Impacts of Recreational Angling on Fish Population Recovery after a Commercial Fishing Ban
Abstract
1. Introduction
2. Materials and Methods
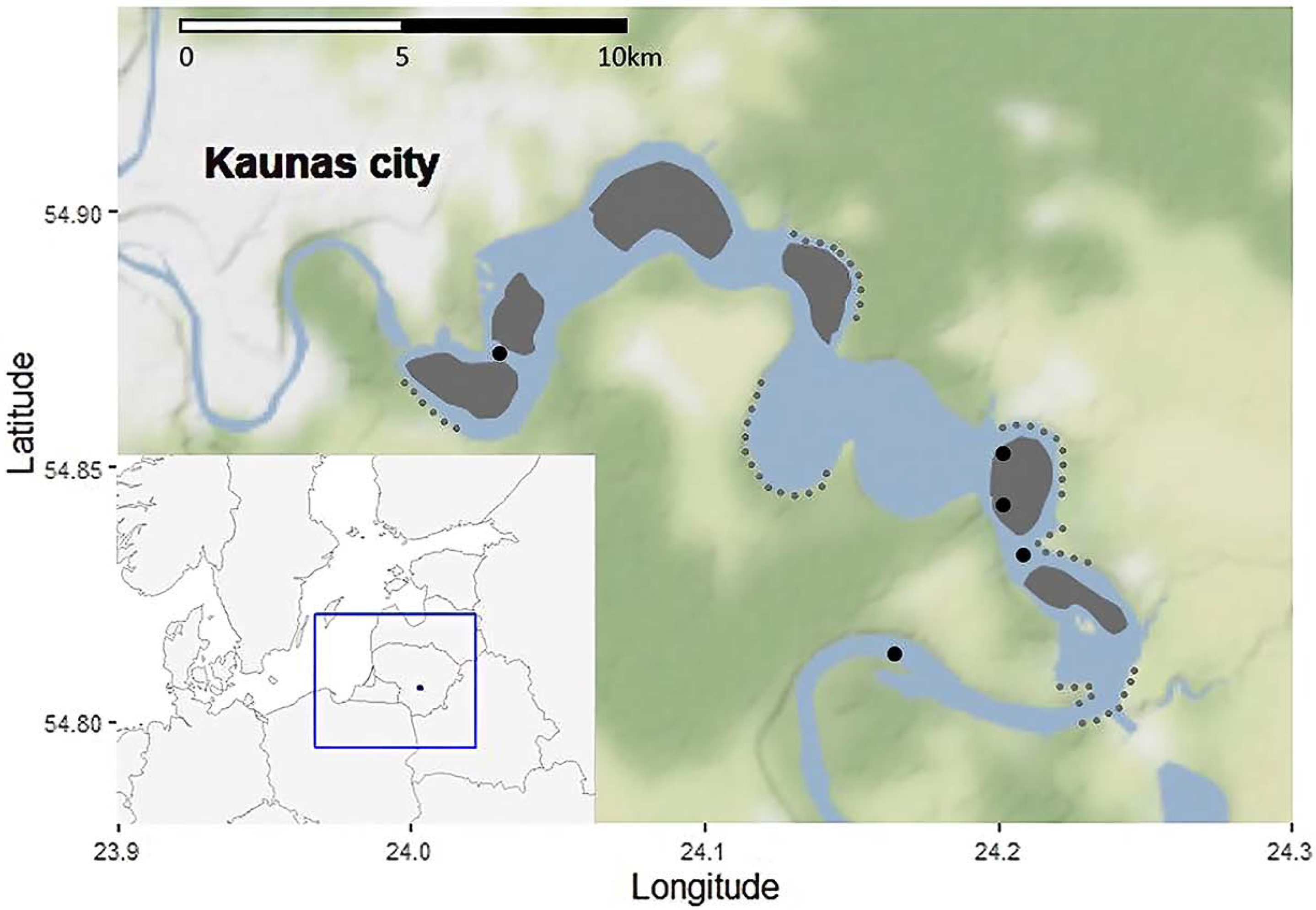
2.1. Research Area and Commercial Catch Data
2.2. Scientific Fish Abundance Data Collection
2.3. Statistical Analyses of Scientific Monitoring Data
2.4. Onsite Recreational Fishing Surveys
2.5. Assessment of Recreational Fishing Effort
2.6. Statistical Analyses for Recreational Catches
3. Results
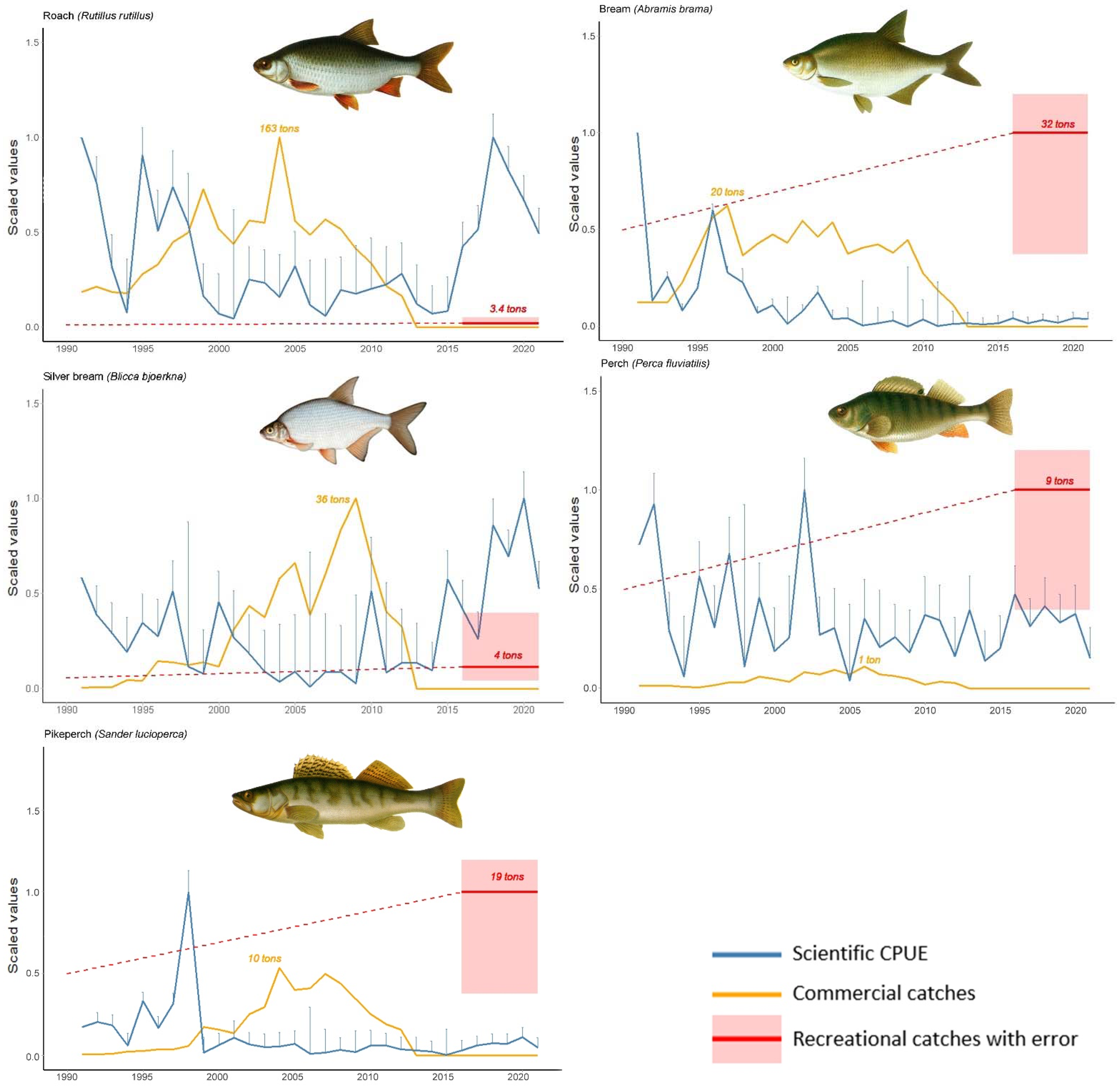
3.1. Scientific Catch-Per-Unit-Effort Trends Show Slow Recovery Rates of Piscivorous Species
3.2. Recreational Angling Mostly Targets Piscivorous Fish and Some Cyprinids
3.3. Estimated Recreational Catches for Some Fish Species Are Much Larger Than Former Commercial Catches
4. Discussion
4.1. Recreational Fishing Has Disproportional Impact on Predatory Species
4.2. Limitations and Future Research Needs
4.3. Suggestions for Recreational Angling Management
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Biggs, J.; von Fumetti, S.; Kelly-Quinn, M. The importance of small waterbodies for biodiversity and ecosystem services: Implications for policy makers. Hydrobiologia 2017, 793, 3–39. [Google Scholar] [CrossRef]
- Tockner, K. Freshwaters: Global Distribution, Biodiversity, Ecosystem Services, and Human Pressures. In Handbook of Water Resources Management: Discourses, Concepts and Examples; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Su, M.; Wang, L.; Xiang, J.; Ma, Y. Adjustment trend of China’s marine fishery policy since 2011. Mar. Policy 2021, 124, 104322. [Google Scholar] [CrossRef]
- FAO. Review of the State of World Fishery Resources: Inland Fisheries; FAO Fisheries Circular No. 942; FAO Fisheries Department: Rome, Italy, 1999. [Google Scholar]
- Hilborn, R.; Branch, T.; Ernst, B.; Magnusson, A.; Minte-Vera, C.; Scheuerell, M.; Valero, J. State of the World’s Fisheries. Ann. Rev. Environ. Resour. 2003, 28, 359–399. [Google Scholar] [CrossRef]
- Kura, Y.; Revenga, C.; Hoshino, E.; Mock, G. Fishing for Answers: Making Sense of the Global Fish Crisis; World Resources Institute: Washington, DC, USA, 2004. [Google Scholar]
- Funge-Smith, S.; Bennett, A. A fresh look at inland fisheries and their role in food security and livelihoods. Fish Fish. 2019, 20, 1176–1195. [Google Scholar] [CrossRef]
