Statolith Microstructure Estimates of the Age, Growth, and Population Structure of Purpleback Flying Squid (Sthenoteuthis oualaniensis) in the Waters of the Xisha Islands of the South China Sea
Abstract
1. Introduction
2. Materials and Methods
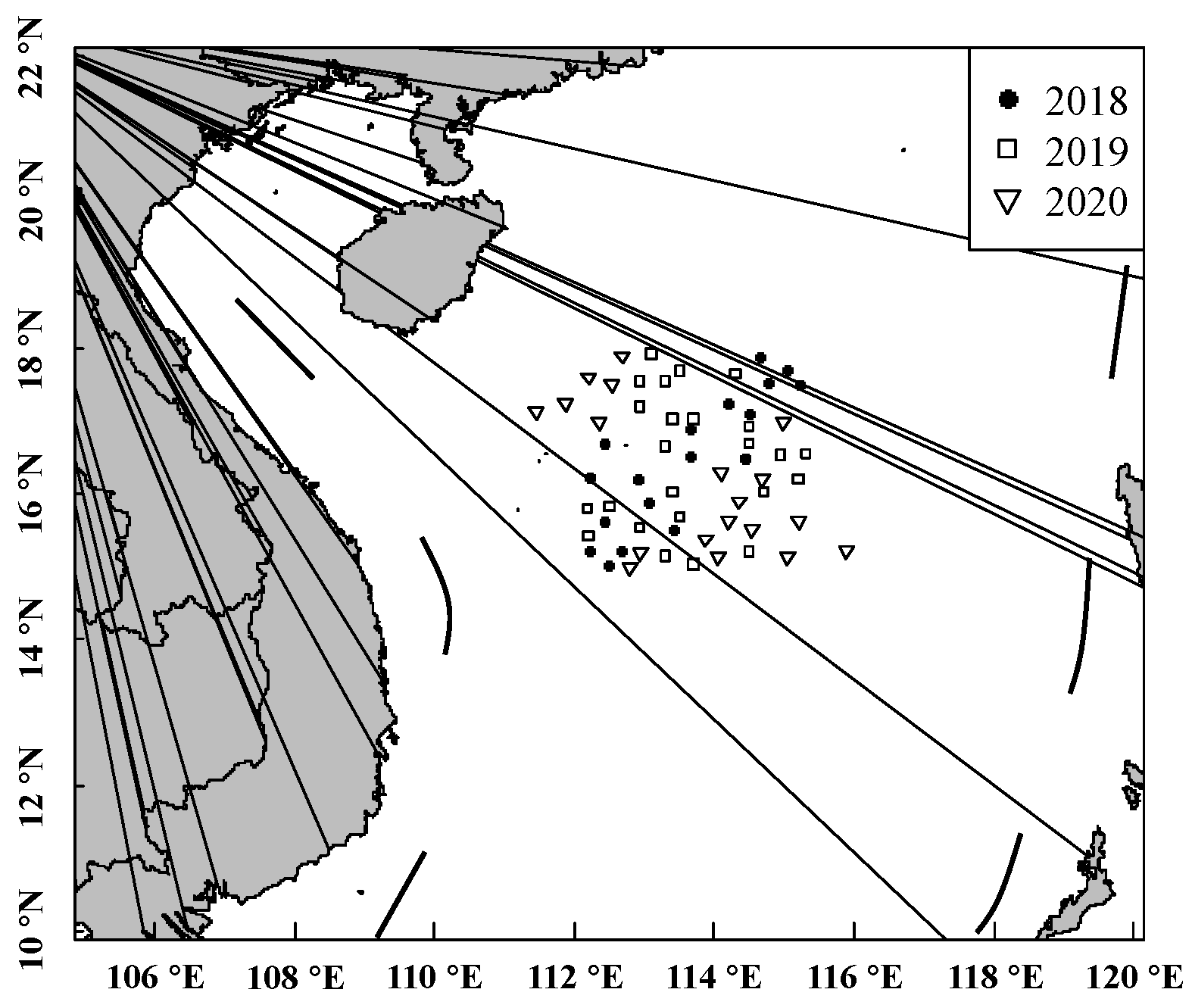
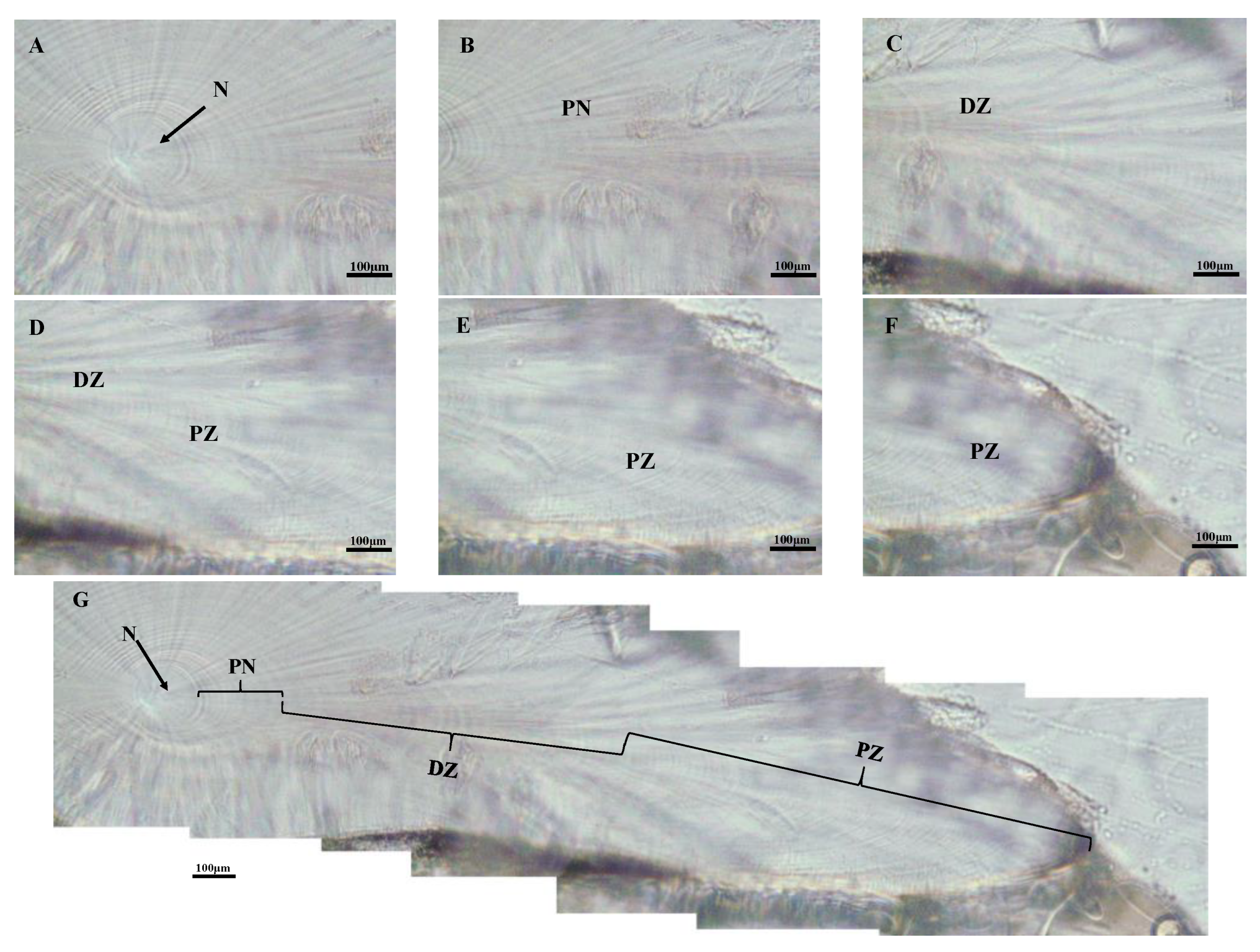
2.1. Sampling and Ageing
2.2. Data Analysis
3. Results
3.1. Size–Structure
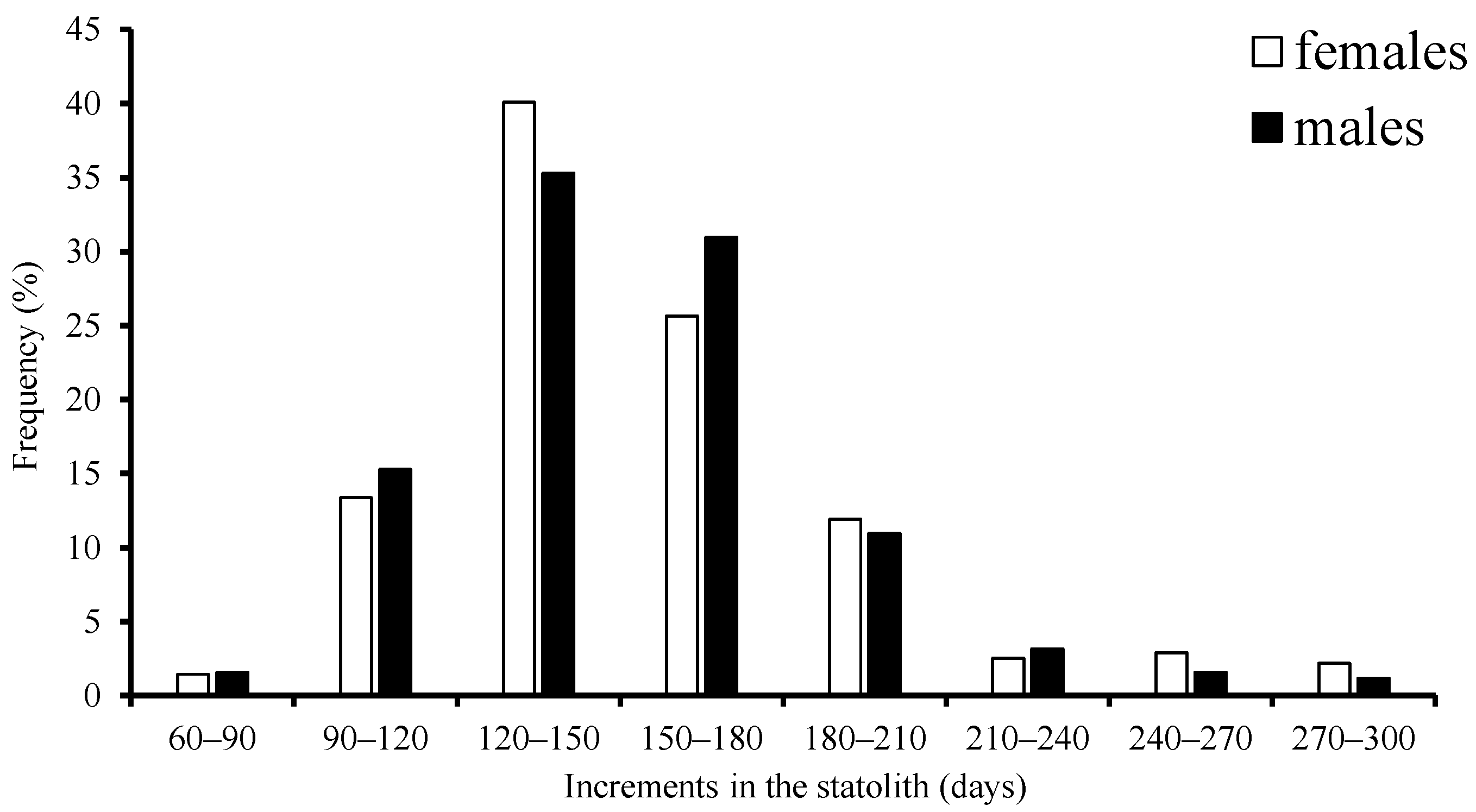
3.2. Age Structure and Population Structure
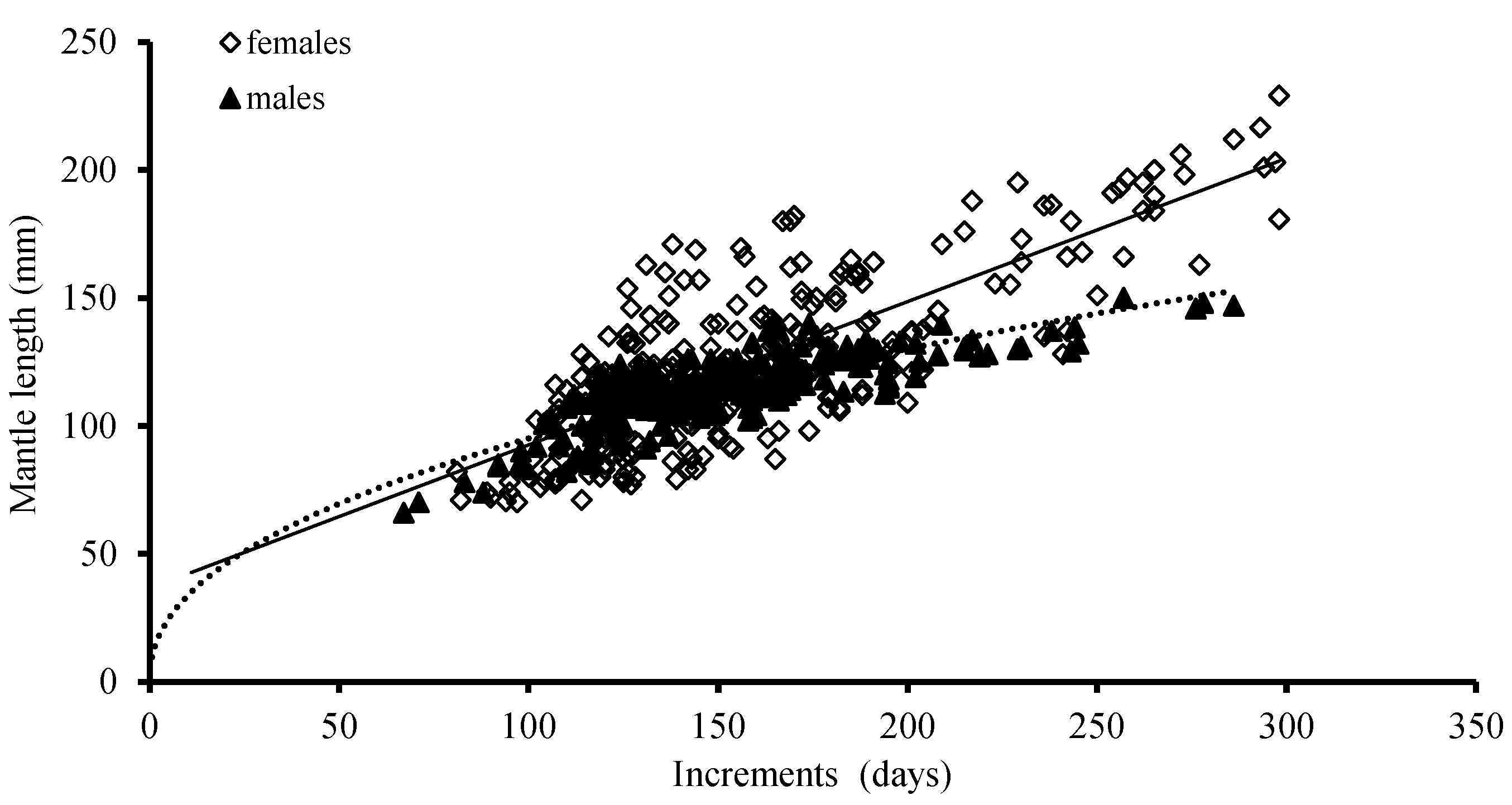
3.3. Growth Models
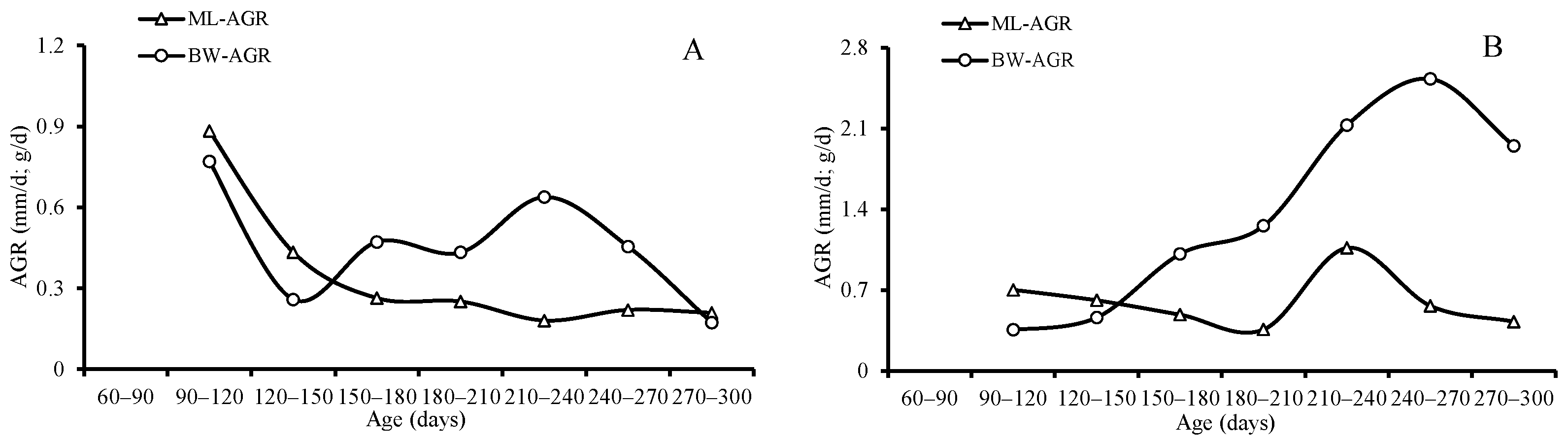
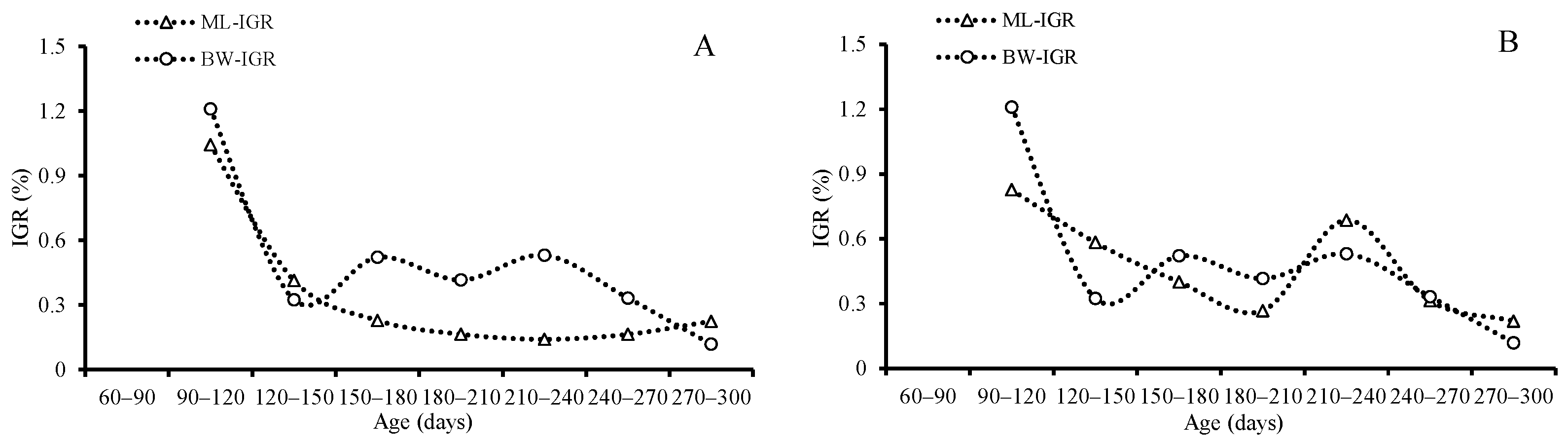
3.4. Growth Patterns
4. Discussion
4.1. Body Size
4.2. Age
4.3. Growth Models
4.4. Population Structure
4.5. Growth Pattern
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jereb, P.; Roper, C.F.E. Cephalopods of the World, Volume 2: Myopsid and Oegopsid Squids An Annotated and Illustrated Catalogue of Cephalopod Species Known to Date; FAO: Rome, Italy, 2010; pp. 315–318. [Google Scholar]
- Chen, Z.Y.; Lu, H.J.; Liu, W.; Liu, K.; Chen, X.J. Beak microstructure estimates of the age, growth, and population structure of purpleback flying squid (Sthenoteuthis oualaniensis) in the Xisha Islands Waters of the South China Sea. Fishes 2022, 7, 187. [Google Scholar] [CrossRef]
- Wang, Y.G.; Chen, X.J. The Resource and Biology of Economic Oceanic Squid in the World; China Ocean Press: Beijing, China, 2005; pp. 190–194. (In Chinese) [Google Scholar]
- Chen, X.J.; Liu, B.L.; Tian, S.Q.; Qian, W.G.; Zhao, X.H. Fishery biology of purpleback squid, Sthenoteuthis oualaniensis, in the northwest Indian Ocean. Fish. Res. 2007, 83, 98–104. [Google Scholar]
- Zhang, P.; Yang, L.; Zhang, X.F.; Tan, Y.G. The present status and prospect on exploitation of tuna and squid fishery resources in South China Sea. South China Fish. Sci. 2010, 6, 68–74, (In Chinese with English abstract). [Google Scholar]
- Lu, H.J.; Tong, Y.H.; Liu, W.; Liu, K.; Dong, Z.X.; Chen, X.; Chen, X.J. Fisheries biological characteristics of Sthenoteuthis oualaniensis in the spring season in the El Nino year of 2016 in the Zhongsha Islands waters of South China Sea. J. Fish. China 2018, 36, 112–121, (In Chinese with English abstract). [Google Scholar]
