Abstract
Alzheimer’s disease (AD) is characterised by progressive neuronal atrophy and the loss of neuronal function as a consequence of multiple pathomechanisms. Current AD treatments primarily operate at a symptomatic level to treat a cholinergic deficiency and can cause side effects. Hence, there is an unmet need for healthier lifestyles to reduce the likelihood of AD as well as improved treatments with fewer adverse reactions. Diets rich in phytochemicals may reduce neurodegenerative risk and limit disease progression. The native South American palm acai berry (Euterpe oleraceae) is a potential source of dietary phytochemicals beneficial to health. This study aimed to screen the nutraceutical potential of the acai berry, in the form of aqueous and ethanolic extracts, for the ability to inhibit acetyl- and butyryl-cholinesterase (ChE) enzymes and scavenge free radicals via 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) or 2,2′-azino-bis-3-ethylbenzthiazoline-6-sulphonic acid (ABTS) assays. In addition, this study aimed to quantify the acai berry’s antioxidant potential via hydrogen peroxide or hydroxyl scavenging, nitric oxide scavenging, lipid peroxidation inhibition, and the ability to reduce ferric ions. Total polyphenol and flavonoid contents were also determined. Acai aqueous extract displayed a concentration-dependent inhibition of acetyl- and butyryl-cholinesterase enzymes. Both acai extracts displayed useful concentration-dependent free radical scavenging and antioxidant abilities, with the acai ethanolic extract being the most potent antioxidant and displaying the highest phenolic and flavonoid contents. In summary, extracts of the acai berry contain nutraceutical components with anti-cholinesterase and antioxidant capabilities and may therefore provide a beneficial dietary component that limits the pathological deficits evidenced in AD.
1. Introduction
Approximately one million people are affected by neurodegenerative diseases (NDDs) in the United Kingdom, and more than 50 million people suffer worldwide from dementia [1,2]. These diseases are of global concern as their prevalence is continuously increasing and is associated with an enormous socioeconomic burden [3]. One widespread NDD is Alzheimer’s disease (AD), which accounts for 60–80% of dementia cases and for which the annual care costs for the USA alone have been estimated at around $244 billion [4,5]. This progressive degenerative brain disease can be identified by clinical and histopathological hallmarks [6]. The early-stage clinical symptoms of AD involve memory loss for new events or conversations, lack of concern, and depression, with advanced symptoms including behavioural changes, the inability to communicate or speak, difficulty swallowing and walking, and a loss of a sense of direction [7]. Histopathological damage to the brain includes the deposition of plaques composed of β-amyloid (Aβ) in the extracellular space around neurons and intraneuronal threads of hyperphosphorylated tau protein (p-tau) that are termed neurofibrillary tangles (NFTs) [6,7,8].
The pathogenicity of AD is complex, with pathomechanisms that include oxidative stress, excitotoxicity, impaired mitochondrial function, and disrupted cholinergic signalling, as well as toxic peptide and protein accumulations [8,9,10,11,12,13,14,15,16,17]. Multiple risk factors contribute to the development of AD, with the greatest risk factor being age itself [18]. Other risk factors include familial genetics [19,20] and sporadic mutations, as well as dietary influences and other environmental factors, such as exposures to metals, pesticides, solvents, and other neurotoxic agents [8,21,22,23].
The currently approved drugs for the symptomatic relief of mild-to-moderate AD are acetylcholinesterase (AChE) inhibitors such as galantamine, rivastigmine, and donepezil, as well as the N-methyl-D-aspartate receptor antagonist memantine [7,24,25]. Even though these medications can potentially slow disease progression, none can prevent or stop the course of the disease [26,27,28]. In addition, these treatments can cause adverse side effects, including headaches, nausea, vomiting, diarrhoea, dizziness, confusion, and cardiac arrhythmias [25,29,30]. The actions of drugs such as cholinesterase inhibitors (ChEIs) and memantine involve the modulation of the levels and activity of neurotransmitters, such as acetylcholine (ACh) and glutamate (Glu), respectively [24,25]. However, these drugs were not originally developed to resolve other pathological mechanisms implicated in the development and/or progression of AD, such as tissue damage from oxidative stress, which has been detected in post-mortem brain tissue from AD patients [16,31,32,33,34,35,36]. Hence, a search continues for other novel treatment strategies with additional activities that target cholinergic deficits as well as other elements of AD pathology, with the expectation of reduced side effects.
Nutraceuticals encompass dietary substances that have physiological benefits or confer resistance to or protection from the development of diseases. The utilisation of nutraceuticals and dietary supplements is expanding, with the proposed benefit of combatting a number of diseases, including neurodegeneration [37,38]. Specifically, the dietary intake of bioactive food compounds has been linked with the prevention of age-dependent memory and cognition decline [39,40,41]. Clinical trials on patients with mild cognitive impairment and healthy people of similar age have indicated that the regular intake of fruits, such as grapes and berries, can have positive effects on cognition [42,43,44,45]. Certainly, there is an actual or perceived assumption that a diet rich in fruit and vegetables is one that is considered ‘healthy’ and has the potential for the improvement or prevention of cognitive decline [46,47,48].
The Euterpe genus has roughly 28 species that are found across the Amazon basin in Central and South America [49]. Two species, E. oleracea and E. precatoria, are widely marketed for their edible fruit [49]. Within the Amazonas River Basin, E. precatoria is a native variety and is commonly known as “acai-do-amazonas” [49], whereas E. oleracea, or “acai-do-pará,” is widely spread throughout the Amazon River estuaries, as well as in Guyana, Venezuela, and the Brazilian estates of Pará, Maranho, Tocantins, and Amapá [49,50]. These species are palm trees with small, rounded, and clustered fruits approximately 0.9–1.3 cm in diameter [49]. The mature fruit is dark purple and globose and has one seed covered with a 2 mm juicy mesocarp layer [49]. Traditional medicines have utilised different parts of this plant to treat a number of illnesses, including fever, gastrointestinal and skin conditions, pain, and infectious diseases [49,51].
Collectively, there have been several clinical, animal, and cell-based studies that have reported the potential health benefits of acai berries, including their antioxidant activity [52,53,54]. Therefore, this study aimed to analyse the nutraceutical potential of Euterpe sp. (in the form of aqueous and ethanolic extracts) for possible development as an AD treatment via the ability to inhibit AChE and butyrylcholinesterase (BuChE); the study also aimed to further delineate its antioxidant capabilities.
2. Results
2.1. Acai Aqueous Extract Is a Cholinesterase Inhibitor
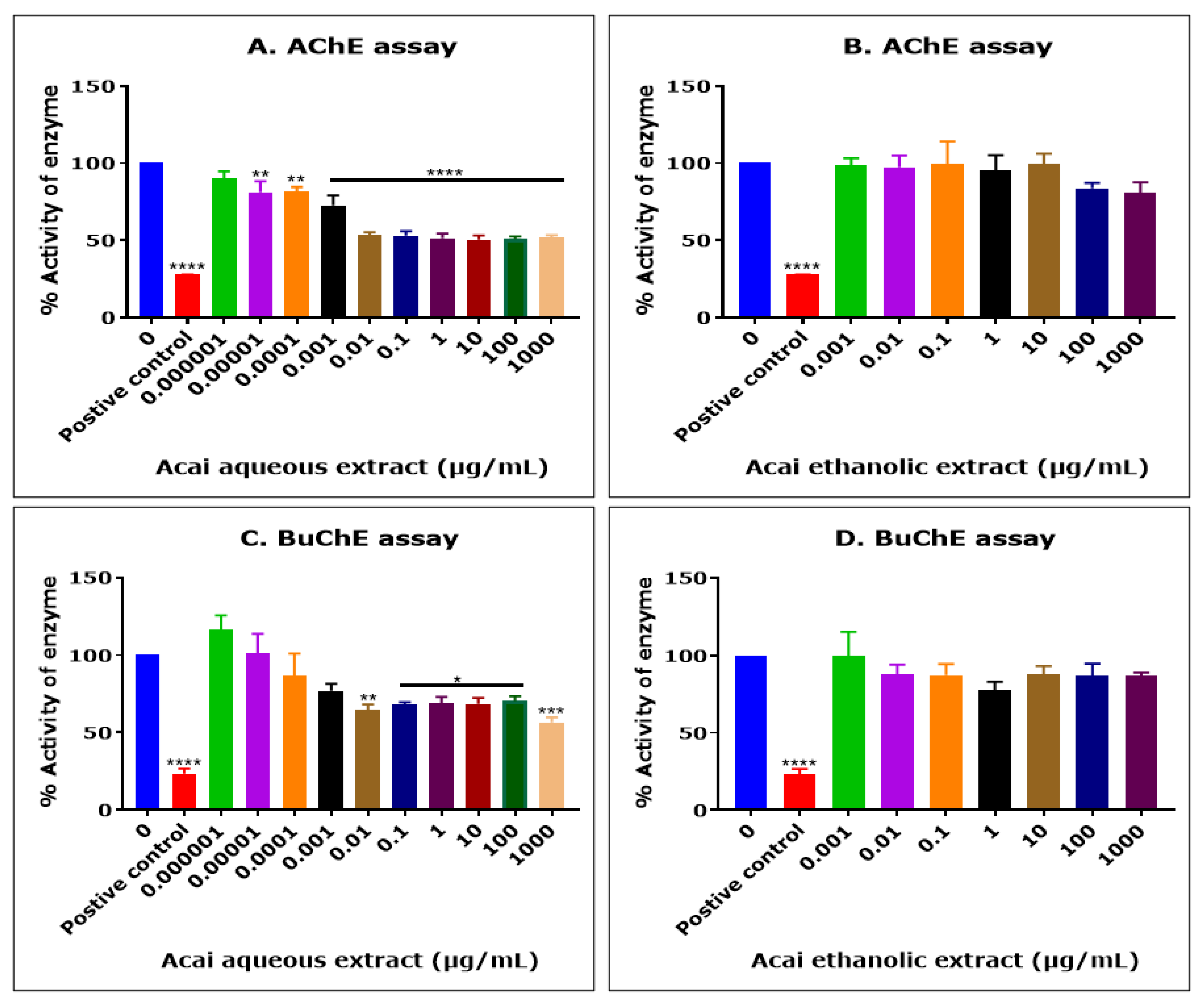
The acai berry aqueous extract significantly inhibited AChE activity in a concentration-dependent manner over concentrations ranging from 1 × 10−5 µg/mL to 0.01 µg/mL, but no further inhibition of AChE activity was observed at concentrations higher than 0.01 µg/mL (Figure 1A). The concentration of acai aqueous extract that produced a 50% inhibition (IC50) of AChE activity was estimated as 13.8 µg/mL using non-linear regression. In contrast, the acai berry ethanolic extract showed a limited inhibition (1–19%) of AChE, and only at the relatively high extract concentrations of 100 and 1000 µg/mL were there inhibition levels of 16% and 19%, respectively; however, this was not statistically significant (Figure 1B).
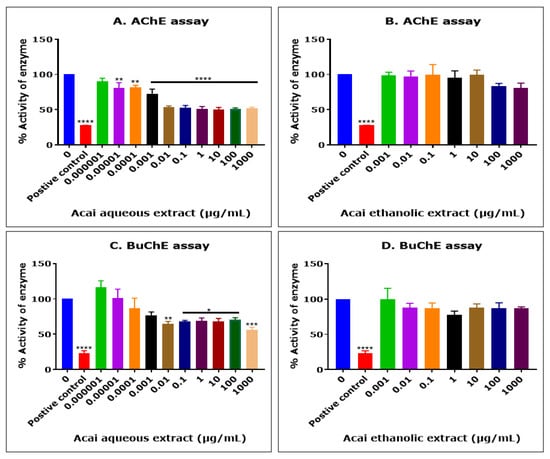
Figure 1.
Cholinesterase inhibition by acai aqueous and ethanolic extracts. AChE inhibitory activity of acai aqueous extract (A) and acai ethanolic extract (B). BuChE inhibitory activity of acai aqueous extract (C) and acai ethanolic extract (D). Histograms represent means ± SEM for at least three replicate assays at each extract concentration (n = 6). For the positive control inhibitors, 5 mM azamethiphos and 5 mM ethopropazine hydrochloride were used for AChE and BuChE, respectively. For marked significance * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001.
The acai berry aqueous extract was also primarily a concentration-dependent inhibitor of BChE activity over concentrations ranging from 1 × 10−5 µg/mL to 0.01 µg/mL (Figure 1C). However, similar to the inhibition of AChE, a point of saturation was detected, with the further inhibition of BuChE observed only at the highest concentration examined, i.e., 1000 µg/mL (43.6%) (Figure 1C). The concentration of acai aqueous extract that inhibited BuChE activity by 50% (IC50) was estimated as 6378 µg/mL using non-linear regression. Similar to the inhibition of AChE, the acai berry ethanolic extract also showed a limited inhibition of BuChE, with only marginal inhibition levels (12–22%) at concentrations of 0.01 µg/mL or higher, and these effects were not statistically significant (Figure 1D).
