Abstract
This study aimed to assess the impacts of dietary supplementation with passionfruit (Passiflora edulis) peel powder (PSPP) on the growth, immune response, and expression of immune and antioxidant-related genes in Nile tilapia (Oreochromis niloticus) maintained in a biofloc system. Fish were fed basal diets supplemented with different doses of PSPP at 10 g kg−1 (PSPP10), 20 g kg−1 (PSPP20), 40 g kg−1 (PSPP40), and 80 g kg−1 (PSPP80). The basal diet, without PSPP-supplementation, was used as a control at 0 g kg−1 (PSPP0). We observed that the dietary supplementation groups fed different levels of PSPP exhibited no substantial difference or only slight increases in growth performance and immunological response in Nile tilapia (p > 0.05), whereas fish fed diets supplemented with PSPP at concentrations of 10 g kg−1, 20 g kg−1, and 40 g kg−1 had significantly higher mRNA transcripts (approximately 1.5–4.5 fold) of immune (il-1, il-8, and lbp) and antioxidant (gst-α, gpx, and gsr) gene expressions than fish in the control treatment group (0 g kg−1). These findings suggest that dietary supplementation with PSPP may effectively stimulate the immune and antioxidant defense system and may function as feed additives in Nile tilapia cultured in a biofloc system.
1. Introduction
Nile tilapia (Oreochromis niloticus) has been extensively produced in more than 100 nations globally, generating around 7.3 million tons in 2021, because of its flexibility, high growth, resistance to stress and disease, and great economic value [1,2,3,4]. Nonetheless, like with any intensively cultured fish, tilapia farming imposes significant strains on the water quality for fish farming and increases the occurrence of pathogenic infections—especially bacterial diseases [5,6]—resulting in a high mortality rate (up to 95%) and massive economic losses [7,8]. Antibiotics and chemotherapeutics have been commonly used by farmers all over the world to control disease outbreaks in fish farming [9]. However, these activities have caused the outgrowth of antibiotic-resistant bacteria [10,11]. Due to restrictions on the use of antibiotics in aquaculture, the development of innovative ways to supply appropriate feed additives and develop cost-effective methods of disease prevention and treatment for fish has become a top concern [12,13]. Consequently, natural immunostimulants (such as prebiotics, probiotics, and synbiotics) are promising alternatives for modifying the bacterial population and attempting to control infectious disease outbreaks in aquaculture by enhancing dietary intake, nutritional absorption, and immune defense systems in aquatic animals [14,15,16,17]. In this respect, fruit by-products have been identified as potential supplements in the diets of aquatic species [18,19,20]. Fruit by-products used as feed additives have the potential to minimize waste, reduce aquafeed costs, and offer raw materials for the nutritional sectors [21]. Additionally, utilizing these by- and co-products would also have a positive influence on the environment and provide additional advantages to farmers [22,23].
Passionfruit (Passiflora edulis) is a species of the Passifloraceae family, which has more than 500 species [24]. It is found mostly in North America, but also in tropical and subtropical Southeast Asia, Australia, and New Zealand due to its economic and medicinal properties [25,26]. The passionfruit extract market is projected to reach USD 1028.6 million by 2029 [27]. Peels are created in great amounts during the processing of passionfruit to produce passionfruit juice [28]. Moreover, passionfruit peel is a by-product of the fruit processing industry that makes up around 50% of the weight of the fruit [29], which is typically thrown away as waste [30,31]. The passionfruit peel includes a variety of bioactive components, including phenolic compounds, flavonoids, cyanogenic chemicals, anthocyanin, minerals, polysaccharides, and vitamins [28,32,33,34,35]. Numerous investigations using passionfruit by-products as feed additives have been conducted on sheep [36], swine [37,38], quail [39,40], and poultry [41,42]. For fish farming, the incorporation of passionfruit seed meal (including its oil residue) in diets for tambaqui (Colossoma macropomum) [43,44] and passionfruit juice in tilapia has been investigated [45]. However, there have been few studies on the influences of passionfruit peel powder (PSPP) on the growth and overall wellbeing of common commercial fish species—particularly Nile tilapia.
Biofloc technology (BFT) is an alternative approach that mixes aggregates of algae, protozoa, or bacteria with particulate organic substances to improve water quality, waste treatment, and disease prevention in intensive aquaculture systems. It has been proposed as a cost-effective alternative to intensive systems since it improves water quality without requiring water exchange and produces microbial protein for aquatic species. Biofloc is a microbial community composed mostly of prokaryotic and eukaryotic microorganisms and different organic particulates [46,47,48]. Biofloc functions as a nutrition supply for aquatic creatures in this system, assisting in growth enhancement, pathogen reduction, and zero-water exchange. Additionally, BFT has a beneficial impact on the host immune system, increasing resistance to diseases and infections [49,50]. Therefore, this study aimed to assess the influence of dietary supplementation with powdered passionfruit peel on growth, immunological responses, and the expression of key immune-antioxidant-related genes in Nile tilapia raised in a biofloc system.
2. Materials and Methods
2.1. Preparation of Powdered Passionfruit Peel and Experimental Diets
Passionfruit was obtained from local markets at Chiang Mai (Thailand). Passionfruit peels were oven-dried at 60 °C for 48 h. The dried peel was then ground into a powder using a mill and stored at 4 °C until used in the fishes’ diets. To prepare the dough, the feedstuffs were blended and then soybean oil and distilled water were added. The dough was then pelleted (2 mm in diameter) in a pelleting machine. Pellets were dried at 50 °C to attain 10% moisture and stored in sealed polyethylene bags at 4 °C until used. The proximate composition of the diets was determined using the techniques recommended by AOAC [51], and the crude fat content was measured using an extractable matter machine (SoxtecTM 8000, Hilleroed, Denmark). The basal diets were modified according to the descriptions reported previously [52], which proved their suitability for Nile tilapia by their different levels of PSPP. The ingredients and elemental composition of the basal diets are shown in Table 1.

Table 1.
Formulation and proximate composition of the experimental diets (g kg−1).
2.2. Culture Conditions
Three weeks before starting the experiment, floc inoculants were generated in each tank (150 L) by adding sea salt (400 g), dolomite (5 g), wheat flour (2 g), and molasses (5 g). After formation, the floc quantity was kept constant throughout the experiment at a level of approximately 8.21 ± 0.15 mL per tank, by maintaining the NH3 concentration at 0.11 ± 0.005 mg L−1 and modifying the C:N ratio (15:1) by adding molasses and probiotics (PondPlus, Bayer Thai Co., Ltd., Bangkok, Thailand) [53]. The C:N ratio was calculated using a diagrammatic representation of residual nitrogen grades and food intake [54].