- Walters, C. Designing fisheries management systems that do not depend upon accurate stock assessment. In Reinventing Fisheries Management; Springer: Dordrecht, The Netherlands, 1998; pp. 279–288. [Google Scholar]
- Mangin, T.; Costello, C.; Anderson, J.; Arnason, R.; Elliott, M.; Gaines, S.D.; Hilborn, R.; Peterson, E.; Sumaila, R. Are fishery management upgrades worth the cost? PLoS ONE 2018, 13, e0204258. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2010; FAO Fisheries and Aquaculture Department: Rome, Italy, 2010; p. 214. [Google Scholar]
- Cowx, I.G. Analysis of Threats to Freshwater Fish Conservation: Past and Present Challenges. In Conservation of Freshwater Fish: Options for the Future; Collares-Pereira, M.J., Cowx, I.G., Coelho, M.M., Eds.; Blackwell Science: Oxford, UK, 2002; pp. 201–220. [Google Scholar]
- Coleman, F.C.; Figueira, W.F.; Ueland, J.S.; Crowder, L.B. The Impact of United States Recreational Fisheries on Marine Fish Populations. Science 2004, 305, 1958–1960. [Google Scholar] [CrossRef] [PubMed]
- Arlinghaus, R.; Cooke, S.J. Recreational Fisheries: Socioeconomic Importance, Conservation Issues and Management Challenges. Recreat. Hunt. Conserv. Rural. Livelihoods 2009, 1, 39–58. [Google Scholar] [CrossRef]
- Arlinghaus, R.; Tillner, R.; Bork, M. Explaining participation rates in recreational fishing across industrialised countries. Fish. Manag. Ecol. 2015, 22, 45–55. [Google Scholar] [CrossRef]
- Kummu, M.; de Moel, H.; Ward, P.J.; Varis, O. How close do we live to water? A global analysis of population distance to freshwater bodies. PLoS ONE 2011, 6, e20578. [Google Scholar] [CrossRef]
- Papenfuss, J.T.; Phelps, N.; Fulton, D.; Venturelli, P.A. Smartphones Reveal Angler Behavior: A Case Study of a Popular Mobile Fishing Application in Alberta, Canada. Fisheries 2015, 40, 318–327. [Google Scholar] [CrossRef]
- Feiner, Z.S.; Latzka, A.W.; Wolter, M.H.; Eslinger, L.D.; Hatzenbeler, G.R. Assessing the Rage Against the Machines: Do Ice Anglers’ Electronics Improve Catch and Harvest Rates? Fisheries 2020, 45, 327–333. [Google Scholar] [CrossRef]
- Cooke, S.J.; Venturelli, P.; Twardek, W.M.; Lennox, R.J.; Brownscombe, J.W.; Skov, C.; Hyder, K.; Suski, C.D.; Diggles, B.K.; Arlinghaus, R.; et al. Technological innovations in the recreational fishing sector: Implications for fisheries management and policy. Rev. Fish Biol. Fish. 2021, 31, 253–288. [Google Scholar] [CrossRef]
- Wadiwel, D. ‘Fishing for Fun’: The Politics of Recreational Fishing. Anim. Stud. J. 2019, 8, 202–228. [Google Scholar] [CrossRef]
