Novel Epigenetic Modulation Chitosan-Based Scaffold as a Promising Bone Regenerative Material
Abstract
1. Introduction
2. Results
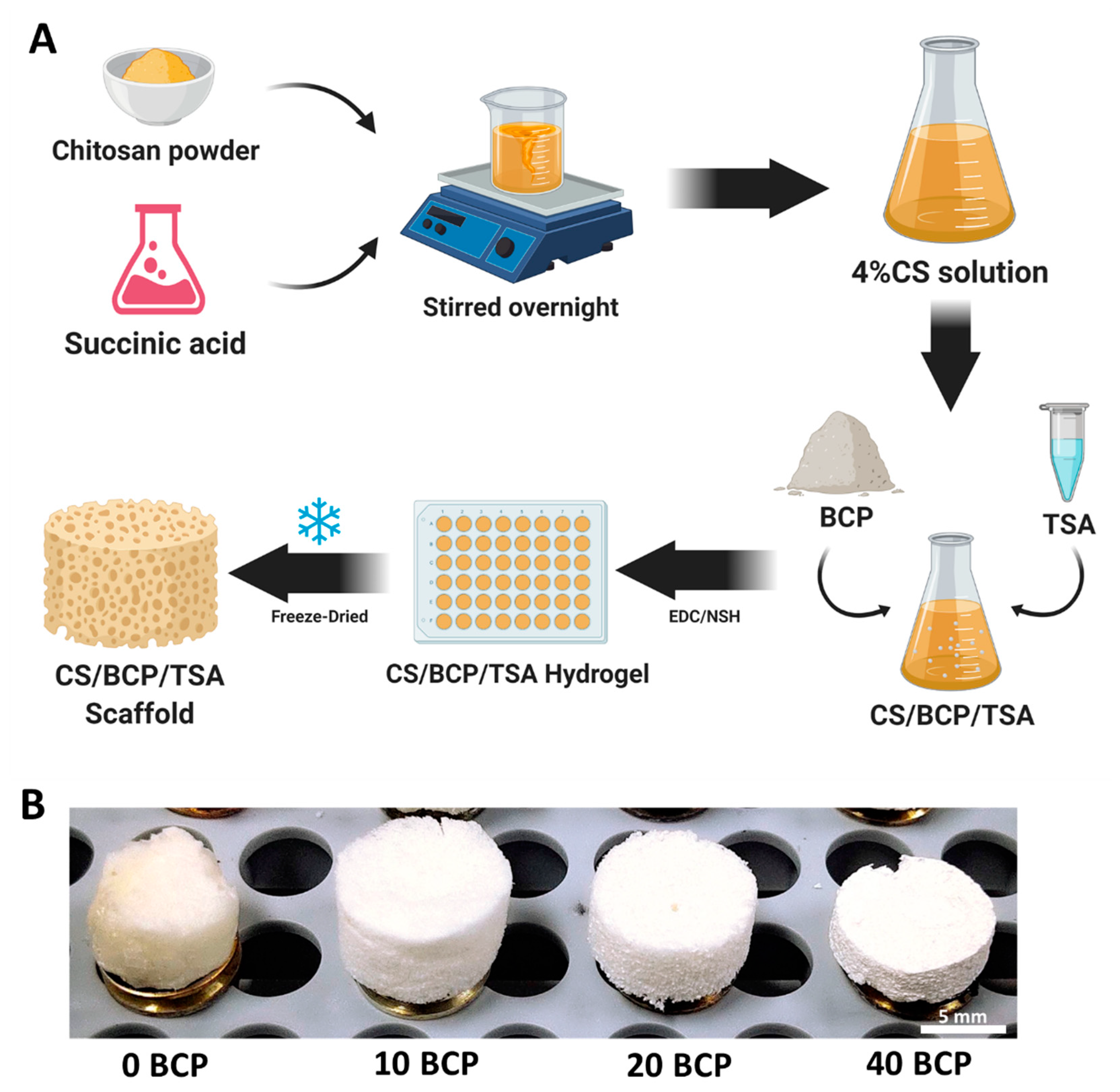
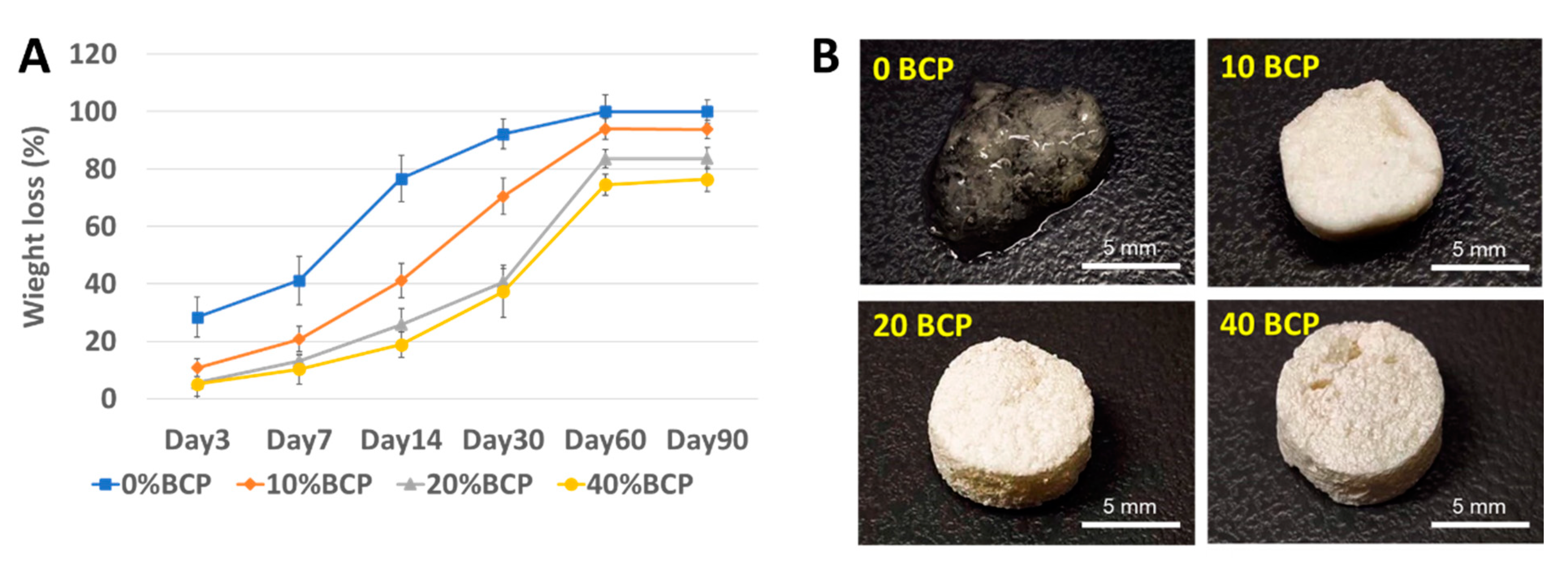
2.1. Selection of Optimal Physicochemical Scaffold
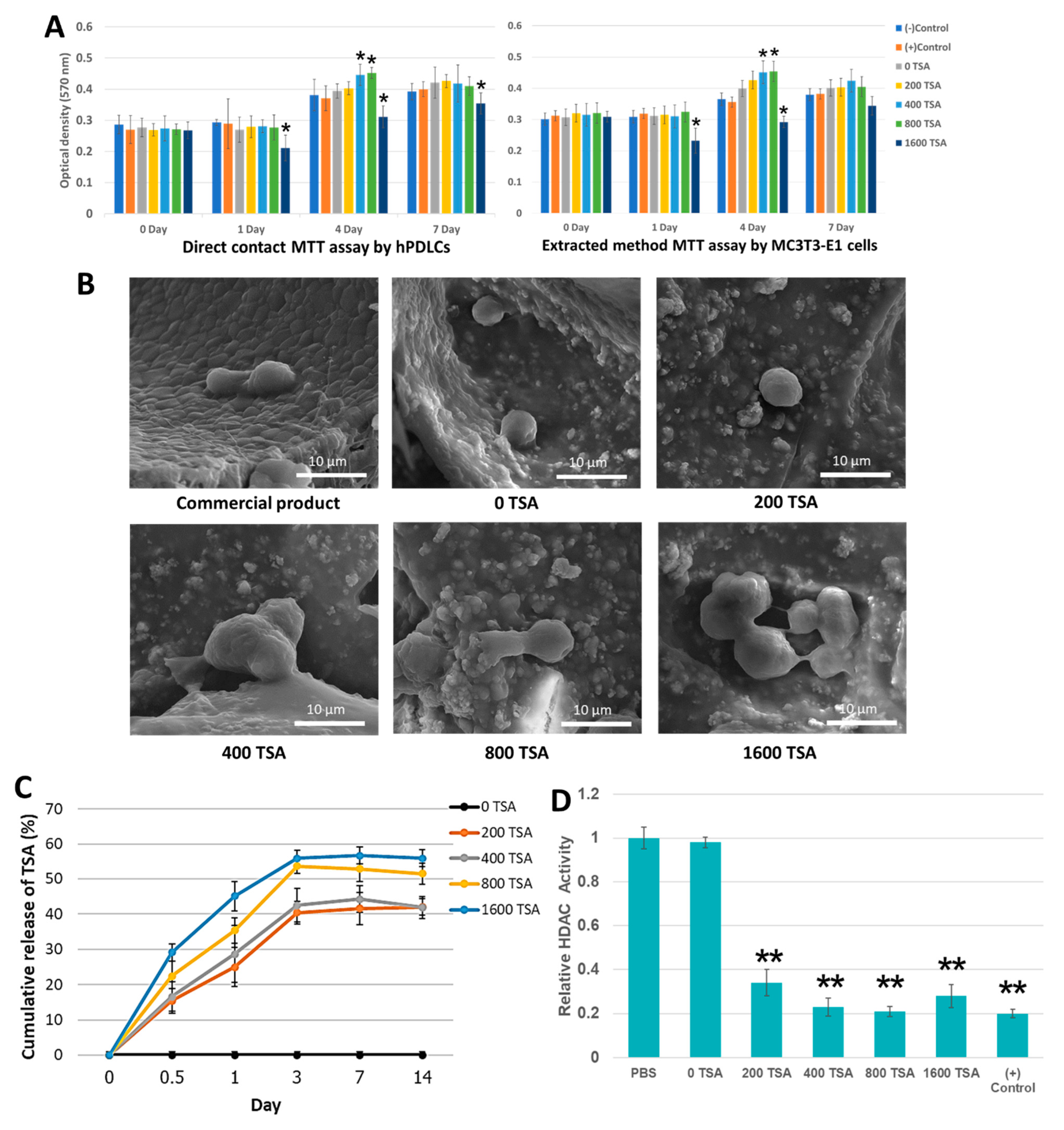
2.2. The Scaffolds Demonstrate Cytocompatibility and Facilitate Cell Attachment
2.3. Incorporated TSA Was Confirmed to Be Released and Stay Bioactive
2.4. The Scaffold Induces Expression of Osteogenic Genes but Not Inflammatory Related Gene
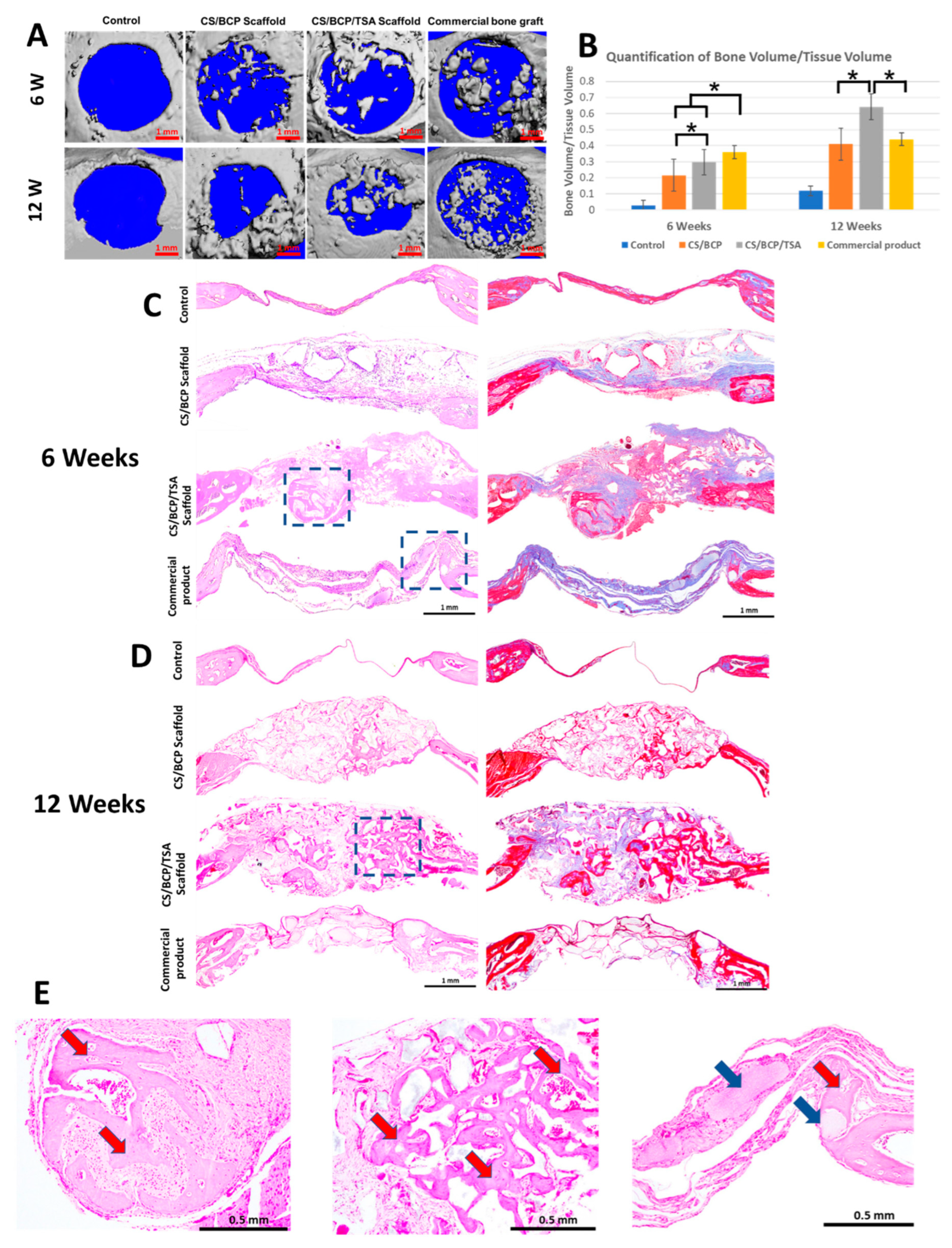
2.5. CS/BCP/TSA Scaffold Promote Excellence Bone Regeneration In Vivo
3. Discussion
4. Materials and Methods
4.1. Fabrication of CS/BCP/TSA Scaffold
4.2. Culture of Primary hPDLCs
4.3. Physical and Chemical Characterization of the Scaffolds
4.4. In Vitro Degradation of the Scaffolds
4.5. Mechanical Properties
4.6. Cellular Biocompatibility
4.7. In Vitro Release and Bioactivity of TSA
4.8. Cells Attachment and Morphology
4.9. Expression of Inflammatory and Osteogenic Genes
4.10. In Vivo Bone Regeneration
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ono, T.; Nakashima, T. Oral bone biology. J. Oral Biosci. 2022, 64, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Raggatt, L.J.; Partridge, N.C. Cellular and molecular mechanisms of bone remodeling. J. Biol. Chem. 2010, 285, 25103–25108. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; You, X.; Zhang, L.; Zhang, C.; Zou, W. Mechanical regulation of bone remodeling. Bone Res. 2022, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Che, D.; Feng, Y.; Wei, C.; Zhou, X.; Zhang, J.; Shi, Y.; Wang, L. Research in Tissue Engineering Scaffold Materials for Alveolar Bone Repair. Crit. Rev. Biomed. Eng. 2021, 49, 29–52. [Google Scholar] [CrossRef]
