Hybrid Mineral/Organic Material Induces Bone Bridging and Bone Volume Augmentation in Rat Calvarial Critical Size Defects
Abstract
:1. Introduction
2. Material and Methods
Preparation of the Functionalized Membrane
3. Morphological and Physicochemical Characterizations
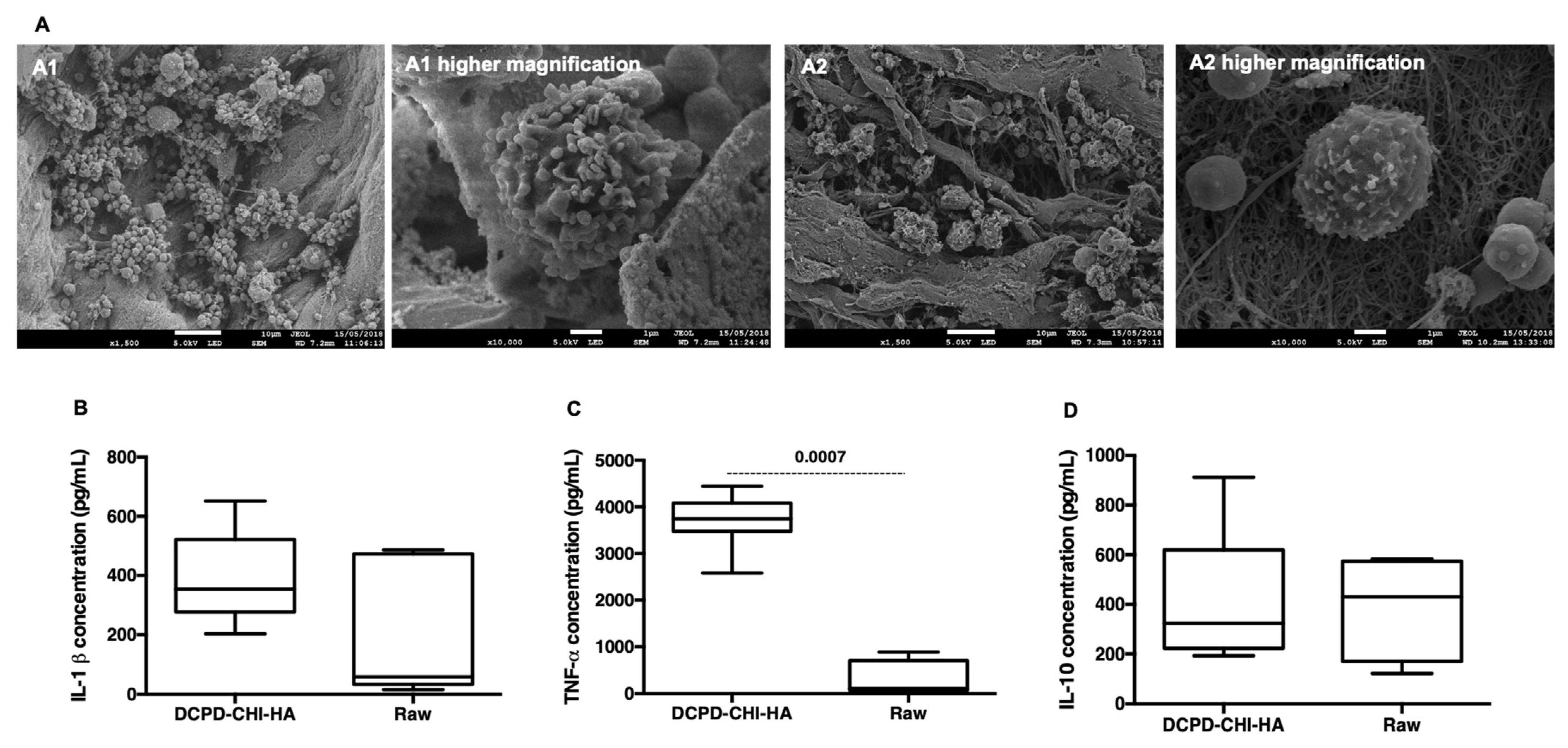
3.1. Scanning Electron Microscopy with a Field Emission Gun (FEG-SEM)
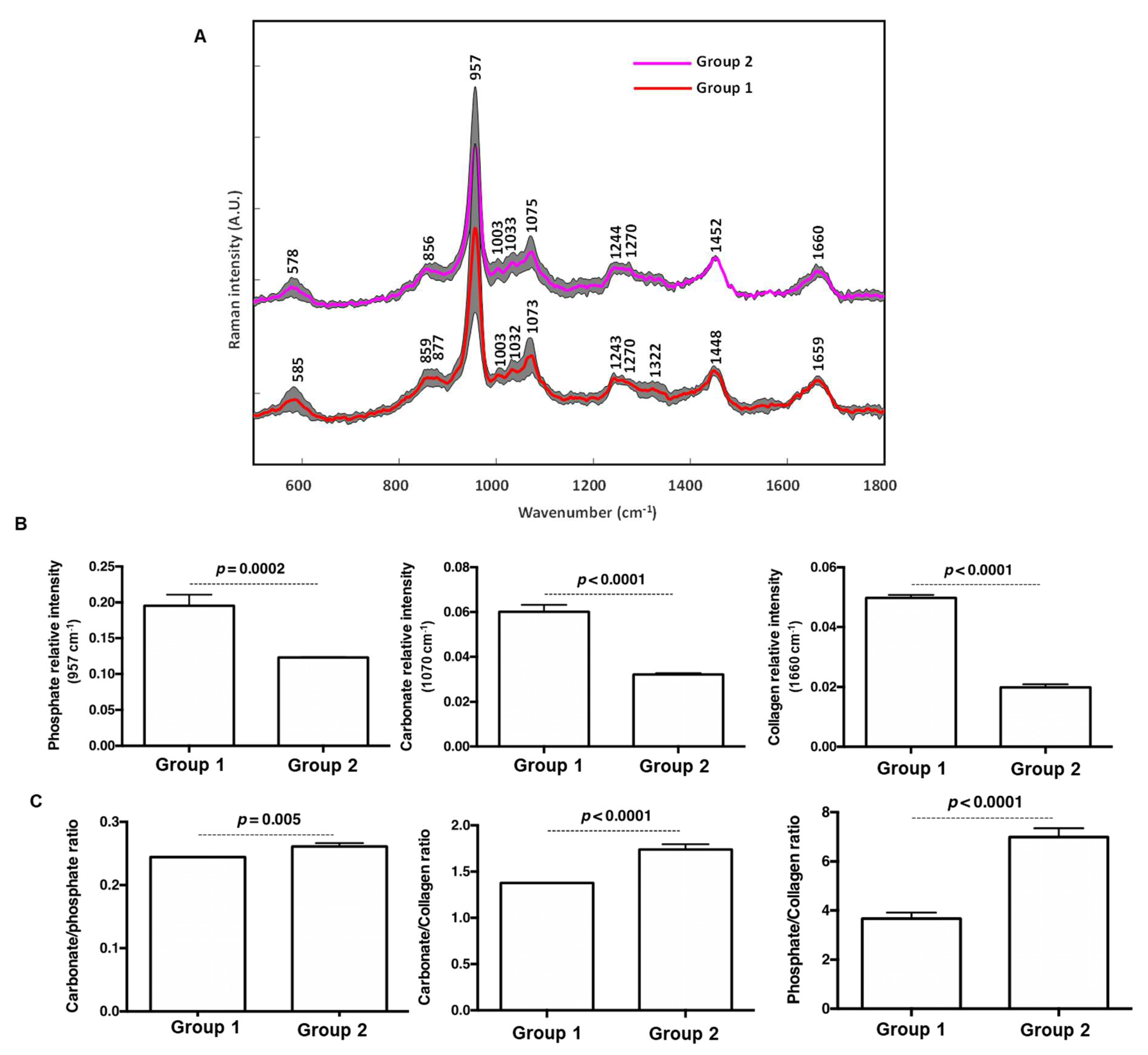
3.2. Confocal Raman Spectroscopy Mapping
3.3. Induced Coupled Plasma-Optical Emission Spectroscopy (ICP-OES)
3.4. Transmission Electron Microscopy (TEM)
4. Biological Assessments
Acute Inflammatory Response
5. In Vivo and Ex Vivo Assessment
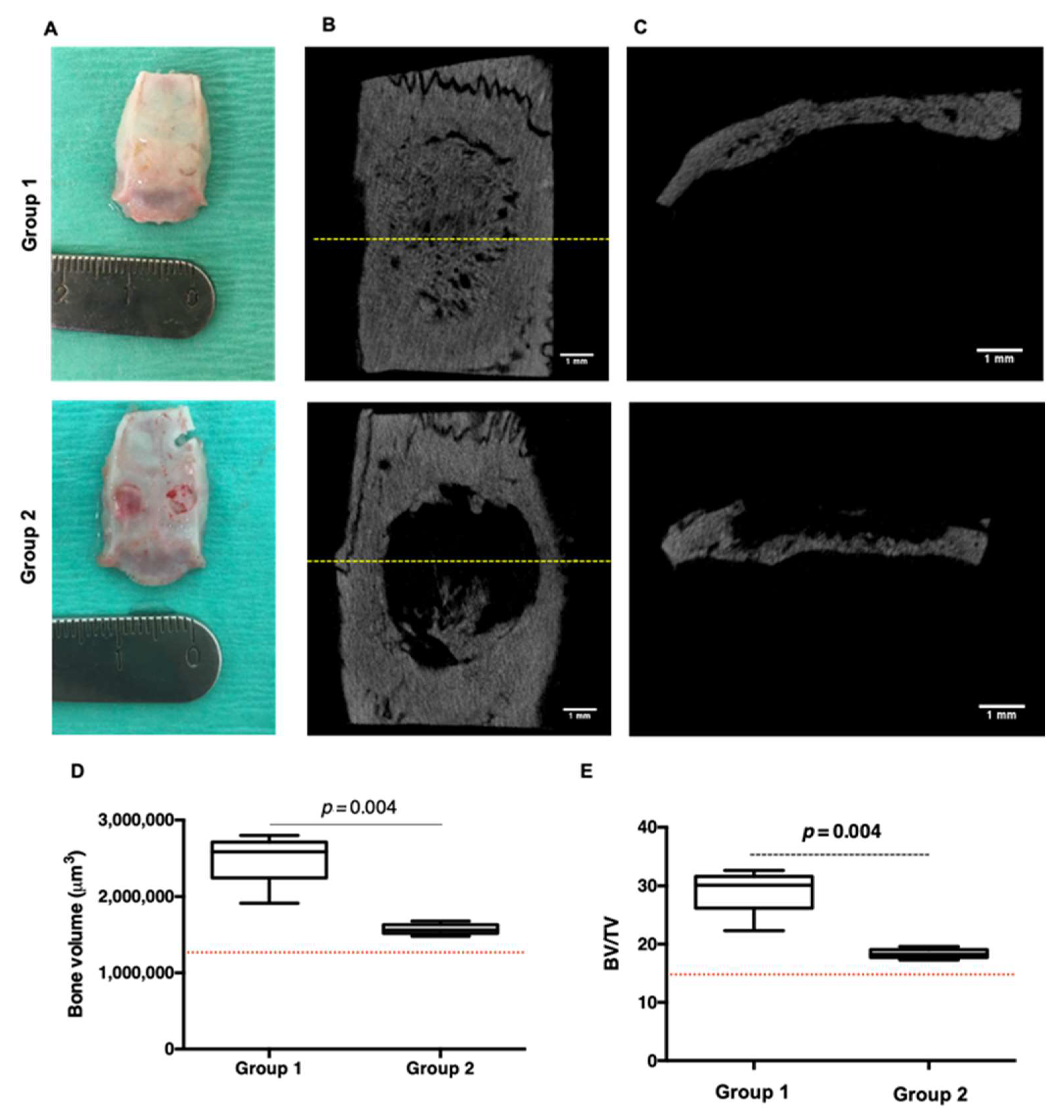
5.1. Parietal Bone Implantation
5.2. Raman
5.3. Micro Tomography
5.4. Histology and Immunohistochemistry
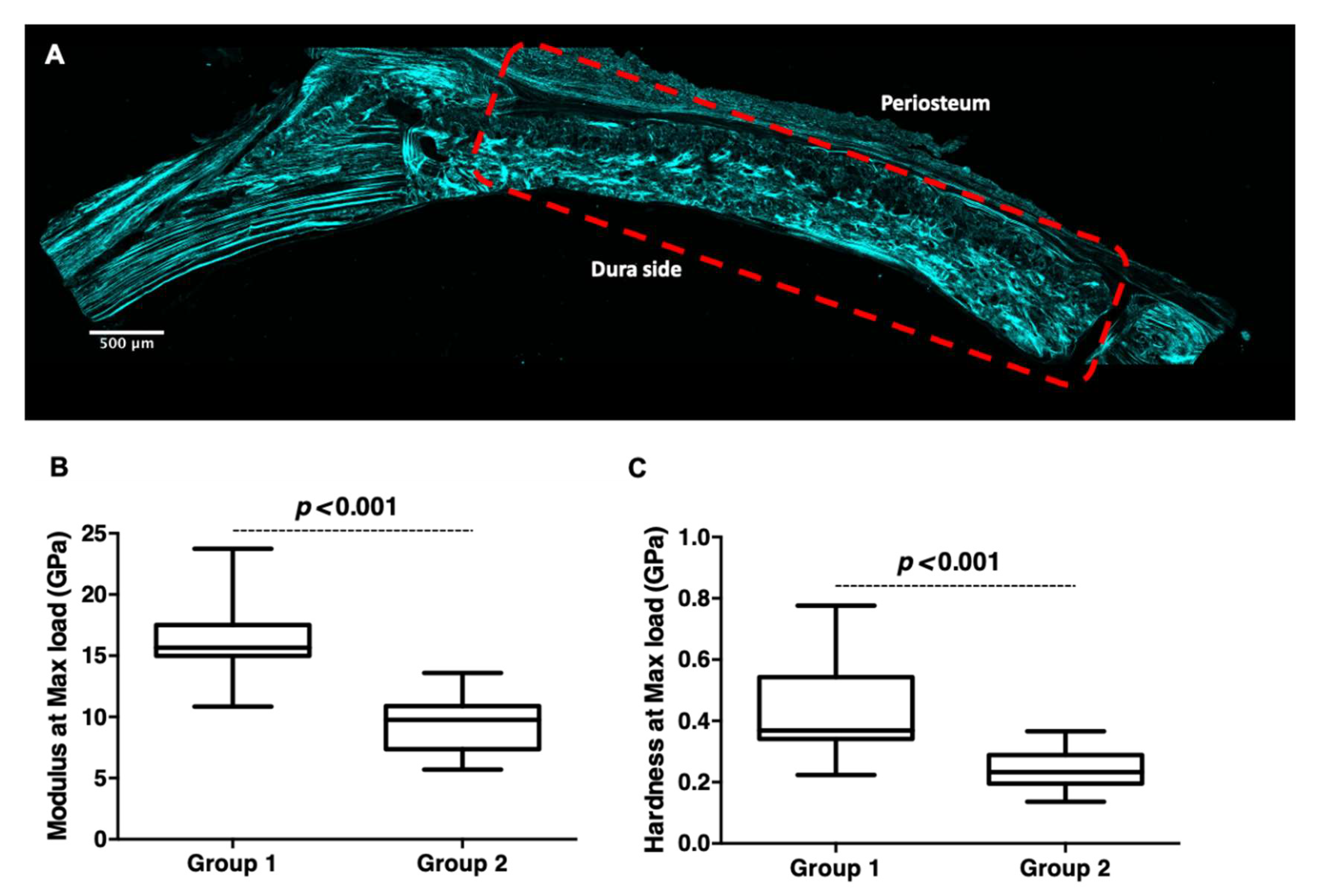
5.5. Confocal Microscopy and Second Harmonic Generation (SHG)
5.6. Nanoindentation
5.7. Statistical Analysis
6. Result and Discussion
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided bone regeneration: Materials and biological mechanisms revisited. Eur. J. Oral Sci. 2017, 125, 315–337. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Yang, L.; Gbureck, U.; Bhaduri, S.B.; Sikder, P. Monetite, an important calcium phosphate compound–Its synthesis, properties and applications in orthopedics. Acta Biomater. 2021, 127, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Rattanachan, S.T.; Srakaew, N.L.