Abstract
Hematopoietic cell transplant (HCT), used for treatment of many malignant and non-malignant pediatric diseases, is associated with serious complications, limiting this therapy’s benefit. Acute kidney injury (AKI), seen often after HCT, can occur at different stages of the transplant process and contributes to morbidity and mortality after HCT. The etiology of AKI is often multifactorial, including kidney hypoperfusion, nephrotoxicity from immunosuppressive and antimicrobial agents, and other transplant-related complications such as transplant-associated thrombotic microangiopathy and sinusoidal obstructive syndrome. Early recognition of AKI is crucial to prevent further AKI and associated complications. Initial management includes identifying the etiology of AKI, preventing further kidney hypoperfusion, adjusting nephrotoxic medications, and preventing fluid overload. Some patients will require further support with kidney replacement therapy to manage fluid overload and AKI. Biomarkers of AKI, such as neutrophil gelatinase-associated lipocalin can aid in detecting AKI before a rise in serum creatinine, allowing earlier intervention. Long-term kidney dysfunction is also prominent in this population. Therefore, long-term follow-up and monitoring of renal function (glomerular filtration rate, microalbuminuria) is required along with management of hypertension, which can contribute to chronic kidney disease.
1. Acute Kidney Injury
AKI is a relatively common complication after HCT, with a reported incidence rate of 21–84% [1,2]. Table 1 summarizes published studies reporting AKI in the pediatric HCT population [3,4,5,6,7,8,9,10]. In a metanalysis including 571 children post HCT, AKI developed in 124 (21.7%) within the first 100 days post-transplant, with a median onset of 4–6 weeks [11]. In a cohort of 408 adult patients post HCT, AKI was observed in 64% within 100 days post-transplant, but most had mild AKI (62% had stage 1 AKI). More importantly, worse outcome was observed in patients with AKI. Compared to patients without AKI, patients in whom AKI developed had inferior 2-year overall survival and higher incidence of grade 3–4 acute graft versus host disease (GVHD) [12].

Table 1.
Summary of studies of AKI in pediatric HCT patients.
Common risk factors for kidney injury include myeloablative conditioning, older age, acute GVHD, and SOS (Table 1). Satwani et al. observed a significant increase in the incidence of kidney injury in children who received myeloablative conditioning versus reduced intensity conditioning (45.7% and 17.1% respectively) [6]. In addition to its contribution to a higher rate of mortality, previous AKI predisposes patients to chronic kidney disease (CKD). In a cohort of 158 adult allogeneic HCT survivors, the risk of CKD ≥ stage 3 was approximately 10-fold higher in patients in whom AKI developed following HCT [13]. AKI is encountered at any stage in the transplant process, although often at the earlier stages. Early in the pre-transplant phase, many children receive myeloablative conditioning regimen with or without total body irradiation (TBI) that can induce kidney injury. Shortly after transplant, nephrotoxic immunosuppressive medications such as calcineurin inhibitors are given to mitigate the risk of GVHD. In addition, children are at a higher risk of infection and subsequent sepsis due to their immune-compromised status. In many instances, the etiology of AKI is multifactorial and includes hypoperfusion in the setting of capillary leak or sepsis resulting in acute tubular necrosis, drug induced nephrotoxicity, thrombotic microangiopathy (TMA), and sinusoidal obstruction syndrome (SOS) (Figure 1) [1]. Drug induced nephrotoxicity is relatively common in HCT patients. Antimicrobials that are often used to treat infections post HCT such as aminoglycoside, vancomycin, or amphotericin can induce direct kidney injury. Nephrotoxicity is also encountered with calcineurin inhibitors such as cyclosporin or tacrolimus that can cause kidney arteriolar vasoconstriction via activation of the renin–angiotensin–aldosterone system [14]. In addition, calcineurin inhibitors may trigger endothelial injury and subsequent TMA [15]. Moreover, kidneys can be a target of GVHD, although less described than other organs like skin, liver, and lungs. Kidney injury related to GVHD is mediated by donor T-cells as well as proinflammatory cytokines. Kidney GVHD can present as AKI, nephrotic syndrome, glomerulonephritis, and TMA [16]. However, the most common presentation is nephrotic syndrome with a high degree of proteinuria, hypoalbuminemia, and edema. Hemorrhagic cystitis can cause obstructive kidney injury when clots in the bladder obstruct the outflow tract. The etiology of hemorrhagic cystitis is usually multifactorial, but often encountered with the use of cyclophosphamide or in the context of reactivation of virus infections such as BK virus, adenovirus, and cytomegalovirus. Treatment include hyperhydration, diuresis, and bladder irrigation with a three-way bladder catheter.
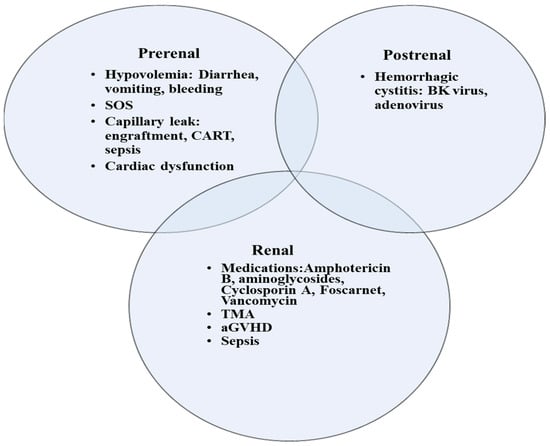
Figure 1.
Etiologies of AKI in children following HCT. SOS, sinusoidal obstructive syndrome; TMA, thrombotic microangiopathy; aGVHD, acute graft versus host disease; CART, Chimeric antigen receptor (CAR) T-cell therapy.
2. Criteria for AKI Diagnosis and Staging
One reason that the incidence of AKI varies so widely (21–84%) is that non-standardized definitions of AKI were used in prior studies. More standardized and widely used AKI scoring systems have now been developed, the most recent and commonly used of which are the KDIGO guidelines. Diagnosis and staging of AKI relies mainly on two factors: serum creatinine (sCr) level and urine output (Table 2). However, many factors affect sCr level, such as muscle mass and age, leading to over- or under-diagnosis of AKI. Volume status also influences creatinine level; for example, fluid overload, which is relatively common in children with AKI post HCT, results in an erroneous lower sCr value, leading to underestimation of the degree of AKI and a delay in diagnosis. Although methods of correcting sCr for particular clinical scenarios have been proposed, such as the following formula for correcting sCr for fluid overload: Corrected cr = sCr × [1 + Net fluid balance/Total body water] where total body water (TBW) = 0.6 × weight (kg), sCr can still have some limitations in accurately assessing eGFR [17].