2.3. Experimental Design
Nile tilapia fingerlings were purchased from a tilapia hatchery in Chiang Mai, Thailand. Fish were acclimatized and fed a commercial meal CP-9950 (Charoen Pokphand Foods Public Co., Ltd., Bangkok, Thailand) for two weeks. Prior to conducting additional experiments, twenty randomly selected fish were subjected to bacterial and parasite examinations to guarantee their health. A total of 300 Nile tilapia (14.22 ± 0.05 g) were randomly assigned into five dietary treatment groups with PSPP supplemented as follows: control-PSPP0 (0 g kg−1), PSPP10 (10 g kg−1), PSPP20 (20 g kg−1), PSPP40 (40 g kg−1), and PSPP80 (80 g kg−1). Fish were maintained in 150 L glass tanks. The experimental trials were conducted in triplicate with 20 fish per tank. Fish were fed twice daily at 8:30 a.m. and 4:30 p.m. at 3% body weight for eight weeks. Temperature, pH, and dissolved oxygen were maintained at 25–29 °C, 7.5–7.9, and 5 mg L−1, respectively.
2.4. Growth Rates
Initial weight (IW), weight gain (WG), survival rate (SR), specific growth rate (SGR), and feed conversion rate (FCR) in Nile tilapia were determined after four and eight weeks of feeding trial as follows [55]:
WG (g) = FW − IW
Weights were measured using a balance with an accuracy of 0.1 g. Additionally, any dead fish were tallied and the mortality rate computed during the experiment.
2.5. Immune Response Analysis
2.5.1. Sample Collection
To examine immunological activities, skin mucus and blood samples (6 fish/treatment) were collected. Before collecting samples, clove oils (5 mL L−1) were used to anesthetize fish. For skin mucus sample collection, the experimental fish were gently rubbed for 2 min in a plastic bag containing 10 mL of 50 mM NaCl (Merck, Germany). The mixture was immediately transferred into new sterile tubes and centrifuged at 1500× g at 4 °C for 10 min. The mucus samples (1 mL) were then kept at −80 °C until used. For blood sampling, blood was collected according to the protocols previously reported [56]. Briefly, approximately 1 mL of fish blood was obtained using a 1 mL syringe at the caudal vein. Blood samples were promptly withdrawn and placed into sterile tubes (without adding anticoagulant). The samples were kept for an hour at room temperature and at 4 °C for a further hour. The samples were then centrifuged, and the serum samples were collected and stored at −80 °C for further analysis.
2.5.2. Lysozyme and Peroxidase Assay
Lysozyme assays were conducted following the procedures reported by Parry, et al. [57], with the minor modifications in Van Doan et al. [55]. The equivalent unit of activity for each sample was calculated in accordance with the standard curve, which was constructed by plotting the decrease in the optical density value against the concentration, ranging from 0–20 µL mL−1 of hen egg-white lysozyme (Sigma-Aldrich, Inc., USA) and represented as µg mL−1 of serum.
Peroxidase activity was determined according to the protocols described in Quade and Roth [58] and Cordero, et al. [59], with minor modifications in Van Doan et al. [55]. The peroxidase assay was represented in units (U) per mg of skin mucus or serum proteins, where a unit was defined as the quantity of serum or mucus proteins that produced a change in absorbance equal to one.
2.6. Total RNA Extraction and Real-Time PCR (qPCR) Analysis
A total of 40–50 mg of fish tissues (gills and liver) was sampled for RNA extraction using TRIzol and an RNA extraction kit (Invitrogen, Waltham, MA, USA). The quality and quantity were measured using spectrophotometers (Thermo Fisher Scientific, Waltham, MA, USA) at wavelengths of 260 and 280 nm. The first-strand complementary DNA (cDNA) was synthesized with 1 μg total RNA using the iScript™ cDNA Synthesis Kit (BIO-RAD, Hercules, CA, USA). The analysis was conducted in triplicate using 100 ng of cDNA and iTaq Universal SYBR Green on a CFX Connect™ real-time PCR (BIO-RAD, Hercules, CA, USA). The qPCR was conducted at 95 °C for 30 s, 40 cycles of 95 °C for 15 s, and 60 °C for 30 s and followed by 95 °C for 15 s, 60 °C for 60 s, and 95 °C for 15 s. Expression levels was analyzed according to the 2−ΔΔCt method [60] The qPCR results were normalized to the 18S rRNA gene. The primers used for the qPCR analysis in this study are presented in Table 2.

Table 2.
Primers used for the qPCR analysis in this study.
2.7. Statistical Analysis
The Kolmogorov–Smirnov test was used to evaluate the normality of the data. Means were compared using Duncan’s multiple range test. Growth rates, immunological responses, and gene expression levels were analyzed using ANOVA analysis. SAS v9.4 statistical software (Cary, NC, USA) was used for all the statistical analyses [62]. p < 0.05 was denoted as a significant difference.
3. Results
3.1. Growth Performance Analysis
In this study, the growth parameters observed in different dietary treatment groups are presented in Table 3. There was no significant difference in final weight (FW), weight gain (WG), feed conversion ratio (FCR), or specific growth rate (SGR) between fish fed PSPP-supplemented diets and those fed only a basal diet (0 g kg−1 PSPP) after four and eight weeks of the experimental trial. The survival rate (SR) of all treatment groups exceeded 95% at the conclusion of the feeding studies. No significant difference in any groups of the dietary PSPP-supplemented diets were detected (p > 0.05).

Table 3.
Growth performances and feed utilization in Nile tilapia with different levels of PSPP-supplemented diets after four and eight weeks of the feeding trial. Different letters in the same row indicate statistically significant differences (p < 0.05). Data are presented as mean ± SE.
3.2. Analysis of Skin Mucus Immune Responses
Lysozyme and peroxidase activities in skin mucus in Nile tilapia after four and eight weeks of feeding are presented in Figure 1. No significant difference (p > 0.05) in lysozyme activity was observed between fish fed PSPP-supplemented diets and those fed only a basal diet (0 g kg−1 PSPP) after four and eight weeks of feeding. Only fish fed the 20 g kg−1 PSPP (PSPP20) diet had significantly (p < 0.05) higher peroxidase activity than those with other treatments after four weeks of feeding trial (Figure 1B). No significant difference was found in peroxidase activity after eight weeks of feeding (p > 0.05).
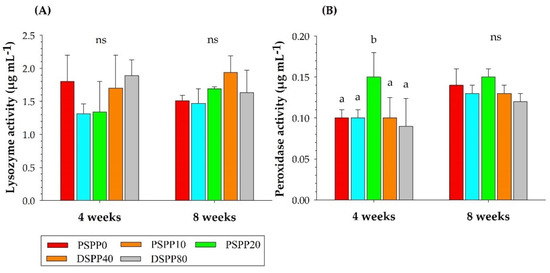
Figure 1.
Lysozyme (A) and peroxidase (B) activity in the skin mucus of Nile tilapia after four and eight weeks of feeding with experimental diets: 0 g kg−1 (PSPP0) control, 10 g kg−1 (PSPP10), 20 g kg−1 (PSPP20), 40 g kg−1 (PSPP40), and 80 g kg−1 (PSPP80) cultivated for eight weeks. Data are presented as mean ± SE. Significantly different levels are denoted by superscript letters (p < 0.05). “ns” denotes no significant difference.