-O.; Thaitalay, P.; Thongsri, O.; Dangviriyakul, R.; Srisuwan, S.; Suksaweang, S.; Widelitz, R.B.; Chuong, C.-M.; Srithunyarat, T.; et al. Development of injectable chitosan/biphasic calcium phosphate bone cement and in vitro and in vivo evaluation. Biomed. Mater. 2020, 15, 055038. [Google Scholar] [CrossRef]
- Ren, W.; Song, W.; Yurgelevic, S.; Markel, D.C. Setting mechanism of a new injectable Dicalcium Phosphate Dihydrate (DCPD) forming cement. J. Mech. Behav. Biomed. Mater. 2018, 79, 226–234. [Google Scholar] [CrossRef]
- Tamimi, F.; Sheikh, Z.; Barralet, J. Dicalcium phosphate cements: Brushite and monetite. Acta Biomater. 2012, 8, 474–487. [Google Scholar] [CrossRef]
- Mechiche Alami, S.; Rammal, H.; Boulagnon-Rombi, C.; Velard, F.; Lazar, F.; Drevet, R.; Laurent Maquin, D.; Gangloff, S.C.; Hemmerlé, J.; Voegel, J.C.; et al. Harnessing Wharton’s jelly stem cell differentiation into bone-like nodule on calcium phosphate substrate without osteoinductive factors. Acta Biomater. 2017, 49, 575–589. [Google Scholar] [CrossRef]
- Li, J.; Baker, B.A.; Mou, X.; Ren, N.; Qiu, J.; Boughton, R.I.; Liu, H. Biopolymer/Calcium phosphate scaffolds for bone tissue engineering. Adv. Healthc. Mater. 2014, 3, 469–484. [Google Scholar] [CrossRef]
- Denry, I.; Kuhn, L.T. Design and characterization of calcium phosphate ceramic scaffolds for bone tissue engineering. Dent. Mater. 2016, 32, 43–53. [Google Scholar] [CrossRef]
- Kim, H.D.; Amirthalingam, S.; Kim, S.L.; Lee, S.S.; Rangasamy, J.; Hwang, N.S. Biomimetic Materials and Fabrication Approaches for Bone Tissue Engineering. Adv. Healthc. Mater. 2017, 6, 1700612. [Google Scholar] [CrossRef] [PubMed]