- McPhee, D.; Leadbitter, D.; Skilleter, A.G. Swallowing the bait: Is recreational fishing in Australia ecologically sustainable? Pac. Conserv. Biol. 2002, 8, 40–51. [Google Scholar] [CrossRef]
- Kearney, R.E. Fisheries property rights and recreational/commercial conflict: Implications of policy development in Australia and New Zealand. Mar. Policy 2001, 25, 49–59. [Google Scholar] [CrossRef]
- Pope, K.L.; Powel, L.A.; Harmon, B.S.; Pegg, M.A.; Chizinski, C.J. Estimating the number of recreational anglers for a given waterbody a given waterbody. Fish. Res. 2017, 191, 69–75. [Google Scholar] [CrossRef][Green Version]
- Winstanley, R. A Wake-Up Call for Recreational Fishing in Australia. SETFIA News, 22 February 2019. Available online: https://setfia.org.au/a-wake-up-call-for-recreational-fishing-in-australia/ (accessed on 7 April 2021).
- Bartholomew, A.; Bohnsack, J.A. A review of catch-and-release angling mortality with implications for no-take reserves. Rev. Fish Biol. Fish. 2005, 15, 129–154. [Google Scholar] [CrossRef]
- Conron, S.; Bridge, N.F.; Oliveiro, P.; Bruce, T.K. Angler Diary Monitoring of Recreational Fishing in Selected Victorian Waters during 2010/11. Recreational Fishing Grant Program Final Report; Department of Primary Industries: St Melbourne, Australia, 2012; 24p. [Google Scholar]
- Vandergoot, C.S. Estimation of Regional Mortality Rates for Lake Erie Walleye Sander vitreus Using Spatial Tag-Recovery Modeling. Ph.D. Thesis, Michigan State University, East Lansing, MI, USA, 2014. [Google Scholar]
- Kristensen, E.; Sand-Jensen, K.; Martinsen, K.T.; Madsen-Østerbye, M.; Kragh, T. Fingerprinting pike: The use of image recognition to identify individual pikes. Fish. Res. 2020, 229, 105622. [Google Scholar] [CrossRef]
- Cline, T.J.; Weidel, B.C.; Kitchell, J.F.; Hodgson, J.R. Growth response of largemouth bass (Micropterus salmoides) to catch-and-release angling: A 27-year mark-recapture study. Can. J. Fish. Aquat. Sci. 2018, 69, 224–230. [Google Scholar] [CrossRef]
- Bennett, B.A. Long-term trends in the catches by shore anglers in False Bay. Trans. R. Soc. S. Afr. 1991, 47, 683–692. [Google Scholar]
- Olson, D.E.; Cunningham, P.K. Sport-fishing trends shown by an annual Minnesota fishing contest over a 58-year period. N. Am. J. Fish. Manag. 1989, 9, 287–297. [Google Scholar] [CrossRef]