3.3. Analysis of Serum Immune Responses
Serum immunological activities (lysozyme and peroxidase) were determined in this study using serum samples obtained after four and eight weeks of feeding. Figure 2 summarizes the impact of the experimental diets on serum immunological activity. Peroxidase and lysozyme activities in serum showed substantially higher levels in fish fed dietary PSPP-supplemented diets than those fed the basal diet without PSPP supplementation after four and eight weeks of the experimental trial. No statistically significant differences in lysozyme activity were detected in any of the dietary supplementation treatments (p > 0.05) after four or eight weeks of feeding. On the other hand, the PSPP10 diet substantially enhanced serum peroxidase activity compared to the control PSPP0 group (p < 0.05). At eight weeks post-feeding, a statistically significant change in the activity of peroxidase was detected between the PSPP-supplemented treatments (PSPP20 and PSPP80) and the PSPP0 control treatment (p < 0.05).
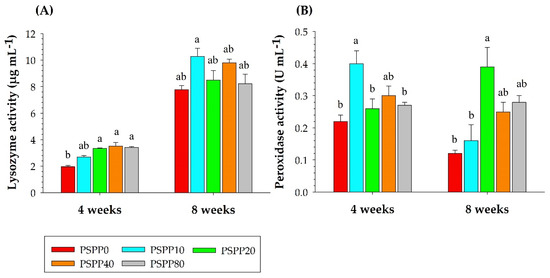
Figure 2.
Lysozyme (A) and peroxidase (B) activity in the serum of Nile tilapia after four and eight weeks of feeding with experimental diets: 0 g kg−1 (PSPP0) control, 10 g kg−1 (PSPP10), 20 g kg−1 (PSPP20), 40 g kg−1 (PSPP40), and 80 g kg−1 (PSPP80) cultivated for eight weeks. Data are presented as mean ± SE. Significantly different levels are denoted by superscript letters (p < 0.05).
3.4. Analysis of Immune and Antioxidant-Related Gene Expression
Fish tissues (gills and liver) were collected to investigate relative immune and antioxidant gene expressions by qPCR. Three relative immune genes (lbp, il-1, and il-8) and three antioxidant genes (gsr, gst-α, and gpx) were investigated in this study. A significant upregulation (approximately 2–2.7 fold) was found in the gill tissues of fish fed with dietary supplementation with PSPP compared to those fed the basal diet without PSPP supplementation (the control group, PSPP0). The greatest level of mRNA transcripts was observed in the PSPP20 diet groups (approximately 3.1–3.7 fold) in lbp, gst-α, and gpx, whereas il-1 and il-8 had the highest levels in the dietary PSPP40 and PSPP10 treatments, respectively (Figure 3).
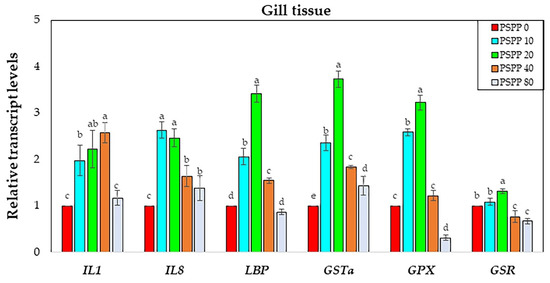
Figure 3.
Expression transcript levels of interleukin-1 (il-1), interleukin-8 (il-8), lipopolysaccharide-binding protein (lbp), glutathione S-transferase-α (GST-α), glutathione peroxidase (gpx), and glutathione reductase (gsr) in gill tissues of Nile tilapia (n = 5) fed diets supplemented 0 g kg−1 PSPP, 10 g kg−1 PSPP, 20 g kg−1 PSPP, 40 g kg−1 PSPP, and 80 g kg−1 PSPP after eight weeks of feeding. The mRNA transcript levels of immune and antioxidant genes were normalized by 18S rRNA. The mRNA transcript level of the 0 g kg−1 PSPP control group was set at 1. Data are presented as mean ± SE. Significantly different levels are denoted by superscript letters (p < 0.05).
A substantial difference in the mRNA transcripts of the examined genes was identified in liver tissues (Figure 4). Fish given the dietary PSPP20 expressed the greatest levels of mRNA transcripts in all target genes (except lbp) compared to the other dietary treatment and the control group (approximately 2–4.3 fold). Lbp expression was considerably upregulated in fish fed with dietary PSPP40 (approximately 2.5-fold), followed by PSPP20 (approximately 2.0-fold) and PSPP10 (approximately 1.8-fold).
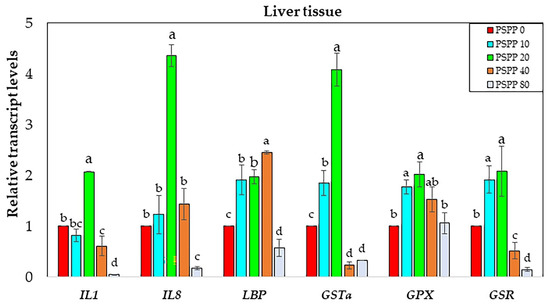
Figure 4.
Expression transcript levels of interleukin-1 (il-1), interleukin-8 (il-8), lipopolysaccharide-binding protein (lbp), glutathione S-transferase-α (gst-α), glutathione peroxidase (gpx), and glutathione reductase (gsr) in liver tissues of Nile tilapia (n = 5) fed diets supplemented 0 g kg−1 PSPP, 10 g kg−1 PSPP, 20 g kg−1 PSPP, 40 g kg−1 PSPP, and 80 g kg−1 PSPP after eight weeks of feeding. The mRNA transcript levels of immune and antioxidant genes were normalized by 18S rRNA. The mRNA transcript level of the 0 g kg−1 PSPP control group was set at 1. Data are presented as mean ± SE. Significantly different levels are denoted by superscript letters (p < 0.05).
4. Discussion
The use of fruit by-products as feed additives in aquaculture is an efficient approach to conserve the environment and generate more income for farmers. By-products, such as peels and seeds, contain many substances with health-promoting effects [63,64,65].