- Chen, Z.Y.; Lu, H.J.; Tong, Y.H.; Tang, Y.; Liu, W.; Cheng, X.; Chen, X.J. Beak growth characteristic of Sthenoteuthis oualaniensis in the waters of Xisha Island in the South China Sea. J. Ocean Univ. Shanghai 2019, 28, 373–383, (In Chinese with English abstract). [Google Scholar]
- Lu, H.J.; Wang, H.H.; Liu, K.; Chen, X.Y.; He, J.R.; Chen, X.J. Growth characteristics of statolith of Sthenoteuthis oualaniensis in the Northwest Indian Ocean in spring and winter in the El Nino year. Chin. J. Ecol. 2020, 39, 3694–3703, (In Chinese with English abstract). [Google Scholar]
- Lu, H.J.; Zhang, X.; Tong, Y.H.; Tang, Y.; Liu, K.; Liu, W.; Chen, X.J. Statolith microstructure and growth characteristics of Sthenoeuthis oualaniensis in the Xisha Islands waters of the South China Sea. J. Fish. China 2020, 44, 767–776, (In Chinese with English abstract). [Google Scholar]
- He, J.R.; Lu, H.J.; Chen, X.Y.; Liu, K.; Wang, H.H.; Chen, X.J. Factors influencing beak morphology of Sthenoteuthis oualaniensis in the northwest Indian Ocean. Chin. J. Appl. Ecol. 2021, 32, 1881–1889, (In Chinese with English abstract). [Google Scholar]
- Bizikov, V.A. Growth of Sthenoteuthis oualaniensis, using a new method based on gladius microstructure. ICES J. Mar. Sci. 1995, 199, 445–458. [Google Scholar]
- Liu, B.L.; Chen, X.J.; Li, J.H.; Chen, Y. Age, growth and maturation of Sthenoteuthis oualaniensis in the eastern tropical Pacific Ocean by statolith analysis. Mar. Freshw. Res. 2016, 67, 1973–1981. [Google Scholar] [CrossRef]
- Takagi, K.; Yatsu, A. Age determination using Statolith microstructure of the purpleback flying squid, Sthenoteuthis oualaniensis, in the North Pacific Ocean. Nippon Suisan Gakkaishi 1996, 65, 98–113. [Google Scholar]
- Takagi, K.; Kitahara, T.; Suzuki, N.; Mori, J. The age and growth of Sthenoteuthis oualaniensis (Cephalopoda: Ommastrephidae) in the Pacific Ocean. Bull. Mar. Sci. 2002, 71, 1105–1108. [Google Scholar]
- Sukramongkol, N.; Promjinda, S.; Prommas, R. Age and reproduction of Sthenoteuthis oualaniensis in the Bay of Bengal. In The Ecosystem-Based Fishery Management in the Bay of Bengal; Department of Fisheries, Ministry of Agriculture and Cooperatives: Phuket, Thailand, 2007; pp. 195–205. [Google Scholar]