- Manzini, B.M.; Machado, L.M.R.; Noritomi, P.Y.; da Silva, J.V.L. Advances in Bone tissue engineering: A fundamental review. J. Biosci. 2021, 46, 17. [Google Scholar] [CrossRef]
- Huo, Y.; Lu, Y.; Meng, L.; Wu, J.; Gong, T.; Zou, J.; Bosiakov, S.; Cheng, L. A Critical Review on the Design, Manufacturing and Assessment of the Bone Scaffold for Large Bone Defects. Front. Bioeng. Biotechnol. 2021, 9, 753715. [Google Scholar] [CrossRef]
- Montoya, C.; Du, Y.; Gianforcaro, A.L.; Orrego, S.; Yang, M.B.; Lelkes, P.I. On the road to smart biomaterials for bone research: Definitions, concepts, advances, and outlook. Bone Res. 2021, 9, 12. [Google Scholar] [CrossRef]
- Safari, B.; Aghanejad, A.; Roshangar, L.; Davaran, S. Osteogenic effects of the bioactive small molecules and minerals in the scaffold-based bone tissue engineering. Colloids Surf. B Biointerfaces 2021, 198, 111462. [Google Scholar] [CrossRef]
- Elieh-Ali-Komi, D.; Hamblin, M.R. Chitin and Chitosan: Production and Application of Versatile Biomedical Nanomaterials. Int. J. Adv. Res. 2016, 4, 411–427. [Google Scholar]
- Islam, S.; Bhuiyan, M.A.R.; Islam, M.N. Chitin and Chitosan: Structure, Properties and Applications in Biomedical Engineering. J. Polym. Environ. 2016, 25, 854–866. [Google Scholar] [CrossRef]
- Sharifianjazi, F.; Khaksar, S.; Esmaeilkhanian, A.; Bazli, L.; Eskandarinezhad, S.; Salahshour, P.; Sadeghi, F.; Rostamnia, S.; Vahdat, S.M. Advancements in Fabrication and Application of Chitosan Composites in Implants and Dentistry: A Review. Biomolecules 2022, 12, 155. [Google Scholar] [CrossRef]
