Abstract
The present trial was conducted to evaluate the supplementation effects of Macleaya cordata extract (MCE) on growth performance, serum biochemical parameters, and intestinal health of the juvenile American eel (Anguilla rostrata). The 480 juvenile American eels (10.93 ± 0.06 g) were randomly divided into four groups. They were fed on diets supplemented with MCE levels of 0, 25 mg/kg, 50 mg/kg, and 100 mg/kg for ten weeks, respectively. The 50 mg/kg or 100 mg/kg MCE could significantly improve growth performance, and increase the activities of acid phosphatase and alkaline phosphatase, as well as the level of high-density lipoprotein cholesterol. These levels of MCE also decreased the levels of D-lactate acid, triglyceride, and total cholesterol and the activities of aspartate aminotransferase, alanine aminotransferase, and diamine oxidase. The antioxidant ability, muscular thickness, and fold height of the intestine were enhanced by 50 mg/kg or 100 mg/kg MCE. There was no significant difference in the above parameters of groups fed with 50 mg/kg or 100 mg/kg of MCE. The beneficial effects on the intestinal microbiota were demonstrated in the group fed with 50 mg/kg MCE. In conclusion, the 50 mg/kg MCE could be used in the diet to improve the growth performance and health status of the juvenile American eels.
1. Introduction
Global aquaculture development increased rapidly in recent years, and the sector has become one of the fastest-growing industries in the animal-derived food production system. However, the intensification and commercialization of aquaculture have been associated with frequent outbreaks of infectious diseases [1,2]. The use of antibiotics and chemotherapeutics is the main strategy in commercial aquaculture for controlling diseases. However, the development of drug-resistant pathogens, drug residues, and environmental pollution have generated great concerns [2]. Recently, growing interest has arisen in the utilization of natural products of the medical plant as an eco-friendly measure to ensure the sustainability of aquaculture. These extracts from the natural plant usually possess multiple biological activities such as antimicrobial, anti-inflammatory, immunostimulant, and antioxidant properties [2,3]. Macleaya cordata is a traditional herb primarily distributed in China, North America, and Europe. The main active ingredients of Macleaya cordata extract (MCE) are isoquinoline alkaloids that are mainly composed of sanguinarine, chelerythrine, some minority of protopine, berberine, coptisine, allocryptopine, etc. [3,4]. Based on the nucleophilic character of the iminium moiety and the polycyclic planar structure, MCE exhibits a broad spectrum of biological activities, such as anti-inflammatory [5,6], antioxidant [7,8], anti-bacterial [9], and anti-parasitic effects [10]. In 2004, compounds containing sanguinarine and chelerythrine from MCE were registered as feed additives in the European Union, and MCE is employed as a feed additive for swine and poultry widely [3,11]. In aquatic animals, previous studies revealed that dietary supplementation with MCE containing 1.5% sanguinarine and 0.75% chelerythrine could promote the growth performance of Caspian roach fry (Rutilus rutilus) and red tilapia (Oreochromis niloticus) [12,13]. Similarly, it was reported that the MCE containing 1.5% sanguinarine could increase weight gain and the average daily gain of common carp (Cyprinus carpio) [14]. However, dietary MCE containing 0.15% sanguinarine supplementation could not improve the growth performance of grass carp (Ctenopharyngodon idellus) fed high cottonseed and rapeseed meal diets [5]. These results indicated that the growth-promotion effects of MCE might vary in different fish species or diets. Little information is available regarding the growth-promotion effects of dietary MCE on other farmed fish species.
Being one of the most common freshwater cultured fish in the world, the eel has made important contributions to the development of the Chinese fisheries economy. With the natural stocks of European eel (Anguilla anguilla) and Japanese eel (Anguilla japonica) declining sharply, the American eel (Anguilla rostrata) has become one of the most popular farmed species in southeastern China [15,16]. Although dietary supplementation with MCE appears to exert beneficial effects on multiple species of fish, no research is available about the application of MCE in the diet of the American eel. The present study is aimed to evaluate the effects of dietary MCE supplementation on the growth performance, serum biochemical parameters, and intestinal health of the American eel.
2. Materials and Methods
2.1. Feeding Trial
One thousand juvenile American eels with similar body weights were obtained from Fujian Jinjiangzhiman Aquatic Technology Co., Ltd., Zhangzhou, China. Before the formal trial, the fish were acclimatized in two PVC tanks with bottom center drains (110 cm diameter, 80 cm height) and about 800 L water volume supplied with 5 L/min of degassed and dechlorinated municipal water. All the eels were fed on a commercial powder feed (Fuzhou Sea Horse Feed Co., Ltd., Fuzhou, Fujian, China) two times daily (6:00 and 18:00). The powder diet was mixed with 1:1.1 volume water to form a dough shape, and then the dough feed was placed on a feeding table for fish. The uneaten feed was siphoned out 20–25 min after feeding. The commercial diet was mainly composed of white fish meal, brown fish meal, pre-gelatinized starch, yeast powder, extruded soybean, and compound premix. The proximate composition of the commercial diet was crude protein 46.58%, crude fat 6.70%, ash 12.35%, and moisture 7.36%. During the acclimation period, the water quality parameters were maintained at 24–26 °C, pH 7.0–7.5, dissolved oxygen 7.0–9.0 mg/L, total ammonia nitrogen 0.2–0.6 mg/L, nitrite 0.03–0.06 mg/L.
