Impact of Tea Processing on Tryptophan, Melatonin, Phenolic and Flavonoid Contents in Mulberry (Morus alba L.) Leaves: Quantitative Analysis by LC-MS/MS
Abstract
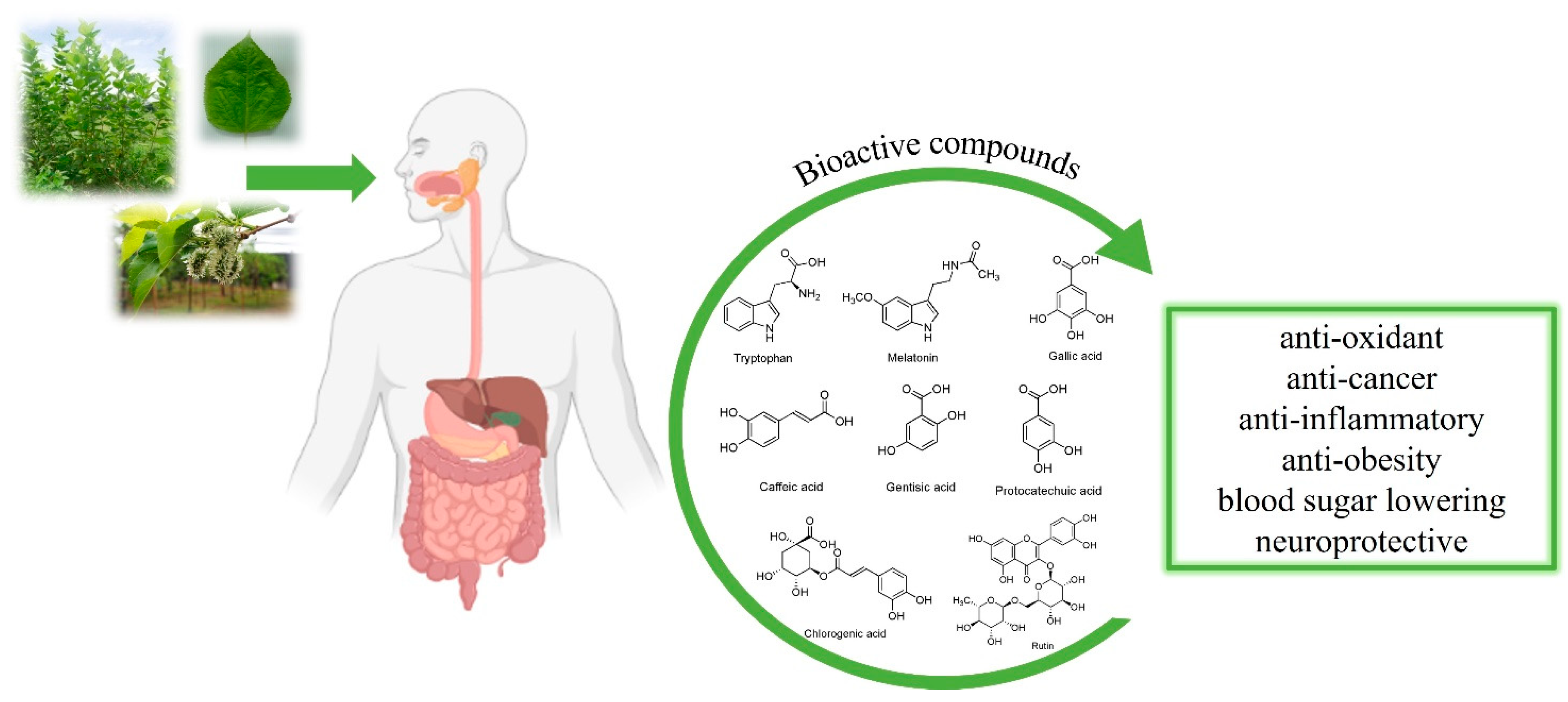
:1. Introduction
2. Results and Discussion
2.1. Total Phenolic and Flavonoid Contents of Mulberry Leaf Extracts
2.2. LC-MS/MS Analysis of Tryptophan, Melatonin, Phenolic and Flavonoid Contents
2.3. Antioxidant Capacities and In Vitro Acetylcholinesterase (AChE) Inhibition of Mulberry Leaf Extracts
3. Materials and Methods
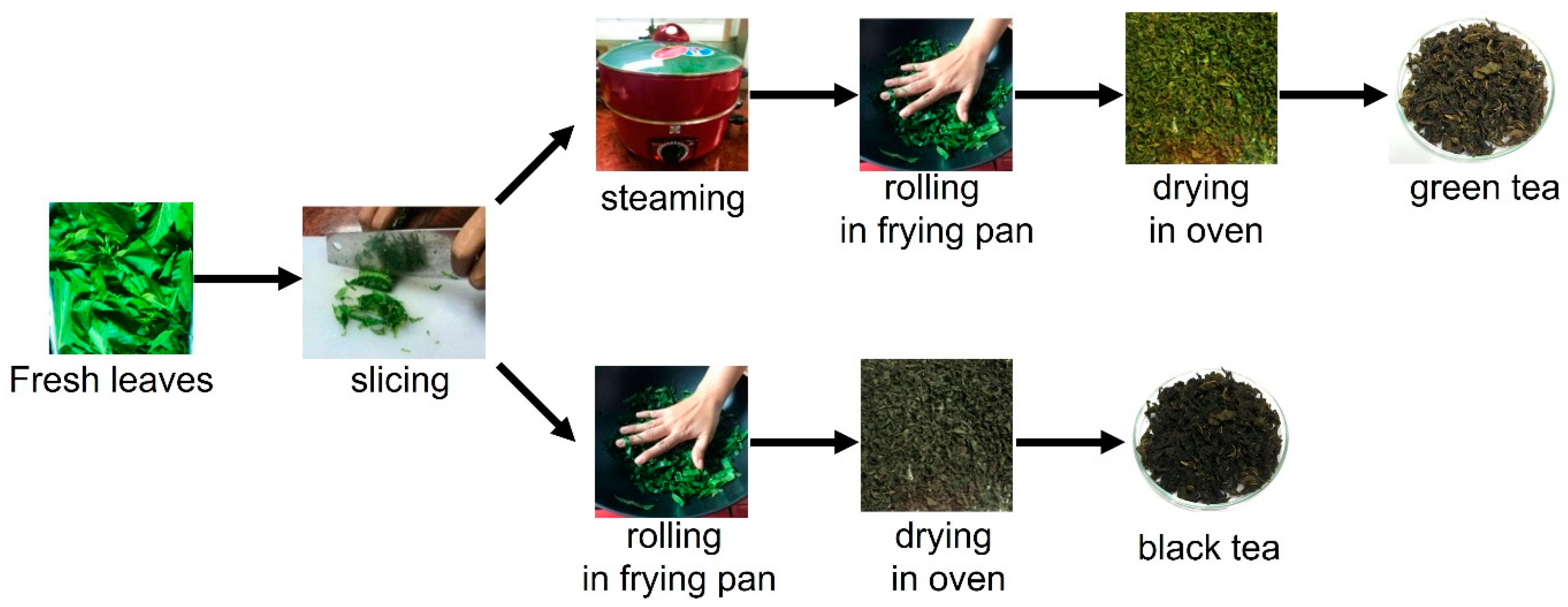
3.1. Sample Preparation and Extraction
3.2. Tryptophan, Melatonin and Phenolic Determination by LC-MS
3.3. Determination of Total Phenolic Content (TPC)
3.4. Determination of Total Flavonoid Content (TFC)
3.5. Antioxidant Activities
3.5.1. 1,1-Diphenyl-2-picrylhydrazyl (DPPH) Radical Scavenging Assay
3.5.2. 2,2-Azinobis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) Radical Scavenging Assay
3.5.3. Ferric Reducing Antioxidant Power (FRAP) Assay
3.6. In Vitro Inhibition Study on Acetylcholinesterase (AChE) Enzyme
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jan, B.; Parveen, B.R.; Zahiruddin, S.; Khan, M.U.; Mohapatra, S.; Ahmad, S. Nutritional constituents of mulberry and their potential applications in food and pharmaceuticals: A review. Saudi J. Biol. Sci. 2021, 28, 3909–3921. [Google Scholar] [CrossRef]
- Ercisli, S.; Orhan, E. Chemical composition of white (Morus alba), red (Morus rubra) and black (Morus nigra) mulberry fruits. Food Chem. 2007, 103, 1380–1384. [Google Scholar] [CrossRef]
- Wen, P.; Hu, T.G.; Linhardt, R.J.; Liao, S.T.; Wu, H.; Zou, Y.X. Mulberry: A review of bioactive compounds and advanced processing technology. Trends Food Sci. Technol. 2019, 83, 138–158. [Google Scholar] [CrossRef]
- Kadam, R.A.; Dhumal, N.D.; Khyade, V.B. The Mulberry, Morus alba (L.): The medicinal herbal source for human health. Int J Curr. Microbiol. Appl. Sci. 2019, 8, 2941–2964. [Google Scholar] [CrossRef]
- Kim, G.N.; Jang, H.D. Flavonol content in the water extract of the mulberry (Morus alba L.) leaf and their antioxidant capacities. J. Food Sci. 2011, 76, C869–C873. [Google Scholar] [CrossRef]
- Wang, Y.; Xiang, L.; Wang, C.; Tang, C.; He, X. Antidiabetic and antioxidant effects and phytochemicals of mulberry fruit (Morus alba L.) polyphenol enhanced extract. PLoS ONE 2013, 8, e71144. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Chang, Y.T.; Tseng, T.H.; Wang, C.J. Mulberry leaf extract inhibit hepatocellular carcinoma cell proliferation via depressing IL-6 and TNF-α derived from adipocyte. J. Food Drug Anal. 2018, 26, 1024–1032. [Google Scholar] [CrossRef]