- Beard, T.D., Jr.; Essington, T.E. Effects of angling and life history processes on bluegill size structure: Insights from an individual-based model. Trans. Am. Fish. Soci. 2000, 129, 561–568. [Google Scholar] [CrossRef]
- Post, J.R.; Sullivan, M.; Cox, S.; Lester, N.P.; Walters, C.J.; Parkinson, E.A.; Paul, A.J.; Jackson, L.; Shuter, B.J. Canada’s recreational fisheries: The invisible collapse? Fisheries 2002, 27, 6–17. [Google Scholar] [CrossRef]
- Embke, H.S.; Rypel, A.L.; Carpenter, S.R.; Sass, G.G.; Ogle, D.; Cichosz, T.; Hennessy, J.; Essington, T.E.; Vander Zanden, M.J. Production dynamics reveal hidden overharvest of inland recreational fisheries. Proc. Natl. Acad. Sci. USA 2019, 116, 24676–24681. [Google Scholar] [CrossRef] [PubMed]
- Cooke, S.J.; Cowx, I.G. The Role of Recreational Fishing in Global Fish Crises. Bioscience 2004, 54, 857–859. [Google Scholar] [CrossRef]
- Morales-Nin, B.; Moranta, J.; García, C.; Tugores, M.P.; Grau, A.M.; Riera, F.; Cerdà, M. The recreational fishery off Majorca Island (western Mediterranean): Some implications for coastal resource management. ICES J. Mar. Sci. 2005, 62, 727–739. [Google Scholar] [CrossRef]
- West, R.J.; Gordon, G.N.G. Commercial and recreational harvest of fish from two Australian coastal rivers. Aust. J. Mar. Freshw. Res. 1994, 45, 1259–1279. [Google Scholar] [CrossRef]
- Ložys, L.; Stanevičius, V.; Pūtys, Ž.; Dainys, J.; Levickienė, D.; Jakimavičius, D.; Akstinas, V.; Adžgauskas, G.; Tomkevičienė, A.; Irbinskas, V. Assessment of the Impact of Water Level Fluctuations on Fish and Waterbird Populations in Kaunas Reservoir (In Lithuanian); Nature research centre: Vilnius, Lithuania, 2020; 98p. [Google Scholar]
- Giner, G.; Smyth, G.K. statmod: Probability calculations for the inverse Gaussian distribution. R J. 2016, 8, 339–351. [Google Scholar] [CrossRef]
- Shono, H. Confidence interval estimation of CPUE year trend in delta-type two-step model. Fish. Sci. 2008, 74, 712–717. [Google Scholar] [CrossRef]
- Dunn, P.K. Tweedie: Evaluation of Tweedie Exponential Family Models; R Package Version 2.3.3; 2017. [Google Scholar]
- Akaike, H. A New Look at the Statistical Model Identification. IEEE Trans. Autom. Control 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Burnham, K.P. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer: New York, NY, USA, 2002; ISBN 0-387-95364-7. [Google Scholar]