After four weeks of acclimation, 480 American eels with similar body weights (10.93 ± 0.06 g/fish) were selected and randomly distributed into 16 tanks. The 16 tanks were randomly divided into four treatment groups fed the diets with MCE levels being 0, 25 mg/kg, 50 mg/kg, and 100 mg/kg, respectively. The four treatment groups were the control group, MCE25 group, MCE50 group, and MCE100 group, respectively. There were four replicates in each treatment group with 30 fish per replicate. The trial period was ten weeks.
MCE (An orange powder product containing 1.5% sanguinarine and 0.75% chelerythrine) was manufactured by Hunan Micolta Bioresource Co., Ltd., Changsha, China. The batch number of the MCE product was 2,103,291. The basal diet for the control group was the commercial feed with no MCE supplementation during the acclimation period, and the diets for the other MCE groups were prepared with the inclusion of MCE at 25 mg/kg, 50 mg/kg, and 100 mg/kg, respectively. The fish in the formal trial were cultured in 16 circular PVC tanks (320 L water) with a water recirculation system and an automatic temperature control device. The fish management and the water quality parameters during the formal trial period were maintained the same as those in the acclimation period. The consumption of diet in each tank was recorded daily.
2.2. Sample Collection
At the end of the feeding trial, all fish in each tank was deprived for 24 h. The fish of each tank were anesthetized with 0.1 mg/L eugenol, weighed, and counted to calculate the growth performance parameters. The blood of nine fish from each tank was sampled, treated, and mixed as one sample before analysis of serum biochemical parameters according to the procedure of Zhang et al. [15]. After the four fish of each tank was dissected, the intestine tissue was sampled and immersed in Bouin’s solution (which consisted of 75 mL saturated picric acid aqueous solution, 25 mL formaldehyde, and 5 mL glacial acetic acid) for the observation of intestinal morphology. The remaining intestine samples were also collected and placed into 1.5 mL sterilization freeze tubes. Intestine samples were homogenized with 10 times the volume (volume/weight) of precooled normal saline (0.86%). Ground the mixture with a grinder (Tissuelyser-24, Shanghai Jingxin Industrial Development Co., Ltd., Shanghai, China), then the homogenate was centrifuged at 4 °C 3000 r/min for 10 min, collected the supernatant in centrifugal tubes, frozen at 80 °C before the analysis of antioxidant parameters. In addition, two intestine samples were selected from each tank for the analysis of the microbial diversity and community structure.
2.3. Growth Performance Calculation
At the end of the trial, the following growth parameters were calculated.
Weight gain rate (WGR, %) = 100 × [final fish weight of each tank (g) − initial fish weight of each tank(g)]/initial fish weight of each tank(g).
Specific growth rate (SGR, %/d) = [Ln final weight of each tank (g) − Ln initial weight of each tank (g) × 100]/trial days (d).
Feeding rate (FR, %) = 100 × feed consumption of each tank (g)/[((initial fish weight of each tank (g) + final fish weight of each tank(g))/2]/trial days (d).
Feed efficiency (FE, %) = 100 × [final fish weight of each tank (g) − the initial fish weight of each tank (g)]/feed intake of each tank (g).
Survival rate (SR, %) = 100 × (final fish number of each tank)/(initial fish number of each tank).
2.4. Measurement of Serum Biochemical Parameters
Total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), glutamic-oxaloacetic transaminase (GOT), glutamic-pyruvic transaminase (GPT), diamine oxidase (DAO), D-lactate (D-lac) in the serum were measured using commercial kits (Nanjing Jiancheng Bioengineering Co., Ltd., Nanjing, China) according to the manufacturer’s instructions manual.
2.5. Measurement of Intestinal Antioxidant Parameters
The activities of superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-PX) and the levels of total antioxidant capacity(T-AOC) and malondialdehyde (MDA) in the intestine were measured by assay kits (Nanjing Jiancheng Biotechnology Co., Ltd., Nanjing, China). The detailed determination steps were referred to in the manufacturer’s instructions manual.
2.6. Observation and Measurement of Intestinal Morphology
The intestinal issues were dehydrated through 75% ethanol, cleared with xylene, and embedded in paraffin. The 5-μm histological section was cut by a microtome (KD-TS3A, Beijing Century Kexin Scientific Instrument Co., Ltd., Beijing, China), then stained with hematoxylin and eosin (H & E). Intestinal slices were observed and photographed with a light microscope (Olympus BX80-JPA) and analyzed using Image-Pro Plus 6.0 (Media Cybernetics, Silver Spring, MD, USA).
2.7. Intestinal Microbiota Profiling
The extraction and quality detection of intestinal total DNA, the design of primers for PCR amplification of bacterial 16S rDNA V3-V4 region, and data analysis were the same as the description by Shi et al. [17]. The high-throughput sequencing analysis was performed on the platform of Illumina Miseq PE300 with the assistance of Beijing Allwegene Tech. Co., Ltd. (Beijing, China). The data of high-throughput sequencing analysis was analyzed using QIIME (version 1.8.0) and Mothur (version 1.31.2) according to the description in the study of Shi et al. [17].