After eight weeks of feeding, we observed that growth performance and feed consumption were unaffected by the PSPP supplement diets. The findings of this study were consistent with those of earlier studies on Jaraqui (Semaprochilodus insignis) and tambaqui fed passionfruit seed cake [66]; silver catfish (Schilbe intermedius) fed grape, orange, guava, and fig residues [67]; Nile tilapia and African catfish (Clarias gariepinus) fed dehydrated lemon peels [68]; Nile tilapia fed passionfruit seed oil and pomegranate peel [69,70]; rainbow trout (Oncorhynchus mykiss) fed dehydrate lemon peel [71]; Asian sea bass (Lates calcarifer) fed fermented lemon peel [72,73]; and giant freshwater prawn (Macrobrachium rosenbergii) fed biogas sludge meal [74], suggesting that the PSPP may not promote the production of digestive enzymes or intestinal absorption due to the large levels of soluble and insoluble fiber in PSPP [75]. Dietary fiber in PSPP has been shown by Vuolo, et al. [76] to decrease glucose and lipid absorption, resulting in less energy storage and increased lipid and glucose excretion. There was no discernible difference between the dietary PSPP-supplemented groups in this study. According to Ramli, et al. [33], PSPP has various valuable active components, including phenolic compounds, flavonoids, cyanogenic chemicals, anthocyanin, minerals, polysaccharides, and vitamins—which may account for its beneficial impact on growth performance [28,34,77]. Additionally, the PSPP contains considerable amounts of vitamin C that can be fortified into fish feed [78]. More investigations are needed to clarify the impact of these extracts on the growth performance of Nile tilapia cultivated in biofloc systems.
Skin mucus plays an important role in fish immune responses [79]. Lysozyme and peroxidase are important indicators of the immune defense system of fish; it has lytic action against bacteria and participates in phagocytic activity, neutrophil activation, and the complement system [80,81]. Lysozyme and peroxidase activities were greater in fish fed PSPP diets than in the control group after four and eight weeks of the feeding experiment. The addition of fruit by-products or extracts to diets, especially powdered passionfruit peel, has a beneficial effect on the immunological activity of Nile tilapia, striped catfish (Pangasianodon hypophthalmus), black rockfish (Sebastes schlegelii), and gilthead seabream (Sparus aurata) [61,82,83,84].
il-1 and il-8 are responsible for regulating inflammatory processes in the innate immune system to stimulate phagocytes to engulf microorganisms [85]. Antioxidant-related genes are involved in the glutathione protection mechanism, responsible for hydrogen peroxide removal (H2O2). A phase II xenobiotic metabolic enzyme, glutathione S-transferase (GST), combines with electrophilic chemicals to produce bigger endogenic complexes known as glutathione S-conjugates, which are then expelled from the body [86]. GPX transforms H2O2 into H2O via the oxidation of glutathione (GSH) to glutathione disulfide (GSSG). GSH is revived by GSR after it has been oxidized by the oxidative reduction of NADPH [87]. Increased immune responses and gene expression levels in fish fed powdered passionfruit peel are likely to be the result of an overall improvement in health and wellbeing due to a combination of several health benefits associated with dietary PSPP. These include (i) greater immunity against pathogens, indicated by elevated lysozyme and peroxidase levels in skin mucus and serum, and by elevated il-1, il-8, and lbp mRNA transcript levels in the gills and liver tissues; (ii) enhanced antioxidant activity, indicated by elevated mRNA transcript levels of gst-α, gpx and gsr; and (iii) PSPP may stimulate the immune defense system in fish, thereby improving survival rates and disease resistance in fish.
The successful application of biofloc in aquaculture depends on the presence of both prebiotics and probiotics. The addition of PSPP in a biofloc aquaculture system may be involved in several processes, such as stimulating the proliferation of favorable bacteria, inhibiting the growth of pathogenic microorganisms, and improving the gastrointestinal condition of fish [61,88,89,90]. On the other hand, the recycling of nitrogen via its conversion to microbial biomass in biofloc increases the populations of favorable bacteria, enhancing host immunity [91].
5. Conclusions
Diets containing powdered passionfruit peel at concentrations of 10 to 20 g kg−1 improved expression levels of innate immune and antioxidant-related genes in Nile tilapia cultured in a biofloc system. However, fish fed PSPP-supplemented feed had no significantly differences in growth performance; further studies should explore this issue to gain a better understanding of the impacts of PSPP in Nile tilapia.
Author Contributions
P.O.; investigation, N.V.L.; writing—original draft preparation, C.L.X.; formal analysis, S.W.; formal analysis, S.T.; resources, C.C.; data curation, N.M.; visualization, H.V.D.; conceptualization, project administration, and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research work was partially supported by Chiang Mai University (CoE2565).
Institutional Review Board Statement
This study was conducted in accordance with international guidelines and approved by the Chiang Mai University Committee (No. RAGIACUC002/2565).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors take this opportunity to thank the National Research Council of Thailand for supporting and helping with the study. This research work was partially supported by Chiang Mai University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Magbanua, T.O.; Ragaza, J.A. Selected dietary plant-based proteins for growth and health response of Nile tilapia Oreochromis niloticus. Aquac. Fish. 2022. [Google Scholar] [CrossRef]