- Dunning, M.; Brandt, S.B. Distribution and life history of deep-water squid of commercial interest from Australia. Aust. J. Mar. Freshw. Res. 1985, 36, 343–359. [Google Scholar] [CrossRef]
- Rancurel, P. Note pour servir á la connaissance de Symplectoteuthis oualaniensis (Lesson, 1830) (Cephalopoda, Oegopsida): Variations ontogéniques du bec supérieur. Cah. De L’indo Pac. 1980, 2, 217–232. [Google Scholar]
- Tung, I. On the Reproduction of Common Squid, Symplectoteuthis oualanrensrs (Lesson); Report of the Institute of Fishery Biology of Ministry of Economic Affairs and National Taiwan University: Taiwan, China, 1976; pp. 6–48. [Google Scholar]
- Young, R.E.; Hirota, J. Review of the ecology of Sthenoteuthis oualaniensis near the Hawaiian Archipelago. In Contributed Papers to International Symposium on Large Pelagic Squids; Okutani, T., Ed.; Japan Marine Fishery Resources Research Center: Tokyo, Japan, 1998; pp. 131–143. [Google Scholar]
- Nesis, K.N. Population structure of oceanic Ommastrephehids, with particular reference to Sthenoteuthis oualaniensis: A review. In Recent Advances in Fisheries Biology; Okutani, K., O’Dor, R.K., Kubodera, T., Eds.; Tokai University Press: Tokyo, Japan, 1993; pp. 375–383. [Google Scholar]
- Clarke, M. A review of the systematics and ecology of oceanic squids. Adv. Mar. Biol. 1966, 4, 91–300. [Google Scholar]
- Lu, H.J.; Wang, C.J.; Chen, X.J. Preliminary study on the biological characteristics of Sthenoteuthis oualaniensis in the high seas nearby the equator of eastern Pacific during April to June. J. Ocean Univ. China 2014, 23, 441–447, (In Chinese with English abstract). [Google Scholar]
- Chen, X.J.; Lu, H.J.; Liu, B.L.; Tian, S.Q. Current exploitation and some scientific issues in the sustainable utilization of Ommastrephidae. J. Ocean Univ. China 2012, 21, 831–840, (In Chinese with English abstract). [Google Scholar]
- Zhang, J.; Chen, G.B.; Zhang, P.; Chen, Z.Z.; Fan, J.T. Estimation of purpleback flying squid (Sthenoteuthis oualaniensis) resource in the central and southern South China Sea based on fisheries acoustics and light-falling net. J. Fish. Sci. China 2014, 10, 822–831, (In Chinese with English abstract). [Google Scholar]