2.8. Statistical Analysis
The results of this trial were presented as means ± SD (n = 4). The data of growth performance, serum biochemical parameters, antioxidant parameters of the intestine, and the parameters of intestinal morphology from the present trial were subjected to Duncan’s multiple comparisons in a one-way ANOVA model to estimate the statistical significance (p < 0.05) by SPSS 17.0 statistical software (SPSS Inc., Chicago, IL, USA). The data expressed as percentages were subjected to square arcsine transformation before statistical analysis. Kruskal Wallis method was used to conduct a non-parametric test for intestinal differential bacteria analysis (p < 0.05).
3. Results
3.1. Growth Performance
As shown in Table 1, the FBW, WGR, and SGR in the MCE50 group and MCE100 group were significantly higher than those of the control group and MCE25 group (p < 0.05). Compared with the other groups, the FR was increased significantly in the MCE50 group (p < 0.05). The FE in the MCE100 group was significantly higher than those of the other groups (p < 0.05). No significant differences in those growth parameters were found between the control group and the MCE25 group (p > 0.05).

Table 1.
Effects of dietary MCE supplementation on growth performance of juvenile American eel.
3.2. Serum Biochemical Parameters
As shown in Table 2, the levels of TC and TG in the MCE100 group were significantly lower than those of the other groups (p < 0.05), and there was no significant difference in TC and TG levels among the other three groups (p > 0.05). The HDL-C level in the MCE50 group and MCE100 group were significantly higher than those of the other two groups (p < 0.05). The activities of GOT and GPT only in the MCE50 group were significantly lower than those of the control group (p < 0.05). Compared with the control group, the DAO activity and D-lac level in MCE groups were significantly decreased (p < 0.05), and no significant differences in the DAO activity and D-lac level (except for the MCE100 group) were observed among the MCE groups (p > 0.05).

Table 2.
Effects of dietary MCE supplementation on the serum biochemical parameters of the juvenile American eel.
3.3. Intestinal Antioxidant Parameters
The antioxidant parameters of juvenile American eels in different treatment groups were shown in Table 3. Compared with the control group, the T-AOC level and the GSH-PX activity in the MCE groups were significantly increased (p < 0.05). The SOD activity was significantly increased only in the MCE100 group (p < 0.05), and no significant difference was presented between the control group and MCE groups (p > 0.05). The CAT activity in the MCE50 group and MCE100 group were significantly higher than those in the control group and MCE25 group (p < 0.05), and there was no significant difference between the MCE50 group and MCE100 group (p > 0.05). The MDA levels in the MCE groups were significantly lower than that in the control group (p < 0.05), and the MDA level of the MCE50 group was significantly lower than that of the MCE25 group (p < 0.05).

Table 3.
Effects of dietary MCE supplementation on intestinal antioxidant parameters of the juvenile American eel.
3.4. Intestinal Morphology
The results of the intestinal morphology of juvenile American eel were shown in Figure 1 and Table 4.
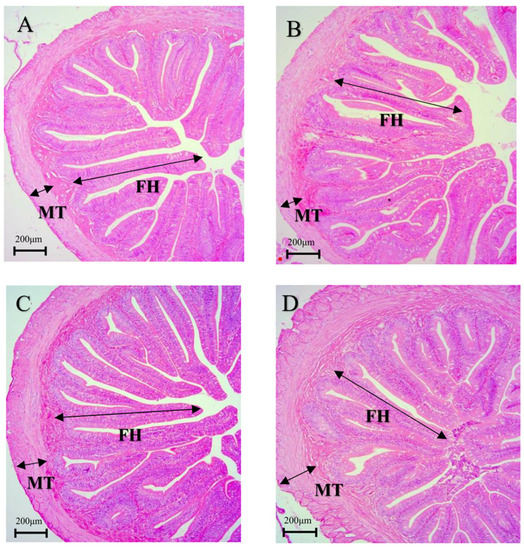
Figure 1.
Effects of dietary Macleaya cordata extract (MCE) supplementation on the intestinal morphology of juvenile American eel. (A) Control group, (B) MCE25 group, (C) MCE50 group, and (D) MCE100 group (Magnification × 100). FH = fold height; MT = muscular thickness.

Table 4.
Effects of dietary MCE supplementation on the muscular thickness and fold height of intestine of the juvenile American eel.
Compared with the control group, the MT and FH of the intestine in the MCE50 group (Figure 1C) and MCE100 group (Figure 1D) were significantly increased (p < 0.05), and the MT in the MCE100 group was significantly higher than that of MCE50 group (p < 0.05). No significant difference was observed between the control group (Figure 1A) and the MCE25 group (Figure 1B) in parameters of intestinal morphology (p > 0.05).
3.5. Intestinal Microbiota
As shown in Table 5, there was no obvious difference in indexes of Shannon and Chao1 between the MCE50 group and the control group. The number of OTUs in the MCE50 group was higher than that in the control group, and the Chao 1 and Shannon indexes were similar between the MCE50 group and the control group. The values of coverage rate were above 98%, suggesting that the majority of intestinal bacteria might be identified.

Table 5.
Comparison of alpha diversity of intestinal microbiota of juvenile American eel between the control group and MCE50 group.
As shown in Figure 2, the predominant bacteria at the phylum level in the intestine of the MCE50 group and the control group were Tenericutes, Fusobacteria, and Firmicutes. Compared with the control group, there was an increasing trend of relative abundance of Fusobacteria and a decreasing trend of the relative abundances of Tenericutes and Firmicutes in the MCE50 group.