- Fialho, N.S.; Valenti, W.C.; David, F.S.; Godoy, E.M.; Proença, D.C.; Roubach, R.; Wolff Bueno, G. Environmental sustainability of Nile tilapia net-cage culture in a neotropical region. Ecol. Indic. 2021, 129, 108008. [Google Scholar] [CrossRef]
- Mai, T.T.; Kayansamruaj, P.; Soontara, C.; Kerddee, P.; Nguyen, D.H.; Senapin, S.; Costa, J.Z.; Del-Pozo, J.; Thompson, K.D.; Rodkhum, C.; et al. Immunization of Nile Tilapia (Oreochromis niloticus) Broodstock with Tilapia Lake Virus (TiLV) Inactivated Vaccines Elicits Protective Antibody and Passive Maternal Antibody Transfer. Vaccines 2022, 10, 167. [Google Scholar] [CrossRef] [PubMed]
- Munguti, J.M.; Nairuti, R.; Iteba, J.O.; Obiero, K.O.; Kyule, D.; Opiyo, M.A.; Abwao, J.; Kirimi, J.G.; Outa, N.; Muthoka, M.; et al. Nile tilapia (Oreochromis niloticus Linnaeus, 1758) culture in Kenya: Emerging production technologies and socio-economic impacts on local livelihoods. Aquac. Fish Fish. 2022, 2, 265–276. [Google Scholar] [CrossRef]
- Paredes-Trujillo, A.; Mendoza-Carranza, M. A systematic review and meta-analysis of the relationship between farm management, water quality and pathogen outbreaks in tilapia culture. J. Fish Dis. 2022, 00, 1–20. [Google Scholar] [CrossRef]
- Alazab, A.; Sadat, A.; Younis, G. Prevalence, antimicrobial susceptibility, and genotyping of Streptococcus agalactiae in Tilapia fish (Oreochromis niloticus) in Egypt. J. Adv. Vet. Anim Res. 2022, 9, 95–103. [Google Scholar] [CrossRef]
- Chen, S.-W.; Liu, C.-H.; Hu, S.-Y. Dietary administration of probiotic Paenibacillus ehimensis NPUST1 with bacteriocin-like activity improves growth performance and immunity against Aeromonas hydrophila and Streptococcus iniae in Nile tilapia (Oreochromis niloticus). Fish Shellfish. Immunol. 2019, 84, 695–703. [Google Scholar] [CrossRef]
- Liu, G.; Zhu, J.; Chen, K.; Gao, T.; Yao, H.; Liu, Y.; Zhang, W.; Lu, C. Development of Streptococcus agalactiae vaccines for tilapia. Dis. Aquat. Org. 2016, 122, 163–170. [Google Scholar] [CrossRef]
- Payne, C.J.; Turnbull, J.F.; MacKenzie, S.; Crumlish, M. Investigating the Effect of an Oxytetracycline Treatment on the Gut Microbiome and Antimicrobial Resistance Gene Dynamics in Nile Tilapia (Oreochromis niloticus). Antibiotics 2021, 10, 1213. [Google Scholar] [CrossRef]
- Sun, B.-Y.; He, W.; Yang, H.-X.; Tian, D.-Y.; Jian, P.-Y.; Wu, K.; Yang, C.-G.; Song, X.-H. Increased susceptibility to Aeromonas hydrophila infection in grass carp with antibiotic-induced intestinal dysbiosis. Aquaculture 2022, 552, 737969. [Google Scholar] [CrossRef]
- Schar, D.; Zhao, C.; Wang, Y.; Larsson, D.G.J.; Gilbert, M.; van Boeckel, T.P. Twenty-year trends in antimicrobial resistance from aquaculture and fisheries in Asia. Nat. Commun. 2021, 12, 5384. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, S.A.; Ramachandran, A.; Perron, G.G. Antibiotic Pollution in the Environment: From Microbial Ecology to Public Policy. Microorganisms 2019, 7, 180. [Google Scholar] [CrossRef] [PubMed]
- Thornber, K.; Verner-Jeffreys, D.; Hinchliffe, S.; Rahman, M.M.; Bass, D.; Tyler, C.R. Evaluating antimicrobial resistance in the global shrimp industry. Rev. Aquac. 2020, 12, 966–986. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Latif, H.M.R.; Yilmaz, E.; Dawood, M.A.O.; Ringø, E.; Ahmadifar, E.; Yilmaz, S. Shrimp vibriosis and possible control measures using probiotics, postbiotics, prebiotics, and synbiotics: A review. Aquaculture 2022, 551, 737951. [Google Scholar] [CrossRef]
- Yilmaz, S.; Yilmaz, E.; Dawood, M.A.O.; Ringø, E.; Ahmadifar, E.; Abdel-Latif, H.M.R. Probiotics, prebiotics, and synbiotics used to control vibriosis in fish: A review. Aquaculture 2022, 547, 737514. [Google Scholar] [CrossRef]
- Iwashita, M.K.P.; Addo, S.; Terhune, J.S. 9—Use of pre- and probiotics in finfish aquaculture. In Feed and Feeding Practices in Aquaculture, 2nd ed.; Davis, D.A., Ed.; Woodhead Publishing: Oxford, UK, 2022; pp. 269–289. [Google Scholar]
- Mugwanya, M.; Dawood, M.A.O.; Kimera, F.; Sewilam, H. Updating the Role of Probiotics, Prebiotics, and Synbiotics for Tilapia Aquaculture as Leading Candidates for Food Sustainability: A Review. Probiotics Antimicrob. Proteins 2022, 14, 130–157. [Google Scholar] [CrossRef]
- Buruiana, C.-T.; Gómez, B.; Vizireanu, C.; Garrote, G. Manufacture and evaluation of xylooligosaccharides from corn stover as emerging prebiotic candidates for human health. LWT 2017, 77, 449–459. [Google Scholar] [CrossRef]
- Yao, W.; Gong, Y.; Li, L.; Hu, X.; You, L. The effects of dietary fibers from rice bran and wheat bran on gut microbiota: An overview. Food Chem. X 2022, 13, 100252. [Google Scholar] [CrossRef]
- Deehan, E.C.; Zhang, Z.; Riva, A.; Armet, A.M.; Perez-Muñoz, M.E.; Nguyen, N.K.; Krysa, J.A.; Seethaler, B.; Zhao, Y.-Y.; Cole, J.; et al. Elucidating the role of the gut microbiota in the physiological effects of dietary fiber. Microbiome 2022, 10, 77. [Google Scholar] [CrossRef]
- Gómez-García, R.; Campos, D.A.; Aguilar, C.N.; Madureira, A.R.; Pintado, M. Valorization of melon fruit (Cucumis melo L.) by-products: Phytochemical and Biofunctional properties with Emphasis on Recent Trends and Advances. Trends Food Sci. Technol. 2020, 99, 507–519. [Google Scholar] [CrossRef]
- Donner, M.; Verniquet, A.; Broeze, J.; Kayser, K.; de Vries, H. Critical success and risk factors for circular business models valorising agricultural waste and by-products. Resour. Conserv. Recycl. 2021, 165, 105236. [Google Scholar] [CrossRef]
- Thakur, N.; Nigam, M.; Tewary, R.; Rajvanshi, K.; Kumar, M.; Shukla, S.K.; Mahmoud, G.A.-E.; Gupta, S. Drivers for the behavioural receptiveness and non-receptiveness of farmers towards organic cultivation system. J. King Saud Univ. Sci. 2022, 34, 102107. [Google Scholar] [CrossRef]