- Forrestal, F.; Goodyear, C.; Schirripa, M. Applications of the longline simulator (LLSIM) using US pelagic longline logbook data and Atlantic blue marlin. Fish. Res. 2019, 211, 331–337. [Google Scholar] [CrossRef]
- Malvestuto, S.P. Sampling the recreational creel. In Fisheries Techniques, 2nd ed.; Murphy, B.R., Willis, D.W., Eds.; American Fisheries Society: Bethesda, MD, USA, 1996; pp. 591–623. [Google Scholar]
- Kaemingk, M.; Chizinski, C.J.; Hurley, K.L.; Pope, K.L. Synchrony—An emergent property of recreational fisheries. J. Appl. Ecol. 2018, 55, 2986–2996. [Google Scholar] [CrossRef]
- VanDeValk, A.J.; Jackson, J.R.; Krueger, S.D.; Brooking, T.E.; Rudstam, L.G. Influence of party size and trip length on angler catch rates on Oneida Lake, New York. N. Am. J. Fish. Manag. 2007, 27, 127–136. [Google Scholar] [CrossRef]
- Dainys, J.; Gorfine, H.; Mateos-González, F.; Skov, C.; Urbonavičius, R.; Audzijonyte, A. Angling counts: Harnessing the power of technological advances for recreational fishing surveys. Fish. Res. 2022, 254, 106410. [Google Scholar] [CrossRef]
- FAO. The role of Recreational Fisheries in the sustainable management of marine resources. In GLOBEFISH Highlights—Issue 2/2017; FAO: Rome, Italy, 2017; pp. 65–68. [Google Scholar]
- Melville-Smith, R.; Thomson, A.W.; Caputi, N. Improved forecasts of recreational western rock lobster (Panulirus cygnus) catches in Western Australia, by predicting licence usage. Fish. Res. 2004, 68, 203–208. [Google Scholar] [CrossRef]
- Muoneke, M.I.; Childress, W.M. Hooking mortality: A review for recreational fisheries. Rev. Fish. Sci. 1994, 2, 123–156. [Google Scholar] [CrossRef]
- Sass, G.G.; Shaw, S.L. Catch-and-Release Influences on Inland Recreational Fisheries. Rev. Fish. Sci. Aquac. 2020, 28, 211–227. [Google Scholar] [CrossRef]
- Cowx, I.G. Characterisation of inland fisheries in Europe. Fish. Manag. Ecol. 2015, 22, 78–87. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, J.; Gorfine, H.; Shan, X.; Shen, L.; Yang, H.; Du, H.; Li, J.; Wang, C.; Zhou, Q.; et al. Inland fisheries development versus aquatic biodiversity conservation in China and its global implications. Rev. Fish Biol. Fish. 2020, 30, 637–655. [Google Scholar] [CrossRef]
- Cooke, S.J.; Cowx, I.G. Contrasting Recreational and Commercial Fishing: Searching for Common Issues to Promote Unified Conservation of Fisheries Resources and Aquatic Environments. Biol. Conserv. 2006, 128, 93–108. [Google Scholar] [CrossRef]