- Derwich, M.; Lassmann, L.; Machut, K.; Zoltowska, A.; Pawlowska, E. General Characteristics, Biomedical and Dental Application, and Usage of Chitosan in the Treatment of Temporomandibular Joint Disorders: A Narrative Review. Pharmaceutics 2022, 14, 305. [Google Scholar] [CrossRef]
- Pitrolino, K.A.; Felfel, R.M.; Pellizzeri, L.M.; McLaren, J.; Popov, A.A.; Sottile, V.; Scotchford, C.A.; Scammell, B.E.; Roberts, G.A.F.; Grant, D.M. Development and in vitro assessment of a bi-layered chitosan-nano-hydroxyapatite osteochondral scaffold. Carbohydr. Polym. 2022, 282, 119126. [Google Scholar] [CrossRef]
- Arnaldi, P.; Di Lisa, D.; Maddalena, L.; Carosio, F.; Fina, A.; Pastorino, L.; Monticelli, O. A facile approach for the development of high mechanical strength 3D neuronal network scaffold based on chitosan and graphite nanoplatelets. Carbohydr. Polym. 2021, 271, 118420. [Google Scholar] [CrossRef]
- Sukpaita, T.; Chirachanchai, S.; Pimkhaokham, A.; Ampornaramveth, R.S. Chitosan-Based Scaffold for Mineralized Tissues Regeneration. Mar. Drugs 2021, 19, 551. [Google Scholar] [CrossRef]
- Szymanska, E.; Winnicka, K. Stability of chitosan-a challenge for pharmaceutical and biomedical applications. Mar. Drugs 2015, 13, 1819–1846. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Botelho, M. Biphasic calcium phosphates (BCP) of hydroxyapatite (HA) and tricalcium phosphate (TCP) as bone substitutes: Importance of physicochemical characterizations in biomaterials studies. Data Brief 2017, 10, 93–97. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, B.; Wan, T.; Zhou, C.; Fan, Y.; Tian, W.; Jing, W. A 3D-printed biphasic calcium phosphate scaffold loaded with platelet lysate/gelatin methacrylate to promote vascularization. J. Mater. Chem B 2022, 10, 3138–3151. [Google Scholar] [CrossRef]