2.2. Acai Aqueous and Ethanolic Extracts Exhibit 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Free Radical Scavenging Activity
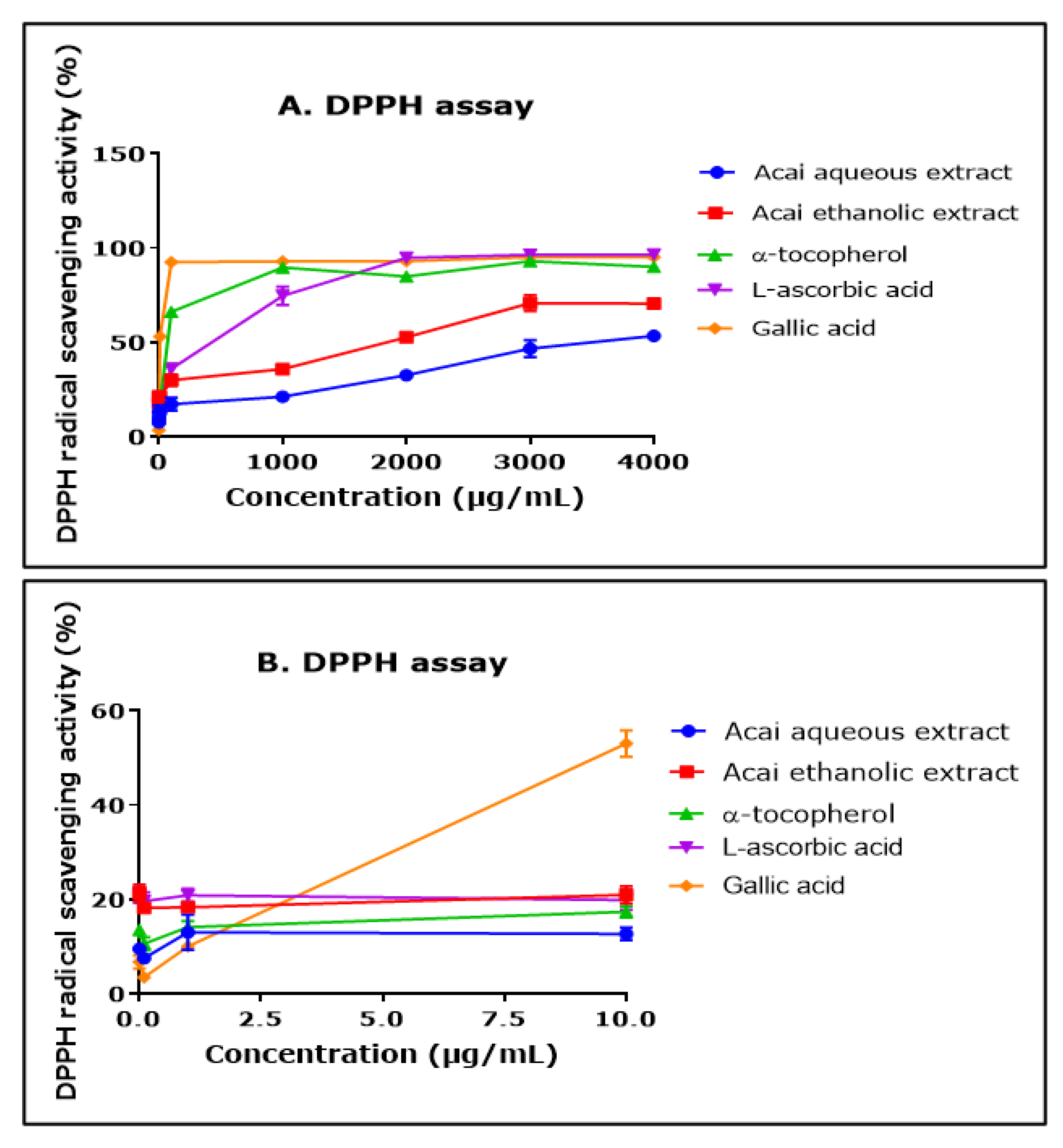
The ability of aqueous and ethanolic extracts of acai berry to act as free radical scavengers was assessed using a DPPH assay. Both extracts displayed DPPH radical scavenging abilities and had similar concentration-dependent curves, although the aqueous extract had a lower percentage of activity than the ethanolic extract over the concentration range of 1000–4000 µg/mL (Figure 2A). The antioxidants α-tocopherol (vitamin E), L-ascorbic acid (vitamin A), and gallic acid all displayed higher free radical scavenging over the concentration range of 1000–4000 µg/mL. The concentration of each of the agents that produced a 50% inhibition of free radical levels (IC50) was calculated by non-linear regression as 11.550 mg/mL for the acai aqueous extract and 791 µg/mL for the ethanolic extract. By comparison, for α-tocopherol, L-ascorbic acid, and gallic acid, the IC50 values were 50 µg/mL, 115 µg/mL, and 8 µg/mL, respectively. At the lower end of the concentrations examined (from 0.01 µg/mL to 10 µg/mL), the aqueous extract displayed a similar antioxidant capability to α-tocopherol, and the ethanolic extract surpassed that of α-tocopherol and gallic acid (Figure 2B).
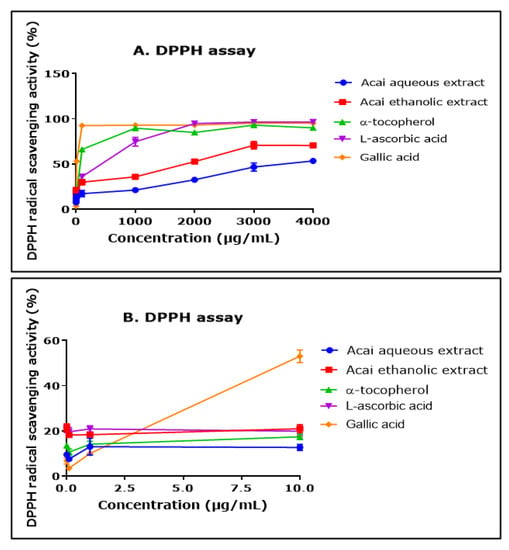
Figure 2.
DPPH radical scavenging activity of acai aqueous and ethanolic extracts. Acai antioxidant activity was assessed via the percentage inhibition (radical scavenging) of DPPH over a concentration range of 0.01–4000 µg/mL (A) and 0.01–10 µg/mL (B). Assays were performed in triplicate at each extract concentration (n = 6).
2.3. Acai Aqueous and Ethanolic Extracts Exhibit 2,2′-Azino-bis-3-ethylbenzthiazoline-6-sulphonic acid Radical Cation (ABTS•+) Scavenging Activity
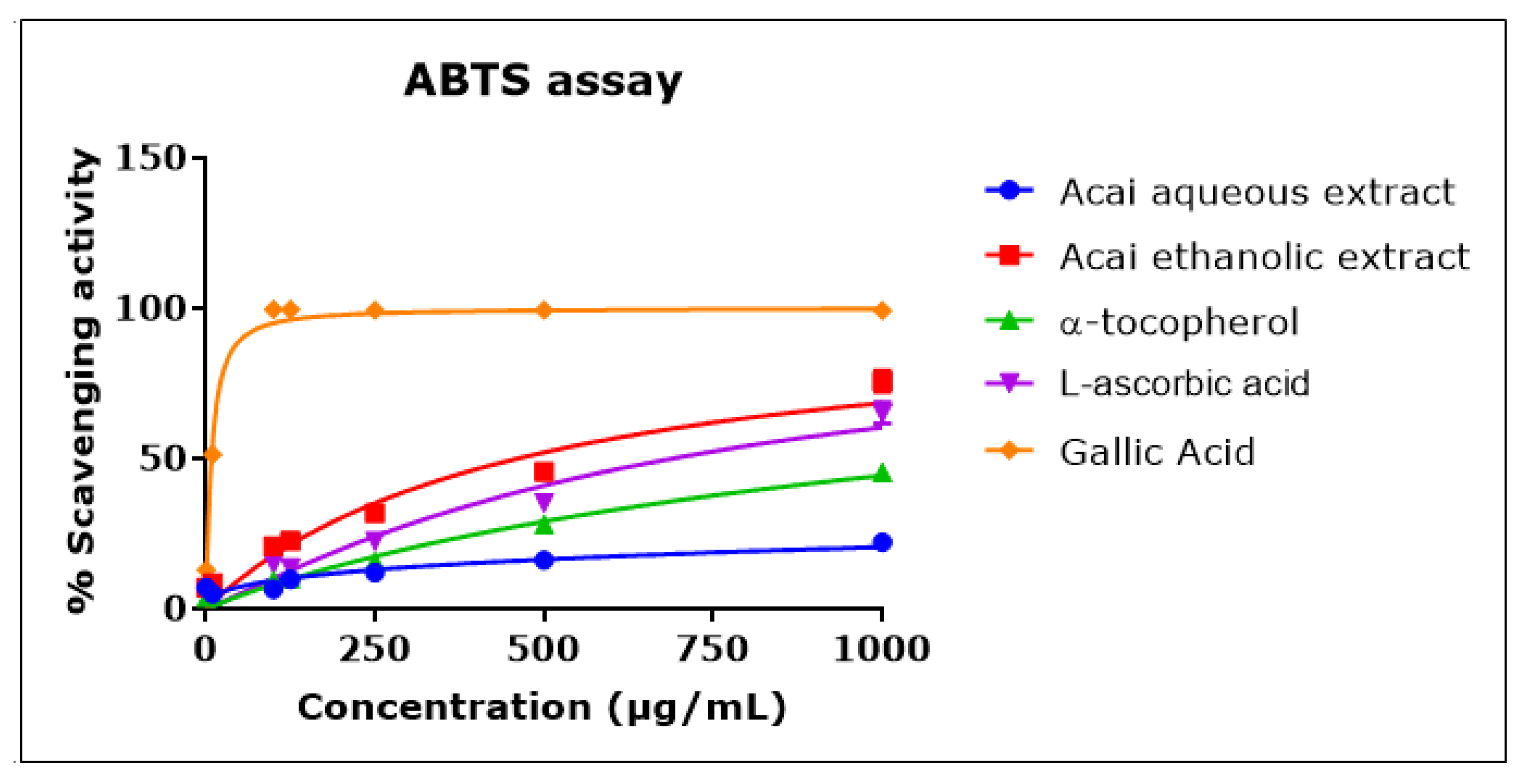
Acai berry aqueous and ethanolic extracts exhibited ABTS•+ scavenging activity in a concentration-dependent manner that was approximately linear over the concentration range of 1–1000 µg/mL (Figure 3). The acai ethanolic extract displayed a greater antioxidant capacity than either L-ascorbic acid or α-tocopherol, with an IC50 of 461.6 µg/mL compared to 690 µg/mL and an estimated 1270 µg/mL for L-ascorbic acid and α-tocopherol, respectively. Gallic acid exhibited the greatest scavenging activity, with an IC50 of 8 µg/mL. The acai aqueous extract showed the lowest antioxidant capacity, with an estimated IC50 of 30.541 mg/mL.
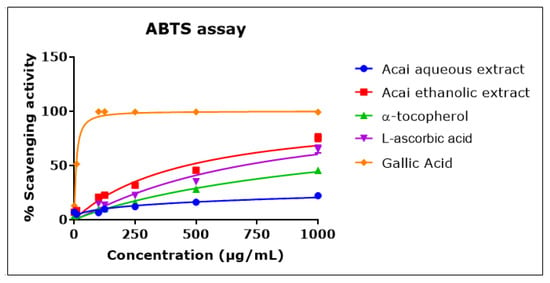
Figure 3.
ABTS•+ scavenging activity of acai aqueous and ethanolic extracts. Acai antioxidant activity was assessed as a percentage inhibition (radical scavenging) of ABTS over a concentration range of 1–1000 µg/mL. Assays were performed in triplicate at each extract concentration (n = 6).
2.4. Acai Aqueous and Ethanolic Extracts Exhibit Hydrogen Peroxide (H2O2) Scavenging Activity
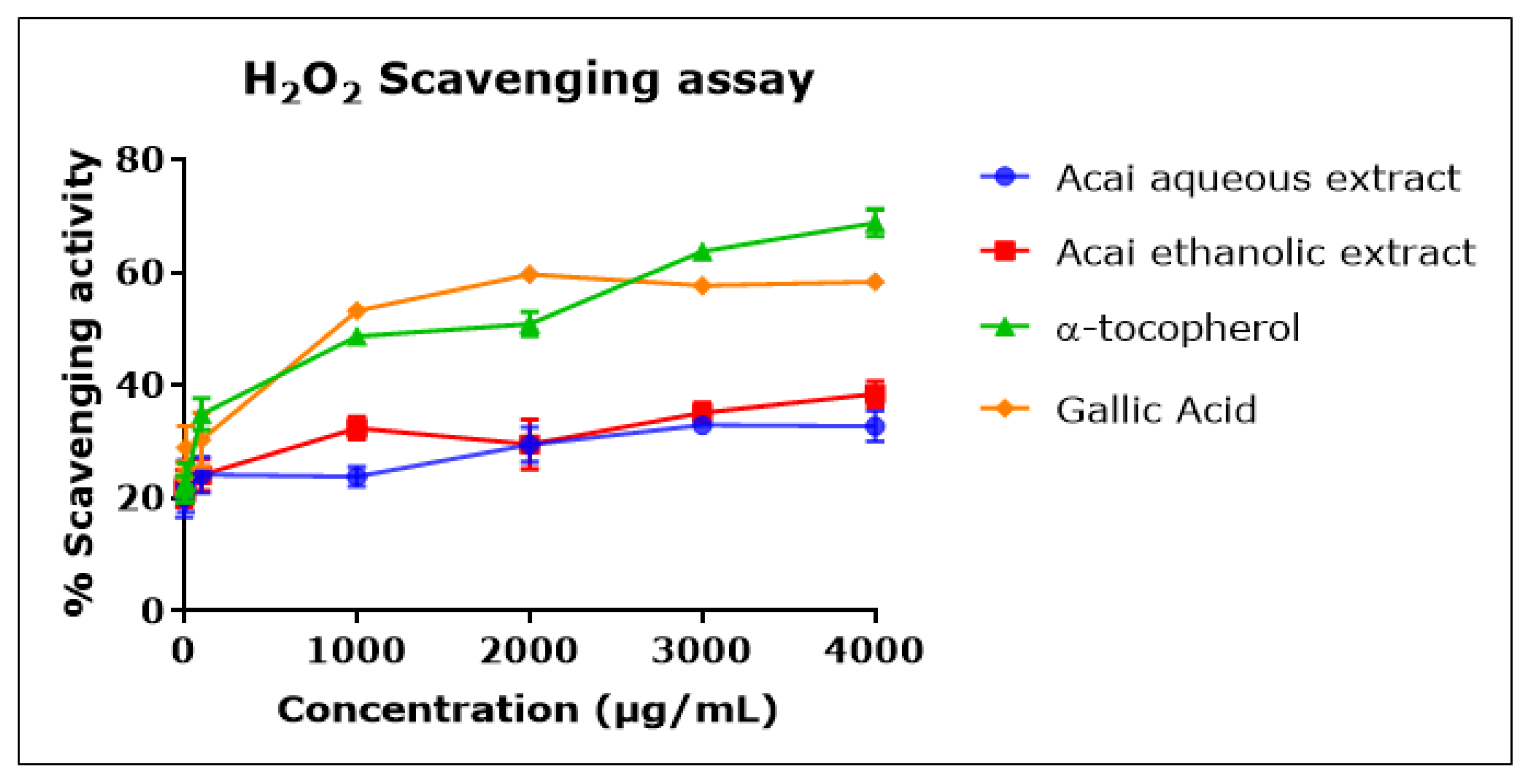
Both acai extracts displayed moderate but concentration-dependent H2O2 scavenging activity (Figure 4), with an inhibition percentage of 20–30% and estimated IC50 values of 7.803 mg/mL for the aqueous extract and 1.479 mg/mL for the ethanolic extract. α-Tocopherol and gallic acid were more potent H2O2 radical scavengers, with IC50 values of 676 µg/mL and 737 µg/mL, respectively.
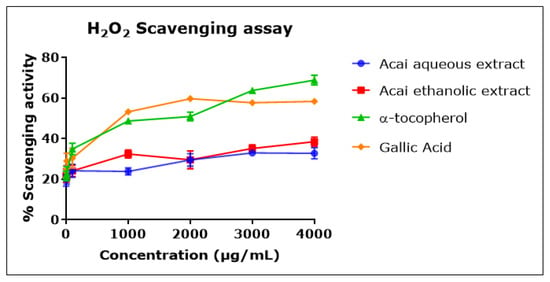
Figure 4.
H2O2 scavenging activity of acai aqueous and ethanolic extracts. Acai antioxidant activity was assessed as a percentage of the scavenging activity of H2O2 over a concentration range of 1–4000 µg/mL. Assays were performed in triplicate at each extract concentration (n = 6).