Table 2.
Staging criteria for AKI.
3. Special Disease Conditions Post HCT That Are Associated with AKI
3.1. Transplant-Associated Thrombotic Microangiopathy
Transplant-associated thrombotic microangiopathy (TA-TMA) is a life-threatening complication that is encountered early in the post-HCT phase. The incidence of TA-TMA in children is 16%, with a median onset of 47 days post-transplant [18]. Risk factors for TA-TMA include acute GVHD, infectious process (especially viral), mismatched donor, multiple HCTs, and myeloablative conditioning [18]. The pathophysiology of TA-TMA involves an initial endothelial injury triggered by factors such as chemotherapy or infection that results in an increase in the proinflammatory cytokines, procoagulant factors, and soluble adhesion molecules. This combination promotes further endothelial injury and initiates and propagates the complement cascade, resulting in platelet aggregation, fibrin deposition, and microthrombi formation. The laboratory characteristics of TA-TMA include elevated lactated dehydrogenase, non-immune mediated hemolytic anemia, presence of schistocytes on the peripheral blood smear, thrombocytopenia, proteinuria, and elevated plasma sC5b-9 (≥244 ng/mL). Renal involvement with microangiopathy is common in patients with TA-TMA. Renal histopathology findings include fibrin deposition in the glomeruli, narrowing of the capillary lumen, presence of fragmented red blood cells, basement membrane duplication, and edema of the endothelium [19]. In a cohort of 98 children in whom TA-TMA developed post HCT, AKI developed in 66% of the cohort, and 15% required renal replacement therapy [20]. Hypertension is one of the earliest manifestations in these patients, occurring as early as 14 days before the diagnosis of TA-TMA [21]. Moreover, proteinuria is common and present in 80% of children with TA-TMA. Proteinuria is an important indicator of renal dysfunction that presents early during TA-TMA and is a marker of severe disease. It is associated with a higher 6-month mortality rate (27% in patients with proteinuria vs 5% in patients without proteinuria [p = 0.04]) [20]. Additionally, the overall survival rate of children with TA-TMA (78%; 76/98) is significantly lower than that of those without TA-TMA (93%; 490/516) (p = 0.001) [20].
Eculizumab is a monoclonal antibody against the complement component C5, which blocks the formation of the membrane attack complex (MAC or C5b-9) and thus prevents endothelial damage. In a cohort of 64 pediatric HCT patients with high-risk TA-TMA and multiorgan dysfunction, the survival rate improved dramatically with the use of eculizumab (66% in 1-year post HCT in treated vs 16.7% in a previously reported untreated cohort) [22]. Notably, 70% of survivors still had proteinuria on long-term follow-up, and their best cystatin C estimated glomerular filtration rate (eGFR) after recovery from TA-TMA was still lower than their pre-transplant baseline value [22].
3.2. Sinusoidal Obstruction Syndrome
Sinusoidal obstruction syndrome (SOS) is associated with multiorgan dysfunction and a high mortality rate [23]. SOS occurs in the early stage post HCT secondary to cytotoxic therapy or radiotherapy [24]. The incidence rate is 20–60%. Diagnosis of SOS is based on the following criteria (two or more criteria present) [25]:
- Consumptive and transfusion-refractory thrombocytopenia;
- Weight gain on 3 consecutive days despite the use of diuretics, or a weight gain of >5% above baseline weight within 72 h;
- Increase in bilirubin from baseline on 3 consecutive days, or bilirubin ≥ 2 mg/dL within 72 h;
- Hepatomegaly (best if supported by imaging) above baseline value;
- Ascites (best if supported by imaging) above baseline.
Kidney injury in SOS is attributed to hypoperfusion and vasoconstriction and is associated with fluid overload. Managing the kidney injury requires fluid restriction and use of diuretics to reduce fluid overload. Renal replacement therapy may be necessary if fluid overload persists, and urine output remains inadequate despite diuretic treatment [26]. In such cases, continuous kidney replacement therapy (CKRT) is typically the preferred modality, enabling more controlled and dynamic adjustment of fluid removal, particularly in hemodynamically unstable patients. Raina et al. described the use of CKRT in six children who had SOS post HCT: Four survived (mortality rate = 34%), and one survivor experienced end-stage renal disease and kidney transplantation [27]. Defibrotide, the drug of choice for treatment of SOS, reduces the mortality rate and reverses organ dysfunction [28].
3.3. Fluid Overload
Fluid overload (FO) is common in critically ill children and negatively affects outcome [29,30]. Additionally, FO can exacerbate kidney injury by worsening kidney venous hypertension, impairing perfusion pressure capacity of the glomerular capillaries. Cumulative fluid balance is often used interchangeably with fluid overload and is calculated as follows: Fluid intake – Fluid output (L)/ICU admission weight (kg) × 100 [31]. FO can also be calculated by comparing current weight to admission weight if fluid balance information is not available. FO > 10% is common in critically ill children and was observed in 33% of a large cohort of 1017 critically ill children [29]. FO was associated with higher risk of mortality, kidney adverse events, and increased duration of mechanical ventilation (MV) and ICU stay. A metanalysis including 44 pediatric studies showed a 6% increase in odds of mortality with each 1% increase in FO [30]. The adverse effect of FO is also prevalent in the pediatric HCT population [32,33]. Children with FO > 10% at CKRT initiation were 6.16 times more likely to die than those with FO ≤ 10% in a cohort of 68 critically ill children with cancer and post HCT (23 patients). In mechanically ventilated pediatric HCT patients, higher cumulative fluid balance is associated with an increased mortality rate [33,34]. In a cohort of 198 children with acute respiratory failure, a 3% increase in ICU mortality occurred for every 1% increase in the cumulative fluid balance on day 3 of the course of mechanical ventilation (adjusted odds ratio = 1.03; 95% CI, 1.00–1.07) [34].