- Salama, A. Cellulose/calcium phosphate hybrids: New materials for biomedical and environmental applications. Int. J. Biol. Macromol. 2019, 127, 606–617. [Google Scholar] [CrossRef]
- Salama, A. Recent progress in preparation and applications of chitosan/calcium phosphate composite materials. Int. J. Biol. Macromol. 2021, 178, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, S.; Taubert, A. Polymer-controlled, bio-inspired calcium phosphate mineralization from aqueous solution. Macromol. Biosci. 2007, 7, 1085–1099. [Google Scholar] [CrossRef] [PubMed]
- Bleek, K.; Taubert, A. New developments in polymer-controlled, bioinspired calcium phosphate mineralization from aqueous solution. Acta Biomater. 2013, 9, 6283–6321. [Google Scholar] [CrossRef]
- Rammal, H.; Dubus, M.; Aubert, L.; Reffuveille, F.; Laurent-Maquin, D.; Terryn, C.; Schaaf, P.; Alem, H.; Francius, G.; Quilès, F.; et al. Bioinspired Nanofeatured Substrates: Suitable Environment for Bone Regeneration. ACS Appl. Mater. Interfaces 2017, 9, 12791–12801. [Google Scholar] [CrossRef] [PubMed]
- Rammal, H.; Entz, L.; Dubus, M.; Moniot, A.; Bercu, N.B.; Sergheraert, J.; Gangloff, S.C.; Mauprivez, C.; Kerdjoudj, H. Osteoinductive Material to Fine-Tune Paracrine Crosstalk of Mesenchymal Stem Cells With Endothelial Cells and Osteoblasts. Front. Bioeng. Biotechnol. 2019, 7, 256. [Google Scholar] [CrossRef] [PubMed]