- Yiemwattana, I.; Chaisomboon, N.; Jamdee, K. Antibacterial and anti-inflammatory potential of Morus alba stem extract. Open Dent. J. 2018, 12, 265–274. [Google Scholar] [CrossRef]
- Khyade, V.B. Influence of leaf decoction of mulberry, Morus alba (L.) on streptozotocin induced diabetes in brown rat, Rattus norvegicus (L.). Int. J. Eng. Res. 2018, 6, 1–23. [Google Scholar]
- Qiao, A.; Wang, Y.; Zhang, W.; He, X. Neuroprotection of brain-targeted bioactive dietary artoindonesianin O (AIO) from mulberry on rat neurons as a novel intervention for Alzheimer’s disease. J. Agric. Food Chem. 2015, 63, 3687–3693. [Google Scholar] [CrossRef]
- Shin, S.K.; Yoo, J.M.; Li, F.Y.; Baek, S.Y.; Kim, M.R. Mulberry fruit improves memory in scopolamine-treated mice: Role of cholinergic function, antioxidant system, and TrkB/Akt signaling. Nutr. Neurosci. 2021, 24, 940–950. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Nie, W.J. Chemical properties in fruits of mulberry species from the Xinjiang province of China. Food Chem. 2015, 174, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Analysis, nutrition, and health benefits of tryptophan. Int. J. Tryptophan Res. 2018, 11, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Salehi, B.; Sharopov, F.; Fokou, P.V.T.; Kobylinska, A.; Jonge, L.D.; Tadio, K.; Iriti, M. Melatonin in medicinal and food plants: Occurrence, bioavailability, and health potential for humans. Cells 2019, 8, 681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bravo, R.; Matito, S.; Cubero, J.; Paredes, S.D.; Franco, L.; Rivero, M.; Barriga, C. Tryptophan-enriched cereal intake improves nocturnal sleep, melatonin, serotonin, and total antioxidant capacity levels and mood in elderly humans. Age 2013, 35, 1277–1285. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Li, H.; Zhang, B.; Wang, J.; Shi, X.; Huang, J.; Deng, Z. Nutritional and functional components of mulberry leaves from different varieties: Evaluation of their potential as food materials. Int. J. Food Prop. 2018, 21, 1495–1507. [Google Scholar] [CrossRef] [Green Version]
- Buhroo, Z.I.; Bhat, M.A.; Malik, M.A.; Kamili, A.S.; Ganai, N.A.; Khan, I.L. Trends in development and utilization of sericulture resources for diversification and value addition. Int. J. Entomol. Res. 2018, 6, 27–47. [Google Scholar] [CrossRef] [Green Version]
- Wanyo, P.; Siriamornpun, S.; Meeso, N. Improvement of quality and antioxidant properties of dried mulberry leaves with combined far-infrared radiation and air convection in Thai tea process. Food Bioprod. Process. 2011, 89, 22–30. [Google Scholar] [CrossRef]