FO is a new post-HCT toxicity category with the following proposed grading system [1]:
Grade 1: AKI Stage 0 or 1; fluid overload < 10%
Grade 2: AKI Stage 0 or 1; fluid overload > 10%
Grade 3: AKI Stage 2 or 3; fluid overload < 10%
Grade 4: AKI Stage 2 or 3; fluid overload > 10%
Close monitoring of daily fluid balance and weight is crucial to prevent FO. This allows for early recognition and prompt management of FO to reduce further kidney injury and improve survival. Unfortunately, even significant FO is often missed. Al-Lawati et al. found that clinicians did not recognize FO > 15% in 30% of pediatric patients [35]. In addition to concentrating the volume of the administered medications, diuretics such as furosemide or bumetanide are administered either intermittently or as a continuous infusion. CKRT may be considered when FO is > 10% if diuretic therapy fails to achieve euvolemia.
3.4. CAR T-Cell Therapy
Chimeric antigen receptor (CAR) T-cell therapy, used for treatment of hematologic malignancies, involves the utilization of engineered cytotoxic T-cell to recognize specific tumor antigen. AKI occurs with this therapy secondary to cytokine release syndrome (CRS), a well described complication of this therapy which can lead to organ dysfunction. Hypoperfusion secondary to capillary leak and proinflammatory cytokines contribute to AKI encountered post CAR-T cell therapy. AKI is usually mild in these cases [36]. In a cohort of 39 children with acute lymphoblastic leukemia treated with an anti-CD19 CAR T-cell therapy, 46% developed AKI with grade 3–4 CRS although none of the patients required CKRT [37]. Similarly, in adults, the reported cumulative incidence of AKI by day 100 was 30% in patients with non-Hodgkin lymphoma and none required CKRT [36]. Prompt management of serious CRS toxicity is important to ameliorate the inflammatory response and reduce organ dysfunction. Such management includes anti-cytokine therapy, such as tocilizumab, and corticosteroids [38].
4. Continuous Kidney Replacement Therapy
Nearly one-third of patients with AKI require kidney replacement therapy (KRT) [1]. CKRT is used often in the ICU to deliver KRT because it is tolerated better than intermittent hemodialysis (IHD) in hemodynamically unstable critically ill children. During CKRT, fluid removal and solute clearance occur continuously, promoting better control of fluid status. Solute clearance occurs by either convection, diffusion, or both, whereas fluid is removed via ultrafiltration. Hemofiltration modes of CKRT can increase removal of small and medium-sized solutes by convection (solute drag); in contrast, hemodialysis modes mainly remove small-sized molecules by diffusion (concentration gradient). Flores et al. reported better survival in children post HCT (51 patients) with convective modes of CKRT than with dialytic mode [39]. However, mortality was similar using the hemofiltration or the hemodialysis modality in a metanalysis including 19 randomized trials in patients with AKI [40].
No consensus exists on the optimal time for initiation of CKRT and whether early initiation can improve outcome. Most evidence is from adult randomized trials that compared the early initiation of CKRT to using a standard strategy. One of the largest adult trials, the STARRT-AKI trial, randomized 3019 critically ill adults with AKI to either an accelerated RRT strategy (initiated within 12 h in adult critically ill patients with Stage 2 or Stage 3 AKI) or a standard strategy. The accelerated RRT strategy did not reduce mortality compared to the standard strategy, and survivors of the accelerated RRT strategy had a higher risk of adverse events and dependence on kidney replacement therapy [41]. In contrast, in the ELAIN trial that included 231 critically ill patients with AKI, a lower mortality in the early RRT group compared to the delayed initiation group was observed (39% versus 54% respectively) [42]. A recent metanalysis that had 5193 critically ill patients with AKI did not demonstrate improved survival or recovery of renal function using the early RRT initiation strategy rather than later RRT [43]. Although the evidence does not support early initiation of KRT in the context of AKI, ample evidence in the literature supports initiating CKRT at the earlier stage of FO to reduce mortality. CKRT should be considered in children with FO > 10%, especially when associated with pulmonary edema or worsening AKI.
The recommended dose of CKRT is 20–25 mL/kg/h, or 2000 mL/1.73 m2/h in children. However, the delivered dose is usually less than prescribed dose due to interruptions that occur during CKRT, such as circuit clotting, scheduled filter changes, and pauses for other procedures. Thus, KDIGO recommends a 25–30 mL/kg/h prescribed dose to achieve a delivered dose of 20–25 mL/kg/h [44]. A CKRT dose > 35 mL/kg/h is not recommended as it does not reduce mortality [45]. Anticoagulation is crucial to prevent circuit clotting and extend the circuit lifespan, thus minimizing treatment interruptions. Systemic heparin infusion or regional citrate anticoagulation (RCA) can be used for anticoagulation. In a cohort of 638 critically ill adults on CRRT, median filter life span was significantly higher with RCA than heparin (47 h vs. 26 h). Bleeding complications were also significantly less in the citrate group than in the heparin group (5.1% vs. 16.9%) [46]. Therefore, RCA may be a better option for anticoagulation than systemic heparin in the HCT population that is at higher risk of bleeding due to thrombocytopenia or coagulopathy.
5. Transition from CKRT to IHD/Discontinuation of CKRT
The optimal timing for successful discontinuation of CKRT or switch to IHD is difficult to predict. Renal recovery is usually preceded by an increase in urine output. Urine output > 500 cc/day is used in some adult patients as a criterion to discontinue KRT [47]. Factors that have been shown to predict successful liberation include the hourly urine output within 12 h before CKRT discontinuation, serum creatinine level within 24 h before liberation, and the cumulative fluid balance (from ICU admission to CKRT discontinuation) [48]. In general, children are switched from CKRT to IHD when FO is resolved and they are hemodynamically stable.
6. Outcomes of KRT
ICU mortality in children post HCT requiring CKRT is estimated to be 52–65% [2,49]. The 1-year overall survival rate is also poor (27.4% (95% CI: 16–40.5%, p < 0.0001)) [1]. Reported factors that are associated with mortality include FO > 10%, mechanical ventilation, vasoactive support, and neutropenia at the end of CKRT [2].
7. Biomarkers of AKI in Children with HCT
Given the potential shortcomings of sCr as a marker of AKI, several additional biomarkers of AKI have been developed and studied. These biomarkers measure either glomerular function or renal tubular damage and can aid in early detection of AKI (Table 3).

Table 3.
Biomarkers in AKI.
