Adaptive Volumetric-Modulated Arc Radiation Therapy for Head and Neck Cancer: Evaluation of Benefit on Target Coverage and Sparing of Organs at Risk
Abstract
1. Introduction
2. Materials and Methods
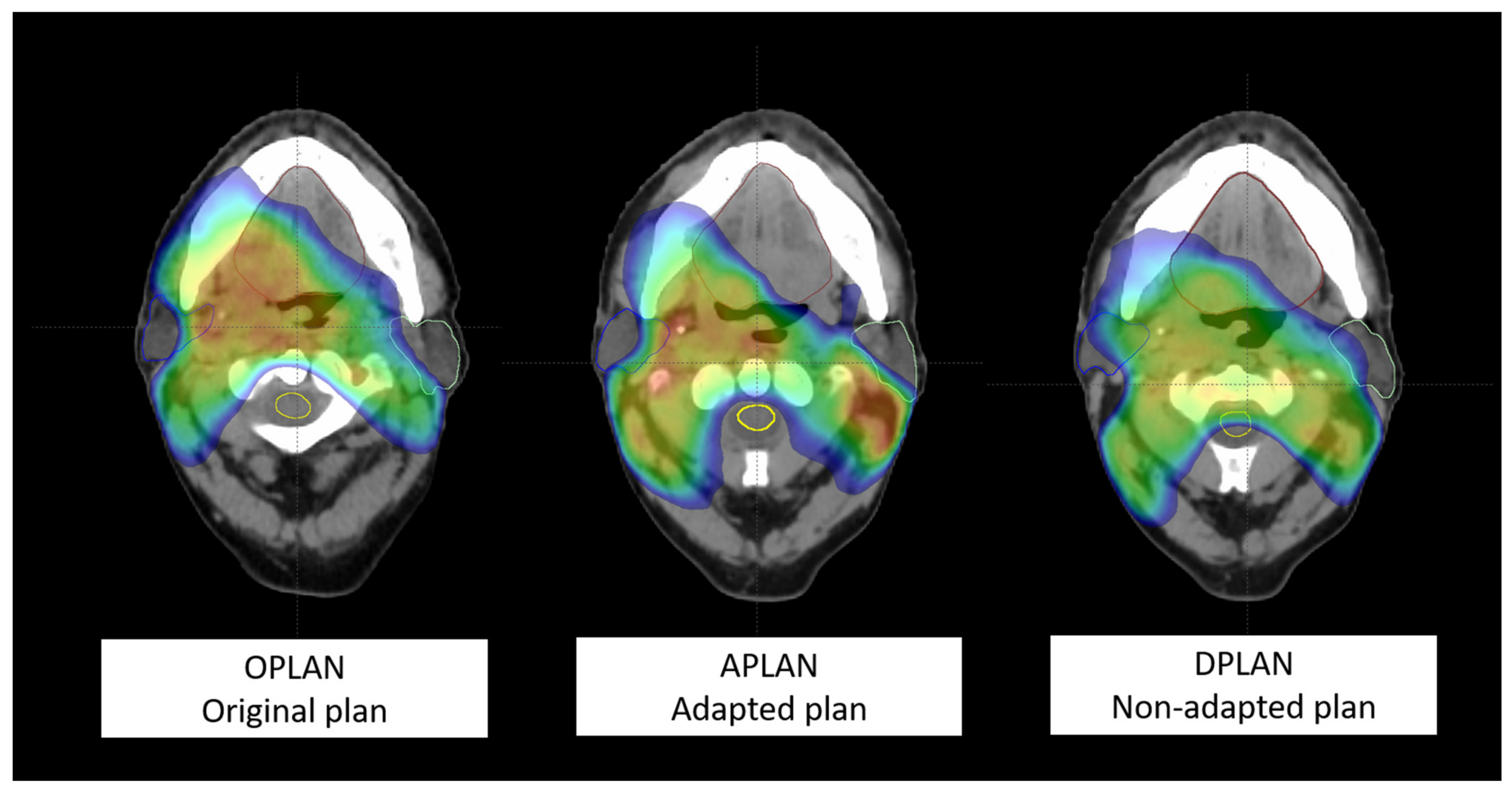
- (1)
- first simulation CT and original plan (OPLAN);
- (2)
- second simulation CT and adapted plan (APLAN);
- (3)
- second simulation CT and original plan (DPLAN).
