Evaluation of Classic and Quantitative Imaging Features in the Differentiation of Benign and Atypical Lipomatous Soft Tissue Tumors Using a Standardized Multiparametric MRI Protocol: A Prospective Single-Centre Study in 45 Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Approval by the Ethical Review Board
2.2. Patient Recruitment
2.3. Histopathological Analysis
2.4. MRI Protocol
2.5. Patient Characteristics and Imaging Features
2.6. Gray Value Analysis of T1, T2, T2*, R2* and ADC Maps
2.7. Statistics
3. Results
3.1. Lesion Characteristics
3.2. Lesion Dimensions
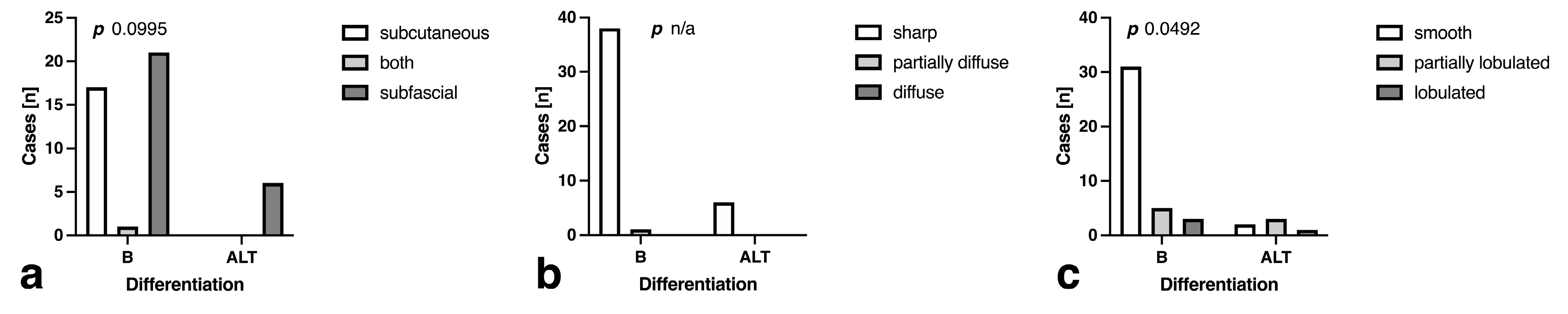
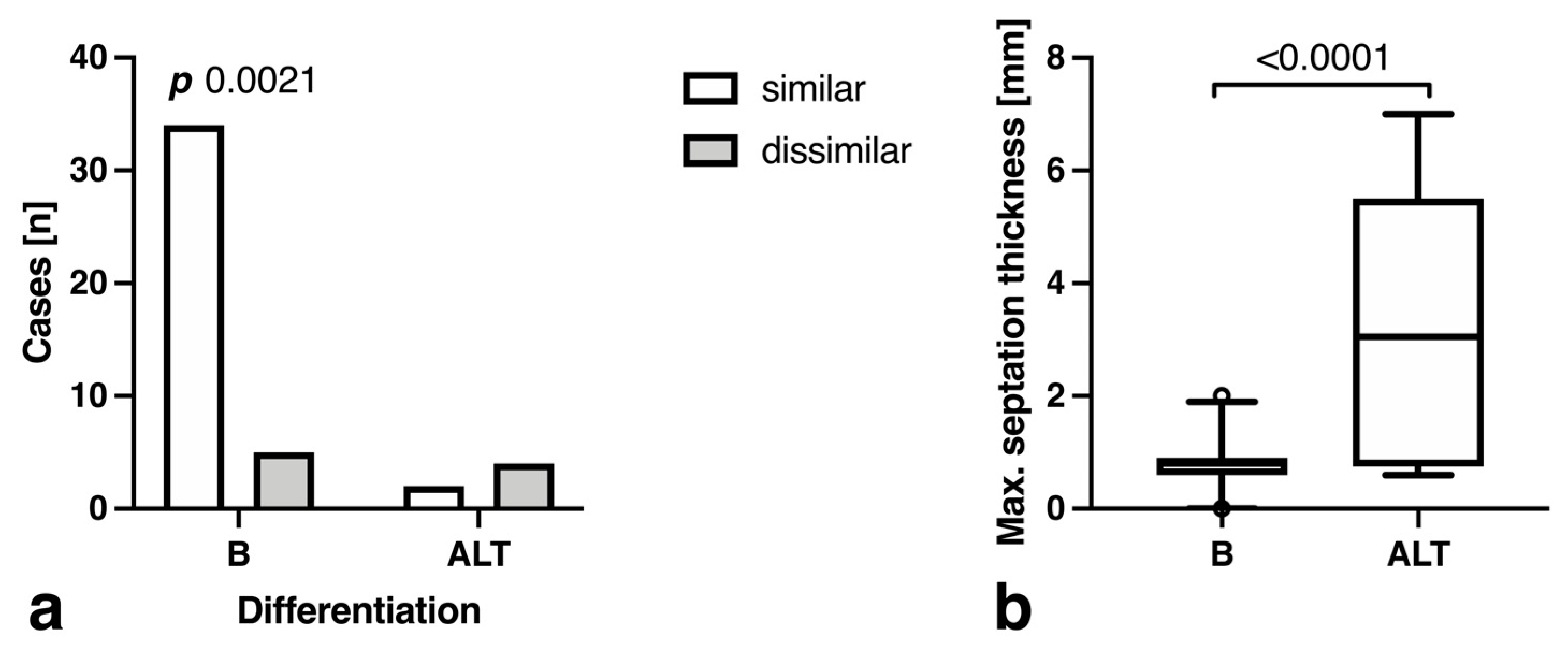
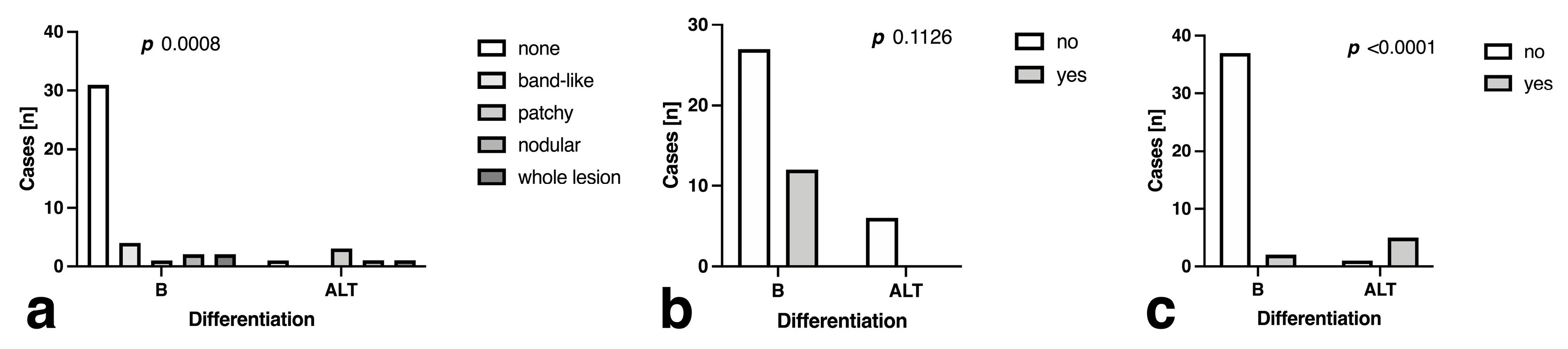
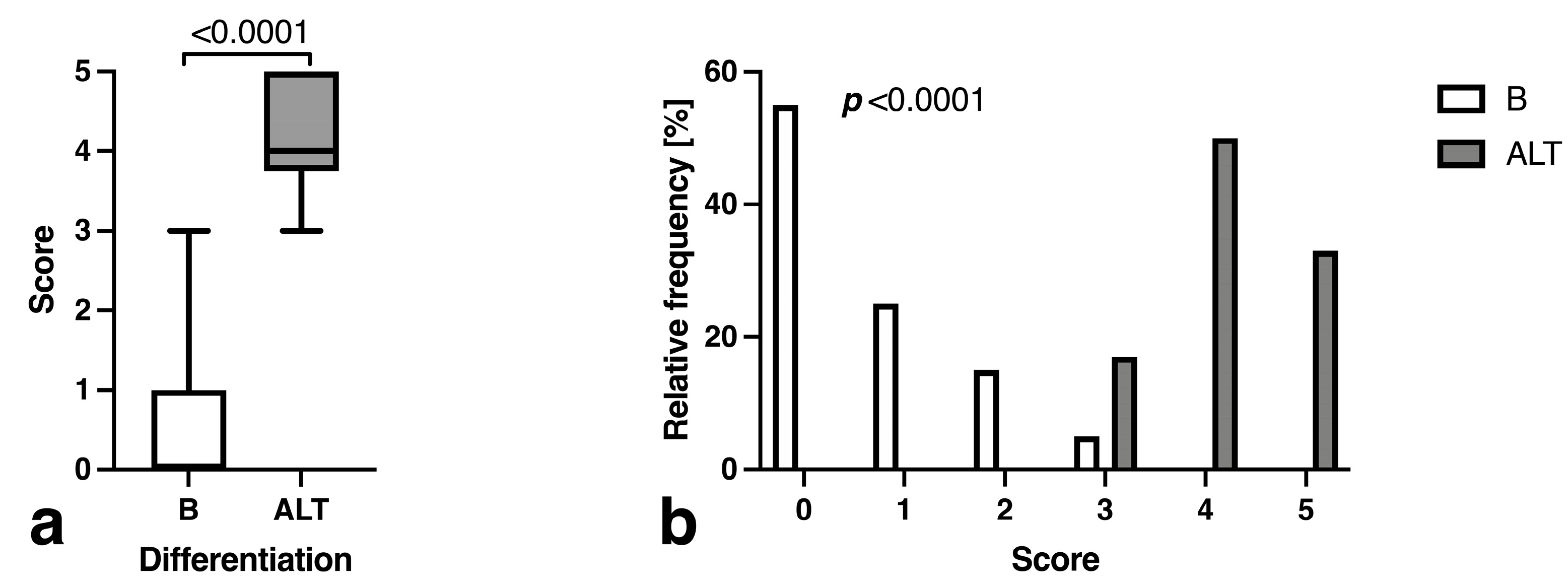
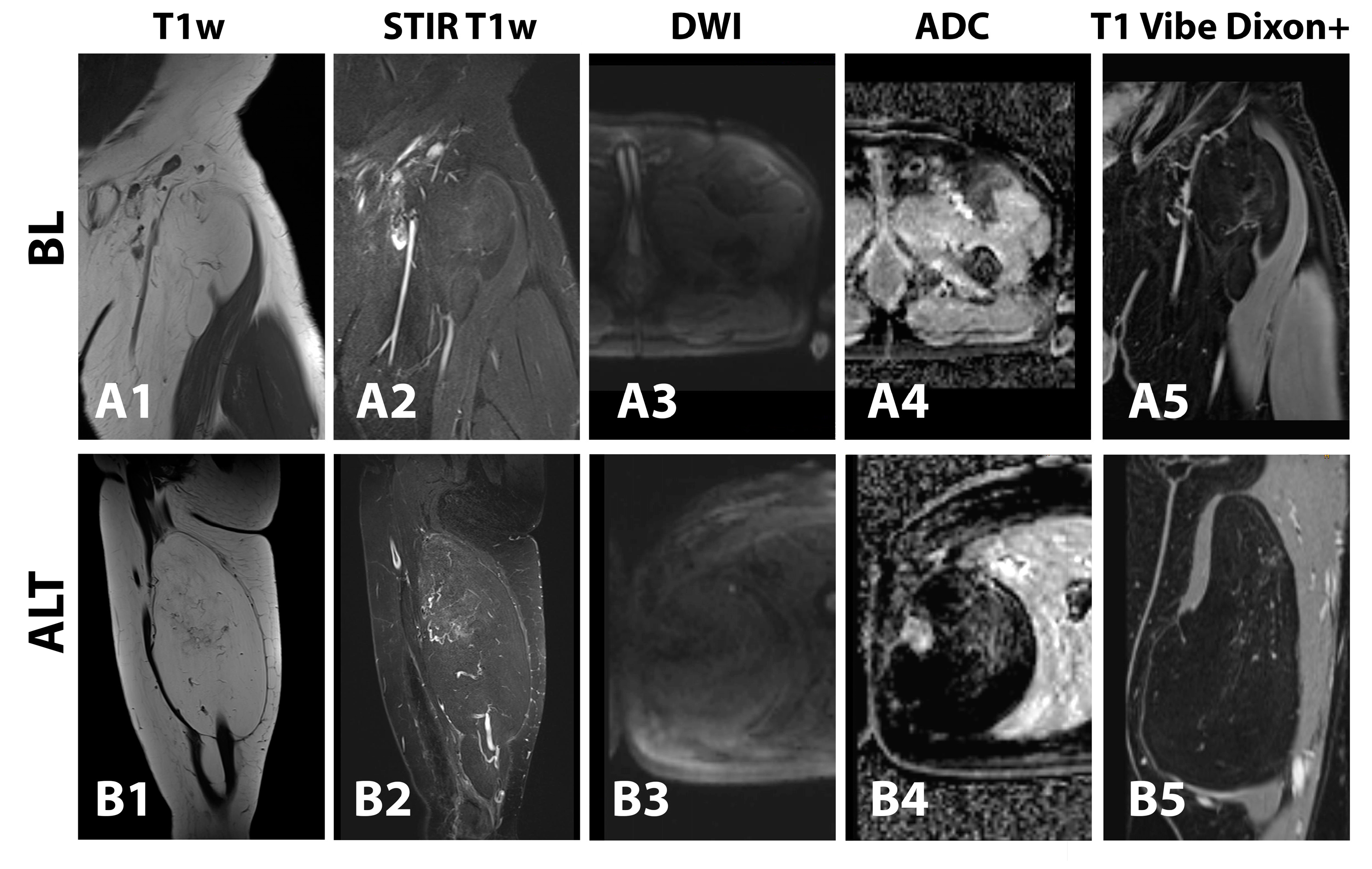
3.3. Morphology and Descriptive Signal Characteristics
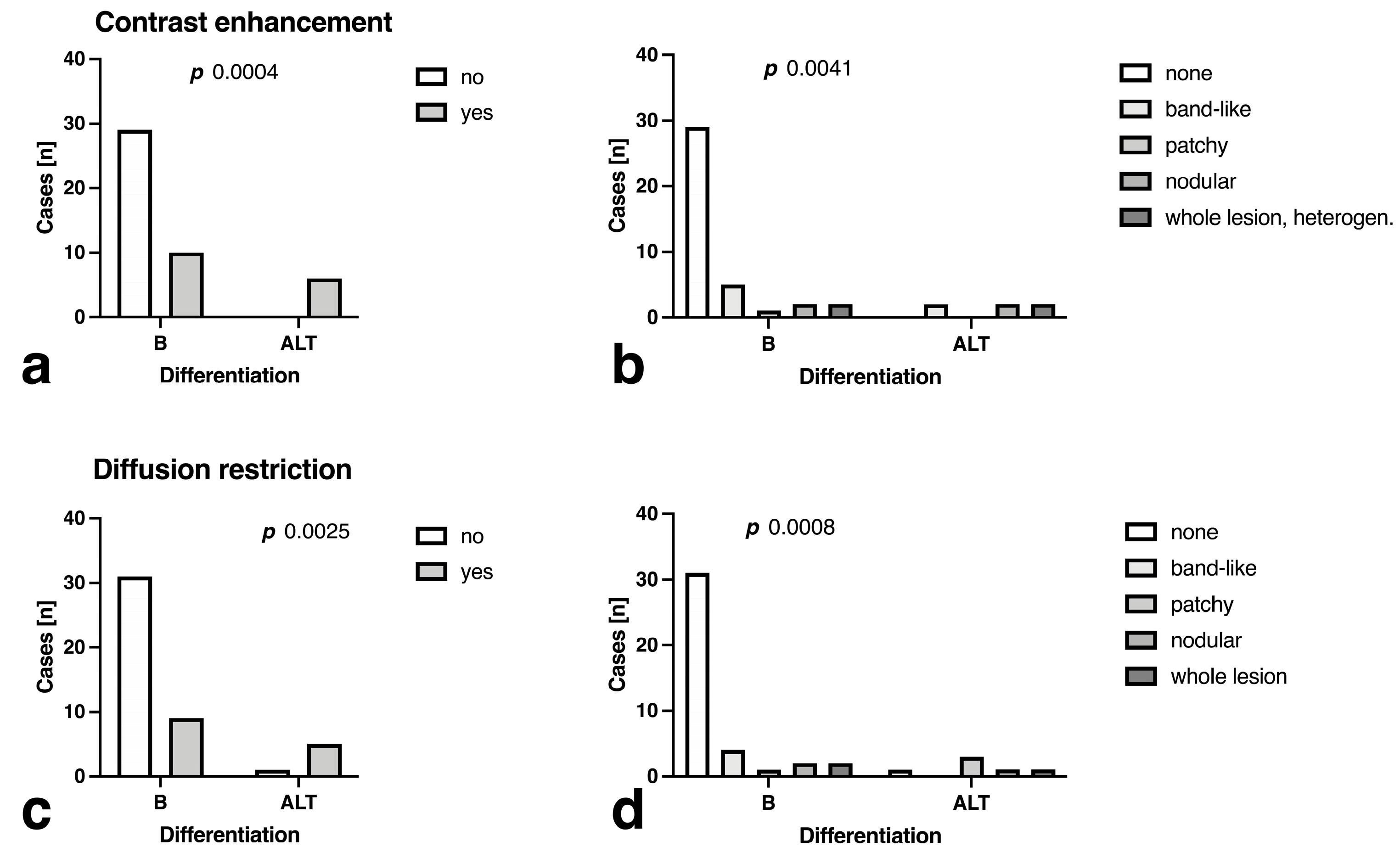
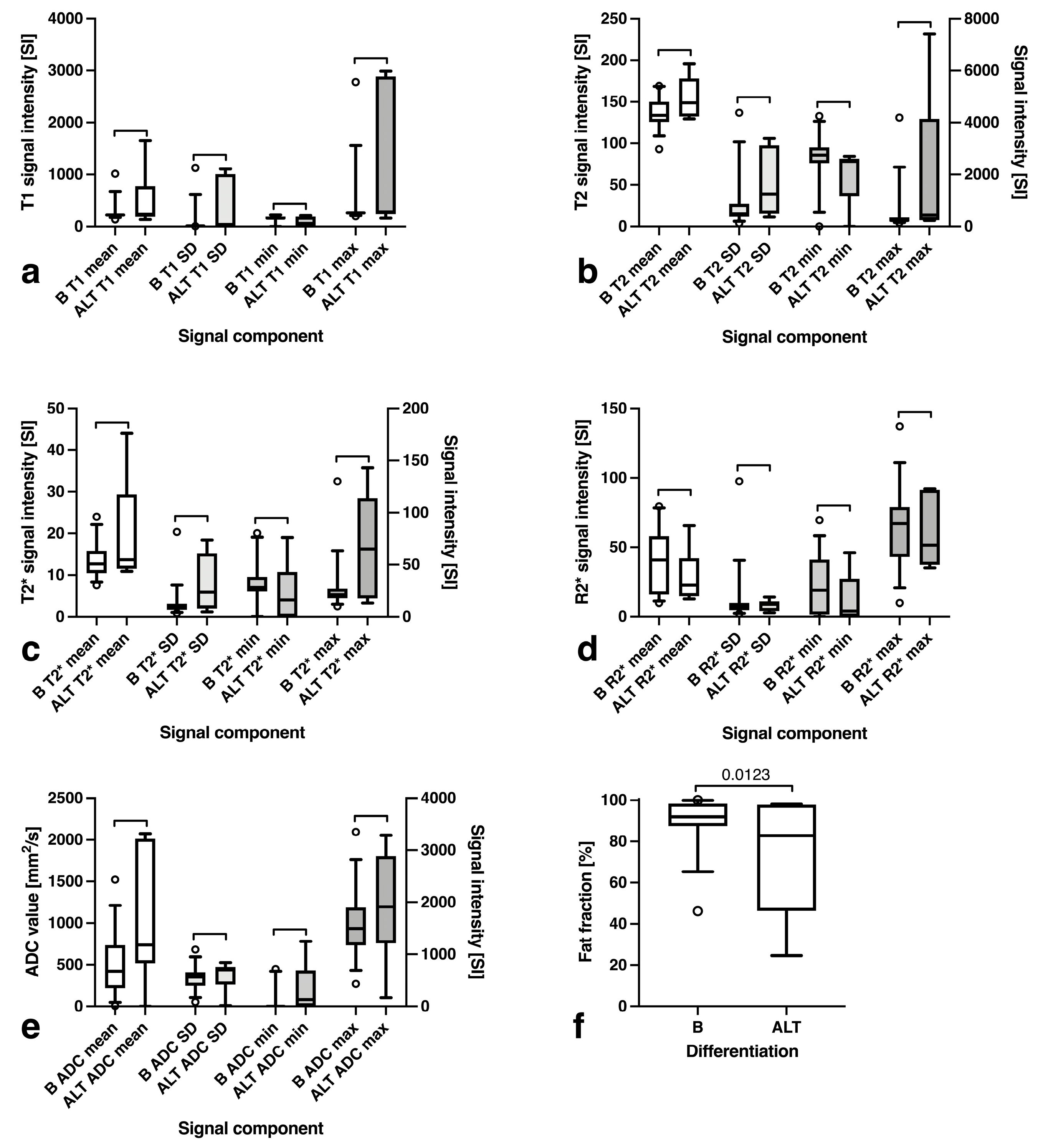
3.4. Fat Fraction, T1, T2, T2*, R2* and ADC Maps Analysis
3.5. Predictor Ranking
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gupta, P.; Potti, T.A.; Wuertzer, S.D.; Lenchik, L.; Pacholke, D.A. Spectrum of Fat-Containing Soft-Tissue Masses at MR Imaging: The Common, the Uncommon, the Characteristic, and the Sometimes Confusing. Radiographics 2016, 36, 753–766. [Google Scholar] [CrossRef]
- Goldblum, J.R.; Folpe, A.L.; Weiss, S.W. (Eds.) Benign Lipomatous Tumors. In Enzinger and Weiss’s Soft Tissue Tumors, 6th ed.; Elsevier: Oxford, UK, 2014; pp. 443–483. ISBN 978-0-323-08834-3. [Google Scholar]
- Goldblum, J.R.; Folpe, A.L.; Weiss, S.W. (Eds.) Liposarcoma. In Enzinger and Weiss’s Soft Tissue Tumors, 6th ed.; Elsevier: Oxford, UK, 2014; pp. 484–523. ISBN 978-0-323-08834-3. [Google Scholar]