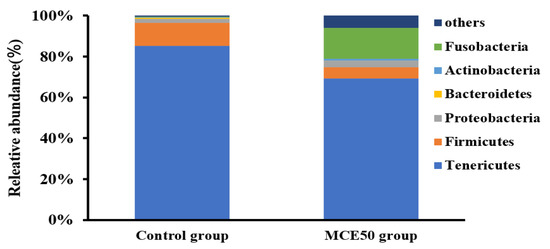
Figure 2.
Comparison of intestinal microbiota at phylum level of juvenile American eels between the control group and MCE50 group.
The differential bacteria at genus level in the intestine of the control group and MCE50 group were presented in Figure 3. Compared with the control group, the lower relative abundance of Acinetobacter and the higher relative abundances of Saccharopolyspora, Thermoactinomyces, and Cronobacter were in the MCE50 group (p < 0.05).
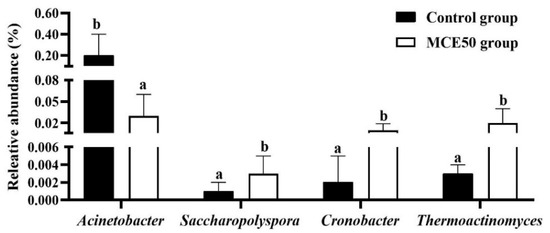
Figure 3.
Comparison of differential bacteria at genus level in the intestine of juvenile American eels between the control group and MCE50 group. a,b Different superscripts on the bar are significantly different (p < 0.05).
4. Discussion
The results of the present trial revealed that dietary MCE supplementation at 50 mg/kg or 100 mg/kg could improve the growth performance of juvenile American eels. Similarly, dietary supplementation with MCE at 500 mg/kg could increase the final weight, weight gain, and specific growth rate of Caspian roach fry [12], as well as weight gain and average daily gain of common carp [14]; dietary supplementation with MCE (Sangrovit®) at 25 mg/kg might improve weight gain and SGR of red tilapia [13]. However, the WGR and other growth parameters were not significantly improved by 0.2–0.8 g/kg MCE supplementation in the grass carp fed high cottonseed and rapeseed meal diets [5]. Those inconsistent results of MCE supplementation in the diets might be caused by the differences in nutrients level, fish species, and feed composition.
The levels of TG, TC, HDL-C, and LDL-C in serum are important parameters of lipid metabolism in animals, and the lipid-lowering effect in serum is considered to be beneficial to health [18]. In this study, the levels of TC and TG were reduced by MCE supplementation. And the HDL-C level was increased by 50 mg/kg or 100 mg/kg MCE supplementation. Those results suggested that MCE might have a hypolipidemic effect in juvenile American eels. Similar results were observed in the previous study of Caspian roach fed diets with MCE supplementation [12]. The lipid-lowering effect of MCE might attribute to altering bile acid metabolism and activating FXR signaling [19].
The GPT and GOT are important biomarkers for liver health and are mainly distributed in the mitochondria of liver cells. The elevated activities of GPT and GOT in the serum mean that there is tissue damage in the liver [20]. In this study, only dietary supplementation of MCE at 50 mg/kg could decrease the activities of GOT and GPT, it indicated that an appropriate level of MCE supplementation might improve the liver health status of the juvenile American eel. This phenomenon was supported by the effect of 375 or 750 μg/kg MCE (in the form of sanguinarine purity > 95%) on alleviating elevation of GOT and GPT activities in serum induced by H2O2 or lipopolysaccharide in rice field eels (Monopterus albus) [6,21]. However, Rawling et al. [13] reported that there was no significant difference in GOT and GPT activities of red tilapia with dietary MCE supplementation.
It was found that the mucosal damage in the intestine might lead to increased permeability and enable a large quantity of D-lac and DAO to get into the peripheral circulation [11,22]. The D-lac and DAO are usually regarded as indicators of damage to the intestinal barrier system [22]. In this study, dietary MCE supplementation decreased the D-lac level and the DAO activity in the serum. This point was confirmed in the study of grass carp fed high cottonseed and rapeseed meal diets on restoring the barrier function of the tightly connected gut by MCE supplementation [5]. In addition, the same results were also found in growing piglets fed diets with MCE supplementation [11]. These results suggested that dietary MCE supplementation could improve the barrier function of the intestine. Further work should be conducted to clarify the specific mechanism for the amelioration of the barrier function.
T-AOC is an index generally for assessing the ability of the nonenzymatic antioxidant defense system of fish [23]. The SOD, GSH-PX, and CAT are the main parameters to assess antioxidant ability in the enzymatic system, and they work together to catalyze free radicals and produce non-toxic compounds [24]. MDA is the product of lipid-peroxidation, which contributes to the production of reactive oxygen radicals. It could be used as an indicator of cellular oxidative damage indirectly [25]. In this study, the higher level of T-AOC and the activities of GSH-PX and CAT were found in certain MCE groups with decreasing MDA levels. The results from this trial were consistent with those of MCE supplemented in grass carp [5], koi carp (Cryprinus carpiod) [25], and pacific white shrimp (Litopenaeus vannamei) [26]. The improvement of antioxidant ability in the intestine might be related to MCE inhibiting phorbol myristate-induced oxidative burst and the NADPH oxidase complex and decreasing the production of reactive oxygen species [7].