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Santuray, R.T.; Johnson, D.E.; Grandis, J.R. New Therapies in Head and Neck Cancer. Trends Cancer 2018, 4, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Veresezan, O.; Troussier, I.; Lacout, A.; Kreps, S.; Maillard, S.; Toulemonde, A.; Marcy, P.-Y.; Huguet, F.; Thariat, J. Adaptive radiation therapy in head and neck cancer for clinical practice: State of the art and practical challenges. Jpn. J. Radiol. 2016, 35, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Eisbruch, A. Clinical aspects of IMRT for head-and-neck cancer. Med. Dosim. 2002, 27, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Ezzell, G.A.; Galvin, J.M.; Low, D.; Palta, J.R.; Rosen, I.; Sharpe, M.B.; Xia, P.; Xiao, Y.; Xing, L.; Yu, C.X. Guidance document on delivery, treatment planning, and clinical implementation of IMRT: Report of the IMRT subcommittee of the AAPM radiation therapy committee. Med. Phys. 2003, 30, 2089–2115. [Google Scholar] [CrossRef]
- Mazzola, R.; Fersino, S.; Ferrera, G.; Targher, G.; Figlia, V.; Triggiani, L.; Pasinetti, N.; Casto, A.L.; Ruggieri, R.; Magrini, S.M.; et al. Stereotactic body radiotherapy for lung oligometastases impacts on systemic treatment-free survival: A cohort study. Int. J. Radiat. Oncol. Biol. Phys. 2018, 127, 121. [Google Scholar] [CrossRef]
- Hansen, E.K.; Bucci, M.K.; Quivey, J.M.; Weinberg, V.; Xia, P. Repeat CT imaging and replanning during the course of IMRT for head-and-neck cancer. Int. J. Radiat. Oncol. 2006, 64, 355–362. [Google Scholar] [CrossRef]
- Ballivy, O.; Parker, W.; Vuong, T.; Shenouda, G.; Patrocinio, H. Impact of Geometric Uncertainties on Dose Distribution During Intensity Modulated Radiotherapy of Head-and-neck Cancer: The Need for a Planning Target Volume and A Planning Organ-at-Risk Volume. Curr. Oncol. 2006, 13, 108–115. [Google Scholar] [CrossRef]
- Wu, Q.; Chi, Y.; Chen, P.Y.; Krauss, D.J.; Yan, D.; Martinez, A. Adaptive Replanning Strategies Accounting for Shrinkage in Head and Neck IMRT. Int. J. Radiat. Oncol. 2009, 75, 924–932. [Google Scholar] [CrossRef]
- Brouwer, C.L.; Steenbakkers, R.J.; Bourhis, J.; Budach, W.; Grau, C.; Grégoire, V.; van Herk, M.; Lee, A.; Maingon, P.; Nutting, C.; et al. CT-based delineation of organs at risk in the head and neck region: Dahanca, eortc, gortec, hknpcsg, ncic ctg, ncri, nrg Oncology and trog consensus guidelines. Radiother. Oncol. 2015, 117, 83–90. [Google Scholar] [CrossRef]