- Kransdorf, M.J.; Bancroft, L.W.; Peterson, J.J.; Murphey, M.D.; Foster, W.C.; Temple, H.T. Imaging of Fatty Tumors: Distinction of Lipoma and Well-Differentiated Liposarcoma. Radiology 2002, 224, 99–104. [Google Scholar] [CrossRef]
- Fletcher, C.D. The Evolving Classification of Soft Tissue Tumours—An Update Based on the New 2013 WHO Classification. Histopathology 2014, 64, 2–11. [Google Scholar] [CrossRef]
- Dei Tos, A.P. Liposarcomas: Diagnostic Pitfalls and New Insights. Histopathology 2014, 64, 38–52. [Google Scholar] [CrossRef]
- Lucas, D.R.; Nascimento, A.G.; Sanjay, B.K.S.; Rock, M.G. Well-Differentiated Liposarcoma: The Mayo Clinic Experience with 58 Cases. Am. J. Clin. Pathol. 1994, 102, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Nardo, L.; Abdelhafez, Y.G.; Acquafredda, F.; Schirò, S.; Wong, A.L.; Sarohia, D.; Maroldi, R.; Darrow, M.A.; Guindani, M.; Lee, S.; et al. Qualitative Evaluation of MRI Features of Lipoma and Atypical Lipomatous Tumor: Results from a Multicenter Study. Skelet. Radiol. 2020, 49, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Rozental, T.D.; Khoury, L.D.; Donthineni-Rao, R.; Lackman, R.D. Atypical Lipomatous Masses of the Extremities: Outcome of Surgical Treatment. Clin. Orthop. Relat. Res. 2002, 398, 203–211. [Google Scholar] [CrossRef]
- Wu, J.S.; Hochman, M.G. Soft-Tissue Tumors and Tumorlike Lesions: A Systematic Imaging Approach. Radiology 2009, 253, 297–316. [Google Scholar] [CrossRef] [PubMed]
- Nagano, S.; Yokouchi, M.; Setoguchi, T.; Ishidou, Y.; Sasaki, H.; Shimada, H.; Komiya, S. Differentiation of Lipoma and Atypical Lipomatous Tumor by a Scoring System: Implication of Increased Vascularity on Pathogenesis of Liposarcoma. BMC Musculoskelet. Disord. 2015, 16, 36. [Google Scholar] [CrossRef]