- Yan, C.; Muhammad Rizwan, H.; Liang, D.; Reichelt, M.; Mithöfer, A.; Scholz, S.S.; Oelmüller, R.; Chen, F. The effect of the root-colonizing Piriformospora indica on passion fruit (Passiflora edulis) development: Initial defense shifts to fitness benefits and higher fruit quality. Food Chem. 2021, 359, 129671. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Luan, F.; Yang, Y.; Wang, Z.; Zhao, Z.; Fang, J.; Wang, M.; Zuo, M.; Li, Y. Passiflora edulis: An Insight Into Current Researches on Phytochemistry and Pharmacology. Front. Pharmacol. 2020, 11, 617. [Google Scholar] [CrossRef]
- Hu, Y.; Jiao, L.; Jiang, M.-H.; Yin, S.; Dong, P.; Zhao, Z.-M.; Yang, D.-P.; Ho, P.-T.; Wang, D.-M. A new C-glycosyl flavone and a new neolignan glycoside from Passiflora edulis Sims peel. Nat. Prod. Res. 2018, 32, 2312–2318. [Google Scholar] [CrossRef] [PubMed]
- FMI. Passion Fruit Extract Market projected to Reach USD 1,028.6 Million by 2029—Comprehensive Research Report by FMI; FMI: Maharashtra, India, 2022. [Google Scholar]
- Chutia, H.; Mahanta, C.L. Green ultrasound and microwave extraction of carotenoids from passion fruit peel using vegetable oils as a solvent: Optimization, comparison, kinetics, and thermodynamic studies. Innov. Food Sci. Emerg. Technol. 2021, 67, 102547. [Google Scholar] [CrossRef]
- Abboud, K.Y.; Iacomini, M.; Simas, F.F.; Cordeiro, L.M.C. High methoxyl pectin from the soluble dietary fiber of passion fruit peel forms weak gel without the requirement of sugar addition. Carbohydr. Polym. 2020, 246, 116616. [Google Scholar] [CrossRef]
- Mohd Basri, M.S.; Abdul Karim Shah, N.N.; Sulaiman, A.; Mohamed Amin Tawakkal, I.S.; Mohd Nor, M.Z.; Ariffin, S.H.; Abdul Ghani, N.H.; Mohd Salleh, F.S. Progress in the Valorization of Fruit and Vegetable Wastes: Active Packaging, Biocomposites, By-Products, and Innovative Technologies Used for Bioactive Compound Extraction. Polymers 2021, 13, 3503. [Google Scholar] [CrossRef]
- Ghada, B.; Pereira, E.; Pinela, J.; Prieto, M.A.; Pereira, C.; Calhelha, R.C.; Stojković, D.; Sokóvić, M.; Zaghdoudi, K.; Barros, L.; et al. Recovery of Anthocyanins from Passion Fruit Epicarp for Food Colorants: Extraction Process Optimization and Evaluation of Bioactive Properties. Molecules 2020, 25, 3203. [Google Scholar] [CrossRef]
- Ramli, A.N.M.; Manap, N.W.A.; Bhuyar, P.; Azelee, N.I.W. Passion fruit (Passiflora edulis) peel powder extract and its application towards antibacterial and antioxidant activity on the preserved meat products. SN Appl. Sci. 2020, 2, 1–11. [Google Scholar] [CrossRef]
- Cao, Q.; Teng, J.; Wei, B.; Huang, L.; Xia, N. Phenolic compounds, bioactivity, and bioaccessibility of ethanol extracts from passion fruit peel based on simulated gastrointestinal digestion. Food Chem. 2021, 356, 129682. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.d.P.; Teixeira Tarley, C.R. Bioaccessibility estimation of metallic macro and micronutrients Ca, Mg, Zn, Fe, Cu and Mn in flours of oat and passion fruit peel. LWT 2021, 150, 111880. [Google Scholar] [CrossRef]
- Júnior, J.E.L.; da Costa, J.M.; Neiva, J.N.M.; Rodriguez, N.M. Physical-chemical characterization of tropical fruit by-products for use in animal feed. Rev. Ciência Agronômica 2006, 37, 70. [Google Scholar]
- Fachinello, M.R.; Pozza, P.C.; Moreira, I.; Carvalho, P.L.O.; Castilha, L.D.; Pasquetti, T.J.; Esteves, L.A.C.; Huepa, L.M.D. Effect of passion fruit seed meal on growth performance, carcass, and blood characteristics in starter pigs. Trop. Anim. Health Prod. 2015, 47, 1397–1403. [Google Scholar] [CrossRef]
- Perondi, D.; Moreira, I.; Pozza, P.C.; Carvalho, P.L.d.O.; Pasquetti, T.J.; Huepa, L.M.D. Passion fruit seed meal at growing and finishing pig (30-90 kg) feeding. Ciência Agrotecnologia 2014, 38, 390–400. [Google Scholar] [CrossRef]
- Wadhwa, M.; Bakshi, M.P.; Makkar, H.P. Waste to worth: fruit wastes and by-products as animal feed. CAB Rev. 2015, 10, 1–26. [Google Scholar] [CrossRef]
- Fachinello, M.R.; Pozza, P.C.; Furlan, A.C.; Paula, V.R.C.d.; Bonagurio, L.P.; Marcato, S.M.; Leal, I.F.; Huepa, L.M.D. Nutritional evaluation of passion fruit seed meal for meat quails. Rev. Bras. De Saúde Produção Anim. 2016, 17, 202–213. [Google Scholar] [CrossRef]
- Togashi, C.K.; Fonseca, J.B.; Soares, R.d.T.R.N.; da Costa, A.P.D.; da Silveira, K.F.; Detmann, E. Subprodutos do maracujá em dietas para frangos de corte. Acta Sci. Anim. Sci. 2008, 30, 395–400. [Google Scholar] [CrossRef]
- Togashi, C.K.; Fonseca, J.B.; Soares, R.d.T.R.N.; Gaspar, A.; Detmann, E. Composição em ácidos graxos dos tecidos de frangos de corte alimentados com subprodutos de maracujá. Rev. Bras. De Zootec. 2007, 36, 2063–2068. [Google Scholar] [CrossRef]
- De Souza, A.M.; Campeche, D.F.B.; Moraes, G.; de Melo, F.; da Cruz Neto, M.A.; Melo, J.F.B. Replacing cornmeal with mango meal in diets for juvenile tambaqui Colossoma macropomum: Growth and metabolic parameters. Bol. Inst. Pesca 2018, 44, e248. [Google Scholar] [CrossRef]
- Wegbecher, F.X. Bactérias Celulolíticas e o Uso de Resíduo de Maracujá (Passiflora edulis) em Rações Extrusadas Para Juvenis de Tambaqui (Colossoma macropomum); Instituto Nacional de Pesquisas da Amazônia: Petrópolis, Brazil, 2010. [Google Scholar]
- dos Santos, E.A.; Ribeiro, A.E.C.; Barcellos, T.T.; Monteiro, M.L.G.; Mársico, E.T.; Caliari, M.; Júnior, M.S.S. Exploitation of byproducts from the passion fruit juice and tilapia filleting industries to obtain a functional meat product. Food Biosci. 2021, 41, 101084. [Google Scholar] [CrossRef]
- Ahmad, I.; Babitha Rani, A.; Verma, A.; Maqsood, M. Biofloc technology: An emerging avenue in aquatic animal healthcare and nutrition. Aquac. Int. 2017, 25, 1215–1226. [Google Scholar] [CrossRef]
- Durigon, E.G.; Lazzari, R.; Uczay, J.; de Alcântara Lopes, D.L.; Jerônimo, G.T.; Sgnaulin, T.; Emerenciano, M.G.C. Biofloc technology (BFT): Adjusting the levels of digestible protein and digestible energy in diets of Nile tilapia juveniles raised in brackish water. Aquac. Fish. 2020, 5, 42–51. [Google Scholar] [CrossRef]
- Wei, G.; Shan, D.; Li, G.; Li, X.; Tian, R.; He, J.; Shao, Z. Prokaryotic communities vary with floc size in a biofloc-technology based aquaculture system. Aquaculture 2020, 529, 735632. [Google Scholar] [CrossRef]