- Dubus, M.; Varin-Simon, J.; Prada, P.; Scomazzon, L.; Reffuveille, F.; Alem, H.; Boulmedais, F.; Mauprivez, C.; Rammal, H.; Kerdjoudj, H. Biopolymers-calcium phosphate antibacterial coating reduces the pathogenicity of internalized bacteria by mesenchymal stromal cells. Biomater. Sci. 2020, 8, 5763–5773. [Google Scholar] [CrossRef]
- Dubus, M.; Rammal, H.; Alem, H.; Bercu, N.B.; Royaud, I.; Quilès, F.; Boulmedais, F.; Gangloff, S.C.; Mauprivez, C.; Kerdjoudj, H. Boosting mesenchymal stem cells regenerative activities on biopolymers-calcium phosphate functionalized collagen membrane. Colloids Surf. B Biointerfaces 2019, 181, 671–679. [Google Scholar] [CrossRef]
- Al-Rifai, R.; Tournois, C.; Kheirallah, S.; Bouland, N.; Poitevin, G.; Nguyen, P.; Beljebbar, A. Subcutaneous and transcutaneous monitoring of murine hindlimb ischemia by in vivo Raman spectroscopy. Analyst 2019, 144, 4677–4686. [Google Scholar] [CrossRef]
- Mittra, E.; Akella, S.; Qin, Y.-X. The effects of embedding material, loading rate and magnitude, and penetration depth in nanoindentation of trabecular bone. J. Biomed. Mater. Res. A 2006, 79, 86–93. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Penel, G.; Leroy, N.; Van Landuyt, P.; Flautre, B.; Hardouin, P.; Lemaître, J.; Leroy, G. Raman microspectrometry studies of brushite cement: In vivo evolution in a sheep model. Bone 1999, 25, 81S–84S. [Google Scholar] [CrossRef]
- Seredin, P.; Goloshchapov, D.; Prutskij, T.; Ippolitov, Y. Phase Transformations in a Human Tooth Tissue at the Initial Stage of Caries. PLoS ONE 2015, 10, e0124008. [Google Scholar] [CrossRef] [PubMed]
