linked tubulin purified on hydroxyapatite and its comparison with intact and dissociated microtubules using limited tryptic digestion. Arch. Biochem. Biophys. 1981, 207, 300–310. [Google Scholar] [CrossRef]
- Lü, X.; Li, D.; Huang, Y.; Zhang, Y. Application of a modified Coomassie brilliant blue protein assay in the study of protein adsorption on carbon thin films. Surf. Coat. Technol. 2007, 201, 6843–6846. [Google Scholar] [CrossRef]
- Luo, W.-h.; Cheng, L.; Yuan, C.; Wu, Z.; Yuan, G.; Hou, M.; Chen, J.Y.; Luo, C.; Li, W. Preparation, characterization and evaluation of cellulose nanocrystal/poly(lactic acid) in situ nanocomposite scaffolds for tissue engineering. Int. J. Biol. Macromol. 2019, 134, 469–479. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Gholami, S.A.; Labbaf, S.; Houreh, A.B.; Ting, H.; Jones, J.R.; Esfahani, M.H.N. Long term effects of bioactive glass particulates on dental pulp stem cells in vitro. Biomed. Glasses 2017, 3, 103–196. [Google Scholar] [CrossRef]
- López-González, I.; Zamora-Ledezma, C.; Sanchez-Lorencio, M.I.; Tristante Barrenechea, E.; Gabaldón-Hernández, J.A.; Meseguer-Olmo, L. Modifications in Gene Expression in the Process of Osteoblastic Differentiation of Multipotent Bone Marrow-Derived Human Mesenchymal Stem Cells Induced by a Novel Osteoinductive Porous Medical-Grade 3D-Printed Poly(ε-caprolactone)/β-tricalcium Phosphate Composite. Int. J. Mol. Sci. 2021, 22, 11216. [Google Scholar]
- Ganesan, V.; Devaraj, M.; Govindan, S.; Kattimani, V.S.; Easwaradas Kreedapathy, G. Eggshell derived mesoporous biphasic calcium phosphate for biomedical applications using rapid thermal processing. Int. J. Appl. Ceram. Technol. 2019, 16, 1932–1943. [Google Scholar] [CrossRef]
- Rohmadi, R.; Harwijayanti, W.; Ubaidillah, U.; Triyono, J.; Diharjo, K.; Utomo, P. In-vitro Degradation and Cytotoxicity of Eggshell-Based Hydroxyapatite: A Systematic Review and Meta-Analysis. Polymers 2021, 13, 3223. [Google Scholar] [CrossRef]
- Cordell, J.M.; Vogl, M.; Wagoner Johnson, A.J. The influence of micropore size on the mechanical properties of bulk hydroxyapatite and hydroxyapatite scaffolds. J. Mech. Behav. Biomed. Mater. 2009, 2, 560–570. [Google Scholar] [CrossRef]
- Mondal, S.; Pal, U.; Dey, A. Natural origin hydroxyapatite scaffold as potential bone tissue engineering substitute. Ceram. Int. 2016, 42, 18338–18346. [Google Scholar] [CrossRef]
- Obada, D.O.; Osseni, S.A.; Sina, H.; Salami, K.A.; Oyedeji, A.N.; Dodoo-Arhin, D.; Bansod, N.D.; Csáki, Š.; Atta, A.Y.; Fasanya, O.O.; et al. Fabrication of novel kaolin-reinforced hydroxyapatite scaffolds with robust compressive strengths for bone regeneration. Appl. Clay Sci. 2021, 215, 106298. [Google Scholar] [CrossRef]
- Osuchukwu, O.A.; Salihi, A.; Abdullahi, I.L.; Abdulkareem, B.; Nwannenna, C.S. Synthesis techniques, characterization and mechanical properties of natural derived hydroxyapatite scaffolds for bone implants: A review. SN Appl. Sci. 2021, 3, 1–23. [Google Scholar] [CrossRef]
- Taşdelen, B.; Erdoğan, S.; Bekar, B. Radiation synthesis and characterization of chitosan/hyraluronic acid/hydroxyapatite hydrogels: Drug uptake and drug delivery systems. Mater. Today Proc. 2018, 5, 15990–15997. [Google Scholar] [CrossRef]
- Wan, Y.Z.; Hong, L.; Jia, S.; Huang, Y.; Zhu, Y.; Wang, Y.L.; Jiang, H.J. Synthesis and characterization of hydroxyapatite–bacterial cellulose nanocomposites. Compos. Sci. Technol. 2006, 66, 1825–1832. [Google Scholar] [CrossRef]
- Wang, Y.; He, W.-J.; Hao, H.; Wu, J.; Qin, N. Eggshell derived Se-doped HA nanorods for enhanced antitumor effect and curcumin delivery. J. Sol-Gel Sci. Technol. 2018, 87, 600–607. [Google Scholar] [CrossRef]