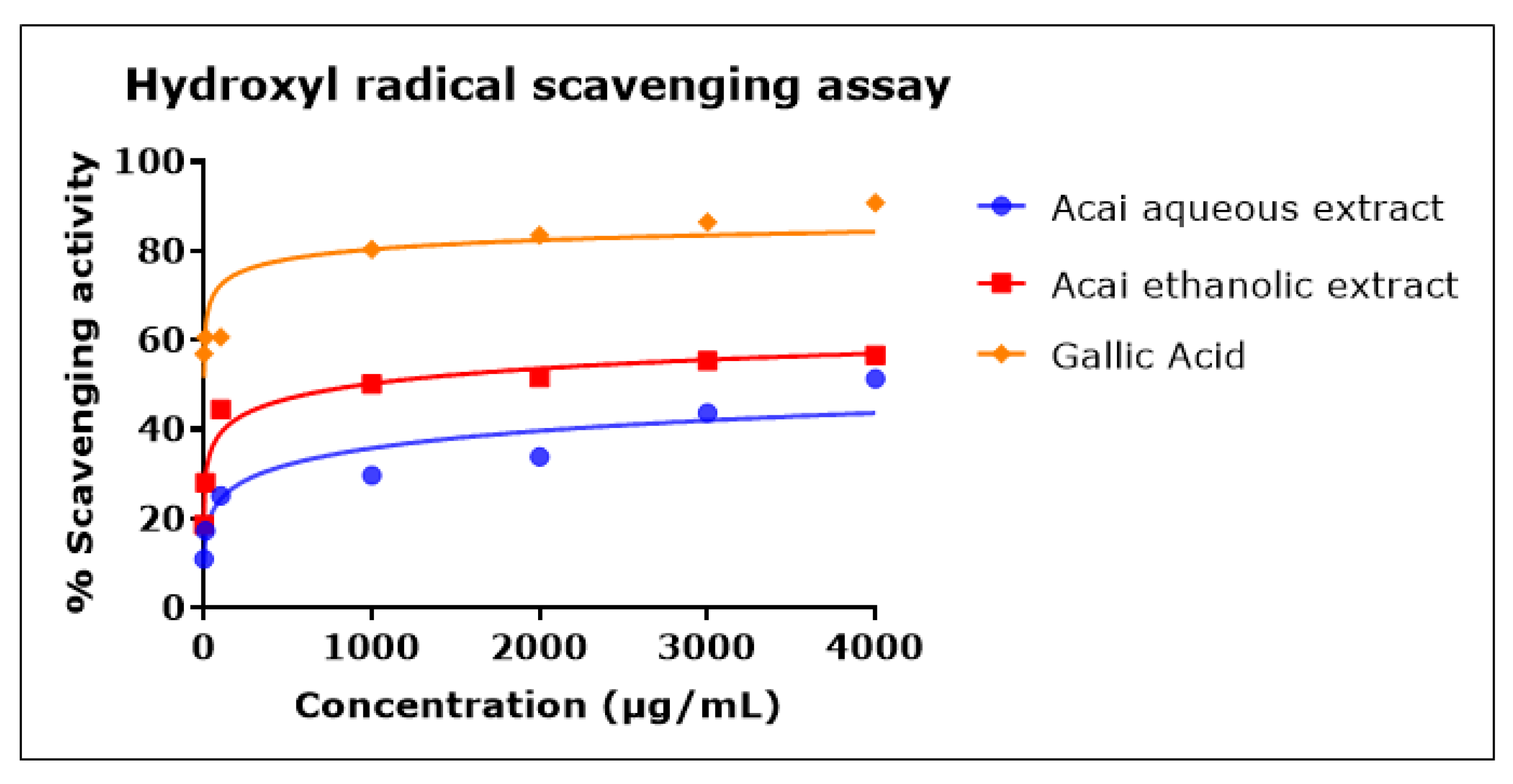
2.5. Acai Aqueous and Ethanolic Extracts Exhibit Hydroxyl Radical (•OH) Scavenging Activity
Both acai extracts exhibited hydroxyl radical (•OH) scavenging activity in a concentration-dependent manner, as shown in Figure 5. In comparison to the acai aqueous extract, the acai ethanolic extract showed higher antioxidant action, with an IC50 of 946 µg/mL, while the IC50 of acai aqueous extract was estimated as 11.604 mg/mL. Gallic acid was a potent •OH radical scavenger, with an IC50 of 0.7 µg/mL.
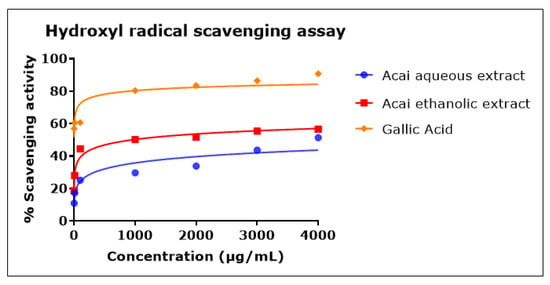
Figure 5.
Hydroxyl radical scavenging activity of acai aqueous and ethanolic extracts. Acai antioxidant activity was assessed via the percentage of the scavenging of •OH. Assays were performed in triplicate at each extract concentration (n = 6).
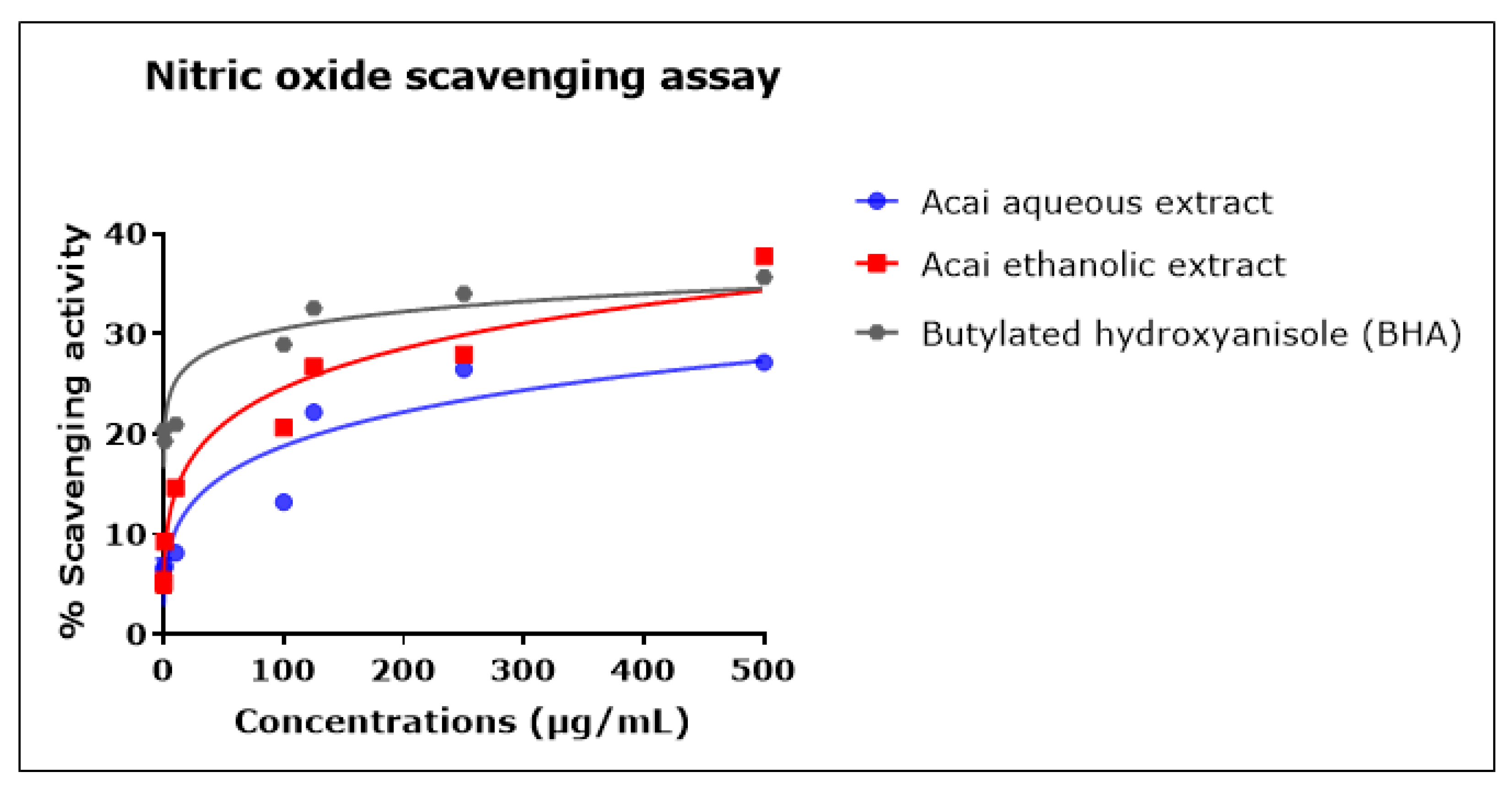
2.6. Acai Aqueous and Ethanolic Extracts Exhibit Nitric Oxide (•NO) Scavenging Activity
The percentage of •NO scavenging increased in proportion to the concentration of the acai extracts, as displayed in Figure 6. The acai ethanolic extract exhibited greater inhibition activity than the aqueous extract; it had an estimated IC50 of 4.544 mg/mL, whereas the estimated IC50 of the aqueous extract was 12.932 mg/mL. The standard BHA displayed a higher scavenging ability at lower concentrations, but these reached saturation such that the estimated IC50 was 135.437 mg/mL, which was higher than either of the two acai extracts.
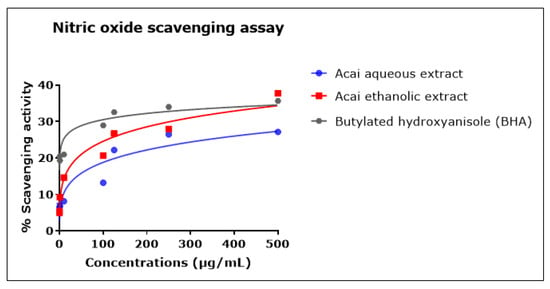
Figure 6.
Nitric oxide (•NO) scavenging activity of acai aqueous and ethanolic extracts. Acai antioxidant activity was assessed via the percentage of the scavenging of •NO. Assays were performed in triplicate at each extract concentration (n = 6).
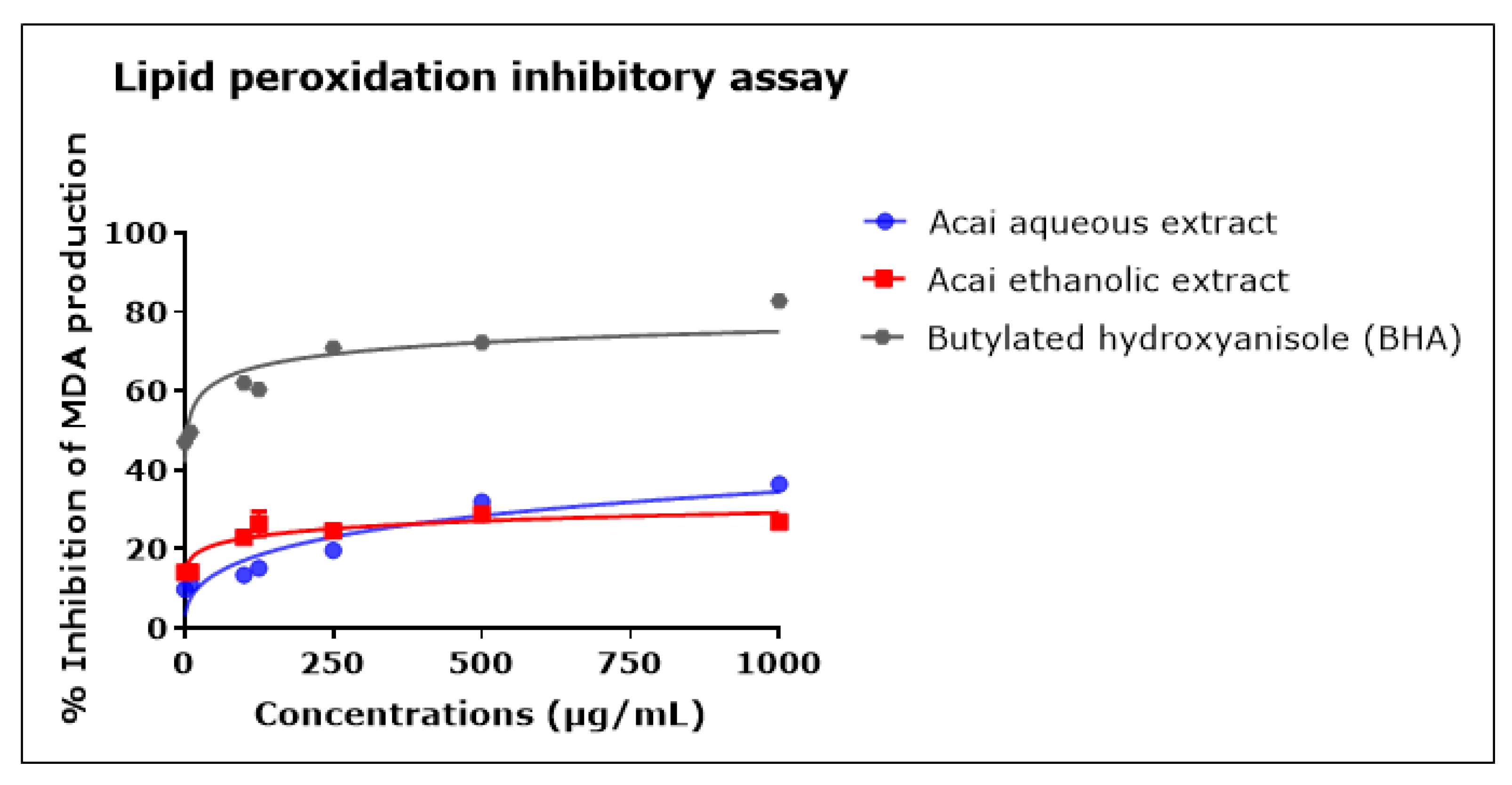
2.7. Acai Aqueous and Ethanolic Extracts Exhibit Lipid Peroxidation Inhibitory Activity
The concentration-dependent inhibition of lipid peroxidation was observed after incubation with either the acai berry aqueous or ethanolic extract, as shown in Figure 7. Both extracts displayed relatively moderate anti-lipid peroxidation in comparison with BHA. The acai aqueous extract displayed greater antioxidant activity than the ethanolic extract, with estimated IC50 values of 4.862 mg/mL and an estimated IC50 of 438.8 mg/mL, respectively; BHA had an IC50 of 4 µg/mL. At the highest concentration examined, i.e., 1000 µg/mL, the inhibition of lipid peroxidation was 36.5% ± 0.51 and 26.8% ± 1.60 for the acai aqueous and ethanolic extracts, respectively, and 82.8% ± 0.16 for BHA.
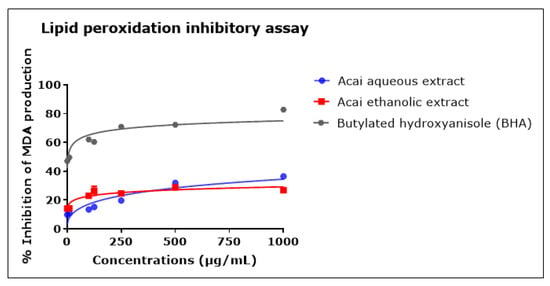
Figure 7.