- Khanjani, M.H.; Sharifinia, M.; Hajirezaee, S. Recent progress towards the application of biofloc technology for tilapia farming. Aquaculture 2022, 552, 738021. [Google Scholar] [CrossRef]
- Abakari, G.; Wu, X.; He, X.; Fan, L.; Luo, G. Bacteria in biofloc technology aquaculture systems: Roles and mediating factors. Rev. Aquac. 2022, 14, 1260–1284. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of Association of Official Analytical Chemists, 18th ed.; Association of Officiating Analytical Chemists: Washington, DC, USA, 2005. [Google Scholar]
- Van Doan, H.; Hoseinifar, S.H.; Harikrishnan, R.; Khamlor, T.; Punyatong, M.; Tapingkae, W.; Yousefi, M.; Palma, J.; El-Haroun, E. Impacts of pineapple peel powder on growth performance, innate immunity, disease resistance, and relative immune gene expression of Nile tilapia, Oreochromis niloticus. Fish Shellfish. Immunol. 2021, 114, 311–319. [Google Scholar] [CrossRef]
- Avnimelech, Y. Biofloc Technology: A Practical Guide Book; World Aquaculture Society: Baton Rouge. LA, USA, 2015. [Google Scholar]
- Panigrahi, A.; Saranya, C.; Sundaram, M.; Vinoth Kannan, S.R.; Das, R.R.; Satish Kumar, R.; Rajesh, P.; Otta, S.K. Carbon: Nitrogen (C:N) ratio level variation influences microbial community of the system and growth as well as immunity of shrimp (Litopenaeus vannamei) in biofloc based culture system. Fish Shellfish. Immunol. 2018, 81, 329–337. [Google Scholar] [CrossRef]
- Van Doan, H.; Lumsangkul, C.; Sringarm, K.; Hoseinifar, S.H.; Dawood, M.A.O.; El-Haroun, E.; Harikrishnan, R.; Jaturasitha, S.; Paolucci, M. Impacts of Amla (Phyllanthus emblica) fruit extract on growth, skin mucosal and serum immunities, and disease resistance of Nile tilapia (Oreochromis niloticus) raised under biofloc system. Aquac. Rep. 2022, 22, 100953. [Google Scholar] [CrossRef]
- Xuan, C.L.; Wannavijit, S.; Outama, P.; Lumsangkul, C.; Tongsiri, S.; Chitmanat, C.; Doan, H.V. Dietary inclusion of rambutan (Nephelium lappaceum L.) seed to Nile tilapia (Oreochromis niloticus) reared in biofloc system: Impacts on growth, immunity, and immune-antioxidant gene expression. Fish Shellfish. Immunol. 2022, 122, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Parry, R.M., Jr.; Chandan, R.C.; Shahani, K.M. A rapid and sensitive assay of muramidase. Proc. Soc. Exp. Biol. Med. 1965, 119, 384–386. [Google Scholar] [CrossRef] [PubMed]
- Quade, M.J.; Roth, J.A. A rapid, direct assay to measure degranulation of bovine neutrophil primary granules. Vet. Immunol. Immunopathol. 1997, 58, 239–248. [Google Scholar] [CrossRef]
- Cordero, H.; Cuesta, A.; Meseguer, J.; Esteban, M.Á. Changes in the levels of humoral immune activities after storage of gilthead seabream (Sparus aurata) skin mucus. Fish Shellfish. Immunol. 2016, 58, 500–507. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Le Xuan, C.; Wannavijit, S.; Outama, P.; Montha, N.; Lumsangkul, C.; Tongsiri, S.; Chitmanat, C.; Hoseinifar, S.H.; van Doan, H. Effects of dietary rambutan (Nephelium lappaceum L.) peel powder on growth performance, immune response and immune-related gene expressions of striped catfish (Pangasianodon hypophthalmus) raised in biofloc system. Fish Shellfish. Immunol. 2022, 124, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Wakai, K.; Hamajima, N.; Okada, R.; Naito, M.; Morita, E.; Hishida, A.; Kawai, S.; Nishio, K.; Yin, G.; Asai, Y. SAS/STAT 9.1 user’s guide SAS/STAT 9.1 user’s guide, 2004. J. Epidemiol. 2009, 19, 72–80. [Google Scholar] [CrossRef]
- Ahmed, M.M.; Badawy, M.T.; Ahmed, F.K.; Kalia, A.; Abd-Elsalam, K.A. Chapter 10—Fruit peel waste-to-wealth: Bionanomaterials production and their applications in agroecosystems. In Agri-Waste and Microbes for Production of Sustainable Nanomaterials; Abd-Elsalam, K.A., Periakaruppan, R., Rajeshkumar, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 231–257. [Google Scholar]
- Samtiya, M.; Aluko, R.E.; Dhewa, T.; Moreno-Rojas, J.M. Potential Health Benefits of Plant Food-Derived Bioactive Components: An Overview. Foods 2021, 10, 839. [Google Scholar] [CrossRef]
- Tlais, A.Z.A.; Fiorino, G.M.; Polo, A.; Filannino, P.; Di Cagno, R. High-Value Compounds in Fruit, Vegetable and Cereal Byproducts: An Overview of Potential Sustainable Reuse and Exploitation. Molecules 2020, 25, 2987. [Google Scholar] [CrossRef]
- Perar, K.; Fonseca, F.; Affonso, E.; Nobre, A. Passion Fruit (Passiflora edulis) Seed Cake as a Feed Ingredient for Jaraqui (Semaprochilodus insignis) and Tambaqui (Colossoma macropomum). J. Aquac. Mar. Biol. 2017, 6, 173. [Google Scholar] [CrossRef]
- Lazzari, R.; Uczay, J.; Henriques, J.; Durigon, E.; Kunz, D.; Peixoto, N.; Fronza, D. Growth and digestive enzymes of silver catfish fed diets containing fruit residue. Arq. Bras. De Med. Veterinária E Zootec. 2019, 71, 323–330. [Google Scholar] [CrossRef]
- Abdel Rahman, A.N.; ElHady, M.; Shalaby, S.I. Efficacy of the dehydrated lemon peels on the immunity, enzymatic antioxidant capacity and growth of Nile tilapia (Oreochromis niloticus) and African catfish (Clarias gariepinus). Aquaculture 2019, 505, 92–97. [Google Scholar] [CrossRef]
- Conrado, A.; Iunes, R.; Neyrão, I. Performance of Nile tilapia (Oreochromis niloticus) juveniles fed diets with different levels of passion fruit seed oil (Passiflora edulis). Livest. Res. Rural. Dev. 2020, 32, 8. [Google Scholar]
- Hamed, H.S.; Abdel-Tawwab, M. Dietary pomegranate (Punica granatum) peel mitigated the adverse effects of silver nanoparticles on the performance, haemato-biochemical, antioxidant, and immune responses of Nile tilapia fingerlings. Aquaculture 2021, 540, 736742. [Google Scholar] [CrossRef]
- Chekani, R.; Akrami, R.; Ghiasvand, Z.; Chitsaz, H.; Jorjani, S. Effect of dietary dehydrated lemon peel (Citrus limon) supplementation on growth, hemato-immunolological and antioxidant status of rainbow trout (Oncorhynchus mykiss) under exposure to crowding stress. Aquaculture 2021, 539, 736597. [Google Scholar] [CrossRef]