Cystatin C
Cystatin C (CysC) is a low molecular weight protein which is present in all nucleated cells and inhibits cysteine proteases. It is freely filtered by the glomerulus, then reabsorbed by the proximal tubule epithelium and catabolized. It is present in urine only in instances of tubular injury. Serum CysC levels can be altered by several factors such as corticosteroids, inflammatory status, and chemotherapy. Levels may also be elevated in patients with leukemia without any documented AKI secondary to increased cell turnover [1]. CysC can detect decline in renal function and GFR 24–48 h before serum creatinine levels. Furthermore, a higher CysC level at discontinuation of KRT was found to be an independent predictor of chronic dialysis [50].
8. Tubular Injury Markers
Several tubular injury markers have been described and investigated for their utility in early recognition of AKI. Neutrophil gelatinase-associated lipocalin (NGAL) levels are elevated in the urine early after ischemic, septic, or toxic injury and precede the rise in serum creatinine levels by 48 h [51]. In addition, urine NGAL can differentiate intrinsic renal damage (where it is elevated) from prerenal acute injury related to hemodynamic alterations due to hypovolemia [52]. N-acetyl-beta-D-glycosaminidase (NAG) urine level is highly specific to tubular injury [53]. Kidney injury molecule-1 (KIM-1) participates in both kidney injury and healing processes [54]. In fact, urinary KIM-1 is highly sensitive and specific for drug-induced kidney injury.
Fatty acid-binding protein 1 is another early biomarker of AKI and can predict the need for dialysis [55]. An increased level of L-FABP at the time of ICU admission is associated with higher risk of AKI and mortality rate [56,57]. The NGAL and L-FABP combination can predict renal recovery after AKI (higher levels associated with non-recovery) [58]. Urinary IL-18 level can predict the initiation of RRT and mortality [59,60]. The combined product of tissue inhibitor of metalloproteinases-2 (TIMP-2) and insulin-like growth factor–binding protein-7 (IGFBP-7), expressed as [TIMP-2] [IGFBP7], predicts the risk of AKI as well as the need for CKRT and death in critically ill adults [61,62].
9. Chronic Kidney Disease
CKD in children post HCT has a reported incidence of 48%. CKD is defined by estimated GFR < 90 mL/min/1.73 BSA or presence of markers of kidney damage for >3 months [63]. CKD can develop as early as 6 months and up to 10 years following transplant. Incidence of CKD in this population is 10-fold higher than in the healthy population. In a cohort of 1635 adult and pediatric HCT patients, CKD developed in 23% [64].
The most common etiologies and risk factors for CKD development are TMA, total body irradiation, nephrotic syndrome, AKI, acute GVHD, and drug toxicity (calcineurin inhibitors) [65]. Estimated GFR at the time of AKI is an important risk factor for the development of CKD. In a cohort of 275 children post allogeneic HCT, CKD developed in 69.5% and 69.8% at 1 and 3 years if GFR was < 80 mL/min/1.73 m2 at the initial AKI episode [66]. Therefore, renal function in these higher-risk children who developed AKI with GFR <80 mL/min/1.73 m2 must be monitored closely for early detection of CKD. Similar to the approach to AKI noted previously, the management of CKD post HCT requires regular monitoring for long term changes in estimated GFR to identify patients with CKD, followed by preventive strategies that minimize nephrotoxic medication exposure, ensure adequate hydration and nutrition, optimize blood pressure control, and minimize proteinuria.
Albuminuria (albumin-to-creatinine ratio over 30 mg/g) is an important parameter to monitor in children post HCT during long-term follow-up for early recognition of CKD. Albuminuria is relatively common (detected in 50% of patients one-year post HCT) [67]. Furthermore, albuminuria at day 100 was associated with CKD at 1 year (OR = 4.0; 95% CI = 1.1 to 14.6). Proteinuria at day 100 conveyed a six-fold increase in the risk of non-relapse mortality by 1 year post HCT. Moreover, hypertension seen in 20–70% of patients post HCT can contribute to the development and progression of CKD [68]. Close monitoring for the development of hypertension is warranted for children post HCT and should follow similar guidelines for the detection and management of hypertension in children in other settings, including obtaining 24-h ambulatory blood pressure monitoring when available [69]. Angiotensin-converting enzyme inhibitors (ACEi) or angiotensin II receptor blockers (ARB) can be used to preserve kidney function, control hypertension, and reduce proteinuria [69,70]. Other antihypertensive medications that can be utilized for optimal control of blood pressure or if ACEi/ARB is not tolerated include long-acting calcium channel blockers and diuretics. In addition to the initiation of antihypertensive medications, other lifestyle modifications including low sodium diet and increased physical activity should be encouraged to reduce long term risk of hypertension. Target BP goals for patients with CKD should be less than or equal to 50th percentile for age, sex, and height unless achieving this is limited by symptomatic hypotension [69,71].
Additional long-term follow-up for HCT patients with CKD includes monitoring and treatment of other consequences of progressive CKD including electrolyte disturbances, such as metabolic acidosis, anemia of CKD, and CKD-related bone disease [65]. Consultation with nephrology is recommended for patients with any degree of CKD to coordinate CKD care, and frequency of follow up is then based on the severity of CKD. These multiple complications of CKD highlight the need for a multidisciplinary approach to post-HCT patients with CKD as these patients may have unique risk factors and needs related to the underlying oncologic diagnosis and post-HCT course to consider when managing their CKD.