- Al-Omar, N.A.; Al-Qutub, M.N.; Ramalingam, S.; Al-Kindi, M.; Nooh, N.; Ar-Regaie, A.; Wang, H.L.; Al-Hezaimi, K. Bone Regeneration Using Bone Morphogenetic Protein-2 and Biphasic Calcium Phosphate with and without Collagen Membrane in Calvarial Standardized Defects: An In Vivo Microcomputed Tomographic Experiment in Rats. Int. J. Periodontics Restor. Dent. 2016, 36, s161–s170. [Google Scholar] [CrossRef]
- Khouly, I.; Braun, R.S.; Ordway, M.; Aouizerat, B.E.; Ghassib, I.; Larsson, L.; Asa’ad, F. The Role of DNA Methylation and Histone Modification in Periodontal Disease: A Systematic Review. Int. J. Mol. Sci. 2020, 21, 6217. [Google Scholar] [CrossRef]
- Algate, K.; Haynes, D.; Fitzsimmons, T.; Romeo, O.; Wagner, F.; Holson, E.; Reid, R.; Fairlie, D.; Bartold, P.; Cantley, M. Histone deacetylases 1 and 2 inhibition suppresses cytokine production and osteoclast bone resorption in vitro. J. Cell. Biochem. 2020, 121, 244–258. [Google Scholar] [CrossRef]
- De Souza, C.; Chatterji, B.P. HDAC Inhibitors as Novel Anti-Cancer Therapeutics. Recent Pat. Anticancer. Drug Discov. 2015, 10, 145–162. [Google Scholar] [CrossRef]
- Sanaei, M.; Kavoosi, F. Effects of trichostatin A on the intrinsic and extrinsic apoptotic pathway, cell viability, and apoptosis induction in hepatocellular carcinoma cell lines. Gastroenterol. Hepatol. Bed Bench 2021, 14, 323–333. [Google Scholar]
- Chodkowska, A.; Bienkowska, A.; Slyk, Z.; Giebultowicz, J.; Malecki, M. Anticancer activity of topical ointments with histone deacetylase inhibitor, trichostatin A. Adv. Clin. Exp. Med. 2020, 29, 1039–1049. [Google Scholar] [CrossRef]