- Shim, E.J.; Yoon, M.A.; Yoo, H.J.; Chee, C.G.; Lee, M.H.; Lee, S.H.; Chung, H.W.; Shin, M.J. An MRI-Based Decision Tree to Distinguish Lipomas and Lipoma Variants from Well-Differentiated Liposarcoma of the Extremity and Superficial Trunk: Classification and Regression Tree (CART) Analysis. Eur. J. Radiol. 2020, 127, 109012. [Google Scholar] [CrossRef]
- Donners, R.; Krieg, A.H.; Baumhoer, D.; Boll, D.T.; Harder, D. Quantitative Signal Intensity Ratios to Distinguish between Subfascial Lipoma and Atypical Lipomatous Tumor/Well-Differentiated Liposarcoma Using Short-Tau Inversion Recovery (STIR) MRI. Diagn. Interv. Imaging 2020, 101, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Drevelegas, A.; Pilavaki, M.; Chourmouzi, D. Lipomatous Tumors of Soft Tissue: MR Appearance with Histological Correlation. Eur. J. Radiol. 2004, 50, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Nikiforaki, K.; Ioannidis, G.S.; Lagoudaki, E.; Manikis, G.H.; de Bree, E.; Karantanas, A.; Maris, T.G.; Marias, K. Multiexponential T2 Relaxometry of Benign and Malignant Adipocytic Tumours. Eur. Radiol. Exp. 2020, 4, 45. [Google Scholar] [CrossRef]
- Nikiforaki, K.; Manikis, G.C.; Kontopodis, E.; Lagoudaki, E.; de Bree, E.; Marias, K.; Karantanas, A.H.; Maris, T.G. T2, T2 * and Spin Coupling Ratio as Biomarkers for the Study of Lipomatous Tumors. Phys. Med. 2019, 60, 76–82. [Google Scholar] [CrossRef]
- Vos, M.; Starmans, M.P.A.; Timbergen, M.J.M.; van der Voort, S.R.; Padmos, G.A.; Kessels, W.; Niessen, W.J.; van Leenders, G.J.L.H.; Grünhagen, D.J.; Sleijfer, S.; et al. Radiomics Approach to Distinguish between Well Differentiated Liposarcomas and Lipomas on MRI. Br. J. Surg. 2019, 106, 1800–1809. [Google Scholar] [CrossRef]