- Siriraksophon, S.; Sukramongkol, N.; Nakamura, Y. Exploration of oceanic squid, Sthenoteuthis oualaniensis resources in the South China Sea, Vietnamese waters. In Proceedings of the SEAFDEC Seminar on Fishery Resources in the South China Sea, Area IV: Vietnamese Waters, Bangkok, Thailand, 18–20 September 2001; pp. 181–197. [Google Scholar]
- Okutani, T.; Tung, I.H. Reviews of biology of commercially important squids in Japanese and adjacent waters, I. Symplectoteuthis oualaniensis (Lesson). Veliger 1978, 21, 87–94. [Google Scholar]
- Labe, L.L. Catch rate of oceanic squid by jigging method in the South China Sea area III: Western Philippines. In Proceedings of the SEAFDEC Seminar on Fishery Resources in the South China Sea: Area III, Bangkok, Thailand, 13–15 July 2000; pp. 19–31. [Google Scholar]
- Qiu., Y.S.; Lin, Z.J.; Wang., Y.Z. Responses of fish production to fishing and climate variability in the northern South China Sea. Prog. Oceanogr. 2010, 85, 197–212. [Google Scholar] [CrossRef]
- Shchetinnikov, A.S. Feeding spectrum of squid Sthenoteuthis oualaniensis (Oegopsida) in the Eastern Pacific. J. Mar. Biol. Assoc. UK 1992, 72, 849–860. [Google Scholar] [CrossRef]
- Young, R.E. A brief review of the biology of the oceanic squid, Symplecttoteuthis oualaniensis (Lesson). Comp. Biochem. Physiol. B 1975, 52, 141–143. [Google Scholar] [CrossRef]
- Wang, M.C.; Walker, W.A.; Shao, K.T.; Chou, L.S. Comparative analysis of the diets of pygmy sperm whales and dwarf sperm whales in Taiwanese waters. Acta Zool. Taiwanica 2002, 13, 53–62. [Google Scholar]
- Chen, X.J.; Lu, H.J.; Liu, B.L.; Chen, Y. Age, growth and population structure of jumbo flying squid, Dosidicus gigas, based on statolith microstructure off the Exclusive Economic Zone of Chilean waters. J. Mar. Biol. Assoc. UK 2011, 91, 229–235. [Google Scholar] [CrossRef]
- Keyl, F.; ArgÜelles, J.; Tafur, R. Interannual variability in size structure, age, and growth of jumbo squid (Dosidicus gigas) assessed by modal progression analysis. ICES J. Mar. Sci. 2011, 68, 507–518. [Google Scholar] [CrossRef]
- Yatsu, A.; Midorikawa, S.; Shimada, T.; Uozumi, Y. Age and growth of the neon flying squid, Ommastrephes bartramii, in the North Pacific Ocean. Fish. Res. 1997, 29, 257–270. [Google Scholar] [CrossRef]