- Zeller, D.; Darcy, M.; Booth, S.; Lowe, M.K.; Martell, S. What about recreational catch?: Potential impact on stock assessment for Hawaii’s bottomfish fisheries? Fish. Res. 2008, 91, 88–97. [Google Scholar] [CrossRef]
- Beard, T.D., Jr.; Kampa, J.M. Changes in bluegill, black crappie, and yellow perch populations in Wisconsin during 1967–1991. N. Am. J. Fish. Manag. 1999, 19, 1037–1043. [Google Scholar] [CrossRef]
- Lewin, W.C.; Arlinghaus, R.; Mehner, T. Documented and Potential Biological Impacts of Recreational Fishing: Insights for Management and Conservation. Rev. Fish. Sci. 2006, 14, 305–367. [Google Scholar] [CrossRef]
- Yount, J.D. Ecology and Management of the Zebra Mussel and Other Introduced Aquatic Nuisance Species; Saginaw Valley State University: Saginaw, MI, USA, 1991. [Google Scholar]
- Carpenter, S.R.; Kitchell, J.F.; Hodgson, J.R. Cascading Trophic Interactions and Lake Productivity: Fish predation and herbivory can regulate lake ecosystems. BioScience 1985, 35, 634–639. [Google Scholar] [CrossRef]
- Vejřík, L.; Matějíčková, I.; Seďa, J.; Blabolil, P.; Jůza, T.; Vašek, M.; Ricard, D.; Matěna, J.; Frouzová, J.; Kubečk, J.; et al. Who Is Who: An Anomalous Predator-Prey Role Exchange between Cyprinids and Perch. PLoS ONE 2016, 11, e0156430. [Google Scholar] [CrossRef]
- Persson, L. Effects of habitat and season on competitive interactions between roach (Rutilus rutilus) and perch (Perca fluviatilis). Oecologia 1987, 73, 170–177. [Google Scholar] [CrossRef]
- Midway, S.R.; Lynch, A.J.; Peoples, B.K.; Dance, M.; Caffey, R. COVID-19 influences on US recreational angler behavior. PLoS ONE 2021, 16, e0254652. [Google Scholar] [CrossRef]
- Barnes, M. A Comparison of A Creel Census to Modeled Access-Point Creel Surveys on Two Small Lakes Managed as Put-and-Take Rainbow Trout Fisheries. Fish. Aquac. J. 2014. [Google Scholar] [CrossRef]
- Jones, C.M.; Robson, D.S.; Lakkis, H.D.; Kressel, J. Properties of catch rates in analysis of angler surveys. Trans. Am. Fish. Soc. 1995, 124, 911–928. [Google Scholar] [CrossRef]
- Lockwood, R.N.; Benjamin, D.M.; Bence, J.R. Estimating Angling Effort and Catch from Michigan Roving and Access Site Angler Survey Data; Fisheries Research Report 2044; Michigan Department of Natural Resources: Lansing, MI, USA, 1999. [Google Scholar]
- Mallison, C.T.; Cichra, C.E. Accuracy of angler-reported harvest in roving creel surveys. N. Am. J. Fish. Manag. 2004, 24, 880–889. [Google Scholar] [CrossRef]