Lipid peroxidation inhibitory activity of acai aqueous and ethanolic extracts. Acai antioxidant activity was assessed via the percentage of the inhibition of malondialdehyde (MDA) production. Assays were performed in triplicate at each extract concentration (n = 6).
The IC50 values for each of the cholinesterase and antioxidant assays are included in Table 1.

Table 1.
The approximate IC50 values (mg/mL) of acai aqueous extract, acai ethanolic extract, α-tocopherol (vitamin E), L-ascorbic acid (vitamin A), gallic acid, and butylated hydroxyanisole (BHA) for the AChE, BuChE, DPPH, ABTS, H2O2, •OH, •NO, and LPO assays.
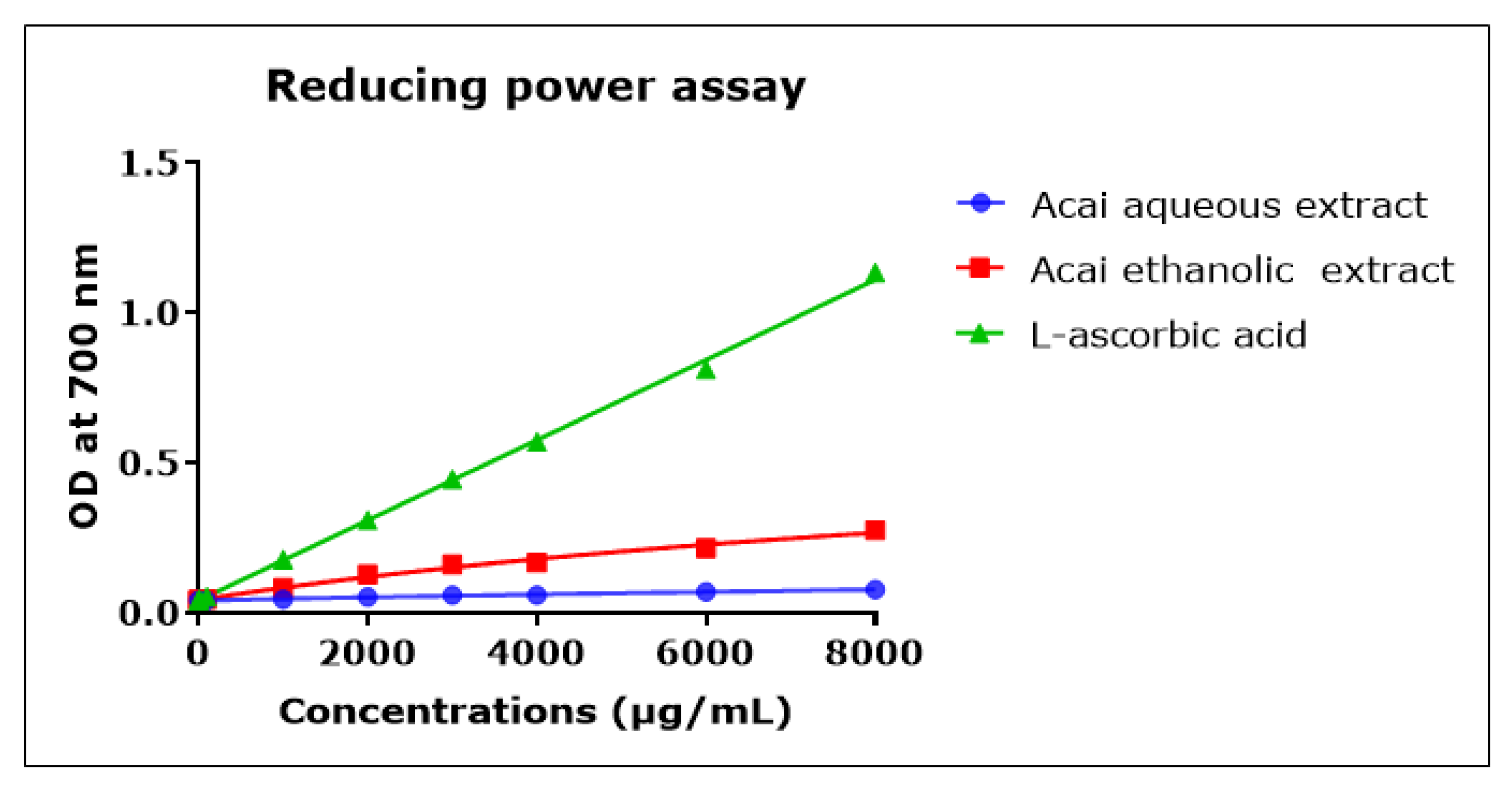
2.8. Acai Aqueous and Ethanolic Extracts Exhibit Reducing Power Activity
The direct reduction of Fe[(CN)6]3 to Fe[(CN)6]2 provides a determination of the reducing capacity of a plant compound [55]. The reducing capacity of acai berry aqueous and ethanolic extracts was concentration-dependent but relatively low compared to L-ascorbic acid over the 1000–8000 µg/mL concentration range, as shown in Figure 8. The acai ethanolic extract exhibited more antioxidant capacity than the aqueous extract. However, at the lower concentrations of 0.001–10 µg/mL the reducing capacities of the two acai extracts were similar and matched that of L-ascorbic acid (results not shown).
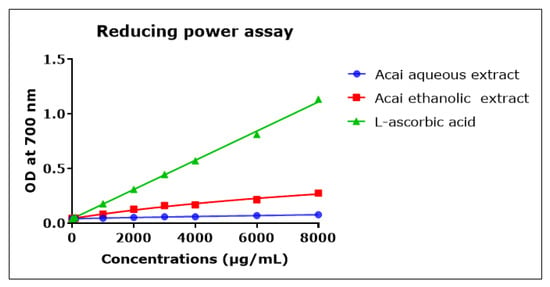
Figure 8.
Reductive capacity of different concentrations of plant extracts from acai berry. Plant reducing power was measured by the ability to reduce ferric (Fe3+) to ferrous (Fe2+) iron (OD density change at 700 nm). The positive control was L-ascorbic acid (vitamin C). Assays were performed in triplicate at each extract concentration (n = 6).
2.9. Total Phenolic and Total Flavonoid Content of Acai Berry Extracts
The total phenolic content (TPC) and total flavonoid content (TFC) of the acai aqueous and ethanolic extracts were quantified and are included in Table 2. Acai ethanolic extract displayed higher levels of phenolic and flavonoid contents.

Table 2.
Total phenolic (TPC) and flavonoid (TFC) contents of acai berry aqueous and ethanolic extracts.
3. Discussion
The present study evaluated the nutraceutical and, hence, the therapeutic potential of acai berry extracts via their ability to act as ChEIs; also assessed were the additional benefits of free radical scavenging and antioxidant activities. The current first-line treatment for mild-to-moderate AD is to treat the cholinergic deficit experienced by AD patients via a transient inhibition of AChE to increase the signal longevity of the neurotransmitter acetylcholine (ACh) [24,25,56]. However, ChEI treatment can induce adverse reactions and only addresses one component of the AD disease pathology (insufficient ACh levels), whereas multiple elements of cellular dysfunction may contribute to the disease, including oxidative stress [25,30,31,32,33,34,35,36,57]. Hence, there is an unmet need to tackle disease aetiology, for example, through a multipronged treatment strategy [57,58]. Indeed, animal models of AD have demonstrated improvements in cognitive function and behavioural defects after antioxidant therapy [59,60].
The aqueous extract from the acai pulp contained an agent(s) capable of inhibiting both AChE (estimated IC50 of 13.8 µg/mL) and, to a lesser extent, BuChE (estimated IC50 of 6.378 mg/mL). Interestingly, the agent(s) binding the cholinesterases presumably reached a point of saturation at an approximate concentration of 0.01 µg/mL, such that further enzymatic inhibition was limited. This may result from finite binding at the esteratic and/or peripheral binding sites of AChE or BuChE [61,62,63,64]; this question can be probed further once the active agent(s) is/are purified. By comparison, the ethanolic extract displayed minimal anti-AChE and anti-BuChE activities; there were only minor reductions in activity evident at the highest concentrations of extract, and these did not reach significance. This may reflect the solvation of the agent(s) within water alone (rather than ethanol) since water is a more polar solvent than ethanol. Ultimately, a range of extraction methods and solvents may be needed to isolate active agents such as polyphenols, with the solvation of specific phytochemical(s) governed by the polarity of the solute of interest [65].
Specifically, for acai berry phytochemical extractions, an independent study reported that water as a solvent produced the highest yields of polyphenols and flavonoids as compared with methanol and ethanol alone [66], although this may be improved further if a hydroalcoholic extraction is undertaken (50% ethanol) [67]. Herein, the benefit of an aqueous extraction as a method for the possible isolation of soluble ChEI(s) and their future purification and identification was evident.
Chemicals able to target and simultaneously inhibit both AChE and BuChE, rather than AChE alone, may offer improved clinical efficacy to combat the increased levels of BuChE in AD patients, with a reduction in side effects [68,69,70,71]. However, neither of the drugs currently approved by the US Food and Drug Administration (FDA), namely donepezil and galantamine, are potent BuChE inhibitors (BuChE IC50 values of 5 µM and 12.6 µM, respectively), whereas rivastigmine, which was originally extracted from a medicinal plant, is a relatively potent AChE and BuChE inhibitor (IC50 values of 4 and 13 nM, respectively) [72,73]. It will be of interest in future studies to determine whether the same agent(s) within the acai aqueous extract is/are responsible for inhibiting both AChE and BuChE, and to determine the relative potency of the inhibitor(s). A comparison of the anti-AChE activity of the extracts/fractions of 54 plant species used guidelines that considered an IC50 < 20 µg/mL as high potency, moderate potency as an IC50 > 20 µg/mL but < 200 µg/mL, and low potency as an IC50 > 200 µg/mL but < 1000 µg/mL [72]. The cut-offs for potency related to the average IC50 value for galantamine, reported in the literature as approximately 2 µM (or 0.575 µg/mL), multiplied by a factor of 10 [74]. Accordingly, the aqueous extract of acai fruits, with an IC50 of 13.8 µg/mL, was an extract of high potency and therefore has potential use as an effective ChEI.
In addition to ChEI activities, the acai extracts displayed useful radical scavenging and antioxidant activities; they were able to scavenge DPPH, ABTS, •OH, H2O2, and •NO free radicals, and they exhibited ferric ion reduction. Similarly, other independent studies have reported the high antioxidant capacity of the acai berry against superoxide (O2•−) and peroxyl radicals (RO2) [75]. Acai also displays useful neuroprotective activity, and it can prevent rotenone-induced oxidative damage [76]. Acai pulp also reduced nitrite radicals (NO2−) in mouse brain BV-2 microglial cells in vitro [53]. Acai flower and spike fractions (as well as the fruit) also contain agents able to inhibit nitric oxide production [77]. In addition, in a pilot study with human volunteers, the antioxidant capacity of plasma was elevated by 2.3- and 3-fold after the consumption of acai juice and pulp, respectively [78]. It is also promising to note that an in vivo study demonstrated that an acai-rich diet reduced markers of oxidative stress in brain regions of aged rats [79].
The current study also demonstrated that acai extracts have anti-lipid peroxidation effects, in support of studies in vitro [75] and in vivo [54]. The significant inhibition of lipid peroxidation was also detected post-consumption of a juice blend (of which acai was the predominant ingredient) in a pilot study with human volunteers [52].
Research has considered the chemical composition of the acai berry and its antioxidant potential and has detected the presence of numerous polyphenols and flavonoids, such as anthocyanins [52,76,77,78,80,81] (refer also to Supplementary Table S1). Phytochemicals such as these may provide the basis for the acai extract to neutralise free radicals and potentially limit oxidative stress, such as that associated with AD aetiology, but further in vitro and in vivo studies are required to confirm the potential use of acai berry extracts as a treatment option for AD. Ultimately, a diet that incorporates acai berries may provide the ongoing benefit of a diet rich in antioxidants along with the possibility of sustained cholinergic signalling that may limit the likelihood of developing or indeed the progression of NDDs such as AD.
4. Materials and Methods
4.1. Chemicals and Reagents
All chemicals were purchased from Sigma (Poole, UK) unless otherwise specified.
4.2. Preparation of Ethanolic and Aqueous Extracts of Acai Berry (Euterpe oleracea)
An ethanolic extract of acai berry was prepared by the maceration of 300 mg/mL of commercially available freeze-dried acai pulp and skin powder purchased from NaturaleBio (Organic product under EU Directive 834/2007, purchased via Amazon.co.uk) in 70% ethanol for 48 h. The macerated sample was shaken 3 times daily to assist solvation and then filtered using a bottle-top filter. Filtrates were dried at 45–50 °C for 24 h in a water bath to obtain the ethanolic dry extracts [76,82]. The aqueous extract (10 mg/mL) was prepared using methods as described by Wong et al. (2013) [83]. The freeze-dried acai pulp and skin powder was weighed and extracted by dissolving in phosphate-buffered saline (PBS) and by vigorous vortexing. The extract was centrifuged at 400 rpm and filtered using a 0.20 µm syringe to obtain a clear solution.
4.3. Cholinesterase Activity Assessments
Based on the method of Ellman et al. (1961) [84], the ability of the acai berry extracts to inhibit the activity of AChE and BuChE was assessed in a 96-well microtiter plate. Ten µL of acai aqueous or ethanolic extracts (concentration range from 1 × 10−6 µg/mL to 1000 µg/mL) was mixed with 150 µL of 0.38 mM 5,5-dithio-bis-(2-nitrobenzoic acid) (DTNB), 3 µL of 0.5 U/mL AChE enzyme from Electrophorus electricus (electric eel) (Sigma, C3389, Irvine, UK) or BuChE enzyme from equine serum (Sigma, C75120, Irvine, UK), and 43 µL of phosphate buffer (Gibco™ PBS, pH 7.4, ThermoFisher, Stafford, UK). Samples were incubated for 20 min at room temperature; then, the reaction was initiated by the addition of 4 µL of 35 mM of acetylthiocholine iodide (ATCI) substrate for AChE or butyrylthiocholine iodide (BTCI) substrate for BuChE, and the absorbance was measured at 412 nm every 30 s for 5 min using a Varioskan™ LUX multimode microplate reader (ThermoFisher, UK). Reagent blanks were performed in the absence of AChE or BuChE. The positive (inhibitor) control for AChE assays was an organophosphate pesticide, azamethiphos (QMX Laboratories Ltd., Thaxted, UK) at 5 mM, capable of the irreversible inhibition of AChE [85]. For BuChE assays, ethopropazine hydrochloride (QMX Laboratories Ltd., Thaxted, UK) at 5 mM was used as a recognised inhibitor of BuChE [86]. The percentage of AChE or BuChE activity remaining after incubation with acai extracts was calculated relative to the enzyme only (the negative control), which was designated as 100% enzymatic activity. The acai extract concentrations producing 50% inhibition (IC50) of AChE or BuChE activity were determined. The assays were performed in duplicate for at least three independent experiments, after which a mean was calculated.