- Brisson, M.; Kashima, T.; Delaney, D.; Tirabosco, R.; Clarke, A.; Cro, S.; Flanagan, A.M.; O’Donnell, P. MRI Characteristics of Lipoma and Atypical Lipomatous Tumor/Well-Differentiated Liposarcoma: Retrospective Comparison with Histology and MDM2 Gene Amplification. Skelet. Radiol. 2013, 42, 635–647. [Google Scholar] [CrossRef]
- Zhang, H.; Erickson-Johnson, M.; Wang, X.; Oliveira, J.L.; Nascimento, A.G.; Sim, F.H.; Wenger, D.E.; Zamolyi, R.Q.; Pannain, V.L.; Oliveira, A.M. Molecular Testing for Lipomatous Tumors: Critical Analysis and Test Recommendations Based on the Analysis of 405 Extremity-Based Tumors. Am. J. Surg. Pathol. 2010, 34, 1304–1311. [Google Scholar] [CrossRef] [PubMed]
- Nandra, R.; Forsberg, J.; Grimer, R. If Your Lump Is Bigger than a Golf Ball and Growing, Think Sarcoma. Eur. J. Surg. Oncol. 2015, 41, 1400–1405. [Google Scholar] [CrossRef]
- World Health Organization. WHO Classification of Tumours: Soft Tissue and Bone Tumours, 4th ed.; International Agency for Research on Cancer: Lyon, France, 2013; Volume 3. [Google Scholar]
- Zhong, X.; Nickel, M.D.; Kannengiesser, S.A.R.; Dale, B.M.; Kiefer, B.; Bashir, M.R. Liver Fat Quantification Using a Multi-Step Adaptive Fitting Approach with Multi-Echo GRE Imaging. Magn. Reson. Med. 2014, 72, 1353–1365. [Google Scholar] [CrossRef]
- Schall, M.; Zimmermann, M.; Iordanishvili, E.; Gu, Y.; Shah, N.J.; Oros-Peusquens, A.-M. A 3D Two-Point Method for Whole-Brain Water Content and Relaxation Time Mapping: Comparison with Gold Standard Methods. PLoS ONE 2018, 13, e0201013. [Google Scholar] [CrossRef]
- Witten, I.H.; Frank, E. Data Mining: Practical Machine Learning Tools and Techniques, 2nd ed.; Elsevier Inc.: San Francisco, CA, USA, 2005; ISBN 9788578110796. [Google Scholar]