- Bigelow, K. Age and Growth of Three Species of Squid Paralarvae from Hawaiian Waters, as Determined by Statolith Microstructures. Master’s Thesis, University of Hawaii, Hilo, HI, USA, 1991. [Google Scholar]
- Arkhipkin, A.I.; Bizikov, A.V.; Doubleday, Z.A.; Laptikhovsky, V.V.; Lishchenko, F.V.; Perales-Raya, C.; Hollyman, P.R. Techniques for estimating the age and growth of molluscs: Cephalopoda. J. Shellfish Res. 2018, 37, 783–792. [Google Scholar] [CrossRef]
- Rodhouse, P.G.; Hatfield, E.M.C. Age determination in squid using statolith growth increments. Fish. Res. 1990, 8, 323–334. [Google Scholar] [CrossRef]
- Jackson, G.D. Application and future potential of statolith increment analysis in squid and sepiolids. Can. J. Fish. Aquat. Sci. 1994, 51, 2612–2625. [Google Scholar] [CrossRef]
- Villanueva, R. Deep-sea cephalopods of the north-western Mediterranean: Indications of up-slope ontogenetic migration in to bathy benthic species. J. Zool. 1992, 227, 267–276. [Google Scholar] [CrossRef]
- Malcolm, H. Modeling and Quantitative Methods in Fisheries; Chapman and Hall/CRC: New York, NY, USA, 2001; pp. 227–232. [Google Scholar]
- Hiramatsu, K. Application of maximum likelihood method and AIC to fish population dynamics. In Fish Population Dynamics and Statistical Models; Matsumiya, Y., Ed.; Koseisha Koseikaku: Tokyo, Japan, 1993; pp. 9–21. (In Japanese) [Google Scholar]
- Imai, C.; Sakai, H.; Katsura, K. Growth model for the endangered cyprinid fish Tribolodon nakamurai based on otolith analyses. Fish. Sci. 2002, 68, 843–848. [Google Scholar] [CrossRef]
- Arkhipkin, A.I.; Roa-Ureta, R. Identification of ontogenetic growth models for squid. Mar. Freshw. Res. 2005, 56, 371–386. [Google Scholar] [CrossRef]
- Dunning, M. A Review of the Systematics, Distribution and Biology of the Arrow Squid Genera Ommastrephes Orbigny, 1835, Sthenoteuthis Verrill, 1880, and Ornithoteuthis Okada, 1927 (Cephalopoda, Ommastrephidae); Voss, N.A., Vecchione, M., Toll, R.B., Sweeney, M.J., Eds.; Systematics and biogeography of cephalopods, Smithsonian Contributions to Zoology 586; Smithsonian Institution Press: Washington, DC, USA, 1998; pp. 425–433. [Google Scholar]