Author Contributions
Conceptualization, L.E.; resources, V.J., J.A. and L.E.; writing—original draft preparation, V.J., J.A. and L.E.; writing—review and editing, V.J., J.A. and L.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
Special thanks to Cherise Guess for editing the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Raina, R.; Abu-Arja, R.; Sethi, S.; Dua, R.; Chakraborty, R.; Dibb, J.T.; Basu, R.K.; Bissler, J.; Felix, M.B.; Brophy, P.; et al. Acute kidney injury in pediatric hematopoietic cell transplantation: Critical appraisal and consensus. Pediatr. Nephrol. 2022, 37, 1179–1203. [Google Scholar] [CrossRef] [PubMed]
- Elbahlawan, L.; Bissler, J.; Morrison, R.R. Continuous Renal Replacement Therapy: A Review of Use and Application in Pediatric Hematopoietic Stem Cell Transplant Recipients. Front. Oncol. 2021, 11, 632263. [Google Scholar] [CrossRef] [PubMed]
- Daraskevicius, J.; Azukaitis, K.; Dziugeviciute-Tupko, J.; Peciulyte, M.; Planciunaite, R.; Vaitkeviciene, G.; Rascon, J.; Jankauskiene, A. Phenotypes and Baseline Risk Factors of Acute Kidney Injury in Children After Allogeneic Hematopoietic Stem Cell Transplantation. Front. Pediatr. 2020, 8, 499. [Google Scholar] [CrossRef] [PubMed]
- Koh, K.N.; Sunkara, A.; Kang, G.; Sooter, A.; Mulrooney, D.A.; Triplett, B.; Onder, A.M.; Bissler, J.; Cunningham, L.C. Acute Kidney Injury in Pediatric Patients Receiving Allogeneic Hematopoietic Cell Transplantation: Incidence, Risk Factors, and Outcomes. Biol. Blood Marrow Transplant. 2018, 24, 758–764. [Google Scholar] [CrossRef]
- Kizilbash, S.J.; Kashtan, C.E.; Chavers, B.M.; Cao, Q.; Smith, A.R. Acute Kidney Injury and the Risk of Mortality in Children Undergoing Hematopoietic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2016, 22, 1264–1270. [Google Scholar] [CrossRef]
- Satwani, P.; Bavishi, S.; Jin, Z.; Jacobson, J.S.; Baker, C.; Duffy, D.; Lowe, L.; Morris, E.; Cairo, M.S. Risk factors associated with kidney injury and the impact of kidney injury on overall survival in pediatric recipients following allogeneic stem cell transplant. Biol. Blood Marrow Transplant. 2011, 17, 1472–1480. [Google Scholar] [CrossRef]
- Yu, Z.P.; Ding, J.H.; Chen, B.A.; Liu, B.C.; Liu, H.; Li, Y.F.; Ding, B.H.; Qian, J. Risk factors for acute kidney injury in patients undergoing allogeneic hematopoietic stem cell transplantation. Chin. J. Cancer 2010, 29, 946–951. [Google Scholar] [CrossRef]
- Ileri, T.; Ertem, M.; Ozcakar, Z.B.; Ince, E.U.; Biyikli, Z.; Uysal, Z.; Ekim, M.; Yalcinkaya, F. Prospective evaluation of acute and chronic renal function in children following matched related donor hematopoietic stem cell transplantation. Pediatr. Transplant. 2010, 14, 138–144. [Google Scholar] [CrossRef]
- Hazar, V.; Gungor, O.; Guven, A.G.; Aydin, F.; Akbas, H.; Gungor, F.; Tezcan, G.; Akman, S.; Yesilipek, A. Renal function after hematopoietic stem cell transplantation in children. Pediatr. Blood Cancer 2009, 53, 197–202. [Google Scholar] [CrossRef]
- Kist-van Holthe, J.E.; Goedvolk, C.A.; Brand, R.; van Weel, M.H.; Bredius, R.G.; van Oostayen, J.A.; Vossen, J.M.; van der Heijden, B.J. Prospective study of renal insufficiency after bone marrow transplantation. Pediatr. Nephrol. 2002, 17, 1032–1037. [Google Scholar] [CrossRef]
- Didsbury, M.S.; Mackie, F.E.; Kennedy, S.E. A systematic review of acute kidney injury in pediatric allogeneic hematopoietic stem cell recipients. Pediatr. Transplant. 2015, 19, 460–470. [Google Scholar] [CrossRef]
- Madsen, K.; Pelletier, K.; Côté, G.; Kitchlu, A.; Chen, S.; Mattsson, J.; Pasic, I. Acute kidney injury within 100 days post allogeneic hematopoietic cell transplantation is associated with increased risk of post-transplant complications and poor transplant outcomes. Bone Marrow Transplant. 2022, 57, 1411–1420. [Google Scholar] [CrossRef]
- Ando, M.; Ohashi, K.; Akiyama, H.; Sakamaki, H.; Morito, T.; Tsuchiya, K.; Nitta, K. Chronic kidney disease in long-term survivors of myeloablative allogeneic haematopoietic cell transplantation: Prevalence and risk factors. Nephrol. Dial. Transplant. 2010, 25, 278–282. [Google Scholar] [CrossRef]
- Prókai, Á.; Csohány, R.; Sziksz, E.; Pap, D.; Balicza-Himer, L.; Boros, S.; Magda, B.; Vannay, Á.; Kis-Petik, K.; Fekete, A.; et al. Calcineurin-inhibition Results in Upregulation of Local Renin and Subsequent Vascular Endothelial Growth Factor Production in Renal Collecting Ducts. Transplantation 2016, 100, 325–333. [Google Scholar] [CrossRef]
- Wanchoo, R.; Bayer, R.L.; Bassil, C.; Jhaveri, K.D. Emerging Concepts in Hematopoietic Stem Cell Transplantation-Associated Renal Thrombotic Microangiopathy and Prospects for New Treatments. Am. J. Kidney Dis. 2018, 72, 857–865. [Google Scholar] [CrossRef]
- Catherine Joseph, J.R.A.; Benjamin, L. Laskin, sangeeta hingorani. Hematopoietic cell transplant associated kidney injury. In Onco-Nephrology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 89–98. [Google Scholar]
- Redant, S.; De Bels, D.; Barbance, O.; Massaut, J.; Honoré, P.M.; Taccone, F.S.; Biarent, D. Creatinine correction to account for fluid overload in children with acute respiratory distress syndrome treated with extracorporeal membrane oxygenation: An initial exploratory report. Pediatr. Nephrol. 2022, 37, 891–898. [Google Scholar] [CrossRef]