- Knebel, C.; Neumann, J.; Schwaiger, B.J.; Karampinos, D.C.; Pfeiffer, D.; Specht, K.; Lenze, U.; Von Eisenhart-Rothe, R.; Rummeny, E.J.; Woertler, K.; et al. Differentiating Atypical Lipomatous Tumors from Lipomas with Magnetic Resonance Imaging: A Comparison with MDM2 Gene Amplification Status. BMC Cancer 2019, 19, 309. [Google Scholar] [CrossRef] [PubMed]
- Gruber, L.; Loizides, A.; Ostermann, L.; Glodny, B.; Plaikner, M.; Gruber, H. Does Size Reliably Predict Malignancy in Soft Tissue Tumours? Eur. Radiol. 2016, 26, 4640–4648. [Google Scholar] [CrossRef] [PubMed]
- Toirkens, J.; De Schepper, A.M.; Vanhoenacker, F.; Van Dyck, P.; Gielen, J.; Creytens, D.; Wouters, K.; Eiber, M.; Wörtler, K.; Parizel, P.M. A Comparison between Histopathology and Findings on Magnetic Resonance Imaging of Subcutaneous Lipomatous Soft-Tissue Tumors. Insights Imaging 2011, 2, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Jung, I.; Gurzu, S.; Turdean, S.; Ciortea, D.; Sahlean, D.I.; Golea, M.; Bara, T. Relationship of Endothelial Area with VEGF-A, COX-2, Maspin, c-KIT, and DOG-1 Immunoreactivity in Liposarcomas versus Non-Lipomatous Soft Tissue Tumors. Int. J. Clin. Exp. Pathol. 2015, 8, 1776–1782. [Google Scholar]
- Bancroft, L.W.; Kransdorf, M.J.; Peterson, J.J.; O’Connor, M.I. Benign Fatty Tumors: Classification, Clinical Course, Imaging Appearance, and Treatment. Skelet. Radiol. 2006, 35, 719–733. [Google Scholar] [CrossRef]
- Wang, S.; Summers, R.M. Machine Learning and Radiology. Med. Image Anal. 2012, 16, 933–951. [Google Scholar] [CrossRef]

| STIR T1w TSE | T1w TSE | PDw/T2w TSE | 3D qDIXON-VIBE + | RESOLVE-DWI | T1 Mapping | 2D meGRE | T1 Vibe Dixon −/+ | |
|---|---|---|---|---|---|---|---|---|
| Orientation | sagittal | sagittal | axial | axial | axial | 3.9 | 200 | axial |
| TR (ms) | 3350 | 644 | 5590 | 15.8 | 4370 | 1.8 | 1.01–19.05 | 6.4 |