- Liu, B.L.; Lin, J.Y.; Feng, C.L.; Li, J.H.; Su, H. Estimation of age, growth and maturation of purpleback flying squid, Sthenoteuthis oualaniensis, in Bashi Channel, central Pacific Ocean. J. Ocean Univ. China 2017, 16, 525–531. [Google Scholar] [CrossRef]
- Clarke, M. Large light organs on the dorsal surfaces of the squids Ommastrephes pteropus, ‘Symplectoteuthis oualaniensis’ and ‘Dosidicus gigas’. J. Molluscan Stud. 1965, 36, 319–321. [Google Scholar] [CrossRef]
- Nigmatullin, C.M.; Tsygankov, V.Y.; Sabirov, R.M. On the taxonomic status of the early-maturing and late-maturing forms of the squid Sthenoteuthis oualaniensis (Lesson). In Taxonomy and Ecology of Cephalopods; Starobogatov, Y.I., Nesis, K.N., Eds.; Zoological Institute of Academy of Sciences USSR: Leningrad, Russia, 1983; pp. 94–96. (In Russian) [Google Scholar]
- Staaf, D.J.; Ruiz-Cooley, R.I.; Elliger, C.; Lebaric, Z.; Campos, B.; Markaida, U.; Gilly, W.F. Ommastrephid squids Sthenoteuthis oualaniensis and Dosidicus gigas in the eastern Pacific show convergent biogeographic breaks but contrasting population structures. Mar. Ecol. Prog. Ser. 2010, 418, 165–178. [Google Scholar] [CrossRef]
- Zuyev, G.; Nigmatullin, C.; Chesalin, M.; Nesis, K.N. Main results of long-term worldwide studies on tropical nektonic oceanic squid genus Sthenoteuthis: An overview of the Soviet investigations. Bull. Mar. Sci. 2002, 71, 1019–1060. [Google Scholar]
- Chembian, J.; Mathew, S. Growth and mortality of the oceanic squid Sthenoteuthis oualaniensis (Lesson, 1830) off south-west coast of India. Indian J. Fish. 2016, 6, 27–34. [Google Scholar] [CrossRef][Green Version]
- Zakaria, M.Z.B. Age and growth studies of oceanic squid, Sthenoteuthis oualaniensis using statoliths in the South China Sea, Area III, western Philippines. In Proceedings of the SEAFDEC Seminar on Fishery Resources in the South China Sea, Area III: Western Philippines, Bangkok, Thailand, 13–15 July 2000; pp. 118–134. [Google Scholar]
- Arkhipkin, A.I. Diversity in growth and longevity in short-lived animals: Squid of the suborder Oegopsina. Mar. Freshw. Res. 2004, 55, 341–355. [Google Scholar] [CrossRef]
- Mohamed, K.S.; Mathew, J.; Alloycious, P.S. Population characteristics and some spects of the biology of oceanic squid Sthenoteuthis oualaniensis (Lesson, 1830). J. Mar. Ecol. Assoc. India 2006, 48, 256–259. [Google Scholar]