The intestinal FH and MT are the well-defined parameters for the health status in the intestine of fish [27]. The absorption of nutrients in the intestine is determined by the villi length of the intestinal fold [28]. In the present study, the FH and MT of the intestine of the American eel were significantly increased in the MCE50 group and MCE100 group. Similarly, the FH and MT of the intestine were also increased in the grass carp fed the high cottonseed and rapeseed meal by dietary MCE supplementation [5]. The improvement of intestinal morphology suggested dietary MCE supplementation might enhance the ability to digest and absorb nutrients, and this might be one of the important factors to improve the growth performance of juvenile American eel. The amelioration of intestinal morphology might be related to MCE inhibiting the colonization of pathogenic bacteria and increasing the colonization of probiotic bacteria in the intestine [29].
Generally, the intestinal microbiota plays the important role in animal growth and nutrition metabolism [30]. The beneficial effects of medical plant-derived extracts on intestinal microbiota are increasingly reported in aquaculture [5,31,32]. The indexes of OTUs, Chao 1, and Shannon are usually used to evaluate the species abundance and richness of the intestine microbiota [27,33]. A high microbiota diversity is generally considered beneficial for intestinal health [34]. In our study, the OTUs number in the MCE50 group was higher than that in the control group, which indicated that dietary MCE supplementation might improve the richness of the intestinal microbiota of American eel. Similar results were observed in koi carp and Trionyx sinensis with dietary MCE supplementation [25,35]. The values of coverage indices were above 98%, which suggested that the majority of intestinal bacteria in this study might be identified [17].
In the present trial, the relative abundances of both Tenericutes and Firmicutes were lowered in the MCE50 group. The decreasing proportion of Tenericutes in the intestine might be beneficial to gut health because the lower abundance of Tenericutes was found in the intestine of the European eel of the fast-growth group in comparison with the stunted-growth group [17,34]. The lower proportion of Firmicutes in the intestine was also found in the American eel fed diet with oligomeric proanthocyanidins supplementation [36]. It was reported that some strains belonging to Firmicutes in the intestine might be associated with the decreasing growth rate of the European eel [17]. The relative abundance of Fusobacteria was increased in the MCE50 group. The Fusobacteria might produce more butyrate and synthesize numerous vitamins [37], which might play essential roles in preventing the development of intestinal inflammation and inflammatory bowel disease [38].
The results of the Lefse analysis in the present study was shown that there was a lower relative abundance of Acinetobacter and higher relative abundances of Saccharopolyspora, Thermoactinomyces, and Cronobacter in the intestine of the MCE50 group. Acinetobacter is a potential pathogen in aquaculture [39]. Saccharopolyspora sp. is reported as the major producer of secondary metabolites that have a broad spectrum of biological activities including anti-microbial, antioxidant, and inhibitory effects of some enzymes to indirectly improve the health status of the animal intestines [40,41]. The Thermoactinomyces species are heat-resistant spore-forming bacteria, and they are capable of producing proteases [42]. Although the Cronobacter turicensis might cause lethal infection in zebrafish larvae [43], some studies indicated that the beneficial or detrimental effects of Cronobacter in aquatic animals might be due to the differences in fish species, individual sizes, living environments, and other factors [44]. The role of Cronobacter in the healthy intestine of American eel fed with MCE is needed to clarify in future research. From the changes in intestinal microbiota, it was obvious that the MCE might have the ability to optimize the bacteria composition in the intestine by promoting the multiplication of some potentially beneficial bacteria and decreasing the relative abundances of the certain potential pathogen. This phenomenon caused by MCE was also found in red tilapia [13], common carp [14], koi carp [25], Pacific white shrimp [26], loach (Misgurnus anguillicaudatus) [45], and even grass carp fed with a higher level of cottonseed and rapeseed meal [5]. The determinant for MCE exerting antibacterial activity is the presence of reactive iminium bond in the molecule of sanguinarine and chelerythrine [46], and the possible mechanisms might be involved in inhibiting the formation of bacteria biofilm or interfering with the bacterial cytokinesis [9,47].
In general, both 50 mg/kg and 100 mg/kg MCE supplementation could have beneficial effects on growth performance, serum biochemical parameters, intestinal antioxidant ability, and intestinal morphology of juvenile American eel. Most of those parameters of growth performance and health status were not improved by 100 mg/kg MCE supplementation. Considering the changes in all the parameters in the present trial and the cost of MCE, the 50 mg/kg MCE should be supplemented in the diet of the juvenile American eel.
5. Conclusions
In conclusion, the appropriate level of dietary MCE supplementation could exert beneficial effects on the growth performance, serum biochemical parameters, antioxidant ability, morphology, and microbiota in the intestine of the juvenile American eel. The 50 mg/kg MCE in the diet was recommended to improve the growth performance and health status of the juvenile American eel. The application effects of MCE under the practical culture conditions should be confirmed in future studies.
Author Contributions
Methodology, L.H. and S.Z.; validation, L.H., R.C. and S.Z.; formal analysis, L.H. and R.C.; investigation, L.H., R.C. and S.Z.; data curation, S.Z.; writing—original draft preparation, R.C.; writing—review and editing, R.C. and S.Z.; project administration, S.Z.; funding acquisition, S.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Outward Cooperation Project in the Science and Technology Plan Program of Fujian Province (2020I0020), the earmarked fund for China Agriculture Research System of MOF and MARA (CARS-46), and the Open Fund of Engineering Research Center of the Modern Industry Technology for Eel, Ministry of Education of China (RE202010).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and was approved by the Animal Care Advisory Committee of Jimei University (Approval No. 2019-0906-003).