4.4. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Free Radical Scavenging Activity
The antioxidant capacities of acai aqueous and ethanolic extracts over the concentration range of 0.01–4000 µg/mL were evaluated by monitoring the ability to reduce the stable free radical di(phenyl)-(2,4,6 trinitrophenyl)iminoazanium (DPPH) according to a previous publication [87]. DPPH was dissolved in ethanol at a final concentration of 0.1 mM, then 160 µL of this solution was added to 20 µL of either acai extract or L-ascorbic acid, α-tocopherol, or gallic acid as positive control antioxidants, and the material was mixed with 20 µL of distilled water. Antioxidant standards were evaluated using the same concentration range as the acai extracts. The mixture was incubated for 40 min in the dark at 37 °C, and then the absorbance was read using a Varioskan™ LUX multimode microplate reader (ThermoFisher, Stafford, UK) at 517 nm as an endpoint measurement. Antioxidant activity was calculated as a percentage of the DPPH radical scavenging activity according to the following equation:
where A0 is the absorbance of the control without extract or positive control, and A1 is the absorbance of the sample.
DPPH scavenging activity (%) = (A0 − A1)/A0 × 100
4.5. Radical 2,2′-Azino-bis-3-ethylbenzthiazoline-6-sulphonic acid Cation (ABTS•+) Scavenging Activity
The ability of the acai extracts to scavenge ABTS•+ was determined according to the procedure of Acharya (2017) [88], with some modifications. Briefly, ABTS (7 mM) and potassium persulfate (2.45 mM) solutions were prepared in distilled water. A working solution was then prepared by combining 3 mL of each stock solution and letting them react for 12–16 h in the dark at room temperature (25 °C). The interaction of ABTS with potassium persulfate led to the formation of the ABTS radical cation (ABTS•+) [88]. The solution was then diluted by mixing 1 mL ABTS radical solution with 25 mL of PBS to obtain an absorbance of 0.70 at 750 nm, as monitored using a Varioskan™ LUX multimode microplate reader (ThermoFisher, Stafford, UK). A total of 200 µL reaction mixture per well was assessed, comprised of 190 µL of radical solution followed by 10 µL of standard or plant extracts at a concentration range of 1–1000 µg/mL. The plate was shaken for 10 s at medium speed and incubated for 5 min in the dark. Then, the absorbance was measured at 750 nm. The activity of the acai extracts was compared with other antioxidant standards, i.e., L-ascorbic acid, α-tocopherol, and gallic acid. The ability of the extracts to scavenge ABTS•+ was calculated using the following equation:
where A0 is the absorbance of the control, and A1 is the absorbance of the sample.
ABTS•+ scavenging activity (%) = (A0 − A1)/A0 × 100
4.6. Hydrogen Peroxide (H2O2) Scavenging Activity
Humans are exposed to H2O2 either directly through mitochondrial metabolism or indirectly from the environment. H2O2 is widely considered a cytotoxic agent, and the rapid breakdown of H2O2 can produce a •OH that can initiate lipid peroxidation and cause protein and DNA damage [89]. The H2O2 scavenging ability of the acai extracts was measured using the method of Alam et al. (2013) [90], with modifications. In 200 µL total solution per well of a 96-well plate, 180 µL of 40 mM H2O2 solution prepared in PBS was added, followed by 20 µL of standard or acai extracts at a concentration range of 1–4000 µg/mL. The mixture was incubated for 10 min, and the absorbance was read at 230 nm using a Varioskan™ LUX multimode microplate reader (ThermoFisher, Stafford, UK). The following equation was used to calculate the percentage of H2O2 scavenging:
where A0 is the absorbance of the control, and A1 is the absorbance of the sample.
H2O2 scavenging activity (%) = (A0 − A1)/A0 × 100
4.7. Hydroxyl Radical (•OH) Scavenging Activity
The acai extracts’ •OH scavenging activity was evaluated using the method described by Bajpai et al. (2015) [91], with modifications. The principle of this experiment is based on a Fenton’s reaction, which involves the Fe3+–ascorbate–ethylenediaminetetraacetic acid–H2O2 system to produce hydroxyl radicals. In a total volume of 200 µL, the reaction mixture contained 50 µL of 2-deoxy2-ribose sugar (12 mM), 20 µL of ferric chloride (FeCl3) (1 mM), 20 µL of ethylenediaminetetraacetic acid (EDTA) (1 mM), 20 µL of L-ascorbic acid (1 mM), 50 µL of H2O2 (8 mM), 30 µL of PBS, and 10 µL of standard or acai extracts at a concentration range of 1–4000 µg/mL. A volume of 40 µL of 2.8% trichloroacetic acid (TCA) and 2-thiobarbituric acid (TBA) (0.5% in 0.025 M sodium hydroxide solution) was added to the reaction mixture after 45 min at 37 °C, and the mixture was incubated at 85 °C for 15 min to generate a pink chromogen that resulted from the reaction of TBA with degraded sugar, i.e., a ‘malondialdehyde-like’ compound [92]. After cooling, 200 µL of the sample was transferred to a 96-well microtiter plate, and the absorbance was measured at 532 nm using a Varioskan™ LUX multimode microplate reader (ThermoFisher, Stafford, UK). Gallic acid was used as a reference standard. The percentage of inhibition activity was determined using the same formula as for the DPPH radical scavenging activity (Section 4.4).
4.8. Nitric Oxide Radical (•NO) Scavenging Activity
The procedures described by Unuofin et al. (2018) and Jimoh et al. (2019) [93,94] were adapted for the determination of the capability of the acai extracts to scavenge •NO radicals. •NO radicals can be produced by sodium nitroprusside (SNP) decomposing in an aqueous solution at pH (7.2) [90]. The Griess reagent can be used to determine •NO quantities under aerobic conditions as NO reacts with oxygen to produce nitrates [90]. Briefly, 2 mL of 10 mM SNP in PBS was combined with 0.5 mL of acai extracts or butylated hydroxyanisole (BHA) at concentrations of 0.1–500 µg/mL. After 150 min of incubation at 25 °C, 0.5 mL of the solution was combined with 0.5 mL of Griess reagent, which was prepared by mixing 1 mL of 0.33% sulphanilamide reagent (in 20% glacial acetic acid) and 1 mL of 0.1% naphthalene diamine dichloride at room temperature for 5 min. Following a 30 min incubation period at room temperature, 150 µL of the mixture was transferred to a 96-well plate, and the absorbance was measured at 540 nm using a Varioskan™ LUX multimode microplate reader (ThermoFisher, Stafford, UK). A negative control was prepared using a water-based solution instead of the extract or standard BHA. Using the same formula as the DPPH radical scavenging activity (Section 4.4), the percentage of the nitric oxide scavenging activity was calculated.
4.9. Lipid Peroxidation (LPO) Inhibitory Activity
Reactive oxygen species (ROS) such as O2•− anion, •OH, and the H2O2 radical, trigger LPO, which damages cell membranes and produces numerous secondary products that are neurotoxic, resulting in neuronal death via necrosis or apoptosis [95]. Moreover, it has been shown that LPO contributes to the development of many NDDs, including AD [95]. The ability of acai extracts to inhibit LPO was assessed using a method modified from that described by Akomolafe et al. (2013) [96]. Briefly, 100 µL of 5 mg/mL bovine brain extract type I, Folch fraction I (Sigma, B1502) was mixed with 30 µL of PBS, 40 µL of distilled water, and 100 µL of acai extracts or standard at a concentration range of 0.1–1000 µg/mL, with 100 µL of 5 mM SNP as the prooxidant. After a 2 h incubation at 37 °C, 300 µL of 8.1% sodium dodecyl sulphate (SDS), 500 µL of acetic acid, and 500 µL of 0.8% thiobarbituric acid (TBA) were added. This mixture was incubated at 85 °C for 45 min to induce the formation of the malondialdehyde (MDA) coloured product. A volume of 200 µL of the samples was transferred to a 96-well microtiter plate after cooling, and the absorbance was measured at 532 nm using a Varioskan™ LUX multimode microplate reader (ThermoFisher, Stafford, UK). The percentage inhibition of the formation of MDA was calculated according to the equation for the DPPH radical scavenging activity (Section 4.4).
4.10. Ferric-Reducing Antioxidant Power (FRAP) Assay
The ability to reduce ferric ions (Fe3+) to ferrous ions (Fe2+) was used to estimate the reducing capacity of acai extracts. The acai extract concentrations were assessed over a concentration range of 0.001–8000 µg/mL. Each assay data point contained 4 µL of each acai extract, 400 µL of phosphate buffer (Gibco™ PBS, pH 7.4, ThermoFisher, Stafford, UK), and 250 µL of 1% potassium ferricyanide. After the incubation of the mixture at 50 °C for 20 min, 250 µL of 10% trichloroacetic acid was added. The samples were centrifuged at 3000 rpm for 10 min. Then, 100 µL of the supernatant was transferred to a 96-well microtiter plate and mixed with 100 µL of double-distilled water and 20 µL of freshly prepared (0.1%) ferric chloride (FeCl3) solution. Then, the formation of Perl’s Prussian blue was read at 700 nm, according to Nwidu et al. (2018) [82] using a Varioskan™ LUX multimode microplate reader (ThermoFisher, Stafford, UK). The positive control was L-ascorbic acid.
4.11. Total Phenolic Content Determination
Based on the Folin–Ciocalteu reagent (FCR) method, the total phenolic content was determined spectrophotometrically at 760 nm according to Nwidu et al. (2018) [82]. In this assay, electrons were transferred from phenolic compounds to phosphomolybdic/phosphotungstic acid complexes (Folin–Ciocalteu reagent) under alkaline conditions, resulting in a detectable colour change [97]. A concentration range of 15.63–3000 µg/mL was used to evaluate the acai extracts. Each assay data point within a 96-well plate contained 20 µL of acai extract, 90 µL of water, and 30 µL of FCR; then, the mixture was shaken vigorously in a plate reader for 8 min. Then, 60 µL 7.5% sodium carbonate solution was added, and the plate was incubated at 40 °C on a shaking incubator for 30 min. The plate was read in a spectrophotometer at 760 nm using a Varioskan™ LUX multimode microplate reader (ThermoFisher, Stafford, UK). The positive control was gallic acid, using a concentration range of 15.63–1000 µg/mL to generate a standard curve for the quantification of the total phenolic content of the acai extracts. The total phenolic content was determined as mg gallic acid equivalents/gram of plant extract (mg GAE/g).
4.12. Total Flavonoid Content Determination
This colourimetric method is based on the principle that aluminium chloride (AlCl3) forms acid-stable complexes with flavone and flavonol keto groups and their hydroxyl groups [98]. Furthermore, AlCl3 produces acidic compounds with orthodihydroxyl groups in flavonoid A- or B-rings [98]. The total flavonoid contents of the plant extracts were assessed via the colourimetric procedure described in Nwidu et al. (2018) [82]. The positive control was quercetin. Within a 96-well plate, 20 µL of acai extracts or quercetin as standard, over a concentration range of 15.63–3000 µg/mL, was mixed with 100 µL of 10% aluminium chloride solution and 100 µL 1 M potassium acetate. After a 30 min incubation at room temperature, the plate was read using a Varioskan™ LUX multimode microplate reader (ThermoFisher, Stafford, UK) at 415 nm. Total flavonoid content was expressed as milligram quercetin equivalents/gram of extract (mg QUER E/g).
4.13. Statistical Analysis
Results were expressed as means ± standard error of the mean (SEM) in each treatment and control group. Non-linear regression analysis was used to calculate the concentration of acai extracts producing 50% inhibition (IC50). The statistical analysis comparing different groups was performed using one-way ANOVA tests with Tukey’s multiple comparisons post-test via PRISM v7 (GraphPad Software Inc., San Diego, CA, USA. www.graphpad.com, accessed on 26 June 2022). A p-value of below 0.05 was defined as the level of statistical significance for all analyses.
5. Conclusions
To summarise, the acai berry contains a range of phytochemicals that likely contribute to its anti-cholinesterase and antioxidant activities. This study suggests that the acai aqueous extract could be further fractionated, and its compounds identified for their potential use as a medication alternative for AD therapy, due to the potent ChEI activity and powerful antioxidant capabilities. However, the limitation of this study is that the work was performed in vitro; future in vivo analyses are required, particularly those that mimic NDDs such as AD.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27154891/s1, Table S1: Chemical compounds detected in acai berry extracts with possible cholinesterase inhibitor activity and recognized antioxidant activity [99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172].
Author Contributions
Conceptualization, M.N.A., I.R.M. and W.G.C.; methodology, M.N.A.; validation, M.N.A., I.R.M. and W.G.C.; formal analysis, M.N.A., I.R.M. and W.G.C.; investigation, M.N.A., I.R.M. and W.G.C.; resources, W.G.C.; data curation, M.N.A.; writing—original draft preparation, M.N.A. and W.G.C.; writing—review and editing, M.N.A., I.R.M. and W.G.C.; supervision, I.R.M. and W.G.C.; project administration, I.R.M. and W.G.C.; funding acquisition, M.N.A., I.R.M. and W.G.C. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support was provided by the Cultural Bureau and King Faisal University PhD Scholarship, Kingdom of Saudi Arabia.
Data Availability Statement
Data supporting the results are available on request from the first author (M.N.A.).
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds (aqueous and ethanolic acai berry extracts) are available from the first author (M.N.A.) on request.
References
- Alzheimer’s-Association. 2019 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2019, 15, 321–387. [Google Scholar] [CrossRef]
- World-Alzheimer-Report. World Alzheimer’s Report 2018: The State of the Art of Dementia Research: New Frontiers. Available online: https://www.alz.co.uk/research/WorldAlzheimerReport2018.pdf (accessed on 26 June 2022).