- Christianen, M.E.; Langendijk, J.A.; Westerlaan, H.E.; van de Water, T.A.; Bijl, H.P. Delineation of organs at risk involved in swallowing for radiotherapy treatment planning. Radiother. Oncol. 2011, 101, 394–402. [Google Scholar] [CrossRef]
- Tukey, J.W. Exploratory Data Analysis; Addison-Wesley: Reading, MA, USA, 1977. [Google Scholar]
- Schwartz, D.L.; Garden, A.S.; Thomas, J.; Chen, Y.; Zhang, Y.; Lewin, J.; Chambers, M.S.; Dong, L. Adaptive Radiotherapy for Head-and-Neck Cancer: Initial Clinical Outcomes from a Prospective Trial. Int. J. Radiat. Oncol. 2012, 83, 986–993. [Google Scholar] [CrossRef]
- Lee, C.; Langen, K.M.; Lu, W.; Haimerl, J.; Schnarr, E.; Ruchala, K.J.; Olivera, G.H.; Meeks, S.L.; Kupelian, P.A.; Shellenberger, T.D.; et al. Assessment of Parotid Gland Dose Changes During Head and Neck Cancer Radiotherapy Using Daily Megavoltage Computed Tomography and Deformable Image Registration. Int. J. Radiat. Oncol. 2008, 71, 1563–1571. [Google Scholar] [CrossRef]
- Han, C.; Chen, Y.-J.; Liu, A.; Schultheiss, T.E.; Wong, J.Y. Actual Dose Variation of Parotid Glands and Spinal Cord for Nasopharyngeal Cancer Patients During Radiotherapy. Int. J. Radiat. Oncol. 2008, 70, 1256–1262. [Google Scholar] [CrossRef]
- Lin, A.; Kim, H.M.; Terrell, J.E.; Dawson, L.A.; Ship, J.A.; Eisbruch, A. Quality of life after parotid-sparing IMRT for head-and-neck cancer: A prospective longitudinal study. Int. J. Radiat. Oncol. 2003, 57, 61–70. [Google Scholar] [CrossRef]
- Nutting, C.M.; Morden, J.P.; Harrington, K.J.; Urbano, T.G.; Bhide, S.A.; Clark, C.; Miles, E.A.; Miah, A.B.; Newbold, K.; Tanay, M.; et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (parsport): A phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011, 12, 127–136. [Google Scholar] [CrossRef]
- Lin, A.; Helgeson, E.S.; Treister, N.S.; Schmidt, B.L.; Patton, L.L.; Elting, L.S.; Lalla, R.V.; Brennan, M.T.; Sollecito, T.P. The impact of head and neck radiotherapy on salivary flow and quality of life: Results of the orarad study. Oral Oncol. 2022, 127, 105783. [Google Scholar] [CrossRef]