| TE (ms) | 23 | 11 | 32/85 | 2.38/4.76/7.14/ 9.52/11.9/14.28 | 46 | 4–3700 | - | 2.39 |
| TI (ms) | 160 | - | - | - | - | none | CHESS | - |
| Fat saturation | STIR | none | none | none | CHESS | 1 | 1 | none |
| Echo train length | 8 | 6 | 6 | 6 | 18 | 4 | 20 | 2 |
| Flip angle (°) | 90 | 90 | 90 | 4 | 90 | 250 × 250 | 250 × 250 | we10 |
| FOV (mm × mm) # | 300 × 300 | 300 × 300 | 170 × 170 | 170 × 143 | 170 × 170 | 128 × 64 | 128 × 128 | 250 × 250 |
| Acquisition matrix | 512 × 410 | 640 × 512 | 384 × 269 | 128 × 101 | 88 × 62 | 128 × 128 | 128 × 128 | 256 × 192 |
| Reconstructed matrix | 512 × 410 | 640 × 512 | 384 × 269 | 256 × 202 | 88 × 88 | 5.0 | 10.0 | 256 × 256 |
| Slice thickness (mm) | 4.0 | 4.0 | 5.0 | 3.5 | 5.0 | 2.0 × 2.0 × 5.0 | 2.0 × 2.0 × 10.0 | 1 |
| Voxel size (mm × mm × mm) | 0.6 × 0.6 × 4.0 | 0.5 × 0.5 × 4.0 | 0.4 × 0.4 × 5.0 | 0.7 × 0.7 × 3.5 | 1.9 × 1.9 × 5.0 | 1 | 1 | 1.0 × 1.0 × 1.0 |
| NEX | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| Parallel imaging mode | GRAPPA $ | GRAPPA $ | GRAPPA $ | CAIPIRINHA * | GRAPPA | none | GRAPPA | GRAPPA |
| Accel. factor | 3 | 3 | 2 | 4 | 2 | - | 2 | 3 |
| Overall | Benign (B) | Atypical Lipomatous Tumors (ALT) | p-Value | |
|---|---|---|---|---|
| Participants [n] | 45 | 39 | 6 | n/a |
| Age [years] (mean ± SD) | 61.2 ± 14.2 | 61.7 ± 14.1 | 57.9 ± 17.2 | 0.546 * |
| Female sex [n,%] | 27 (60.0%) | 23 (59.0%) | 4 (66.7%) | >0.999 § |
| Predictor | Avg. Rank | Gain Ratio Merit | Lipoma | ALT | Sensitivity (%) | Specificity (%) | PPV $ | NPV $ | p-Value $ |
|---|---|---|---|---|---|---|---|---|---|
| Septation thickness (cut-off 1.3 mm) | 1.6 ± 0.49 | 0.623 ± 0.025 | 0.8 ± 0.4 mm | 3.3 ± 2.6 mm | 90.0 [77.0 to 96.0] | 66.7 [30.0 to 94.1] | 94.7 [82.7 to 99.1] | 50.0 [21.5 to 78.5] | 0.0055 |