- Liu, B.L.; Chen, X.J.; Zhong, J.S. Age, growth and population structure of squid Sthenoteuthis oualaniensis in northwest Indian Ocean by statolith microstructure. J. Dalian Fish. Univ. 2009, 24, 206–212, (In Chinese with English abstract). [Google Scholar]
- Pecl, G.T.; Moltschaniwskyj, N.A.; Tracey, S.R.; Jordan, A.R. Interannual plasticity of squid life history and population structure: Ecological and management implications. Oecologia 2004, 139, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Sajikumar, K.K.; Ragesh, N.; Venkatesan, V.; Said-Koya, K.P.; Sasikumar, G.; Kripa, V.; Mohamed, K.S. Morphological development and distribution of paralarvae juveniles of purple back flying squid Sthenoteuthis oualaniensis (Ommastrephidae), in the south eastern Arabian Sea. Vie Et Milieu 2018, 68, 75–86. [Google Scholar]
- Jackson, G.D. Advances in defining the life histories of myopsid squid. Mar. Freshw. Res. 2004, 55, 357–365. [Google Scholar] [CrossRef]
- Harman, R.F.; Young, R.E.; Reid, S.B.; Mangold, K.M.; Hixon, R.F. Evidence for multiple spawning in the tropical oceanic squid Stenoteuthis oualaniensis. Mar. Biol. 1989, 101, 513–519. [Google Scholar] [CrossRef]




| Growth Functions | References | |
|---|---|---|
| Linear (1) | L = a + bt | [37] |
| Power (2) | L = atb | [38] |
| Exponential (3) | L = aebt | [35,39] |
| Logarithmic (4) | L = aln(t) + b | [32] |
| Logistic (5) | Lt = L∞/(1 + exp[−K(ti − t0)]) | [12] |
| von Bertalanffy (6) | Lt = L∞ × {1 − exp[−K(ti − t0)]} | [32] |
| Gompertz (7) | Lt = L∞ × exp{1 − exp[−K(ti − t0]} | [32] |
| Growth Model | a | b | AIC | R2 |
|---|---|---|---|---|
| Female—ML (Linear) | 0.5606 | 2.8759 | 1940.3 | 0.62 |
| Male—ML (Power) | 5.3251 | 0.5733 | 1310.43 | 0.64 |
| Female—BW (Exponential) | 6.5606 | 0.0019 | 2241.16 | 0.85 |
| Male—ML (Power) | 0.2629 | 1.0946 | 1470.33 | 0.71 |
| Age–Class (d) | Sample Number | Mantle Length | Body Weight | |||||
|---|---|---|---|---|---|---|---|---|
| Average of ML (mm) | IGR | AGR (mm−1) | Average of BW (g) | IGR | AGR (g−1) | |||
| females | 60–90 | 4 | 74.75 | \ | \ | 42.50 | \ | \ |
| 90–120 | 42 | 95.81 | 0.83 | 0.70 | 47.18 | 0.36 | 0.35 | |