- El-Hayek, Y.; Wiley, R.; Khoury, C.; Daya, R.; Ballard, C.; Evans, A.; Karran, M.; Molinuevo, J.; Norton, M.; Atri, A. Tip of the iceberg: Assessing the global socioeconomic costs of Alzheimer’s disease and related dementias and strategic implications for stakeholders. J. Alzheimers Dis. 2019, 70, 323–341. [Google Scholar] [CrossRef] [PubMed]
- Alzhemier’s-Association-Report. 2021 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2021, 17, 327–406. [Google Scholar] [CrossRef] [PubMed]
- Alzhemier’s-Association-Report. 2020 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2020, 16, 391–460. [Google Scholar] [CrossRef] [PubMed]
- Trejo-Lopez, J.; Yachnis, A.; Prokop, S. Neuropathology of Alzheimer’s disease. Neurotherapeutics 2021, 19, 173–185. [Google Scholar] [CrossRef]
- Grand, J.; Caspar, S.; Macdonald, S. Clinical features and multidisciplinary approaches to dementia care. J. Multidiscip. Healthc. 2011, 4, 125–147. [Google Scholar] [CrossRef]
- Reitz, C.; Mayeux, R. Alzheimer disease: Epidemiology, diagnostic criteria, risk factors and biomarkers. Biochem. Pharmacol. 2014, 88, 640–651. [Google Scholar] [CrossRef]
- Chen, X.; Guo, C.; Kong, J. Oxidative stress in neurodegenerative diseases. Neural. Regen. Res. 2012, 7, 376. [Google Scholar] [CrossRef] [PubMed]
- Denzer, I.; Muench, G.; Friedland, K. Modulation of mitochondrial dysfunction in neurodegenerative diseases via activation of nuclear factor erythroid-2-related factor 2 by food-derived compounds. Pharmacol. Res. 2016, 103, 80–94. [Google Scholar] [CrossRef]
- Kovacs, G. Molecular pathological classification of neurodegenerative diseases: Turning towards precision medicine. Int. J. Mol. Sci. 2016, 17, 189. [Google Scholar] [CrossRef]
- Lewerenz, J.; Maher, P. Chronic glutamate toxicity in neurodegenerative diseases—what is the evidence? Front. Neurosci. 2015, 9, 469. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Jiang, L. Neuroinflammation in Alzheimer’s disease. Neuropsychiatr. Dis. Treat. 2015, 11, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Mufson, E.; Counts, S.; Perez, S.; Ginsberg, S. Cholinergic system during the progression of Alzheimer’s disease: Therapeutic implications. Expert Rev. Neurother. 2008, 8, 1703–1718. [Google Scholar] [CrossRef]
- Guo, T.; Zhang, D.; Zeng, Y.; Huang, T.; Xu, H.; Zhao, Y. Molecular and cellular mechanisms underlying the pathogenesis of Alzheimer’s disease. Mol. Neurodegener. 2020, 15, 40. [Google Scholar] [CrossRef] [PubMed]
- Misrani, A.; Tabassum, S.; Yang, L. Mitochondrial dysfunction and oxidative stress in Alzheimer’s disease. Front. Aging Neurosci. 2021, 13, 617588. [Google Scholar] [CrossRef]
- ALNasser, M.; Mellor, I.; Carter, W. Is L-Glutamate toxic to neurons and thereby contributes to neuronal loss and neurodegeneration? A systematic review. Brain Sci. 2022, 12, 577. [Google Scholar] [CrossRef]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.; Croteau, D.; Bohr, V. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef]
- Loy, C.; Schofield, P.; Turner, A.; Kwok, J. Genetics of dementia. Lancet 2014, 383, 828–840. [Google Scholar] [CrossRef]
- Green, R.; Cupples, A.; Go, R.; Benke, K.; Edeki, T.; Griffith, P.; Williams, M.; Hipps, Y.; Graff-Radford, N.; Bachman, D. Risk of dementia among white and African American relatives of patients with Alzheimer disease. JAMA 2002, 287, 329–336. [Google Scholar] [CrossRef]
- Jakaria, M.; Park, S.; Haque, M.; Karthivashan, G.; Kim, I.; Ganesan, P.; Choi, D. Neurotoxic agent-induced injury in neurodegenerative disease model: Focus on involvement of glutamate receptors. Front. Mol. Neurosci. 2018, 11, 307. [Google Scholar] [CrossRef]
- Modgil, S.; Lahiri, D.; Sharma, V.; Anand, A. Role of early life exposure and environment on neurodegeneration: Implications on brain disorders. Transl. Neurodegener. 2014, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R. Risk factors for Alzheimer’s disease. Folia Neuropathol 2019, 57, 87–105. [Google Scholar] [CrossRef]
- Massoud, F.; Léger, G. Pharmacological treatment of Alzheimer disease. Can. J. Psychiatry 2011, 56, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Winslow, B.; Onysko, M.; Stob, C.; Hazlewood, K. Treatment of Alzheimer disease. Am Fam Physician 2011, 83, 1403–1412. [Google Scholar] [PubMed]
- Yiannopoulou, K.; Papageorgiou, S. Current and future treatments for Alzheimer’s disease. Ther. Adv. Neurol. Disord. 2013, 6, 19–33. [Google Scholar] [CrossRef]
- Féger, J.; Hirsch, E. In search of innovative therapeutics for neuropsychiatric disorders: The case of neurodegenerative diseases. Ann. Pharm. Fr. 2015, 73, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-D.; Zhang, Y.-H.; Zhang, W.; Zhao, P. Meta-analysis of randomized controlled trials on the efficacy and safety of donepezil, galantamine, rivastigmine, and memantine for the treatment of Alzheimer’s disease. Front. Neurosci. 2019, 13, 472. [Google Scholar] [CrossRef] [PubMed]
- Atri, A. Current and future treatments in Alzheimer’s disease. Semin. Neurol. 2019, 39, 227–240. [Google Scholar] [CrossRef]
- Yiannopoulou, K.; Papageorgiou, S. Current and future treatments in Alzheimer disease: An update. J. Cent. Nerv. Syst. Dis. 2020, 12, 1179573520907397. [Google Scholar] [CrossRef]
- Sutherland, G.; Chami, B.; Youssef, P.; Witting, P. Oxidative stress in Alzheimer’s disease: Primary villain or physiological by-product? Redox. Rep. 2013, 18, 134–141. [Google Scholar] [CrossRef]
- Barbagallo, M.; Marotta, F.; Dominguez, L. Oxidative stress in patients with Alzheimer’s disease: Effect of extracts of fermented papaya powder. Mediators Inflamm. 2015, 2015, 624801. [Google Scholar] [CrossRef]
- Su, B.; Wang, X.; Nunomura, A.; Moreira, P.; Lee, H.; Perry, G.; Smith, M.; Zhu, X. Oxidative stress signaling in Alzheimer’s disease. Curr. Alzheimer. Res. 2008, 5, 525–532. [Google Scholar] [CrossRef]
- Cai, Z.; Zhao, B.; Ratka, A. Oxidative stress and β-Amyloid protein in Alzheimer’s disease. Neuromolecular. Med. 2011, 13, 223–250. [Google Scholar] [CrossRef]
- Youssef, P.; Chami, B.; Lim, J.; Middleton, T.; Sutherland, G.; Witting, P. Evidence supporting oxidative stress in a moderately affected area of the brain in Alzheimer’s disease. Sci. Rep. 2018, 8, 11553. [Google Scholar] [CrossRef]
- Lovell, M.; Markesbery, W. Oxidative DNA damage in mild cognitive impairment and late-stage Alzheimer’s disease. Nucleic. Acids Res. 2007, 35, 7497–7504. [Google Scholar] [CrossRef]
- Nasri, H.; Baradaran, A.; Shirzad, H.; Rafieian-Kopaei, M. New concepts in nutraceuticals as alternative for pharmaceuticals. Int. J. Prev. Med. 2014, 5, 1487–1499. [Google Scholar]
- AlAli, M.; Alqubaisy, M.; Aljaafari, M.; AlAli, A.; Baqais, L.; Molouki, A.; Abushelaibi, A.; Lai, K.; Lim, S. Nutraceuticals: Transformation of conventional foods into health promoters/disease preventers and safety considerations. Molecules 2021, 26, 2540. [Google Scholar] [CrossRef] [PubMed]
- Bigford, G.; Del Rossi, G. Supplemental substances derived from foods as adjunctive therapeutic agents for treatment of neurodegenerative diseases and disorders. Adv. Nutr. 2014, 5, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Macready, A.; Kennedy, O.; Ellis, J.; Williams, C.; Spencer, J.; Butler, L. Flavonoids and cognitive function: A review of human randomized controlled trial studies and recommendations for future studies. Genes Nutr. 2009, 4, 227. [Google Scholar] [CrossRef]
- Van de Rest, O.; Berendsen, A.; Haveman-Nies, A.; de Groot, L. Dietary patterns, cognitive decline, and dementia: A systematic review. Adv. Nutr. 2015, 6, 154–168. [Google Scholar] [CrossRef]
- Krikorian, R.; Nash, T.; Shidler, M.; Shukitt-Hale, B.; Joseph, J. Concord grape juice supplementation improves memory function in older adults with mild cognitive impairment. Br. J. Nutr. 2010, 103, 730–734. [Google Scholar] [CrossRef] [PubMed]
- Kean, R.; Lamport, D.; Dodd, G.; Freeman, J.; Williams, C.; Ellis, J.; Butler, L.; Spencer, J. Chronic consumption of flavanone-rich orange juice is associated with cognitive benefits: An 8-wk, randomized, double-blind, placebo-controlled trial in healthy older adults. Am. J. Clin. Nutr. 2015, 101, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, A.; Salo, I.; Plaza, M.; Björck, I. Effects of a mixed berry beverage on cognitive functions and cardiometabolic risk markers; A randomized cross-over study in healthy older adults. PLoS ONE 2017, 12, e0188173. [Google Scholar] [CrossRef] [PubMed]
- Bird, R.J.; Hoggard, N.; Aceves-Martins, M. The effect of grape interventions on cognitive and mental performance in healthy participants and those with mild cognitive impairment: A systematic review of randomized controlled trials. Nutr. Rev. 2021, 80, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, J.; Zafrilla, M.; Marhuenda, J. Cognitive function and consumption of fruit and vegetable polyphenols in a young population: Is there a relationship? Foods 2019, 8, 507. [Google Scholar] [CrossRef]
- Grodzicki, W.; Dziendzikowska, K. The role of selected bioactive compounds in the prevention of Alzheimer’s disease. Antioxidants 2020, 9, 229. [Google Scholar] [CrossRef]
- Jiang, X.; Huang, J.; Song, D.; Deng, R.; Wei, J.; Zhang, Z. Increased consumption of fruit and vegetables is related to a reduced risk of cognitive impairment and dementia: Meta-analysis. Front. Aging Neurosci. 2017, 9, 18. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Pereira, L.; Lamarão, C.; Lima, E.; da Veiga-Junior, V. Amazon acai: Chemistry and biological activities: A review. Food Chem. 2015, 179, 137–151. [Google Scholar] [CrossRef]
- De Souza, F.; de Araújo, F.; de Paulo Farias, D.; Zanotto, A.; Neri-Numa, I.; Pastore, G. Brazilian fruits of Arecaceae family: An overview of some representatives with promising food, therapeutic and industrial applications. Food Res. Int. 2020, 138, 109690. [Google Scholar] [CrossRef] [PubMed]
- Benatrehina, A.; Pan, L.; Naman, B.; Li, J.; Kinghorn, D. Usage, biological activity, and safety of selected botanical dietary supplements consumed in the United States. J. Tradit. Complement Med. 2018, 8, 267–277. [Google Scholar] [CrossRef]
- Jensen, G.; Wu, X.; Patterson, K.; Barnes, J.; Carter, S.; Scherwitz, L.; Beaman, R.; Endres, J.; Schauss, A. In Vitro and in vivo antioxidant and anti-inflammatory capacities of an antioxidant-rich fruit and berry juice blend. results of a pilot and randomized, double-blinded, placebo-controlled, crossover study. J. Agric. Food Chem. 2008, 56, 8326–8333. [Google Scholar] [CrossRef] [PubMed]
- Poulose, S.; Fisher, D.; Larson, J.; Bielinski, D.; Rimando, A.; Carey, A.; Schauss, A.; Shukitt-Hale, B. Anthocyanin-rich açai (Euterpe oleracea Mart.) fruit pulp fractions attenuate inflammatory stress signaling in mouse brain BV-2 microglial cells. J. Agric. Food Chem. 2012, 60, 1084–1093. [Google Scholar] [CrossRef] [PubMed]
- Spada, P.; Dani, C.; Bortolini, G.; Funchal, C.; Henriques, J.; Salvador, M. Frozen fruit pulp of Euterpe oleraceae Mart. (acai) prevents hydrogen peroxide-induced damage in the cerebral cortex, cerebellum, and hippocampus of rats. J. Med. Food 2009, 12, 1084–1088. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, İ. Fe3+–Fe2+ transformation method: An important antioxidant assay. In Advanced Protocols in Oxidative Stress III; Springer: Berlin/Heidelberg, Germany, 2015; pp. 233–246. [Google Scholar]
- Ferreira-Vieira, T.; Guimaraes, I.; Silva, F.; Ribeiro, F. Alzheimer’s disease: Targeting the cholinergic system. Curr Neuropharmacol 2016, 14, 101–115. [Google Scholar] [CrossRef]
- Cummings, J.; Tong, G.; Ballard, C. Treatment combinations for Alzheimer’s disease: Current and future pharmacotherapy options. J. Alzheimers. Dis. 2019, 67, 779–794. [Google Scholar] [CrossRef]
- Amat-Ur-Rasool, H.; Ahmed, M.; Hasnain, S.; Carter, W. Anti-cholinesterase combination drug therapy as a potential treatment for Alzheimer’s disease. Brain Sci. 2021, 11, 184. [Google Scholar] [CrossRef]
- Collins, A.; Saleh, T.; Kalisch, B. Naturally occurring antioxidant therapy in Alzheimer’s disease. Antioxidants 2022, 11, 213. [Google Scholar] [CrossRef]
- Wang, E.; Wu, M.; Lu, J. Ferulic acid in animal models of Alzheimer’s disease: A systematic review of preclinical studies. Cells 2021, 10, 2653. [Google Scholar] [CrossRef]
- Dvir, H.; Silman, I.; Harel, M.; Rosenberry, T.; Sussman, J. Acetylcholinesterase: From 3D structure to function. Chem. Biol. Interact. 2010, 187, 10–22. [Google Scholar] [CrossRef]
- Rosenberry, T. Strategies to resolve the catalytic mechanism of acetylcholinesterase. J. Mol. Neurosci. 2010, 40, 32–39. [Google Scholar] [CrossRef]
- Bajda, M.; Więckowska, A.; Hebda, M.; Guzior, N.; Sotriffer, C.; Malawska, B. Structure-based search for new inhibitors of cholinesterases. Int. J. Mol. Sci. 2013, 14, 5608–5632. [Google Scholar] [CrossRef]
- Silva, M.; Kiametis, A.; Treptow, W. Donepezil inhibits acetylcholinesterase via multiple binding modes at room temperature. J. Chem. Inf. Model 2020, 60, 3463–3471. [Google Scholar] [CrossRef]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.; Lightfoot, D. Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef]