| STIR discrepancy (intralesional vs. surrounding) | 3.0 ± 0.37 | 0.458 ± 0.046 | 5.1% (2) | 83.3% (5) | 95.0 [83.5 to 99.1] | 83.3 [43.7 to 99.2] | 97.4 [86.8 to 99.9] | 71.4 [35.9 to 94.9] | <0.0001 |
| Size (Y) (cut-off 39.5 mm) | 3.0 ± 5.02 | 0.554 ± 0.188 | 37.6 ± 18.1 mm | 56.3 ± 39.3 mm | 95.0 [83.5 to 99.1] | 66.7 [30.0 to 94.1] | 95.0 [83.5 to 99.1] | 66.7 [30.0 to 94.1] | 0.0013 |
| Contrast enhancement [yes/no] | 4.8 ± 0.72 | 0.235 ± 0.015 | 25.6% (10) | 100.0% (6) | 74.4 [58.9 to 85.4] | 100.0 [61.0 to 100.0] | 100.0 [88.3 to 100.0] | 37.5 [18.5 to 61.4] | 0.0010 |
| Size (X) (cut-off 125.5 mm) | 5.5 ± 3.53 | 0.221 ± 0.075 | 94.3 ± 49.2 mm | 171.2 ± 43.4 mm | 75.0 [59.8 to 85.8] | 100.0 [61.0 to 100.0] | 100.0 [88.7 to 100.0] | 37.5 [18.5 to 61.4] | 0.0009 |
| Differing septation morphology | 6.4 ± 0.99 | 0.168 ± 0.026 | 12.8% (5) | 66.7% (4) | 87.5 [73.9 to 94.5] | 66.7 [30.0 to 94.1] | 94.6 [82.3 to 99.0] | 44.4 [18.9 to 73.3] | 0.0095 |
| Contrast enhancement pattern (patchy, nodular, whole lesion) | 6.9 ± 0.85 | 0.158 ± 0.012 | 12.8% (5) | 66.7% (4) | 72.5 [57.2 to 83.9] | 100.0 [61.0 to 100.0] | 100.0 [88.3 to 100.0] | 35.3 [17.3 to 58.7] | 0.0013 |
| ADC pattern | 7.6 ± 0.91 | 0.155 ± 0.019 | 23.1% (9) | 83.3% (5) | 75.0 [59.8 to 85.8] | 100.0 [61.0 to 100.0] | 100.0 [88.7 to 100.0] | 37.5 [18.5 to 61.4] | 0.0009 |
| Subfascial localization | 9.1 ± 0.6 | 0.099 ± 0.006 | 53.9% (21) | 100.0% (6) | 75.0 [59.8 to 85.8] | 100.0 [61.0 to 100.0] | 100.0 [887 to 100.0] | 37.5 [18.5 to 61.4] | 0.0009 |