- Van Benschoten, V.; Roy, C.; Gupta, R.; Ouellette, L.; Hingorani, S.; Li, A. Incidence and Risk Factors of Transplantation-Associated Thrombotic Microangiopathy: A Systematic Review and Meta-Analysis. Transplant. Cell Ther. 2022, 28, 266.e261–266.e268. [Google Scholar] [CrossRef]
- Siami, K.; Kojouri, K.; Swisher, K.K.; Selby, G.B.; George, J.N.; Laszik, Z.G. Thrombotic microangiopathy after allogeneic hematopoietic stem cell transplantation: An autopsy study. Transplantation 2008, 85, 22–28. [Google Scholar] [CrossRef]
- Dandoy, C.E.; Rotz, S.; Alonso, P.B.; Klunk, A.; Desmond, C.; Huber, J.; Ingraham, H.; Higham, C.; Dvorak, C.C.; Duncan, C.; et al. A pragmatic multi-institutional approach to understanding transplant-associated thrombotic microangiopathy after stem cell transplant. Blood Adv. 2021, 5, 1–11. [Google Scholar] [CrossRef]
- Jodele, S.; Davies, S.M.; Lane, A.; Khoury, J.; Dandoy, C.; Goebel, J.; Myers, K.; Grimley, M.; Bleesing, J.; El-Bietar, J.; et al. Diagnostic and risk criteria for HSCT-associated thrombotic microangiopathy: A study in children and young adults. Blood 2014, 124, 645–653. [Google Scholar] [CrossRef]
- Jodele, S.; Dandoy, C.E.; Lane, A.; Laskin, B.L.; Teusink-Cross, A.; Myers, K.C.; Wallace, G.; Nelson, A.; Bleesing, J.; Chima, R.S.; et al. Complement blockade for TA-TMA: Lessons learned from a large pediatric cohort treated with eculizumab. Blood 2020, 135, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Coppell, J.A.; Richardson, P.G.; Soiffer, R.; Martin, P.L.; Kernan, N.A.; Chen, A.; Guinan, E.; Vogelsang, G.; Krishnan, A.; Giralt, S.; et al. Hepatic veno-occlusive disease following stem cell transplantation: Incidence, clinical course, and outcome. Biol. Blood Marrow Transplant. 2010, 16, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Bonifazi, F.; Barbato, F.; Ravaioli, F.; Sessa, M.; Defrancesco, I.; Arpinati, M.; Cavo, M.; Colecchia, A. Diagnosis and Treatment of VOD/SOS After Allogeneic Hematopoietic Stem Cell Transplantation. Front. Immunol. 2020, 11, 489. [Google Scholar] [CrossRef] [PubMed]
- Mohty, M.; Malard, F.; Abecassis, M.; Aerts, E.; Alaskar, A.S.; Aljurf, M.; Arat, M.; Bader, P.; Baron, F.; Bazarbachi, A.; et al. Sinusoidal obstruction syndrome/veno-occlusive disease: Current situation and perspectives-a position statement from the European Society for Blood and Marrow Transplantation (EBMT). Bone Marrow Transplant. 2015, 50, 781–789. [Google Scholar] [CrossRef]
- Mahadeo, K.M.; McArthur, J.; Adams, R.H.; Radhi, M.; Angelo, J.; Jeyapalan, A.; Nicol, K.; Su, L.; Rabi, H.; Auletta, J.J.; et al. Consensus Report by the Pediatric Acute Lung Injury and Sepsis Investigators and Pediatric Blood and Marrow Transplant Consortium Joint Working Committees on Supportive Care Guidelines for Management of Veno-Occlusive Disease in Children and Adolescents: Part 2-Focus on Ascites, Fluid and Electrolytes, Renal, and Transfusion Issues. Biol. Blood Marrow Transplant. 2017, 23, 2023–2033. [Google Scholar] [CrossRef]
- Raina, R.; Abusin, G.A.; Vijayaraghavan, P.; Auletta, J.J.; Cabral, L.; Hashem, H.; Vogt, B.A.; Cooke, K.R.; Abu-Arja, R.F. The role of continuous renal replacement therapy in the management of acute kidney injury associated with sinusoidal obstruction syndrome following hematopoietic cell transplantation. Pediatr. Transplant. 2018, 22, e13139. [Google Scholar] [CrossRef]
- Mohty, M.; Battista, M.L.; Blaise, D.; Calore, E.; Cesaro, S.; Maximova, N.; Perruccio, K.; Renard, C.; Wynn, R.; Zecca, M.; et al. A multicentre, multinational, prospective, observational registry study of defibrotide in patients diagnosed with veno-occlusive disease/sinusoidal obstruction syndrome after haematopoietic cell transplantation: An EBMT study. Bone Marrow Transplant. 2021, 56, 2454–2463. [Google Scholar] [CrossRef]
- Alobaidi, R.; Basu, R.K.; DeCaen, A.; Joffe, A.R.; Lequier, L.; Pannu, N.; Bagshaw, S.M. Fluid Accumulation in Critically Ill Children. Crit. Care Med. 2020, 48, 1034–1041. [Google Scholar] [CrossRef]
- Alobaidi, R.; Morgan, C.; Basu, R.K.; Stenson, E.; Featherstone, R.; Majumdar, S.R.; Bagshaw, S.M. Association Between Fluid Balance and Outcomes in Critically Ill Children: A Systematic Review and Meta-analysis. JAMA Pediatr. 2018, 172, 257–268. [Google Scholar] [CrossRef]
- Goldstein, S.L.; Currier, H.; Graf, C.; Cosio, C.C.; Brewer, E.D.; Sachdeva, R. Outcome in children receiving continuous venovenous hemofiltration. Pediatrics 2001, 107, 1309–1312. [Google Scholar] [CrossRef]
- Raymakers-Janssen, P.; Lilien, M.R.; Tibboel, D.; Kneyber, M.C.J.; Dijkstra, S.; van Woensel, J.B.M.; Lemson, J.; Cransberg, K.; van den Heuvel-Eibrink, M.M.; Wosten-van Asperen, R.M. Epidemiology and Outcome of Critically Ill Pediatric Cancer and Hematopoietic Stem Cell Transplant Patients Requiring Continuous Renal Replacement Therapy: A Retrospective Nationwide Cohort Study. Crit. Care Med. 2019, 47, e893–e901. [Google Scholar] [CrossRef]