Data Availability Statement
The data used during the current study are available from the corresponding author on reasonable request.
Acknowledgments
The authors thank Keding Xia for providing the trial fish.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Naylor, R.L.; Hardy, R.W.; Buschmann, A.H.; Bush, S.R.; Cao, L.; Klinger, D.H.; Little, D.C.; Lubchenco, J.; Shumway, S.E.; Troell, M. A 20-year retrospective review of global aquaculture. Nature 2021, 591, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Tadese, D.A.; Song, C.; Sun, C.; Liu, B.; Liu, B.; Zhou, Q.; Xu, P.; Ge, X.; Liu, M.; Xu, X.; et al. The role of currently used medicinal plants in aquaculture and their action mechanisms: A review. Rev. Aquac. 2022, 14, 816–847. [Google Scholar] [CrossRef]
- Hou, B.; Zeng, J. Biological activities of sanguinarine and application of Macleaya cordata extract in animal production. Chin. J. Anim. Nutr. 2018, 30, 413–420. [Google Scholar] [CrossRef]
- Lin, L.; Liu, Y.C.; Huang, J.L.; Liu, X.B.; Qing, Z.X.; Zeng, J.G.; Liu, Z.Y. Medicinal plants of the genus Macleaya (Macleaya cordata, Macleaya microcarpa): A review of their phytochemistry, pharmacology, and toxicology. Phytother. Res. 2017, 32, 19–48. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Zhong, L.; Chen, T.; Shi, Y.; Xu, S.D. Dietary sanguinarine supplementation on the growth performance, immunity and intestinal health of grass carp (Ctenopharyngodon idellus) fed cottonseed and rapeseed meal diets. Aquaculture 2020, 528, 735521. [Google Scholar] [CrossRef]
- Shi, Y.; Zhong, L.; Chen, K.J.; Fan, Y.D.; Xie, K.; Zhang, J.Z.; Dai, J.; Hu, Y. Sanguinarine attenuates hydrogen peroxide-induced toxicity in liver of Monopterus albus: Role of oxidative stress, inflammation and apoptosis. Fish Shellfish Immun. 2022, 125, 190–199. [Google Scholar] [CrossRef]
- Qin, F.; Patel, R.; Yan, C.; Liu, W. NADPH oxidase is involved in angiotensin II induced apoptosis in H9C2 cardiac muscle cells: Effects of apocynin. Free Radical Bio. Med. 2006, 40, 236–246. [Google Scholar] [CrossRef]
- Guan, G.; Ding, S.; Yin, Y.; Duraipandiyan, V.; AlDhabi, N.A.; Liu, G. Macleaya cordata extract alleviated oxidative stress and altered innate immune response in mice challenged with enterotoxigenic Escherichia coli. Sci. China Life Sci. 2019, 62, 1019–1027. [Google Scholar] [CrossRef]
- Cushnie, T.P.; Cushnie, B.; Lamb, A.J. Alkaloids: An overview of their antibacterial, antibiotic-enhancing and anti-virulence activities. Int. J. Antimicrob. Ag. 2014, 44, 377–386. [Google Scholar] [CrossRef]
- Zhou, S.; Li, W.X.; Wang, Y.Q.; Zou, H.; Wu, S.G.; Wang, G.T. Anthelmintic efficacies of three common disinfectants and extracts of four traditional Chinese medicinal plants against Gyrodactylus kobayashii (Monogenea) in goldfish (Carassius auratus). Aquaculture 2017, 466, 72–77. [Google Scholar] [CrossRef]
- Liu, G.; Guan, G.P.; Fang, J.; Martinez, Y.; Chen, S.; Bin, P.; Duraipandiyan, V.; Gong, T.; Tossou, M.C.; Al-Dhabi, N.A.; et al. Macleaya cordata extract decreased diarrhea score and enhanced intestinal barrier function in growing piglets. Biomed Res. Int. 2016, 2016, 1069585. [Google Scholar] [CrossRef] [PubMed]
- Imanpoor, M.R.; Roohi, Z. Effects of Sangrovit®-supplemented diet on growth performance, blood biochemical parameters, survival and stress resistance to salinity in the Caspian roach (Rutilus rutilus) fry. Aquac. Res. 2015, 47, 2874–2880. [Google Scholar] [CrossRef]
- Rawling, M.D.; Merrifield, D.L.; Davies, S.J. Preliminary assessment of dietary supplementation of Sangrovit® on red tilapia (Oreochromis niloticus) growth performance and health. Aquaculture 2009, 294, 118–122. [Google Scholar] [CrossRef]
- Abdelnaby, E.A.; Mohamed, M.F.; Gammazh, A.K. Pharmacological studies of feed additives (Sanguinarine and Saccharomyces cerevisiae) on growth performance, hematological and intestinal bacterial count with challenge test by Aeromonas hydrophila in Cyprinus carpio. Global. Anim. Sci. J. 2013, 11, 1154–1172. [Google Scholar] [CrossRef]
- Zhang, M.L.; Wang, Y.; Zhai, S.W. Effects of dietary compound acidifiers supplementation on growth performance and intestinal health of juvenile American eels (Anguilla rostrata) cultured in cement tanks. Isr. J. Aquacult-Bamid. 2021, 73, 1520998. [Google Scholar] [CrossRef]