| Region (thigh/calf) | 10.6 ± 0.99 | 0.084 ± 0.006 | 7.7% (3) | 50.0% (3) | 90.0 [77.0 to 96.0] | 50.0 [18.8 to 81.2] | 92.3 [79.7 to 97.4] | 42.9 [15.8 to 75.0] | 0.0370 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gruber, L.; Kremser, C.; Zelger, B.; Schwabegger, A.; Josip, E.; Dammerer, D.; Thaler, M.; Henninger, B. Evaluation of Classic and Quantitative Imaging Features in the Differentiation of Benign and Atypical Lipomatous Soft Tissue Tumors Using a Standardized Multiparametric MRI Protocol: A Prospective Single-Centre Study in 45 Patients. Curr. Oncol. 2023, 30, 3315-3328. https://doi.org/10.3390/curroncol30030252
Gruber L, Kremser C, Zelger B, Schwabegger A, Josip E, Dammerer D, Thaler M, Henninger B. Evaluation of Classic and Quantitative Imaging Features in the Differentiation of Benign and Atypical Lipomatous Soft Tissue Tumors Using a Standardized Multiparametric MRI Protocol: A Prospective Single-Centre Study in 45 Patients. Current Oncology. 2023; 30(3):3315-3328. https://doi.org/10.3390/curroncol30030252
Chicago/Turabian StyleGruber, Leonhard, Christian Kremser, Bettina Zelger, Anton Schwabegger, Ena Josip, Dietmar Dammerer, Martin Thaler, and Benjamin Henninger. 2023. "Evaluation of Classic and Quantitative Imaging Features in the Differentiation of Benign and Atypical Lipomatous Soft Tissue Tumors Using a Standardized Multiparametric MRI Protocol: A Prospective Single-Centre Study in 45 Patients" Current Oncology 30, no. 3: 3315-3328. https://doi.org/10.3390/curroncol30030252
APA StyleGruber, L., Kremser, C., Zelger, B., Schwabegger, A., Josip, E., Dammerer, D., Thaler, M., & Henninger, B. (2023). Evaluation of Classic and Quantitative Imaging Features in the Differentiation of Benign and Atypical Lipomatous Soft Tissue Tumors Using a Standardized Multiparametric MRI Protocol: A Prospective Single-Centre Study in 45 Patients. Current Oncology, 30(3), 3315-3328. https://doi.org/10.3390/curroncol30030252