- Bohner, M. Bioresorbable ceramics. In Degradation Rate of Bioresorbable Materials; Buchanan, F., Ed.; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Sawston, UK, 2008; pp. 95–114. [Google Scholar]
- Place, E.S.; Evans, N.D.; Stevens, M.M. Complexity in biomaterials for tissue engineering. Nat. Mater. 2009, 8, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Beevers, C.A. The crystal structure of dicalcium phosphate dihydrate, CaHPO4.2H2O. Acta Crystallogr. 1958, 11, 273–277. [Google Scholar] [CrossRef]
- Renaudin, G.; Laquerrière, P.; Filinchuk, Y.; Jallot, E.; Nedelec, J.M. Structural characterization of sol–gel derived Sr-substituted calcium phosphates with anti-osteoporotic and anti-inflammatory properties. J. Mater. Chem. 2008, 18, 3593–3600. [Google Scholar] [CrossRef]
- He, J.; Chen, G.; Liu, M.; Xu, Z.; Chen, H.; Yang, L.; Lv, Y. Scaffold strategies for modulating immune microenvironment during bone regeneration. Mater. Sci. Eng. C 2020, 108, 110411. [Google Scholar] [CrossRef]
- Xie, Y.; Hu, C.; Feng, Y.; Li, D.; Ai, T.; Huang, Y.; Chen, X.; Huang, L.; Tan, J. Osteoimmunomodulatory effects of biomaterial modification strategies on macrophage polarization and bone regeneration. Regen. Biomater. 2020, 7, 233–245. [Google Scholar] [CrossRef]
- Jayasree, R.; Kumar, T.S.S.; Venkateswari, R.; Nankar, R.P.; Doble, M. Eggshell derived brushite bone cement with minimal inflammatory response and higher osteoconductive potential. J. Mater. Sci. Mater. Med. 2019, 30, 113. [Google Scholar] [CrossRef]