- Pothinuch, P.; Tongchitpakdee, S. Melatonin contents in mulberry (Morus spp.) leaves: Effects of sample preparation, cultivar, leaf age and tea processing. Food Chem. 2011, 128, 415–419. [Google Scholar] [CrossRef]
- Ross, C.F.; Hoye Jr., C.; Fernandez-Plotka, V.C. Influence of heating on the polyphenolic content and antioxidant activity of grape seed flour. J. Food Sci. 2011, 76, C884–C890. [Google Scholar] [CrossRef]
- Przeor, M.; Flaczyk, E.; Beszterda, M.; Szymandera-Buszka, K.E.; Piechocka, J.; Kmiecik, D.; Tylewicz, U. Air-drying temperature changes the content of the phenolic acids and flavonols in white mulberry (Morus alba L.) leaves. Cienc. Rural 2019, 49, 1–4. [Google Scholar] [CrossRef]
- Chaaban, H.; Ioannou, I.; Chebil, L.; Slimane, M.; Gérardin, C.; Paris, C.; Ghoul, M. Effect of heat processing on thermal stability and antioxidant activity of six flavonoids. J. Food Process. Preserv. 2017, 41, e13203. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, Z.; Liu, Z.; Feng, Z.; Zhang, L.; Wan, X.; Yang, X. Identification of d-amino acids in tea leaves. Food Chem. 2020, 317, 126428. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Stepp, J.R. Green tea: The plants, processing, manufacturing and production. In Tea in Health and Disease Prevention; Preedy, V.R., Ed.; Academic Press: London, UK, 2013; pp. 19–31. [Google Scholar]
- Yılmaz, C.; Özdemir, F.; Gökmen, V. Investigation of free amino acids, bioactive and neuroactive compounds in different types of tea and effect of black tea processing. LWT-Food Sci. Technol. 2020, 117, 108655. [Google Scholar] [CrossRef]
- Fernstrom, J.D.; Langham, K.A.; Marcelino, L.M.; Irvine, Z.L.; Fernstrom, M.H.; Kaye, W.H. The ingestion of different dietary proteins by humans induces large changes in the plasma tryptophan ratio, a predictor of brain tryptophan uptake and serotonin synthesis. Clin. Nutr. 2013, 32, 1073–1076. [Google Scholar] [CrossRef] [Green Version]
- Hunyadi, A.; Martins, A.; Hsieh, T.J.; Seres, A.; Zupkó, I. Chlorogenic acid and rutin play a major role in the in vivo anti-diabetic activity of Morus alba leaf extract on type II diabetic rats. PLoS ONE 2012, 7, e50619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeni, A.L.B.; Moreira, T.D.; Dalmagro, A.P.; Camargo, A.; Bini, L.A.; Simionatto, E.L.; Scharf, D.R. Evaluation of phenolic compounds and lipid-lowering effect of Morus nigra leaves extract. An. Acad. Bras. Cienc. 2017, 89, 2805–2815. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.J.; Choi, S.W. Quantitative changes of polyphenolic compounds in mulberry (Morus alba L.) leaves in relation to varieties, harvest period, and heat processing. Prev. Nutr. Food Sci. 2012, 17, 280–285. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Guo, J.; Yuan, J. In vitro antioxidant properties of rutin. LWT-Food Sci. Technol. 2008, 41, 1060–1066. [Google Scholar] [CrossRef]