- Huynh, N.C.; Everts, V.; Ampornaramveth, R.S. Histone deacetylases and their roles in mineralized tissue regeneration. Bone Rep. 2017, 7, 33–40. [Google Scholar] [CrossRef]
- Li, Q.; Liu, F.; Dang, R.; Feng, C.; Xiao, R.; Hua, Y.; Wang, W.; Jia, Z.; Liu, D. Epigenetic modifier trichostatin A enhanced osteogenic differentiation of mesenchymal stem cells by inhibiting NF-kappaB (p65) DNA binding and promoted periodontal repair in rats. J. Cell. Physiol. 2020, 235, 9691–9701. [Google Scholar] [CrossRef]
- El-Serafi, A.T.; Oreffo, R.O.; Roach, H.I. Epigenetic modifiers influence lineage commitment of human bone marrow stromal cells: Differential effects of 5-aza-deoxycytidine and trichostatin A. Differentiation 2011, 81, 35–41. [Google Scholar] [CrossRef]
- Cho, Y.D.; Kim, W.J.; Ryoo, H.M.; Kim, H.G.; Kim, K.H.; Ku, Y.; Seol, Y.J. Current advances of epigenetics in periodontology from ENCODE project: A review and future perspectives. Clin. Epigenet. 2021, 13, 92. [Google Scholar] [CrossRef]
- Huynh, N.C.; Everts, V.; Pavasant, P.; Ampornaramveth, R.S. Inhibition of Histone Deacetylases Enhances the Osteogenic Differentiation of Human Periodontal Ligament Cells. J. Cell. Biochem. 2016, 117, 1384–1395. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, X.; Zhang, E.; Yang, L.; Yuan, H.; Tu, W.; Zhang, H.; Yin, Z.; Shen, W.; Chen, X.; et al. An epigenetic bioactive composite scaffold with well-aligned nanofibers for functional tendon tissue engineering. Acta Biomater. 2018, 66, 141–156. [Google Scholar] [CrossRef]