- Elbahlawan, L.; Morrison, R.; Li, Y.; Huang, S.; Cheng, C.; Avent, Y.; Madden, R. Outcome of Acute Respiratory Failure Secondary to Engraftment in Children After Hematopoietic Stem Cell Transplant. Front. Oncol. 2020, 10, 584269. [Google Scholar] [CrossRef]
- Sallee, C.J.; Smith, L.S.; Rowan, C.M.; Heckbert, S.R.; Angelo, J.R.; Daniel, M.C.; Gertz, S.J.; Hsing, D.D.; Mahadeo, K.M.; McArthur, J.A.; et al. Early Cumulative Fluid Balance and Outcomes in Pediatric Allogeneic Hematopoietic Cell Transplant Recipients With Acute Respiratory Failure: A Multicenter Study. Front. Oncol. 2021, 11, 705602. [Google Scholar] [CrossRef]
- Al-Lawati, Z.H.; Sur, M.; Kennedy, C.E.; Akcan Arikan, A. Profile of Fluid Exposure and Recognition of Fluid Overload in Critically Ill Children. Pediatr. Crit. Care Med. 2020, 21, 760–766. [Google Scholar] [CrossRef]
- Gutgarts, V.; Jain, T.; Zheng, J.; Maloy, M.A.; Ruiz, J.D.; Pennisi, M.; Jaimes, E.A.; Perales, M.A.; Sathick, J. Acute Kidney Injury after CAR-T Cell Therapy: Low Incidence and Rapid Recovery. Biol. Blood Marrow Transplant. 2020, 26, 1071–1076. [Google Scholar] [CrossRef]
- Fitzgerald, J.C.; Weiss, S.L.; Maude, S.L.; Barrett, D.M.; Lacey, S.F.; Melenhorst, J.J.; Shaw, P.; Berg, R.A.; June, C.H.; Porter, D.L.; et al. Cytokine Release Syndrome After Chimeric Antigen Receptor T Cell Therapy for Acute Lymphoblastic Leukemia. Crit. Care Med. 2017, 45, e124–e131. [Google Scholar] [CrossRef]
- Brudno, J.N.; Kochenderfer, J.N. Recent advances in CAR T-cell toxicity: Mechanisms, manifestations and management. Blood Rev. 2019, 34, 45–55. [Google Scholar] [CrossRef]
- Flores, F.X.; Brophy, P.D.; Symons, J.M.; Fortenberry, J.D.; Chua, A.N.; Alexander, S.R.; Mahan, J.D.; Bunchman, T.E.; Blowey, D.; Somers, M.J.; et al. Continuous renal replacement therapy (CRRT) after stem cell transplantation. A report from the prospective pediatric CRRT Registry Group. Pediatr. Nephrol. 2008, 23, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, J.O.; Wald, R.; Bagshaw, S.M.; Burns, K.E.; Adhikari, N.K. Hemofiltration compared to hemodialysis for acute kidney injury: Systematic review and meta-analysis. Crit. Care 2012, 16, 1–16. [Google Scholar] [CrossRef]
- Bagshaw, S.M.; Wald, R.; Adhikari, N.K.J.; Bellomo, R.; da Costa, B.R.; Dreyfuss, D.; Du, B.; Gallagher, M.P.; Gaudry, S.; Hoste, E.A.; et al. Timing of Initiation of Renal-Replacement Therapy in Acute Kidney Injury. N. Engl. J. Med. 2020, 383, 240–251. [Google Scholar] [CrossRef]
- Zarbock, A.; Kellum, J.A.; Schmidt, C.; Van Aken, H.; Wempe, C.; Pavenstädt, H.; Boanta, A.; Gerß, J.; Meersch, M. Effect of Early vs Delayed Initiation of Renal Replacement Therapy on Mortality in Critically Ill Patients With Acute Kidney Injury: The ELAIN Randomized Clinical Trial. Jama 2016, 315, 2190–2199. [Google Scholar] [CrossRef] [PubMed]
- Ostermann, M.; Bagshaw, S.M.; Lumlertgul, N.; Wald, R. Indications for and Timing of Initiation of KRT. Clin. J. Am. Soc. Nephrol. 2023, 18, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Khwaja, A. KDIGO clinical practice guidelines for acute kidney injury. Nephron. Clin. Pract. 2012, 120, c179–c184. [Google Scholar] [CrossRef] [PubMed]
- Fayad, A.I.; Buamscha, D.G.; Ciapponi, A. Intensity of continuous renal replacement therapy for acute kidney injury. Cochrane Database Syst. Rev. 2016, 10, Cd010613. [Google Scholar] [CrossRef]
- Zarbock, A.; Küllmar, M.; Kindgen-Milles, D.; Wempe, C.; Gerss, J.; Brandenburger, T.; Dimski, T.; Tyczynski, B.; Jahn, M.; Mülling, N.; et al. Effect of Regional Citrate Anticoagulation vs Systemic Heparin Anticoagulation During Continuous Kidney Replacement Therapy on Dialysis Filter Life Span and Mortality Among Critically Ill Patients With Acute Kidney Injury: A Randomized Clinical Trial. Jama 2020, 324, 1629–1639. [Google Scholar] [CrossRef]
- Mendu, M.L.; Ciociolo, G.R., Jr.; McLaughlin, S.R.; Graham, D.A.; Ghazinouri, R.; Parmar, S.; Grossier, A.; Rosen, R.; Laskowski, K.R.; Riella, L.V.; et al. A Decision-Making Algorithm for Initiation and Discontinuation of RRT in Severe AKI. Clin. J. Am. Soc. Nephrol. 2017, 12, 228–236. [Google Scholar] [CrossRef]
- Liu, C.; Peng, Z.; Dong, Y.; Li, Z.; Andrijasevic, N.M.; Albright, R.C., Jr.; Kashani, K.B. Predicting successful continuous renal replacement therapy liberation in critically ill patients with acute kidney injury. J. Crit. Care 2021, 66, 6–13. [Google Scholar] [CrossRef]
- Elbahlawan, L.; Morrison, R.R. Continuous renal replacement therapy in children post-hematopoietic stem cell transplantation: The present and the future. Curr. Stem. Cell Res. Ther. 2012, 7, 381–387. [Google Scholar] [CrossRef]
- Yang, T.; Sun, S.; Lin, L.; Han, M.; Liu, Q.; Zeng, X.; Zhao, Y.; Li, Y.; Su, B.; Huang, S.; et al. Predictive Factors Upon Discontinuation of Renal Replacement Therapy for Long-Term Chronic Dialysis and Death in Acute Kidney Injury Patients. Artif. Organs 2017, 41, 1127–1134. [Google Scholar] [CrossRef]
- Charlton, J.R.; Portilla, D.; Okusa, M.D. A basic science view of acute kidney injury biomarkers. Nephrol. Dial. Transplant. 2014, 29, 1301–1311. [Google Scholar] [CrossRef]