- Umar, S.; Mishra, N.K.; Pal, K.; Sajad, M.; Ansari, M.M.; Ahmad, S.; Khan, H.A. Protective effect of rutin in attenuation of collagen-induced arthritis in Wistar rat by inhibiting inflammation and oxidative stress. Indian J. Rheumatol. 2012, 7, 191–198. [Google Scholar] [CrossRef]
- Chua, L.S. A review on plant-based rutin extraction methods and its pharmacological activities. J. Ethnopharmacol. 2013, 150, 805–817. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.C.; Li, S.L.; Zhang, X.Q.; Ye, W.C.; Zhang, Q.W. Flavonoids with α-glucosidase inhibitory activities and their contents in the leaves of Morus atropurpurea. Chin. Med. 2013, 8, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Pothinuch, P.; Tongchitpakdee, S. Phenolic analysis for classification of mulberry (Morus spp.) leaves according to cultivar and leaf age. J. Food Qual. 2019, 2019, 1–11. [Google Scholar] [CrossRef]
- Fish, P.V.; Steadman, D.; Bayle, E.D.; Whiting, P. New approaches for the treatment of Alzheimer’s disease. Bioorg. Med. Chem. Lett. 2019, 29, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Oboh, G.; Agunloye, O.M.; Akinyemi, A.J.; Ademiluyi, A.O.; Adefegha, S.A. Comparative study on the inhibitory effect of caffeic and chlorogenic acids on key enzymes linked to Alzheimer’s disease and some pro-oxidant induced oxidative stress in rats’ brain-in vitro. Neurochem. Res. 2013, 38, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Szwajgier, D. Anticholinesterase activity of phenolic acids and their derivatives. Z. Naturforsch. C J. Biosci. 2013, 68, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Ademosun, A.O.; Oboh, G.; Bello, F.; Ayeni, P.O. Antioxidative properties and effect of quercetin and its glycosylated form (Rutin) on acetylcholinesterase and butyrylcholinesterase activities. Evid. Based Complementary Altern. Med. 2016, 21, NP11–NP17. [Google Scholar] [CrossRef]
- Oh, J.; Jo, H.; Cho, A.R.; Kim, S.J.; Han, J. Antioxidant and antimicrobial activities of various leafy herbal teas. Food Control. 2013, 31, 403–409. [Google Scholar] [CrossRef]
- Kim, T.K.; Kleszczyński, K.; Janjetovic, Z.; Sweatman, T.; Lin, Z.; Li, W.; Slominski, A.T. Metabolism of melatonin and biological activity of intermediates of melatoninergic pathway in human skin cells. FASEB J. 2013, 27, 2742–2755. [Google Scholar] [CrossRef]