- Shi, C.; Zhu, Y.; Ran, X.; Wang, M.; Su, Y.; Cheng, T. Therapeutic potential of chitosan and its derivatives in regenerative medicine. J. Surg. Res. 2006, 133, 185–192. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, L.; Liu, R.; Lin, Y.; Chen, S.; Lu, S.; Lin, Z.; Chen, Z.; Wu, C.; Xiao, Y. The osteoimmunomodulatory property of a barrier collagen membrane and its manipulation via coating nanometer-sized bioactive glass to improve guided bone regeneration. Biomater. Sci. 2018, 6, 1007–1019. [Google Scholar] [CrossRef]
- Visser, R.; Arrabal, P.M.; Santos-Ruiz, L.; Fernandez-Barranco, R.; Becerra, J.; Cifuentes, M. A collagen-targeted biomimetic RGD peptide to promote osteogenesis. Tissue Eng. Part A 2014, 20, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.; Law, A.W.L.; Cheung, T.W.; Lau, C. Raman spectroscopy of bone composition during healing of subcritical calvarial defects. Biomed. Opt. Express 2018, 9, 1704–1716. [Google Scholar] [CrossRef] [PubMed]
- Hadjab, I.; Farlay, D.; Crozier, P.; Douillard, T.; Boivin, G.; Chevalier, J.; Meille, S.; Follet, H. Intrinsic properties of osteomalacia bone evaluated by nanoindentation and FTIRM analysis. J. Biomech. 2021, 117, 110247. [Google Scholar] [CrossRef] [PubMed]