| 120–150 | 104 | 114.14 | 0.58 | 0.61 | 61.04 | 0.46 | 0.86 | |
| 150–180 | 65 | 128.73 | 0.40 | 0.49 | 91.42 | 1.01 | 1.35 | |
| 180–210 | 28 | 139.44 | 0.27 | 0.36 | 135.14 | 1.26 | 1.30 | |
| 210–240 | 10 | 171.41 | 0.69 | 1.07 | 254.70 | 2.13 | 2.11 | |
| 240–270 | 15 | 188.29 | 0.31 | 0.56 | 325.20 | 2.53 | 0.82 | |
| 270–300 | 9 | 201.07 | 0.22 | 0.43 | 385.78 | 1.95 | 0.57 | |
| males | 60–90 | 4 | 72.00 | \ | \ | 52.75 | \ | \ |
| 90–120 | 39 | 98.48 | 1.04 | 0.88 | 75.85 | 1.21 | 0.77 | |
| 120–150 | 90 | 111.49 | 0.41 | 0.43 | 83.60 | 0.33 | 0.26 | |
| 150–180 | 77 | 119.39 | 0.23 | 0.26 | 97.75 | 0.52 | 0.47 | |
| 180–210 | 27 | 125.41 | 0.16 | 0.25 | 110.74 | 0.42 | 0.43 | |
| 210–240 | 8 | 130.81 | 0.14 | 0.18 | 129.88 | 0.53 | 0.64 | |
| 240–270 | 4 | 137.40 | 0.16 | 0.22 | 143.50 | 0.33 | 0.45 | |
| 270–300 | 3 | 146.97 | 0.22 | 0.21 | 148.67 | 0.12 | 0.17 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, H.; Chen, Z.; Liu, K.; Ou, Y.; Zhao, M.; Sun, T. Statolith Microstructure Estimates of the Age, Growth, and Population Structure of Purpleback Flying Squid (Sthenoteuthis oualaniensis) in the Waters of the Xisha Islands of the South China Sea. Fishes 2022, 7, 234. https://doi.org/10.3390/fishes7050234
Lu H, Chen Z, Liu K, Ou Y, Zhao M, Sun T. Statolith Microstructure Estimates of the Age, Growth, and Population Structure of Purpleback Flying Squid (Sthenoteuthis oualaniensis) in the Waters of the Xisha Islands of the South China Sea. Fishes. 2022; 7(5):234. https://doi.org/10.3390/fishes7050234
Chicago/Turabian StyleLu, Huajie, Ziyue Chen, Kai Liu, Yuzhe Ou, Maolin Zhao, and Tianzi Sun. 2022. "Statolith Microstructure Estimates of the Age, Growth, and Population Structure of Purpleback Flying Squid (Sthenoteuthis oualaniensis) in the Waters of the Xisha Islands of the South China Sea" Fishes 7, no. 5: 234. https://doi.org/10.3390/fishes7050234
APA StyleLu, H., Chen, Z., Liu, K., Ou, Y., Zhao, M., & Sun, T. (2022). Statolith Microstructure Estimates of the Age, Growth, and Population Structure of Purpleback Flying Squid (Sthenoteuthis oualaniensis) in the Waters of the Xisha Islands of the South China Sea. Fishes, 7(5), 234. https://doi.org/10.3390/fishes7050234