- Yuan, Y.; Yuan, Y.M.; Dai, Y.Y.; Gong, Y.C.; Yuan, Y.Q. Development status and trends in the eel farming industry in Asia. N. Am. J. Aquacult. 2021, 84, 3–17. [Google Scholar] [CrossRef]
- Shi, Y.; Ma, D.Y.; Zhai, S.W. Revealing the difference of intestinal microbiota composition of cultured European eels (Anguilla anguilla) with different growth rates. Isr. J. Aquacult-Bamid. 2020, 72, 959575. [Google Scholar] [CrossRef]
- Guo, D.; Xie, M.; Xiao, H.; Xu, L.; Zhang, S.; Chen, X.; Wu, Z. Bacillus subtilis supplementation in a high-fat diet modulates the gut microbiota and ameliorates hepatic lipid accumulation in grass carp (Ctenopharyngodon idella). Fishes 2022, 7, 94. [Google Scholar] [CrossRef]
- Tian, Y.; Cai, J.; Gui, W.; Nichols, R.G.; Koo, I.; Zhang, J.; Patterson, A.D. Berberine directly impacts the gut microbiota to promote intestinal farnesoid X receptor activation. Drug Metab. Dispos. 2019, 47, 86–93. [Google Scholar] [CrossRef]
- Farris, N.W.; Kim, D.; Hamidoghli, A.; Won, S.; Lee, S.; Bae, J. Dietary α-Tocopheryl acetate and arachidonic acid synergistically improves superoxide dismutase activity in female Japanese eel broodstock, Anguilla japonica. Aquaculture 2020, 522, 735100. [Google Scholar] [CrossRef]
- Shi, Y.; Hu, Y.; Liu, Y.Y.; Che, C.B. Effects of sanguinarine on immune and intestinal inflammation related to gene expression in rice field eels (Monopterus albus) induced by LPS. J. Fish. Sci. China 2020, 27, 125–137. [Google Scholar] [CrossRef]
- Fukudome, I.; Kobayashi, M.; Dabanaka, K.; Maeda, H.; Okamoto, K.; Okabayashi, T.; Hanazaki, K. Diamine oxidase as a marker of intestinal mucosal injury and the effect of soluble dietary fiber on gastrointestinal tract toxicity after intravenous 5-fluorouracil treatment in rats. Med. Mol. Morphol. 2013, 47, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Mourente, G.; Tocher, D.R.; Diaz, E.; Grau, A.; Pastor, E. Relationships between antioxidants, antioxidant enzyme activities and lipid peroxidation products during early development in dentex dentex eggs and larvae. Aquaculture 1999, 179, 309–324. [Google Scholar] [CrossRef]
- Shi, Q.; Xiong, X.; Wen, Z.; Qin, C.; Li, R.; Zhang, Z.; Gong, Q.; Wu, X. Cu/Zn superoxide dismutase and catalase of Yangtze sturgeon, Acipenser dabryanus: Molecular cloning, tissue distribution and response to fasting and refeeding. Fishes 2022, 7, 35. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, X.W.; Zhu, J.Y.; Liu, L.L.; Liu, Y.C.; Zhu, H. Dietary sanguinarine affected immune response, digestive enzyme activity and intestinal microbiota of koi carp (Cryprinus carpiod). Aquaculture 2019, 502, 72–79. [Google Scholar] [CrossRef]
- Bussabong, P.; Rairat, T.; Chuchird, N.; Keetanon, A.; Phansawat, P.; Cherdkeattipol, K.; Pichitkul, P.; Kraitavin, W. Effects of isoquinoline alkaloids from Macleaya cordata on growth performance, survival, immune response, and resistance to Vibrio parahaemolyticus infection of Pacific white shrimp (Litopenaeus vannamei). PLoS ONE 2021, 16, e0251343. [Google Scholar] [CrossRef]
- Su, X.; Ji, D.; Yao, J.; Zou, Y.; Yan, M. Comparative analysis of intestinal characteristics of Largemouth Bass (Micropterus salmoides) and intestinal flora with different growth rates. Fishes 2022, 7, 65. [Google Scholar] [CrossRef]
- Saravanan, K.; Sivaramakrishnan, T.; Praveenraj, J.; Kiruba, S.R.; Haridas Harsha, K.S.; Varghese, B. Effects of single and multi-strain probiotics on the growth, hemato-immunological, enzymatic activity, gut morphology and disease resistance in Rohu, Labeo rohita. Aquaculture 2021, 540, 736749. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Y.; Xue, M.; Xiao, Z.; Fan, Y.; Zeng, L.; Zhou, Y. Effects of dietary Enterococcus faecalis YFI-G720 on the growth, immunity, serum biochemical, intestinal morphology, intestinal microbiota, and disease resistance of Crucian Carp (Carassius auratus). Fishes 2022, 7, 18. [Google Scholar] [CrossRef]
- Ingerslev, H.C.; Jørgensen, L.V.G.; Strube, M.L.; Larsen, N.; Dalsgaard, I.; Boye, M. The development of the gut microbiota in rainbow trout (Oncorhynchus mykiss) is affected by first feeding and diet type. Aquaculture 2014, 424, 24–34. [Google Scholar] [CrossRef] [Green Version]