- Chung, H.-J. Physiological activity of acai berry (Euterpe oleracea Mart.) extracted with different solvents. J. Korean Soc. Food Cult. 2012, 27, 75–81. [Google Scholar] [CrossRef]
- López de Dicastillo, C.; Piña, C.; Garrido, L.; Arancibia, C.; Galotto, M. Enhancing thermal stability and bioaccesibility of Açaí fruit polyphenols through electrohydrodynamic encapsulation into zein electrosprayed particles. Antioxidants 2019, 8, 464. [Google Scholar] [CrossRef]
- Zhou, S.; Huang, G. The biological activities of butyrylcholinesterase inhibitors. Biomed. Pharmacother. 2022, 146, 112556. [Google Scholar] [CrossRef]
- Li, Q.; Yang, H.; Chen, Y.; Sun, H. Recent progress in the identification of selective butyrylcholinesterase inhibitors for Alzheimer’s disease. Eur. J. Med. Chem. 2017, 132, 294–309. [Google Scholar] [CrossRef]
- Amat-ur-Rasool, H.; Ahmed, M.; Hasnain, S.; Ahmed, A.; Carter, W. In silico design of dual-binding site anti-cholinesterase phytochemical heterodimers as treatment options for Alzheimer’s disease. Curr. Issues Mol. Biol. 2022, 44, 152–175. [Google Scholar] [CrossRef]
- Mushtaq, G.; Greig, N.; Khan, J.; Kamal, M. Status of acetylcholinesterase and butyrylcholinesterase in Alzheimer’s disease and type 2 diabetes mellitus. CNS Neurol. Disord. Drug Targets 2014, 13, 1432–1439. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Han, Y. Pharmacological profile of huperzine a, a novel acetylcholinesterase inhibitor from Chinese herb. CNS Drug Rev. 2006, 5, 281–300. [Google Scholar] [CrossRef]
- Ogura, H.; Kosasa, T.; Kuriya, Y.; Yamanishi, Y. Comparison of inhibitory activities of donepezil and other cholinesterase inhibitors on acetylcholinesterase and butyrylcholinesterase in vitro. Methods Find Exp. Clin. Pharmacol. 2000, 22, 609–613. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, T.; Gomes, T.; Pinto, B.; Camara, A.; Paes, A. Naturally occurring acetylcholinesterase inhibitors and their potential use for Alzheimer’s disease therapy. Front. Pharmacol. 2018, 9, 1192. [Google Scholar] [CrossRef]
- Schauss, A.; Wu, X.; Prior, R.; Ou, B.; Huang, D.; Owens, J.; Agarwal, A.; Jensen, G.; Hart, A.; Shanbrom, E. Antioxidant capacity and other bioactivities of the freeze-dried Amazonian palm berry, Euterpe oleraceae Mart. (acai). J. Agric. Food Chem. 2006, 54, 8604–8610. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.; Andreazza, A.; da Silva, T.; Boligon, A.; do Nascimento, V.; Scola, G.; Duong, A.; Cadona, F.; Ribeiro, E.; da Cruz, I. Neuroprotective effects of acai (Euterpe oleracea Mart.) against rotenone in vitro exposure. Oxid. Med. Cell Longev. 2016, 2016, 8940850. [Google Scholar] [CrossRef] [PubMed]
- Matheus, M.; Fernandes, S.; Silveira, C.; Rodrigues, V.; Menezes, F.; Fernandes, P. Inhibitory effects of Euterpe oleracea Mart. on nitric oxide production and iNOS expression. J. Ethnopharmacol. 2006, 107, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Mertens-Talcott, S.; Rios, J.; Jilma-Stohlawetz, P.; Pacheco-Palencia, L.; Meibohm, B.; Talcott, S.; Derendorf, H. Pharmacokinetics of anthocyanins and antioxidant effects after the consumption of anthocyanin-rich açai juice and pulp (Euterpe oleracea Mart.) in human healthy volunteers. J. Agric. Food Chem. 2008, 56, 7796–7802. [Google Scholar] [CrossRef] [PubMed]
- Poulose, S.; Bielinski, D.; Carey, A.; Schauss, A.; Shukitt-Hale, B. Modulation of oxidative stress, inflammation, autophagy and expression of Nrf2 in hippocampus and frontal cortex of rats fed with açaí-enriched diets. Nutr. Neurosci. 2017, 20, 305–315. [Google Scholar] [CrossRef]
- Kang, J.; Xie, C.; Li, Z.; Nagarajan, S.; Schauss, A.; Wu, T.; Wu, X. Flavonoids from acai (Euterpe oleracea Mart.) pulp and their antioxidant and anti-inflammatory activities. Food Chem. 2011, 128, 152–157. [Google Scholar] [CrossRef]
- Pacheco-Palencia, L.; Mertens-Talcott, S.; Talcott, S. Chemical composition, antioxidant properties, and thermal stability of a phytochemical enriched oil from açai (Euterpe oleracea Mart.). J. Agric. Food Chem. 2008, 56, 4631–4636. [Google Scholar] [CrossRef]
- Nwidu, L.; Elmorsy, E.; Aprioku, J.; Siminialayi, I.; Carter, W. In Vitro anti-Cholinesterase and antioxidant activity of extracts of Moringa oleifera plants from Rivers State, Niger Delta, Nigeria. Medicines 2018, 5, 71. [Google Scholar] [CrossRef]
- Wong, D.; Musgrave, I.; Harvey, B.; Smid, S. Açaí (Euterpe oleraceae Mart.) berry extract exerts neuroprotective effects against β-amyloid exposure in vitro. Neurosci. Lett. 2013, 556, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.; Courtney, K.; Andres, V.; Feather-Stone, R. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Carter, W.; Tarhoni, M.; Rathbone, A.; Ray, D. Differential protein adduction by seven organophosphorus pesticides in both brain and thymus. Hum. Exp. Toxicol. 2007, 26, 347–353. [Google Scholar] [CrossRef]
- Dorling, J.; Clayton, D.; Jones, J.; Carter, W.; Thackray, A.; King, J.; Pucci, A.; Batterham, R.; Stensel, D. A randomized crossover trial assessing the effects of acute exercise on appetite, circulating ghrelin concentrations, and butyrylcholinesterase activity in normal-weight males with variants of the obesity-linked FTO rs9939609 polymorphism. Am. J. Clin. Nutr. 2019, 110, 1055–1066. [Google Scholar] [CrossRef]
- Nwidu, L.; Elmorsy, E.; Thornton, J.; Wijamunige, B.; Wijesekara, A.; Tarbox, R.; Warren, A.; Carter, W. Anti-acetylcholinesterase activity and antioxidant properties of extracts and fractions of Carpolobia lutea. Pharm. Biol. 2017, 55, 1875–1883. [Google Scholar] [CrossRef] [PubMed]
- Acharya, K. Simplified methods for microtiter based analysis of in vitro antioxidant activity. Asian J. Pharm. 2017, 11, S327–S335. [Google Scholar] [CrossRef]
- Phaniendra, A.; Jestadi, D.; Periyasamy, L. Free radicals: Properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef]
- Alam, M.; Bristi, N.; Rafiquzzaman, M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef]
- Bajpai, V.; Park, Y.; Agrawal, P. Studies on phytochemical analysis, antioxidant and lipid peroxidation inhibitory effects of a medicinal plant, Coleus forskohlii. Front. Life Sci. 2015, 8, 139–147. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J. Formation of a thiobarbituric-acid-reactive substance from deoxyribose in the presence of iron salts. FEBS Lett. 1981, 128, 347–352. [Google Scholar] [CrossRef]
- Unuofin, J.; Otunola, G.; Afolayan, A. Polyphenolic content, antioxidant and antimicrobial activities of Vernonia mespilifolia Less. Used in folk medicine in the Eastern Cape Province, South Africa. J. Evid. Based Integr. Med. 2018, 23, 2515690X18773990. [Google Scholar] [CrossRef] [PubMed]
- Jimoh, M.; Afolayan, A.; Lewu, F. Antioxidant and phytochemical activities of Amaranthus caudatus L. harvested from different soils at various growth stages. Sci. Rep. 2019, 9, 12965. [Google Scholar] [CrossRef] [PubMed]
- Angelova, P.; Esteras, N.; Abramov, A. Mitochondria and lipid peroxidation in the mechanism of neurodegeneration: Finding ways for prevention. Med. Res. Rev. 2021, 41, 770–784. [Google Scholar] [CrossRef]
- Akomolafe, S.; Oboh, G.; Akindahunsi, A.; Akinyemi, A.; Tade, O. Inhibitory effect of aqueous extract of stem bark of Cissus populnea on ferrous sulphate- and sodium nitroprusside-induced oxidative stress in rat’s testes in vitro. ISRN Pharmacol. 2013, 2013, 130989. [Google Scholar] [CrossRef] [PubMed]
- Ford, L.; Theodoridou, K.; Sheldrake, G.; Walsh, P. A critical review of analytical methods used for the chemical characterisation and quantification of phlorotannin compounds in brown seaweeds. Phytochem. Anal. 2019, 30, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-C.; Yang, M.-H.; Wen, H.-M.; Chern, J.-C. Estimation of total flavonoid content in propolis by two complementary colometric methods. J. Food Drug Anal. 2002, 10, 3. [Google Scholar] [CrossRef]
- Da Silveira, T.; de Souza, T.; Carvalho, A.; Ribeiro, A.; Kuhnle, G.; Godoy, H. White açaí juice (Euterpe oleracea): Phenolic composition by LC-ESI-MS/MS, antioxidant capacity and inhibition effect on the formation of colorectal cancer related compounds. J. Funct. Foods 2017, 36, 215–223. [Google Scholar] [CrossRef]
- ALNasser, M.; Mellor, I. Neuroprotective activities of acai berries (Euterpe sp.): A review. J. Herbmed. Pharmacol. 2022, 11, 166–181. [Google Scholar] [CrossRef]
- Alqurashi, R.; Commane, D.; Rowland, I. Açai fruit as a source of bioactive phytochemicals. J. Life Sci. 2016, 10, 391–404. [Google Scholar] [CrossRef]
- Schauss, A.; Wu, X.; Prior, R.; Ou, B.; Patel, D.; Huang, D.; Kababick, J. Phytochemical and nutrient composition of the freeze-dried Amazonian palm berry, Euterpe oleraceae Mart. (acai). J. Agric. Food Chem. 2006, 54, 8598–8603. [Google Scholar] [CrossRef]
- Ahmed, S.; Khan, S.; Zargaham, M.; Khan, A.; Khan, S.; Hussain, A.; Uddin, J.; Khan, A.; Al-Harrasi, A. Potential therapeutic natural products against Alzheimer’s disease with reference of acetylcholinesterase. Biomed. Pharmacother. 2021, 139, 111609. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Marya; Amin, S.; Kamal, M.A.; Patel, S. Flavonoids as acetylcholinesterase inhibitors: Current therapeutic standing and future prospects. Biomed. Pharmacother. 2018, 101, 860–870. [Google Scholar] [CrossRef]
- Kobus-Cisowska, J.; Szymanowska, D.; Maciejewska, P.; Kmiecik, D.; Gramza-Michałowska, A.; Kulczyński, B.; Cielecka-Piontek, J. In Vitro screening for acetylcholinesterase and butyrylcholinesterase inhibition and antimicrobial activity of chia seeds (Salvia hispanica). Electron. J. Biotechnol. 2019, 37, 1–10. [Google Scholar] [CrossRef]
- Birsan, R.; Wilde, P.; Waldron, K.; Rai, D. Anticholinesterase activities of different solvent extracts of brewer’s spent grain. Foods 2021, 10, 930. [Google Scholar] [CrossRef]
- Budryn, G.; Majak, I.; Grzelczyk, J.; Szwajgier, D.; Rodríguez-Martínez, A.; Pérez-Sánchez, H. Hydroxybenzoic acids as acetylcholinesterase inhibitors: Calorimetric and docking simulation studies. Nutrients 2022, 14, 2476. [Google Scholar] [CrossRef]
- Orhan, I.; Kartal, M.; Tosun, F.; Sener, B. Screening of various phenolic acids and flavonoid derivatives for their anticholinesterase potential. J. Biosci. 2007, 62, 829–832. [Google Scholar] [CrossRef]
- Woo, Y.-J.; Lee, B.-H.; Yeun, G.-H.; Kim, H.-J.; Won, M.-H.; Kim, S.-H.; Lee, B.-H.; Park, J.-H. Selective butyrylcholinesterase inhibitors using polyphenol-polyphenol hybrid molecules. Bull Korean Chem. Soc. 2011, 32, 2593–2598. [Google Scholar] [CrossRef]
- Salau, V.; Erukainure, O.; Ibeji, C.; Olasehinde, T.; Koorbanally, N.; Islam, M. Vanillin and vanillic acid modulate antioxidant defense system via amelioration of metabolic complications linked to Fe2+-induced brain tissues damage. Metab. Brain Dis. 2020, 35, 727–738. [Google Scholar] [CrossRef]
- Jabir, N.; Khan, F.; Tabrez, S. Cholinesterase targeting by polyphenols: A therapeutic approach for the treatment of Alzheimer’s disease. CNS Neurosci. Ther. 2018, 24, 753–762. [Google Scholar] [CrossRef]
- Oh, J.; Jang, H.; Kang, M.; Song, S.; Kim, D.; Kim, J.; Noh, J.; Park, J.; Park, D.; Yee, S.; et al. Acetylcholinesterase and monoamine oxidase-B inhibitory activities by ellagic acid derivatives isolated from Castanopsis cuspidata var. sieboldii. Sci. Rep. 2021, 11, 13953. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Kwon, H.; Cho, E.; Jeon, J.; Kang, R.; Youn, K.; Jun, M.; Lee, Y.; Ryu, J.; Kim, D. The effects of pinoresinol on cholinergic dysfunction-induced memory impairments and synaptic plasticity in mice. Food Chem. Toxicol. 2019, 125, 376–382. [Google Scholar] [CrossRef] [PubMed]
- El-Hassan, A.; El-Sayed, M.; Hamed, A.; Rhee, I.; Ahmed, A.; Zeller, K.; Verpoorte, R. Bioactive constituents of Leptadenia arborea. Fitoterapia 2003, 74, 184–187. [Google Scholar] [CrossRef]
- Tang, H.-Y.; Bai, M.-M.; Tian, J.-M.; Pescitelli, G.; Ivšić, T.; Huang, X.-H.; Lee, H.; Son, Y.N.; Kim, J.H.; Kim, Y. Chemical components from the seeds of Catalpa bungei and their inhibitions of soluble epoxide hydrolase, cholinesterase and nuclear factor kappa B activities. RSC Adv. 2016, 6, 40706–40716. [Google Scholar] [CrossRef]
- Geiss, J.; Sagae, S.; Paz, E.; de Freitas, M.; Souto, N.; Furian, A.; Oliveira, M.; Guerra, G. Oral administration of lutein attenuates ethanol-induced memory deficit in rats by restoration of acetylcholinesterase activity. Physiol. Behav. 2019, 204, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Xu, J.; Zhao, H.; Jiang, W.; Guo, X.; Zhao, M.; Sun-Waterhouse, D.; Zhao, Q.; Su, G. Antioxidant and anti-acetylcholinesterase activities of anchovy (Coilia mystus) protein hydrolysates and their memory-improving effects on scopolamine-induced amnesia mice. Int. J. Food Sci. Technol. 2017, 52, 504–510. [Google Scholar] [CrossRef]
- Cásedas, G.; Les, F.; González-Burgos, E.; Gómez-Serranillos, M.; Smith, C.; López, V. Cyanidin-3-O-glucoside inhibits different enzymes involved in central nervous system pathologies and type-2 diabetes. S. Afr. J. Bot. 2019, 120, 241–246. [Google Scholar] [CrossRef]