- Mechiche Alami, S.; Gangloff, S.C.; Laurent-Maquin, D.; Wang, Y.; Kerdjoudj, H. Concise Review: In Vitro Formation of Bone-Like Nodules Sheds Light on the Application of Stem Cells for Bone Regeneration. Stem Cells Transl. Med. 2016, 5, 1587–1593. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dubus, M.; Scomazzon, L.; Ledouble, C.; Braux, J.; Beljebbar, A.; Van Gulick, L.; Baldit, A.; Gorin, C.; Alem, H.; Bouland, N.; et al. Hybrid Mineral/Organic Material Induces Bone Bridging and Bone Volume Augmentation in Rat Calvarial Critical Size Defects. Cells 2022, 11, 2865. https://doi.org/10.3390/cells11182865
Dubus M, Scomazzon L, Ledouble C, Braux J, Beljebbar A, Van Gulick L, Baldit A, Gorin C, Alem H, Bouland N, et al. Hybrid Mineral/Organic Material Induces Bone Bridging and Bone Volume Augmentation in Rat Calvarial Critical Size Defects. Cells. 2022; 11(18):2865. https://doi.org/10.3390/cells11182865
Chicago/Turabian StyleDubus, Marie, Loïc Scomazzon, Charlotte Ledouble, Julien Braux, Abdelilah Beljebbar, Laurence Van Gulick, Adrien Baldit, Caroline Gorin, Halima Alem, Nicole Bouland, and et al. 2022. "Hybrid Mineral/Organic Material Induces Bone Bridging and Bone Volume Augmentation in Rat Calvarial Critical Size Defects" Cells 11, no. 18: 2865. https://doi.org/10.3390/cells11182865
APA StyleDubus, M., Scomazzon, L., Ledouble, C., Braux, J., Beljebbar, A., Van Gulick, L., Baldit, A., Gorin, C., Alem, H., Bouland, N., Britton, M., Schiavi, J., Vaughan, T. J., Mauprivez, C., & Kerdjoudj, H. (2022). Hybrid Mineral/Organic Material Induces Bone Bridging and Bone Volume Augmentation in Rat Calvarial Critical Size Defects. Cells, 11(18), 2865. https://doi.org/10.3390/cells11182865