- Feher, M.; Fauszt, P.; Tolnai, E.; Fidler, G.; Pesti, A.G.; Stagel, A.; Szucs, I.; Biro, S.; Remenyik, J.; Paholcsek, M.; et al. Effects of phytonutrient-supplemented diets on the intestinal microbiota of Cyprinus carpio. PLoS ONE 2021, 16, e0248537. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.J.; Xiao, S.Y.; Chen, S.Y.; Xu, Q.; Yang, Z.; Liu, J.; Wang, H.Q.; Lan, S.L. Effects of fermented mulberry leaves on growth, serum antioxidant capacity, digestive enzyme activities and microbial compositions of the intestine in Crucian (Carassius carassius). Aquac. Res. 2021, 52, 6356–6366. [Google Scholar] [CrossRef]
- Burtseva, O.; Kublanovskaya, A.; Fedorenko, T.; Lobakova, E.; Chekanov, K. Gut microbiome of the white sea fish revealed by 16s rRNA metabarcoding. Aquaculture 2020, 533, 736175. [Google Scholar] [CrossRef]
- Lin, M.; Zeng, C.X.; Jia, X.Q.; Zhai, S.W.; Li, Z.Q.; Ma, Y. The composition and structure of the intestinal microflora of Anguilla marmorata at different growth rates: A deep sequencing study. J. Appl. Microbiol. 2019, 126, 1340–1352. [Google Scholar] [CrossRef]
- Chen, Z.N.; Wang, X.Q.; Luo, L.T.; Wang, P.; Tu, K.F.; Yang, T.; Hu, Y.Z.; Xiong, G. High-throughput sequencing analysis of the effects of sanguinarine on Trionyx sinensis intestinal microbiota. Prog. Fish. Sci. 2021, 42, 177–185. [Google Scholar] [CrossRef]
- Zhai, S.; Wang, Y.; He, Y.; Chen, X.H. Oligomeric proanthocyanidins counteracts the negative effects of high level of dietary histamine on American Eel (Anguilla rostrata). Front. Mar. Sci. 2020, 7, 549145. [Google Scholar] [CrossRef]
- Kostic, A.D.; Gevers, D.; Pedamallu, C.S.; Michaud, M.; Duke, F.; Ear, A.M.; Ojesina, A.I.; Jung, J.; Bass, A.J.; Tabernero, J.; et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 2012, 22, 292–298. [Google Scholar] [CrossRef]
- Lobionda, S.; Sittipo, P.; Kwon, H.Y.; Lee, Y.K. The role of gut microbiota in intestinal inflammation with respect to diet and extrinsic stressors. Microorganisms 2019, 7, 271. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, Z.; Liu, X.; Xu, Y.; Bussabong, B.; Wang, B. Structural characteristics and succession of intestinal microbiota for Paralichthys olivaceus during the early life stage. Aquac. Res. 2019, 50, 529–540. [Google Scholar] [CrossRef]
- Azman, A.S.; Mawang, C.I.; Khairat, J.E.; Abubakar, S. Actinobacteria-a promising natural source of anti-biofilm agents. Int. Microbiol. 2019, 22, 403–409. [Google Scholar] [CrossRef]
- Krishnamoorthy, M.; Dharmaraj, D.; Rajendran, K.; Karuppiah, K.; Ethiraj, K. Pharmacological activities of coral reef associated actinomycetes, Saccharopolyspora sp. IMA1. Biocatal. Agr. Biotechnol. 2020, 28, 101748. [Google Scholar] [CrossRef]
- Gupta, T.B.; Risson, A.N.; Brightwell, G.; Maclean, P.; Jauregui, R. Draft genome sequence of Thermoactinomyces vulgaris strain AGRTWHS02, isolated from pasture soil of a sheep dairy farm in New Zealand. Microbiol. Resour. Announc. 2022, 11, e0007622. [Google Scholar] [CrossRef] [PubMed]
- Fehr, A.; Eshwar, A.K.; Neuhauss, S.C.; Ruetten, M.; Vaughan, L. Evaluation of zebrafish as a model to study the pathogenesis of the opportunistic pathogen Cronobacter turicensis. Emerg. Microbes. Infec. 2015, 4, e29. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Kumar, S.H.; Nayak, B.B.; Lekshmi, M. Isolation and identification of Cronobacter spp. from fish and shellfish sold in retail markets. Curr. Microbiol. 2021, 78, 1973–1980. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.H.; Liu Gao, P.W.; Xiao, L.C.; Shuang, S.Y.; Cong, H.; Du, Q.Y.; Zhang, X.W. Effects of Macleaya cordata extract on TLR20 and the proinflammatory cytokines in acute spleen injury of loach (Misgurnus anguillicaudatus) against Aeromonas hydrophila infection. Aquaculture 2021, 544, 737105. [Google Scholar] [CrossRef]
- Miao, F.; Yang, X.J.; Zhou, L.; Hu, H.J.; Zheng, F.; Ding, X.D.; Sun, W. Structural modification of sanguinarine and chelerythrine and their antibacterial activity. Natural Product Res. 2011, 25, 863–875. [Google Scholar] [CrossRef]
- Beuria, T.K.; Santra, M.K.; Panda, D. Sanguinarine blocks cytokinesis in bacteria by inhibiting FtsZ assembly and bundling. Biochemistry 2005, 44, 16584–16593. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).