- Ahn, P.H.; Chen, C.-C.; Ahn, A.I.; Hong, L.; Scripes, P.G.; Shen, J.; Lee, C.-C.; Miller, E.; Kalnicki, S.; Garg, M.K. Adaptive Planning in Intensity-Modulated Radiation Therapy for Head and Neck Cancers: Single-Institution Experience and Clinical Implications. Int. J. Radiat. Oncol. 2011, 80, 677–685. [Google Scholar] [CrossRef]
- Beltran, M.; Ramos, M.; Rovira, J.J.; Pérez-Hoyos, S.; Sancho, M.; Puertas, E.; Benavente, S.; Ginjaume, M.; Giralt, J. Dose variations in tumor volumes and organs at risk during IMRT for head-and-neck cancer. J. Appl. Clin. Med. Phys. 2012, 13, 101–111. [Google Scholar] [CrossRef]
- Berwouts, D.; Olteanu, L.A.M.; Duprez, F.; Vercauteren, T.; De Gersem, W.; De Neve, W.; Van de Wiele, C.; Madani, I. Three-phase adaptive dose-painting-by-numbers for head-and-neck cancer: Initial results of the phase I clinical trial. Radiother. Oncol. 2013, 107, 310–316. [Google Scholar] [CrossRef]
- Hunter, K.U.; Fernandes, L.L.; Vineberg, K.A.; McShan, D.; Antonuk, A.E.; Cornwall, C.; Feng, M.; Schipper, M.J.; Balter, J.M.; Eisbruch, A. Parotid Glands Dose–Effect Relationships Based on Their Actually Delivered Doses: Implications for Adaptive Replanning in Radiation Therapy of Head-and-Neck Cancer. Int. J. Radiat. Oncol. 2013, 87, 676–682. [Google Scholar] [CrossRef]
- Nishi, T.; Nishimura, Y.; Shibata, T.; Tamura, M.; Nishigaito, N.; Okumura, M. Volume and dosimetric changes and initial clinical experience of a two-step adaptive intensity modulated radiation therapy (IMRT) scheme for head and neck cancer. Radiother. Oncol. 2013, 106, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Castelli, J.; Simon, A.; Louvel, G.; Henry, O.; Chajon, E.; Nassef, M.; Haigron, P.; Cazoulat, G.; Ospina, J.D.; Jegoux, F.; et al. Impact of head and neck cancer adaptive radiotherapy to spare the parotid glands and decrease the risk of xerostomia. Radiat. Oncol. 2015, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jensen, A.D.; Nill, S.; Huber, P.E.; Bendl, R.; Debus, J.; Münter, M.W. A Clinical Concept for Interfractional Adaptive Radiation Therapy in the Treatment of Head and Neck Cancer. Int. J. Radiat. Oncol. 2012, 82, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Duma, M.; Kampfer, S.; Schuster, T.; Winkler, C.; Geinitz, H. Adaptive radiotherapy for soft tissue changes during helical tomotherapy for head and neck cancer. Strahlenther. Und. Onkol. 2012, 188, 243–247. [Google Scholar] [CrossRef]