- Venohr, M.; Langhans, S.D.; Peters, O.; Hölker, F.; Arlinghaus, R.; Mitchell, L.; Wolter, C. The underestimated dynamics and impacts of water-based recreational activities on freshwater ecosystems. Environ. Rev. 2018, 26, 199–213. [Google Scholar] [CrossRef]
- Ahrens, R.N.; Allen, M.S.; Walters, C.; Arlinghaus, R. Saving large fish through harvest slots outperforms the classical minimum-length limit when the aim is to achieve multiple harvest and catch-related fisheries objectives. Fish Fish. 2020, 2, 483–510. [Google Scholar] [CrossRef]
- Stoll-Kleemann, S.; de la Vega-Leinert, A.; Schultz, L. The role of community participation in the effectiveness of UNESCO Biosphere Reserve management: Evidence and reflections from two parallel global surveys. Environ. Conserv. 2010, 37, 227–238. [Google Scholar] [CrossRef]
- Abell, R.; Allan, J.D.; Lehner, B. Unlocking the potential of protected areas for freshwaters. Biol. Conserv. 2007, 134, 48–63. [Google Scholar] [CrossRef]
- Manning, R.E. Studies in Outdoor Recreation: Search and Research for Satisfaction; Oregon State University Press: Corvallis, OR, USA, 2010. [Google Scholar]
- Cooke, S.; Schreer, J.; Wahl, D.; Philipp, D. Physiological impacts of catch-and-release angling practices on largemouth and smallmouth bass. Am. Fish. Soc. Symp. 2002, 31, 489–512. [Google Scholar]
| Species | Mean | Min | Max |
|---|---|---|---|
| Prussian carp | 43.63 | 19.62 | 101.44 |
| Bream | 31.98 | 11.94 | 89.52 |
| Pikeperch * | 19.36 | 7.29 | 54.79 |
| Common carp | 17.24 | 5.53 | 72.06 |
| Catfish | 11.49 | 4.12 | 33.37 |
| Eurasian perch | 9.09 | 3.61 | 27.9 |
| Silver bream | 4.14 | 1.55 | 14.32 |
| Roach | 3.38 | 1.32 | 8.45 |
| Vimba | 1.29 | 0.36 | 13.45 |
| Asp | 1.07 | 0.32 | 4.08 |
| Northern pike * | 1.07 | 0.22 | 6.06 |
| Total catch | 142.91 | 70.08 | 304.65 |
| Parameter | Mean Catch | 2.5% CI | 97.5% CI | p |
|---|---|---|---|---|
| Total catch | ||||
| Autumn | 991.32 | 752.25 | 1306.37 | 0.00 |
| Spring | 1396.70 | 985.08 | 1980.31 | 0.13 |
| Summer | 1975.64 | 1551.60 | 2515.58 | 0.00 |
| Winter | 299.76 | 211.98 | 423.89 | 0.00 |
| Roach Rutillus rutillus | ||||
| Total average (intercept) | 31.13 | 18.22 | 49.75 | 0.00 |
| Pikeperch Sander lucioperca | ||||
| Autumn | 20.48 | 5.39 | 77.73 | 0.00 |
| Spring | 238.11 | 123.95 | 457.43 | 0.00 |
| Summer | 302.84 | 188.53 | 486.46 | 0.00 |
| Winter | 0.47 | 0.00 | 99.85 | 0.18 |
| Eurasian perch Perca fluviatilis | ||||