- Wang, L.; Halquist, M.S.; Sweet, D.H. Simultaneous determination of gallic acid and gentisic acid in organic anion transporter expressing cells by liquid chromatography–tandem mass spectrometry. J. Chromatogr. B 2013, 937, 91–96. [Google Scholar] [CrossRef]
- Um, M.; Shin, G.J.; Lee, J.W. Extraction of total phenolic compounds from yellow poplar hydrolysate and evaluation of their antioxidant activities. Ind. Crops Prod. 2017, 97, 574–581. [Google Scholar] [CrossRef]
- Copra-Janićijević, A.; Culum, D.; Vidic, D.; Tahirović, A.; Klepo, L.; Bašić, N. Chemical composition and antioxidant activity of the endemic Crataegus microphylla Koch subsp. malyana K. I. Chr. & Janjić from Bosnia. Ind. Crops Prod. 2018, 113, 75–79. [Google Scholar]
- Arabshahi-Delouee, S.; Urooj, A. Antioxidant properties of various solvent extracts of mulberry (Morus indica L.) leaves. Food Chem. 2007, 102, 1233–1240. [Google Scholar] [CrossRef]
- Shalaby, E.A.; Shanab, S.M. Comparison of DPPH and ABTS assays for determining antioxidant potential of water and methanol extracts of Spirulina platensis. Indian J. Mar. Sci. 2013, 42, 556–564. [Google Scholar]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Byrne, D.H. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compost. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Petrachaianan, T.; Chaiyasirisuwan, S.; Athikomkulchai, S.; Sareedenchai, V. Screening of acetylcholinesterase inhibitory activity in essential oil from Myrtaceae. Thai J. Pharm. Sci. 2019, 43, 63–68. [Google Scholar]
| Samples | TPC (mg GAE/g Extract) | TFC (mg QE/g Extract) | Antioxidant Capacities | AChE Inhibition IC50 (µg/mL) | ||
|---|---|---|---|---|---|---|
| DPPH IC50 (µg/mL) | ABTS IC50 (µg/mL) | FRAP (mmole/100 g Extract) | ||||
| YB-FL | 390.89 ± 3.90 a | 10.15 ± 0.21 c | 96.17 ± 2.28 b | 86.05 ± 1.40 b | 163.99 ± 9.26 b | 165.24 ± 5.11 c |
| YB-GT | 194.69 ± 2.81 c | 29.42 ± 1.06 b | 253.70 ± 1.71 d | 145.22 ± 6.20 c | 143.15 ± 0.44 c | 160.66 ± 6.51 c |
| YB-BT | 261.10 ± 1.69 b | 39.09 ± 0.48 a | 117.69 ± 0.90 c | 90.40 ± 2.18 b | 187.54 ± 0.94 a | 146.53 ± 2.66 b |
| KP-FL | 290.94 ± 4.56 a | 17.59 ± 0.23 b | 128.97 ± 1.17 d | 88.71 ± 1.47 d | 111.62 ± 1.96 c | 163.07 ± 2.30 c |
| KP-GT | 129.93 ± 2.37 c | 30.76 ± 0.05 a | 121.55 ± 1.23 c | 76.89 ± 1.32 c | 173.52 ± 1.29 b | 156.01 ± 3.19 b |
| KP-BT | 252.15 ± 4.72 b | 30.24 ± 0.17 a | 117.75 ± 1.42 b | 67.35 ± 1.15 b | 239.26 ± 1.78 a | 159.51 ± 4.24 b,c |
| Trolox | - | - | 3.40 ± 0.01 a | 4.49 ± 0.04 a | 40.89 ± 0.51 d | - |
| Galantamine | - | - | - | - | - | 1.06 ± 0.08 a |
| Samples | Tryptophan (µg/g Extract) | Melatonin (µg/g Extract) | Phenolics (mg/g Extract) | Flavonoids (mg/g Extract) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Gallic Acid | Caffeic Acid | Gentisic Acid | Protocatechuic Acid | Chlorogenic Acid | Rutin | Myricetin | |||