- Murphy, C.M.; Haugh, M.G.; O’Brien, F.J. The effect of mean pore size on cell attachment, proliferation and migration in collagen-glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 2010, 31, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Ghassemi, T.; Shahroodi, A.; Ebrahimzadeh, M.H.; Mousavian, A.; Movaffagh, J.; Moradi, A. Current Concepts in Scaffolding for Bone Tissue Engineering. Arch. Bone Jt. Surg. 2018, 6, 90–99. [Google Scholar] [PubMed]
- Polo-Corrales, L.; Latorre-Esteves, M.; Ramirez-Vick, J.E. Scaffold design for bone regeneration. J. Nanosci. Nanotechnol. 2014, 14, 15–56. [Google Scholar] [CrossRef] [PubMed]
- Aslam Khan, M.U.; Abd Razak, S.I.; Al Arjan, W.S.; Nazir, S.; Sahaya Anand, T.J.; Mehboob, H.; Amin, R. Recent Advances in Biopolymeric Composite Materials for Tissue Engineering and Regenerative Medicines: A Review. Molecules 2021, 26, 619. [Google Scholar] [CrossRef]
- Sareethammanuwat, M.; Boonyuen, S.; Arpornmaeklong, P. Effects of beta-tricalcium phosphate nanoparticles on the properties of a thermosensitive chitosan/collagen hydrogel and controlled release of quercetin. J. Biomed. Mater. Research Part A 2021, 109, 1147–1159. [Google Scholar] [CrossRef]
- Dasgupta, S.; Maji, K.; Nandi, S.K. Investigating the mechanical, physiochemical and osteogenic properties in gelatin-chitosan-bioactive nanoceramic composite scaffolds for bone tissue regeneration: In vitro and in vivo. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 94, 713–728. [Google Scholar] [CrossRef]
- Young, D.A.; Barter, M.J.; Soul, J. Osteoarthritis year in review: Genetics, genomics, epigenetics. Osteoarthr. Cartil. 2022, 30, 216–225. [Google Scholar] [CrossRef]