| Autumn | 140.87 | 88.28 | 224.80 | 0.00 |
| Spring | 24.90 | 5.53 | 112.12 | 0.00 |
| Summer | 75.37 | 35.98 | 157.87 | 0.00 |
| Winter | 170.61 | 124.60 | 233.61 | 0.5 |
| Bream Abramis brama | ||||
| Autumn | 322.90 | 207.51 | 502.45 | 0.00 |
| Spring | 430.57 | 242.66 | 763.98 | 0.44 |
| Summer | 230.56 | 124.73 | 426.20 | 0.38 |
| Winter | 50.22 | 23.75 | 106.18 | 0.00 |
| Silver bream Blica bjoerkna | ||||
| Autumn | 41.37 | 22.43 | 76.31 | 0.00 |
| Spring | 3.77 | 0.27 | 53.67 | 0.08 |
| Summer | 98.93 | 60.37 | 162.10 | 0.03 |
| Winter | 3.17 | 0.76 | 13.20 | 0.00 |
| Prussian carp Carassius gibelio | ||||
| Autumn | 110.61 | 62.77 | 194.90 | 0.00 |
| Spring | 643.87 | 440.97 | 940.13 | 0.00 |
| Summer | 500.49 | 355.19 | 705.24 | 0.00 |
| Winter | 0.00 | 0.00 | Inf | 0.98 |
| Common carp Cyprinus carpio | ||||
| Autumn | 220.78 | 112.12 | 434.75 | 0.00 |
| Spring | 28.30 | 2.27 | 352.14 | 0.12 |
| Summer | 370.37 | 195.08 | 703.16 | 0.28 |
| Winter | 0.00 | 0.00 | Inf | 0.99 |
| Catfish Silurus glanis | ||||
| Autumn | 0.00 | 0.00 | Inf | 0.99 |
| Spring | 0.00 | 0.00 | Inf | 1.00 |
| Summer | 370.37 | 201.49 | 680.79 | 0.99 |
| Winter | 0.00 | 0.00 | Inf | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dainys, J.; Jakubavičiūtė, E.; Gorfine, H.; Kirka, M.; Raklevičiūtė, A.; Morkvėnas, A.; Pūtys, Ž.; Ložys, L.; Audzijonyte, A. Impacts of Recreational Angling on Fish Population Recovery after a Commercial Fishing Ban. Fishes 2022, 7, 232. https://doi.org/10.3390/fishes7050232
Dainys J, Jakubavičiūtė E, Gorfine H, Kirka M, Raklevičiūtė A, Morkvėnas A, Pūtys Ž, Ložys L, Audzijonyte A. Impacts of Recreational Angling on Fish Population Recovery after a Commercial Fishing Ban. Fishes. 2022; 7(5):232. https://doi.org/10.3390/fishes7050232
Chicago/Turabian StyleDainys, Justas, Eglė Jakubavičiūtė, Harry Gorfine, Mindaugas Kirka, Alina Raklevičiūtė, Augustas Morkvėnas, Žilvinas Pūtys, Linas Ložys, and Asta Audzijonyte. 2022. "Impacts of Recreational Angling on Fish Population Recovery after a Commercial Fishing Ban" Fishes 7, no. 5: 232. https://doi.org/10.3390/fishes7050232
APA StyleDainys, J., Jakubavičiūtė, E., Gorfine, H., Kirka, M., Raklevičiūtė, A., Morkvėnas, A., Pūtys, Ž., Ložys, L., & Audzijonyte, A. (2022). Impacts of Recreational Angling on Fish Population Recovery after a Commercial Fishing Ban. Fishes, 7(5), 232. https://doi.org/10.3390/fishes7050232