- Uriarte-Pueyo, I.; Calvo, M. Flavonoids as acetylcholinesterase inhibitors. Curr. Med. Chem. 2011, 18, 5289–5302. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, S.-H.; Lee, H.W.; Sun, Y.; Jang, W.-H.; Yang, S.-Y.; Jang, H.-D.; Kim, Y. (−)-Epicatechin derivate from Orostachys japonicus as potential inhibitor of the human butyrylcholinesterase. Int. J. Biol. Macromol. 2016, 91, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Okello, E.; Mather, J. Comparative kinetics of acetyl- and butyryl-cholinesterase inhibition by green tea catechins|relevance to the symptomatic treatment of Alzheimer’s disease. Nutrients 2020, 12, 1090. [Google Scholar] [CrossRef] [PubMed]
- Kucukboyacı, N.; Orhan, I.; Şener, B.; Nawaz, S.; Choudhary, M. Assessment of Enzyme Inhibitory and Antioxidant Activities of Lignans from Taxus baccata L. J. Biosci. 2010, 65, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Szwajgier, D.; Borowiec, K. Phenolic acids from malt are efficient acetylcholinesterase and butyrylcholinesterase inhibitors. J. Inst. Brew. 2012, 118, 40–48. [Google Scholar] [CrossRef]
- Chunhakant, S.; Chaicharoenpong, C. Antityrosinase, Antioxidant, and Cytotoxic Activities of Phytochemical Constituents from Manilkara zapota L. Bark. Molecules 2019, 24, 2798. [Google Scholar] [CrossRef] [PubMed]
- Karaoglan, E.; Koca, M. Tyrosinase and cholinesterase inhibitory activities and molecular docking studies on apigenin and vitexin. J. Pharm. Istanbul. Univ. 2020, 50, 268–272. [Google Scholar] [CrossRef]
- Choi, J.; Nurul Islam, M.; Yousof Ali, M.; Kim, E.; Kim, Y.; Jung, H. Effects of C-glycosylation on anti-diabetic, anti-Alzheimer’s disease and anti-inflammatory potential of apigenin. Food Chem. Toxicol. 2014, 64, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Conforti, F.; Rigano, D.; Menichini, F.; Loizzo, M.; Senatore, F. Protection against neurodegenerative diseases of Iris pseudopumila extracts and their constituents. Fitoterapia 2009, 80, 62–67. [Google Scholar] [CrossRef]
- Khaw, K.-Y.; Murugaiyah, V.; Khairuddean, M.; Tan, W.-N. Garcinexanthone G, a Selective Butyrylcholinesterase Inhibitor from the Stem Bark of Garcinia atroviridis. Nat. Prod. Sci. 2018, 24, 88–92. [Google Scholar] [CrossRef]
- Fang, Z.; Jeong, S.; Jung, H.; Choi, J.; Min, B.; Woo, M. Anticholinesterase and antioxidant constituents from Gloiopeltis furcata. Chem. Pharm. Bull. 2010, 58, 1236–1239. [Google Scholar] [CrossRef]
- Wu, Y.; Su, X.; Lu, J.; Wu, M.; Yang, S.; Mai, Y.; Deng, W.; Xue, Y. In Vitro and in silico analysis of phytochemicals from fallopia dentatoalata as dual functional cholinesterase inhibitors for the treatment of Alzheimer’s disease. Front. Pharmacol. 2022, 13, 905708. [Google Scholar] [CrossRef]
- Ayaz, M.; Junaid, M.; Ullah, F.; Subhan, F.; Sadiq, A.; Ali, G.; Ovais, M.; Shahid, M.; Ahmad, A.; Wadood, A.; et al. Anti-Alzheimer’s Studies on β-Sitosterol Isolated from Polygonum hydropiper L. Front. Pharmacol. 2017, 8, 697. [Google Scholar] [CrossRef]
- Sultana, N.; Khalid, A. Phytochemical and enzyme inhibitory studies on indigenous medicinal plant Rhazya stricta. Nat. Prod. Res. 2010, 24, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Kissling, J.; Ioset, J.R.; Marston, A.; Hostettmann, K. Bio-guided isolation of cholinesterase inhibitors from the bulbs of Crinum x powellii. Phytother. Res. 2005, 19, 984–987. [Google Scholar] [CrossRef] [PubMed]
- Pohanka, M. Copper, aluminum, iron and calcium inhibit human acetylcholinesterase in vitro. Environ. Toxicol. Pharmacol. 2014, 37, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Kikuzaki, H.; Hisamoto, M.; Hirose, K.; Akiyama, K.; Taniguchi, H. Antioxidant properties of ferulic acid and its related compounds. J. Agric. Food Chem. 2002, 50, 2161–2168. [Google Scholar] [CrossRef] [PubMed]
- Bernal-Mercado, A.T.; Vazquez-Armenta, F.J.; Tapia-Rodriguez, M.R.; Islas-Osuna, M.A.; Mata-Haro, V.; Gonzalez-Aguilar, G.A.; Lopez-Zavala, A.A.; Ayala-Zavala, J.F. Comparison of Single and Combined Use of Catechin, Protocatechuic, and Vanillic Acids as Antioxidant and Antibacterial Agents against Uropathogenic Escherichia Coli at Planktonic and Biofilm Levels. Molecules 2018, 23, 2813. [Google Scholar] [CrossRef] [PubMed]
- Platzer, M.; Kiese, S.; Herfellner, T.; Schweiggert-Weisz, U.; Miesbauer, O.; Eisner, P. Common Trends and Differences in Antioxidant Activity Analysis of Phenolic Substances Using Single Electron Transfer Based Assays. Molecules 2021, 26, 1244. [Google Scholar] [CrossRef] [PubMed]
- Tung, Y.-T.; Wu, J.-H.; Kuo, Y.-H.; Chang, S.-T. Antioxidant activities of natural phenolic compounds from Acacia confusa bark. Bioresour. Technol. 2007, 98, 1120–1123. [Google Scholar] [CrossRef]
- Kawabata, J.; Okamoto, Y.; Kodama, A.; Makimoto, T.; Kasai, T. Oxidative Dimers Produced from Protocatechuic and Gallic Esters in the DPPH Radical Scavenging Reaction. J. Agric. Food Chem. 2002, 50, 5468–5471. [Google Scholar] [CrossRef]
- Alcalde, B.; Granados, M.; Saurina, J. Exploring the Antioxidant Features of Polyphenols by Spectroscopic and Electrochemical Methods. Antioxidants 2019, 8, 523. [Google Scholar] [CrossRef]
- McCann, M.J.; Dalziel, J.E.; Bibiloni, R.; Barnett, M.P. An integrated approach to assessing the bio-activity of nutrients in vitro: The anti-oxidant effects of catechin and chlorogenic acid as an example. Integr. Food Nutr. Metab. 2015, 2, 197–204. [Google Scholar] [CrossRef]
- Xu, J.-G.; Hu, Q.-P.; Liu, Y. Antioxidant and DNA-Protective Activities of Chlorogenic Acid Isomers. J. Agric. Food Chem. 2012, 60, 11625–11630. [Google Scholar] [CrossRef]
- Spagnol, C.M.; Assis, R.P.; Brunetti, I.L.; Isaac, V.L.B.; Salgado, H.R.N.; Corrêa, M.A. In Vitro methods to determine the antioxidant activity of caffeic acid. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2019, 219, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.; Allen, P.; Brunton, N.; O’Grady, M.; Kerry, J. Phenolic composition and in vitro antioxidant capacity of four commercial phytochemical products: Olive leaf extract (Olea europaea L.), lutein, sesamol and ellagic acid. Food Chem. 2010, 126, 948–955. [Google Scholar] [CrossRef]
- Kikuzaki, H.; Masuda, M.; Kishi, E.; Ozaki, M.; Kondo, K.; Kanai, A.; Shiomi, K.; Furuta, T.; Mizu, M.; Nagai, Y. Components for Inhibiting Lipid Oxidation Related to Discoloration of Carotenoid Contained in Sugarcane Extract. Food Sci. Technol. Res. 2019, 25, 715–725. [Google Scholar] [CrossRef]
- Veselova, M.V.; Fedoreev, S.A.; Vasilevskaya, N.A.; Denisenko, V.A.; Gerasimenko, A.V. Antioxidant activity of polyphenols from the far-east plant Taxus cuspidata. Pharm. Chem. J. 2007, 41, 88–93. [Google Scholar] [CrossRef]
- Chambers, C.S.; Biedermann, D.; Valentová, K.; Petrásková, L.; Viktorová, J.; Kuzma, M.; Křen, V. Preparation of Retinoyl-Flavonolignan Hybrids and Their Antioxidant Properties. Antioxidants 2019, 8, 236. [Google Scholar] [CrossRef] [PubMed]
- Ambigaipalan, P.; Oh, W.Y.; Shahidi, F. Epigallocatechin (EGC) esters as potential sources of antioxidants. Food Chem. 2019, 309, 125609. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, X.; Liu, G.; Li, J.; Zhang, J.; Cao, Y.; Miao, J. Antioxidant Activity and Mechanism of Resveratrol and Polydatin Isolated from Mulberry (Morus alba L.). Molecules 2021, 26, 7574. [Google Scholar] [CrossRef]
- Wei, C.-C.; Yen, P.-L.; Chang, S.-T.; Cheng, P.-L.; Lo, Y.-C.; Liao, V.H.-C. Antioxidative Activities of Both Oleic Acid and Camellia tenuifolia Seed Oil Are Regulated by the Transcription Factor DAF-16/FOXO in Caenorhabditis elegans. PLoS ONE 2016, 11, e0157195. [Google Scholar] [CrossRef]
- Yoshida, Y.; Niki, E. Antioxidant Effects of Phytosterol and Its Components. J. Nutr. Sci. Vitaminol. 2003, 49, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Gulaboski, R.; Bogeski, I.; Mirčeski, V.; Saul, S.; Pasieka, B.; Haeri, H.H.; Stefova, M.; Stanoeva, J.P.; Mitrev, S.; Hoth, M.; et al. Hydroxylated derivatives of dimethoxy-1,4-benzoquinone as redox switchable earth-alkaline metal ligands and radical scavengers. Sci. Rep. 2013, 3, srep01865. [Google Scholar] [CrossRef]
- Rohmah, M.; Rahmadi, A.; Raharjo, S. Bioaccessibility and antioxidant activity of β-carotene loaded nanostructured lipid carrier (NLC) from binary mixtures of palm stearin and palm olein. Heliyon 2022, 8, e08913. [Google Scholar] [CrossRef]
- Müller, L.; Fröhlich, K.; Böhm, V. Comparative antioxidant activities of carotenoids measured by ferric reducing antioxidant power (FRAP), ABTS bleaching assay (αTEAC), DPPH assay and peroxyl radical scavenging assay. Food Chem. 2011, 129, 139–148. [Google Scholar] [CrossRef]
- Murillo, A.G.; Hu, S.; Fernandez, M.L. Zeaxanthin: Metabolism, Properties, and Antioxidant Protection of Eyes, Heart, Liver, and Skin. Antioxidants 2019, 8, 390. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Song, J.; Zhang, M.; Wang, H.; Zhang, Y.; Suo, H. Comparison of In Vitro and In Vivo Antioxidant Activities of Six Flavonoids with Similar Structures. Antioxidants 2020, 9, 732. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yang, W.; Liu, J.; Liu, H.; Lv, Z.; Zhang, C.; Chen, D.; Jiao, Z. Identification of Six Flavonoids as Novel Cellular Antioxidants and Their Structure-Activity Relationship. Oxidative Med. Cell. Longev. 2020, 2020, 4150897. [Google Scholar] [CrossRef]
- Sordon, S.; Popłoński, J.; Milczarek, M.; Stachowicz, M.; Tronina, T.; Kucharska, A.Z.; Wietrzyk, J.; Huszcza, E. Structure–Antioxidant–Antiproliferative Activity Relationships of Natural C7 and C7–C8 Hydroxylated Flavones and Flavanones. Antioxidants 2019, 8, 210. [Google Scholar] [CrossRef]
- Mishra, B.; Priyadarsini, K.; Kumar, M.; Unnikrishnan, M.; Mohan, H. Effect of O -glycosilation on the antioxidant activity and free radical reactions of a plant flavonoid, chrysoeriol. Bioorganic Med. Chem. 2003, 11, 2677–2685. [Google Scholar] [CrossRef]
- He, J.-W.; Yang, L.; Mu, Z.-Q.; Zhu, Y.-Y.; Zhong, G.-Y.; Liu, Z.-Y.; Zhou, Q.-G.; Cheng, F. Anti-inflammatory and antioxidant activities of flavonoids from the flowers of Hosta plantaginea. RSC Adv. 2018, 8, 18175–18179. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fang, X.; Ge, L.; Cao, F.; Zhao, L.; Wang, Z.; Xiao, W. Antitumor, antioxidant and anti-inflammatory activities of kaempferol and its corresponding glycosides and the enzymatic preparation of kaempferol. PLoS ONE 2018, 13, e0197563. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, X.; Cheng, J.; Jin, C.; Zhang, Y. The reduction effect of dietary flavone C- and O-glycosides on the formation of acrylamide and its correlation and prediction with the antioxidant activity of Maillard reaction products. RSC Adv. 2014, 4, 24147–24155. [Google Scholar] [CrossRef]
- Jung, Y.H.; Chae, C.W.; Choi, G.E.; Shin, H.C.; Lim, J.R.; Chang, H.S.; Park, J.; Cho, J.H.; Park, M.R.; Lee, H.J.; et al. Cyanidin 3-O-arabinoside suppresses DHT-induced dermal papilla cell senescence by modulating p38-dependent ER-mitochondria contacts. J. Biomed. Sci. 2022, 29, 17. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Kwak, H.; Hwang, K.T. Antioxidant and anti-inflammatory activities of cyanidin-3-glucoside and cyanidin-3-rutinoside in hydrogen peroxide and lipopolysaccharide treated RAW264.7 cells (830.23). FASEB J. 2014, 28, 830.23. [Google Scholar] [CrossRef]
- Tena, N.; Martín, J.; Asuero, A.G. State of the Art of Anthocyanins: Antioxidant Activity, Sources, Bioavailability, and Therapeutic Effect in Human Health. Antioxidants 2020, 9, 451. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.-D.; Wu, D.-G.; Cai, X.-H.; Kennelly, E.J. New Antioxidant Phenolic Glycosides fromWalsura yunnanensis. Chem. Biodivers. 2006, 3, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, V.K.; Alam, M.B.; Quan, K.T.; Kwon, K.-R.; Ju, M.-K.; Choi, H.-J.; Lee, J.S.; Yoon, J.-I.; Majumder, R.; Rather, I.A.; et al. Antioxidant efficacy and the upregulation of Nrf2-mediated HO-1 expression by (+)-lariciresinol, a lignan isolated from Rubia philippinensis, through the activation of p38. Sci. Rep. 2017, 7, srep46035. [Google Scholar] [CrossRef]
- Saito, S.; Okamoto, Y.; Kawabata, J. Effects of Alcoholic Solvents on Antiradical Abilities of Protocatechuic Acid and Its Alkyl Esters. Biosci. Biotechnol. Biochem. 2004, 68, 1221–1227. [Google Scholar] [CrossRef]
- Li, L.; Seeram, N.P. Further Investigation into Maple Syrup Yields 3 New Lignans, a New Phenylpropanoid, and 26 Other Phytochemicals. J. Agric. Food Chem. 2011, 59, 7708–7716. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Tani, S.; Baba, C.; Hashimoto, T. Phenylpropanoid, Sapnol A, Lignan and Neolignan Sophorosides, Saposides A and B, Isolated from Canadian Sugar Maple Sap. Molecules 2013, 18, 9641–9649. [Google Scholar] [CrossRef]
- Xu, N.; Chen, G.; Liu, H. Antioxidative Categorization of Twenty Amino Acids Based on Experimental Evaluation. Molecules 2017, 22, 2066. [Google Scholar] [CrossRef]
- XueJiao, Z.; DengYong, L.; DavidWang, H.J.S.K.F.S. Antioxidant activity in vitro of hydroxyproline peptides. Shipin Kexue/Food Sci. 2021, 42, 55–60. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).