- Man, K.; Joukhdar, H.; Manz, X.D.; Brunet, M.Y.; Jiang, L.H.; Rnjak-Kovacina, J.; Yang, X.B. Bone tissue engineering using 3D silk scaffolds and human dental pulp stromal cells epigenetic reprogrammed with the selective histone deacetylase inhibitor MI192. Cell Tissue Res. 2022, 388, 565–581. [Google Scholar] [CrossRef]
- Xiong, A.; He, Y.; Gao, L.; Li, G.; Weng, J.; Kang, B.; Wang, D.; Zeng, H. Smurf1-targeting miR-19b-3p-modified BMSCs combined PLLA composite scaffold to enhance osteogenic activity and treat critical-sized bone defects. Biomater. Sci. 2020, 8, 6069–6081. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, T.; Peng, L.; Sun, Q.; Wei, Y.; Han, B. Advancements in Hydrogel-Based Drug Sustained Release Systems for Bone Tissue Engineering. Front. Pharmacol. 2020, 11, 622. [Google Scholar] [CrossRef]
- Khalaf, A.T.; Wei, Y.; Wan, J.; Zhu, J.; Peng, Y.; Abdul Kadir, S.Y.; Zainol, J.; Oglah, Z.; Cheng, L.; Shi, Z. Bone Tissue Engineering through 3D Bioprinting of Bioceramic Scaffolds: A Review and Update. Life 2022, 12, 903. [Google Scholar] [CrossRef]
- Bozorgi, A.; Khazaei, M.; Soleimani, M.; Jamalpoor, Z. Application of nanoparticles in bone tissue engineering; a review on the molecular mechanisms driving osteogenesis. Biomater. Sci. 2021, 9, 4541–4567. [Google Scholar] [CrossRef]
- Huynh, N.C.; Everts, V.; Akira, N.; Pavasant, P.; Ampornaramveth, R.S. Histone deacetylase inhibition enhances in-vivo bone regeneration induced by human periodontal ligament cells. Bone 2016, 95, 76–84. [Google Scholar] [CrossRef]
- Sukpaita, T.; Chirachanchai, S.; Suwattanachai, P.; Everts, V.; Pimkhaokham, A.; Ampornaramveth, R.S. In Vivo Bone Regeneration Induced by a Scaffold of Chitosan/Dicarboxylic Acid Seeded with Human Periodontal Ligament Cells. Int. J. Mol. Sci. 2019, 20, 883. [Google Scholar] [CrossRef]