- Horta, M.K.d.S.; Moura, F.J.; Aguilar, M.S.; Westin, C.B.; Campos, J.B.d.; Peripolli, S.B.; Ramos, V.S.; Navarro, M.I.; Archanjo, B.S. Nanostructured hydroxyapatite from Hen´ s eggshells using sucrose as a template. Mater. Res. 2020, 23, 45–51. [Google Scholar] [CrossRef]
- Zhang, C.; Yi, Y.; Yang, H.-s.; Yi, Z.; Chen, X.; Zhou, Z.; Yi, Y.; Li, H.; Chen, J.; Liu, C. Wide spectrum solar energy absorption based on germanium plated ZnO nanorod arrays: Energy band regulation, Finite element simulation, Super hydrophilicity, Photothermal conversion. Appl. Mater. Today 2022, 28, 101531. [Google Scholar] [CrossRef]
- Zhu, X.; Ohtsubo, M.; Böhmer, R.M.; Roberts, J.M.D.; Assoian, R.K. Adhesion-dependent cell cycle progression linked to the expression of cyclin D1, activation of cyclin E-cdk2, and phosphorylation of the retinoblastoma protein. J. Cell Biol. 1996, 133, 391–403. [Google Scholar] [CrossRef]
- Steele, J.G.; Dalton, B.A.; Johnson, G.; Underwood, P.A. Adsorption of fibronectin and vitronectin onto Primaria and tissue culture polystyrene and relationship to the mechanism of initial attachment of human vein endothelial cells and BHK-21 fibroblasts. Biomaterials 1995, 16, 1057–1067. [Google Scholar] [CrossRef]
- Kim, H.; Yang, G.H.; Kim, G. Three-dimensional gelatin/PVA scaffold with nanofibrillated collagen surface for applications in hard-tissue regeneration. Int. J. Biol. Macromol. 2019, 135, 21–28. [Google Scholar] [CrossRef]
- Ho, M.L.; Fu, Y.C.; Wang, G.J.; Chen, H.-T.; Chang, J.-K.; Tsai, T.H.; Wang, C.-K. Controlled release carrier of BSA made by W/O/W emulsion method containing PLGA and hydroxyapatite. J. Control. Release 2008, 128, 142–148. [Google Scholar] [CrossRef]
- Tripathi, G.; Basu, B. A porous hydroxyapatite scaffold for bone tissue engineering: Physico-mechanical and biological evaluations. Ceram. Int. 2012, 38, 341–349. [Google Scholar] [CrossRef]
- Gutiérrez-Prieto, S.J.; Fonseca, L.F.; Sequeda-Castañeda, L.G.; Díaz, K.J.; Castañeda, L.Y.; Leyva-Rojas, J.A.; Salcedo-Reyes, J.C.; Acosta, A.P. Elaboration and Biocompatibility of an Eggshell-Derived Hydroxyapatite Material Modified with Si/PLGA for Bone Regeneration in Dentistry. Int. J. Dent. 2019, 2019, 5949232. [Google Scholar] [CrossRef]
- Patel, D.K.; Kim, M.-H.; Lim, K.-T. Synthesis and Characterization of Eggshell-Derived Hydroxyapatite Bioceramics. J. Biosyst. Eng. 2019, 44, 128–133. [Google Scholar] [CrossRef]
- Ingole, V.H.; Vuherer, T.; Maver, U.; Vinchurkar, A.S.; Ghule, A.V.; Kokol, V. Mechanical Properties and Cytotoxicity of Differently Structured Nanocellulose-hydroxyapatite Based Composites for Bone Regeneration Application. Nanomaterials 2019, 10, 25. [Google Scholar] [CrossRef]
- Kumar, G.S.; Girija, E.K. Flower-like hydroxyapatite nanostructure obtained from eggshell: A candidate for biomedical applications. Ceram. Int. 2013, 39, 8293–8299. [Google Scholar] [CrossRef]
- Neacşu, I.; Serban, A.P.; Nicoară, A.I.; Trusca, R.; Ene, V.L.; Iordache, F. Biomimetic Composite Scaffold Based on Naturally Derived Biomaterials. Polymers 2020, 12, 1161. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Li, Y.; Luo, W.; Jiang, J.; Zhao, J.; Liu, C. Controllable Synthesis of Biomimetic Hydroxyapatite Nanorods with High Osteogenic Bioactivity. ACS Biomater. Sci. Eng. 2020, 6, 320–328. [Google Scholar] [CrossRef]
- Roopavath, U.K.; Sah, M.K.; Panigrahi, B.B.; Rath, S.N. Mechanochemically synthesized phase stable and biocompatible β-tricalcium phosphate from avian eggshell for the development of tissue ingrowth system. Ceram. Int. 2019, 45, 12910–12919. [Google Scholar] [CrossRef]