| YB-FL | 29.54 ± 4.03 c | 9.77 ± 0.32 | 1.84 ± 0.08 c | 7.67 ± 0.15 b | 17.58 ± 0.27 c | 4.91 ± 0.45 c | 6580.60 ± 35.28 a | 1009.75 ± 11.29 a | ND |
| YB-GT | 113.03 ± 2.6 b | ND | 5.95 ± 0.32 a | 4.78 ± 0.88 c | 50.84 ± 1.84 b | 22.51 ± 1.06 a | 5832.83 ± 15.82 b | 109.48 ± 1.55 c | ND |
| YB-BT | 673.72 ± 4.43 a | ND | 3.23 ± 0.47 b | 11.91 ± 1.00 a | 54.00 ± 0.79 a | 13.73 ± 0.38 b | 19.63 ± 0.92 c | 751.33 ± 13.80 b | ND |
| KP-FL | 209.40 ± 11.87 b | ND | 2.49 ± 0.27 a | 5.80 ± 0.14 c | 16.13 ± 0.54 c | 6.18 ± 0.13 c | 11907.10 ± 151.80 a | 773.76 ± 26.20 a | ND |
| KP-GT | 210.86 ± 1.38 b | ND | 2.54 ± 0.13 a | 7.77 ± 0.74 b | 36.49 ± 2.35 b | 7.75 ± 0.14 b | 5122.08 ± 50.75 b | 372.66 ± 6.90 c | ND |
| KP-BT | 481.49 ± 10.11 a | ND | 2.65 ± 0.24 a | 11.17 ± 0.80 a | 46.93 ± 1.31 a | 10.73 ± 0.53 a | 1330.69 ± 11.36 c | 543.14 ± 4.04 b | ND |
| Samples | Fresh Weights (g) | Extracts (g) | Yields (%) |
|---|---|---|---|
| YB-FL | 500.00 | 23.12 | 4.63 |
| YB-GT | 100.00 | 3.40 | 3.40 |
| YB-BT | 100.00 | 2.13 | 2.13 |
| KP-FL | 500.00 | 26.43 | 5.30 |
| KP-GT | 100.00 | 3.07 | 3.07 |
| KP-BT | 100.00 | 2.93 | 2.93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panyatip, P.; Padumanonda, T.; Yongram, C.; Kasikorn, T.; Sungthong, B.; Puthongking, P. Impact of Tea Processing on Tryptophan, Melatonin, Phenolic and Flavonoid Contents in Mulberry (Morus alba L.) Leaves: Quantitative Analysis by LC-MS/MS. Molecules 2022, 27, 4979. https://doi.org/10.3390/molecules27154979
Panyatip P, Padumanonda T, Yongram C, Kasikorn T, Sungthong B, Puthongking P. Impact of Tea Processing on Tryptophan, Melatonin, Phenolic and Flavonoid Contents in Mulberry (Morus alba L.) Leaves: Quantitative Analysis by LC-MS/MS. Molecules. 2022; 27(15):4979. https://doi.org/10.3390/molecules27154979
Chicago/Turabian StylePanyatip, Panyada, Tanit Padumanonda, Chawalit Yongram, Tiantip Kasikorn, Bunleu Sungthong, and Ploenthip Puthongking. 2022. "Impact of Tea Processing on Tryptophan, Melatonin, Phenolic and Flavonoid Contents in Mulberry (Morus alba L.) Leaves: Quantitative Analysis by LC-MS/MS" Molecules 27, no. 15: 4979. https://doi.org/10.3390/molecules27154979
APA StylePanyatip, P., Padumanonda, T., Yongram, C., Kasikorn, T., Sungthong, B., & Puthongking, P. (2022). Impact of Tea Processing on Tryptophan, Melatonin, Phenolic and Flavonoid Contents in Mulberry (Morus alba L.) Leaves: Quantitative Analysis by LC-MS/MS. Molecules, 27(15), 4979. https://doi.org/10.3390/molecules27154979