| IL-1β | TGGACCTTCCAGGATGAGGACA | GTTCATCTCGGAGCCTGTAGTG |
| COL-1 | GTGCTAAAGGTGCCAATGGT | ACCAGGTTCACCGCTGTTAC |
| RUNX2 | CCCCACGACAACCGCACCAT | CACTCCGGCCCACAAATC |
| ALP | CGAGATACAAGCACTCCCACTTC | CTGTTCAGCTCGTACTGCATGTC |
| OPN | AGGAGGAGGCAGAGCACA | CTGGTATGGCACAGGTGATG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sukpaita, T.; Chirachanchai, S.; Chanamuangkon, T.; Nampuksa, K.; Monmaturapoj, N.; Sumrejkanchanakij, P.; Pimkhaokham, A.; Ampornaramveth, R.S. Novel Epigenetic Modulation Chitosan-Based Scaffold as a Promising Bone Regenerative Material. Cells 2022, 11, 3217. https://doi.org/10.3390/cells11203217
Sukpaita T, Chirachanchai S, Chanamuangkon T, Nampuksa K, Monmaturapoj N, Sumrejkanchanakij P, Pimkhaokham A, Ampornaramveth RS. Novel Epigenetic Modulation Chitosan-Based Scaffold as a Promising Bone Regenerative Material. Cells. 2022; 11(20):3217. https://doi.org/10.3390/cells11203217
Chicago/Turabian StyleSukpaita, Teerawat, Suwabun Chirachanchai, Theerapat Chanamuangkon, Katanchalee Nampuksa, Naruporn Monmaturapoj, Piyamas Sumrejkanchanakij, Atiphan Pimkhaokham, and Ruchanee Salingcarnboriboon Ampornaramveth. 2022. "Novel Epigenetic Modulation Chitosan-Based Scaffold as a Promising Bone Regenerative Material" Cells 11, no. 20: 3217. https://doi.org/10.3390/cells11203217
APA StyleSukpaita, T., Chirachanchai, S., Chanamuangkon, T., Nampuksa, K., Monmaturapoj, N., Sumrejkanchanakij, P., Pimkhaokham, A., & Ampornaramveth, R. S. (2022). Novel Epigenetic Modulation Chitosan-Based Scaffold as a Promising Bone Regenerative Material. Cells, 11(20), 3217. https://doi.org/10.3390/cells11203217