- Zhuo, L.-C.; Chen, C.-F.; Lin, Y.-H. Dietary supplementation of fermented lemon peel enhances lysozyme activity and susceptibility to Photobacterium damselae for orange-spotted grouper, Epinephelus coioides. Fish Shellfish. Immunol. 2021, 117, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, L.-C.; Abang Zamhari, D.N.J.b.; Yong, A.S.K.; Shapawi, R.; Lin, Y.-H. Effects of fermented lemon peel supplementation in diet on growth, immune responses, and intestinal morphology of Asian sea bass, Lates calcarifer. Aquac. Rep. 2021, 21, 100801. [Google Scholar] [CrossRef]
- Whangchai, N.; Yaibouathong, D.; Junluthin, P.; Balakrishnan, D.; Unpaprom, Y.; Ramaraj, R.; Pimpimol, T. Effect of biogas sludge meal supplement in feed on growth performance molting period and production cost of giant freshwater prawn culture. Chemosphere 2022, 301, 134638. [Google Scholar] [CrossRef]
- Abboud, K.Y.; da Luz, B.B.; Dallazen, J.L.; Werner, M.F.d.P.; Cazarin, C.B.B.; Maróstica Junior, M.R.; Iacomini, M.; Cordeiro, L.M.C. Gastroprotective effect of soluble dietary fibres from yellow passion fruit (Passiflora edulis f. flavicarpa) peel against ethanol-induced ulcer in rats. J. Funct. Foods 2019, 54, 552–558. [Google Scholar] [CrossRef]
- Vuolo, M.M.; Lima, G.C.; Batista, Â.G.; Carazin, C.B.B.; Cintra, D.E.; Prado, M.A.; Júnior, M.R.M. Passion fruit peel intake decreases inflammatory response and reverts lipid peroxidation and adiposity in diet-induced obese rats. Nutr. Res. 2020, 76, 106–117. [Google Scholar] [CrossRef]
- Pertuzatti, P.B.; Sganzerla, M.; Jacques, A.C.; Barcia, M.T.; Zambiazi, R.C. Carotenoids, tocopherols and ascorbic acid content in yellow passion fruit (Passiflora edulis) grown under different cultivation systems. LWT Food Sci. Technol. 2015, 64, 259–263. [Google Scholar] [CrossRef]
- Shabir, U.; Dar, J.S.; Bhat, A.H.; Ganai, B.A.; Khan, I.A. Isolation and characterization of β-defensin-like protein 1 from epidermal mucus of fungal infected fish (Cyprinus carpio) and assessment of its antimicrobial potencies. Aquac. Rep. 2022, 23, 101056. [Google Scholar] [CrossRef]
- Alvarez-Pellitero, P. Fish immunity and parasite infections: From innate immunity to immunoprophylactic prospects. Vet. Immunol. Immunopathol. 2008, 126, 171–198. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.Y.; Ding, L.G.; Huang, Z.Y.; Xu, H.Y.; Xu, Z. Commensal bacteria-immunity crosstalk shapes mucosal homeostasis in teleost fish. Rev. Aquac. 2021, 13, 2322–2343. [Google Scholar] [CrossRef]
- Wannavijit, S.; Outama, P.; Le Xuan, C.; Lumsangkul, C.; Lengkidworraphiphat, P.; Tongsiri, S.; Chitmanat, C.; Doan, H.V. Modulatory effects of longan seed powder on growth performance, immune response, and immune-antioxidant related gene expression in Nile tilapia (Oreochromis niloticus) raised under biofloc system. Fish Shellfish. Immunol. 2022, 123, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-Y.; Lee, C.-H.; Kim, K.-D.; Lim, H.J.; Kim, H.S. Effects of diet supplementation with plant juice processing by-products on juvenile black rockfish (Sebastes schlegelii) growth performance, feed utilization, non-specific immunity, and disease resistance against Vibrio harveyi. Aquac. Rep. 2021, 21, 100831. [Google Scholar] [CrossRef]
- Silva-Brito, F.; Cardoso, A.; Machado, M.; Ramos-Pinto, L.; Hinzmann, M.; Abreu, H.; Costas, B.; Magnoni, L. Dietary supplementation with Gracilaria gracilis by-products modulates the immune status and oxidative stress response of gilthead seabream (Sparus aurata) stimulated with Photobacterium damselae subsp. piscicida. Fish Shellfish. Immunol. 2022, 126, 164–177. [Google Scholar] [CrossRef]
- Oliva-Teles, A. Nutrition and health of aquaculture fish. J. Fish Dis. 2012, 35, 83–108. [Google Scholar] [CrossRef]
- Lall, S.P.; Dumas, A. 3—Nutritional requirements of cultured fish: Formulating nutritionally adequate feeds. In Feed and Feeding Practices in Aquaculture, 2nd ed.; Davis, D.A., Ed.; Woodhead Publishing: Oxford, UK, 2022; pp. 65–132. [Google Scholar]
- Cruvinel, W.d.M.; Mesquita Júnior, D.; Araújo, J.A.P.; Catelan, T.T.T.; Souza, A.W.S.d.; Silva, N.P.d.; Andrade, L.E.C. Immune system: Part I. Fundamentals of innate immunity with emphasis on molecular and cellular mechanisms of inflammatory response. Rev. Bras. De Reumatol. 2010, 50, 434–447. [Google Scholar] [CrossRef]
- Ahlf, W.; Heise, S. Sediment Toxicity Assessment: Rationale for effect classes (5 pp). J. Soils Sediments 2005, 5, 16–20. [Google Scholar] [CrossRef]
- Imai, H.; Nakagawa, Y. Biological significance of phospholipid hydroperoxide glutathione peroxidase (PHGPx, GPx4) in mammalian cells. Free. Radic. Biol. Med. 2003, 34, 145–169. [Google Scholar] [CrossRef]
- Panigrahi, A.; Esakkiraj, P.; Das, R.R.; Saranya, C.; Vinay, T.; Otta, S.K.; Shekhar, M.S. Bioaugmentation of biofloc system with enzymatic bacterial strains for high health and production performance of Penaeus indicus. Sci. Rep. 2021, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Van Doan, H.; Lumsangkul, C.; Hoseinifar, S.H.; Harikrishnan, R.; Balasundaram, C.; Jaturasitha, S. Effects of coffee silverskin on growth performance, immune response, and disease resistance of Nile tilapia culture under biofloc system. Aquaculture 2021, 543, 736995. [Google Scholar] [CrossRef]
- Van Doan, H.; Lumsangkul, C.; Jaturasitha, S.; Meidong, R.; Hoseinifar, S.H.; Dawood, M.A. Modulation of growth, skin mucus and serum immunities, and disease resistance of Nile tilapia fed host-associated probiotic (Lactobacillus paracasei l61-27b). Aquac. Nutr. 2021, 27, 3–12. [Google Scholar] [CrossRef]
- Mohammadi, G.; Rafiee, G.; Tavabe, K.R.; Abdel-Latif, H.M.; Dawood, M.A. The enrichment of diet with beneficial bacteria (single-or multi-strain) in biofloc system enhanced the water quality, growth performance, immune responses, and disease resistance of Nile tilapia (Oreochromis niloticus). Aquaculture 2021, 539, 736640. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).