- Huang, K.; Hou, J.; Gu, Z.; Wu, J. Egg-White-/Eggshell-Based Biomimetic Hybrid Hydrogels for Bone Regeneration. ACS Biomater. Sci. Eng. 2019, 5, 5384–5391. [Google Scholar] [CrossRef]
- Yilmaz, P.; Öztürk Er, E.; Bakırdere, S.; Ülgen, K.Ö.; Özbek, B. Application of supercritical gel drying method on fabrication of mechanically improved and biologically safe three-component scaffold composed of graphene oxide/chitosan/hydroxyapatite and characterization studies. J. Mater. Res. Technol. 2019, 8, 5201–5216. [Google Scholar] [CrossRef]
- McDonnell, L.P.; Viner, J.J.S.; Ruiz-Tijerina, D.A.; Rivera, P.; Xu, X.; Fal’ko, V.I.; Smith, D.C. Superposition of intra- and inter-layer excitons in twistronic MoSe2/WSe2 bilayers probed by resonant Raman scattering. 2D Mater. 2020, 8, 035009. [Google Scholar] [CrossRef]
- Mondal, S.; Bardhan, R.; Mondal, B.; Dey, A.; Mukhopadhyay, S.S.; Roy, S.; Guha, R.; Roy, K. Synthesis, characterization and in-vitro cytotoxicity assessment of hydroxyapatite from different bioresources for tissue engineering application. Bull. Mater. Sci. 2012, 35, 683–691. [Google Scholar] [CrossRef]
- Jayasree, R.; Kumar, T.S.S.; Venkateswari, R.; Nankar, R.P.; Doble, M. Eggshell derived brushite bone cement with minimal inflammatory response and higher osteoconductive potential. J. Mater. Sci. Mater. Med. 2019, 30, 113. [Google Scholar] [CrossRef]
- Manakhov, A.M.; Solovieva, A.O.; Permyakova, E.S.; Sitnikova, N.A.; Klyushova, L.S.; Kiryukhantsev-Korneev, P.V.; Konopatsky, A.S.; Shtansky, D.V. Adhesion and Proliferation of Mesenchymal Stem Cells on Plasma-Coated Biodegradable Nanofibers. J. Compos. Sci. 2022, 6, 193. [Google Scholar] [CrossRef]
- Costa, B.N.L.; Adão, R.M.R.; Maibohm, C.; Accardo, A.; Cardoso, V.F.; Nieder, J.B. Cellular Interaction of Bone Marrow Mesenchymal Stem Cells with Polymer and Hydrogel 3D Microscaffold Templates. ACS Appl. Mater. Interfaces 2022, 14, 13013–13024. [Google Scholar] [CrossRef]




| Ca (atomic%) | P (atomic%) | Ge (atomic%) | |
|---|---|---|---|
| HA | 62.41 | 37.60 | - |
| HA-1%Ge | 61.78 | 37.22 | 1.01 |
| HA-3%Ge | 60.71 | 36.35 | 3.02 |
| HA-5%Ge | 59.3 | 35.72 | 5.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elango, J.; Bushin, R.; Lijnev, A.; De Aza, P.N.; Martínez, C.P.-A.; Marín, J.M.G.; Hernandez, A.B.; Olmo, L.R.M.; Val, J.E.M.S.D. The Effect of Germanium-Loaded Hydroxyapatite Biomaterials on Bone Marrow Mesenchymal Stem Cells Growth. Cells 2022, 11, 2993. https://doi.org/10.3390/cells11192993
Elango J, Bushin R, Lijnev A, De Aza PN, Martínez CP-A, Marín JMG, Hernandez AB, Olmo LRM, Val JEMSD. The Effect of Germanium-Loaded Hydroxyapatite Biomaterials on Bone Marrow Mesenchymal Stem Cells Growth. Cells. 2022; 11(19):2993. https://doi.org/10.3390/cells11192993
Chicago/Turabian StyleElango, Jeevithan, Rodion Bushin, Artiom Lijnev, Piedad N. De Aza, Carlos Pérez-Albacete Martínez, José Manuel Granero Marín, Ana Belen Hernandez, Luis Ramón Meseguer Olmo, and José Eduardo Maté Sánchez De Val. 2022. "The Effect of Germanium-Loaded Hydroxyapatite Biomaterials on Bone Marrow Mesenchymal Stem Cells Growth" Cells 11, no. 19: 2993. https://doi.org/10.3390/cells11192993
APA StyleElango, J., Bushin, R., Lijnev, A., De Aza, P. N., Martínez, C. P.-A., Marín, J. M. G., Hernandez, A. B., Olmo, L. R. M., & Val, J. E. M. S. D. (2022). The Effect of Germanium-Loaded Hydroxyapatite Biomaterials on Bone Marrow Mesenchymal Stem Cells Growth. Cells, 11(19), 2993. https://doi.org/10.3390/cells11192993