- Nickolas, T.L.; Schmidt-Ott, K.M.; Canetta, P.; Forster, C.; Singer, E.; Sise, M.; Elger, A.; Maarouf, O.; Sola-Del Valle, D.A.; O’Rourke, M.; et al. Diagnostic and prognostic stratification in the emergency department using urinary biomarkers of nephron damage: A multicenter prospective cohort study. J. Am. Coll. Cardiol. 2012, 59, 246–255. [Google Scholar] [CrossRef]
- Augustynowicz, M.; Bargenda-Lange, A.; Kałwak, K.; Zwolińska, D.; Musiał, K. Markers of acute kidney injury in children undergoing hematopoietic stem cell transplantation. Adv. Clin. Exp. Med. 2019, 28, 1111–1118. [Google Scholar] [CrossRef]
- Shao, X.; Tian, L.; Xu, W.; Zhang, Z.; Wang, C.; Qi, C.; Ni, Z.; Mou, S. Diagnostic value of urinary kidney injury molecule 1 for acute kidney injury: A meta-analysis. PLoS ONE 2014, 9, e84131. [Google Scholar] [CrossRef]
- Susantitaphong, P.; Siribamrungwong, M.; Doi, K.; Noiri, E.; Terrin, N.; Jaber, B.L. Performance of urinary liver-type fatty acid-binding protein in acute kidney injury: A meta-analysis. Am. J. Kidney Dis. 2013, 61, 430–439. [Google Scholar] [CrossRef]
- Suzuki, G.; Ichibayashi, R.; Yamamoto, S.; Nakamichi, Y.; Watanabe, M.; Honda, M. Clinical significance of urinary L-FABP in the emergency department. Int. J. Emerg. Med. 2019, 12, 24. [Google Scholar] [CrossRef]
- Suzuki, G.; Ichibayashi, R.; Yamamoto, S.; Serizawa, H.; Nakamichi, Y.; Watanabe, M.; Honda, M. Urinary liver-type fatty acid-binding protein variation as a predictive value of short-term mortality in intensive care unit patients. Ren. Fail. 2021, 43, 1041–1048. [Google Scholar] [CrossRef]
- Zeng, X.F.; Li, J.M.; Tan, Y.; Wang, Z.F.; He, Y.; Chang, J.; Zhang, H.; Zhao, H.; Bai, X.; Xie, F.; et al. Performance of urinary NGAL and L-FABP in predicting acute kidney injury and subsequent renal recovery: A cohort study based on major surgeries. Clin. Chem. Lab. Med. 2014, 52, 671–678. [Google Scholar] [CrossRef]
- Gauer, S.; Sichler, O.; Obermüller, N.; Holzmann, Y.; Kiss, E.; Sobkowiak, E.; Pfeilschifter, J.; Geiger, H.; Mühl, H.; Hauser, I.A. IL-18 is expressed in the intercalated cell of human kidney. Kidney Int. 2007, 72, 1081–1087. [Google Scholar] [CrossRef]
- Gonzalez, F.; Vincent, F. Biomarkers for acute kidney injury in critically ill patients. Minerva Anestesiol 2012, 78, 1394–1403. [Google Scholar]
- Kashani, K.; Al-Khafaji, A.; Ardiles, T.; Artigas, A.; Bagshaw, S.M.; Bell, M.; Bihorac, A.; Birkhahn, R.; Cely, C.M.; Chawla, L.S.; et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit. Care 2013, 17, 1–12. [Google Scholar] [CrossRef]
- Xie, Y.; Ankawi, G.; Yang, B.; Garzotto, F.; Passannante, A.; Breglia, A.; Digvijay, K.; Ferrari, F.; Brendolan, A.; Raffaele, B.; et al. Tissue inhibitor metalloproteinase-2 (TIMP-2) • IGF-binding protein-7 (IGFBP7) levels are associated with adverse outcomes in patients in the intensive care unit with acute kidney injury. Kidney Int. 2019, 95, 1486–1493. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Eckardt, K.U.; Dorman, N.M.; Christiansen, S.L.; Cheung, M.; Jadoul, M.; Winkelmayer, W.C. Nomenclature for Kidney Function and Disease: Executive Summary and Glossary from a Kidney Disease: Improving Global Outcomes (KDIGO) Consensus Conference. Kidney Dis. Basel. 2020, 6, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Hingorani, S.; Guthrie, K.A.; Schoch, G.; Weiss, N.S.; McDonald, G.B. Chronic kidney disease in long-term survivors of hematopoietic cell transplant. Bone Marrow Transplant. 2007, 39, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Hingorani, S. Chronic kidney disease after pediatric hematopoietic cell transplant. Biol. Blood Marrow Transplant. 2008, 14, 84–87. [Google Scholar] [CrossRef]
- Prasad, M.; Jain, N.G.; Radhakrishnan, J.; Jin, Z.; Satwani, P. Risk factors for chronic kidney disease following acute kidney injury in pediatric allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2021, 56, 1665–1673. [Google Scholar] [CrossRef]
- Hingorani, S.R.; Seidel, K.; Lindner, A.; Aneja, T.; Schoch, G.; McDonald, G. Albuminuria in hematopoietic cell transplantation patients: Prevalence, clinical associations, and impact on survival. Biol. Blood Marrow Transplant. 2008, 14, 1365–1372. [Google Scholar] [CrossRef]
- Hingorani, S. Renal Complications of Hematopoietic-Cell Transplantation. N. Engl. J. Med. 2016, 374, 2256–2267. [Google Scholar] [CrossRef]
- Flynn, J.T.; Kaelber, D.C.; Baker-Smith, C.M. Subcommittee on screening and management of high blood pressure in children. Clinical Practice Guideline for Screening and Management of High Blood Pressure in Children and Adolescents. Pediatrics. Pediatrics 2017, 140, e20171904. [Google Scholar] [CrossRef]
- Baltatzi, M.; Savopoulos, C.; Hatzitolios, A. Role of angiotensin converting enzyme inhibitors and angiotensin receptor blockers in hypertension of chronic kidney disease and renoprotection. Study results. Hippokratia 2011, 15, 27–32. [Google Scholar]
- Wühl, E.; Trivelli, A.; Picca, S.; Litwin, M.; Peco-Antic, A.; Zurowska, A.; Testa, S.; Jankauskiene, A.; Emre, S.; Caldas-Afonso, A.; et al. Strict blood-pressure control and progression of renal failure in children. N. Engl. J. Med. 2009, 361, 1639–1650. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).