- Capelle, L.; Mackenzie, M.; Field, C.; Parliament, M.; Ghosh, S.; Scrimger, R. Adaptive Radiotherapy Using Helical Tomotherapy for Head and Neck Cancer in Definitive and Postoperative Settings: Initial Results. Clin. Oncol. 2012, 24, 208–215. [Google Scholar] [CrossRef]
- Dewan, A.; Sharma, S.; Dewan, A.; Srivastava, H.; Rawat, S.; Kakria, A.; Mishra, M.; Suresh, T.; Mehrotra, K. Impact of Adaptive Radiotherapy on Locally Advanced Head and Neck Cancer-A Dosimetric and Volumetric Study. Asian Pac. J. Cancer Prev. 2016, 17, 985–992. [Google Scholar] [CrossRef]
- Olteanu, L.A.M.; Berwouts, D.; Madani, I.; De Gersem, W.; Vercauteren, T.; Duprez, F.; De Neve, W. Comparative dosimetry of three-phase adaptive and non-adaptive dose-painting IMRT for head-and-neck cancer. Radiother. Oncol. 2014, 111, 348–353. [Google Scholar] [CrossRef]
- Zhao, L.; Wan, Q.; Zhou, Y.; Deng, X.; Xie, C.; Wu, S. The role of replanning in fractionated intensity modulated radiotherapy for nasopharyngeal carcinoma. Radiother. Oncol. 2011, 98, 23–27. [Google Scholar] [CrossRef]
| Organs at Risk | Dose-Volume Histogram Metric | Constraint |
|---|---|---|
| Spinal cord | D2% | <40–45 Gy |
| Parotid glands | V45Gy V30Gy V15Gy Dmean | <24% <45% <67% <26 Gy |
| Oral cavity | V40Gy | <35% |
| Larynx (whole organ) | V40Gy Dmean | <50% <35 Gy |
| N. (%) | |
|---|---|
| Age at Diagnosis, Median (Range) | 69 years (39–95) |
| Sex Male Female | 36 (64%) 20 (36%) |
| ECOG Performance status 0 1 | 15 (27%) 41 (73%) |
| Smoking Yes Ex-smoker No | 22 (39%) 5 (9%) 29 (52%) |
| Primary tumor site Oral cavity Larynx Nasopharynx Oropharynx Hypopharynx Salivary glands Thyroid Paranasal sinus Unknown primary | 14 (25%) 8 (14%) 2 (4%) 17 (30%) 5 (9%) 6 (11%) 2 (4%) 1 (2%) 1 (2%) |
| Histology Squamous cell carcinoma Other histologies | 44 (79%) 12 (21%) |
| Radiotherapy aim Radical radiotherapy Adjuvant radiotherapy | 35 (62.5%) 21 (37.5%) |
| Concomitant systemic therapy Yes No | 36 (%) 20 (35%) |
| Organs at Risk | DVH Metric | S * | Median (P25–P75) | Difference with S1 * | % | p ** |
|---|---|---|---|---|---|---|
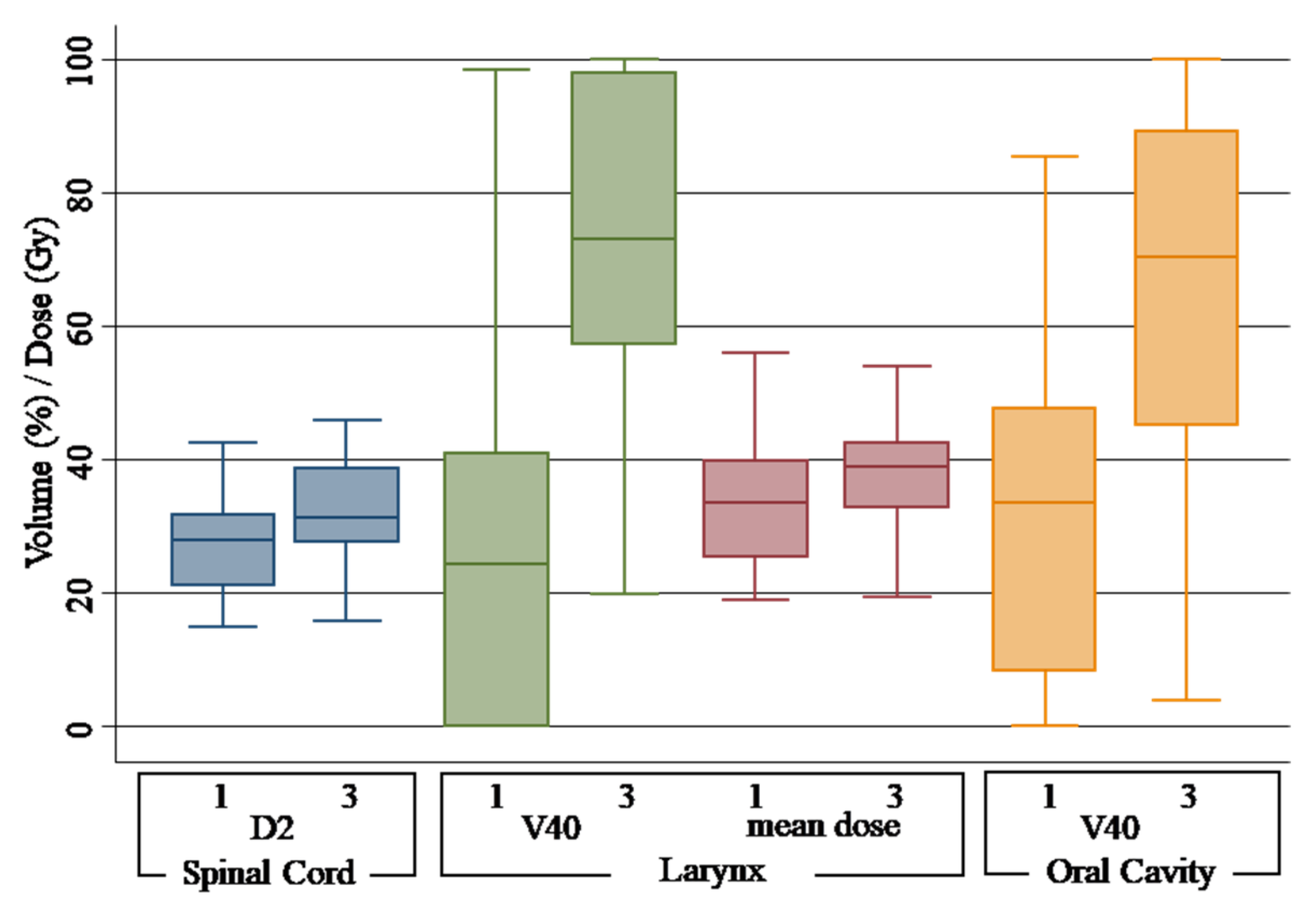
| Spinal cord | D2% | 1 | 27.91 Gy (21.06–31.76) | |||
| 2 | 26.48 Gy (22.10–32.51) | −1.43 Gy | −5.12% | 0.66 | ||
| 3 | 31.39 Gy (27.66–38.79) | 3.48 Gy | 12.46% | 0.00 | ||
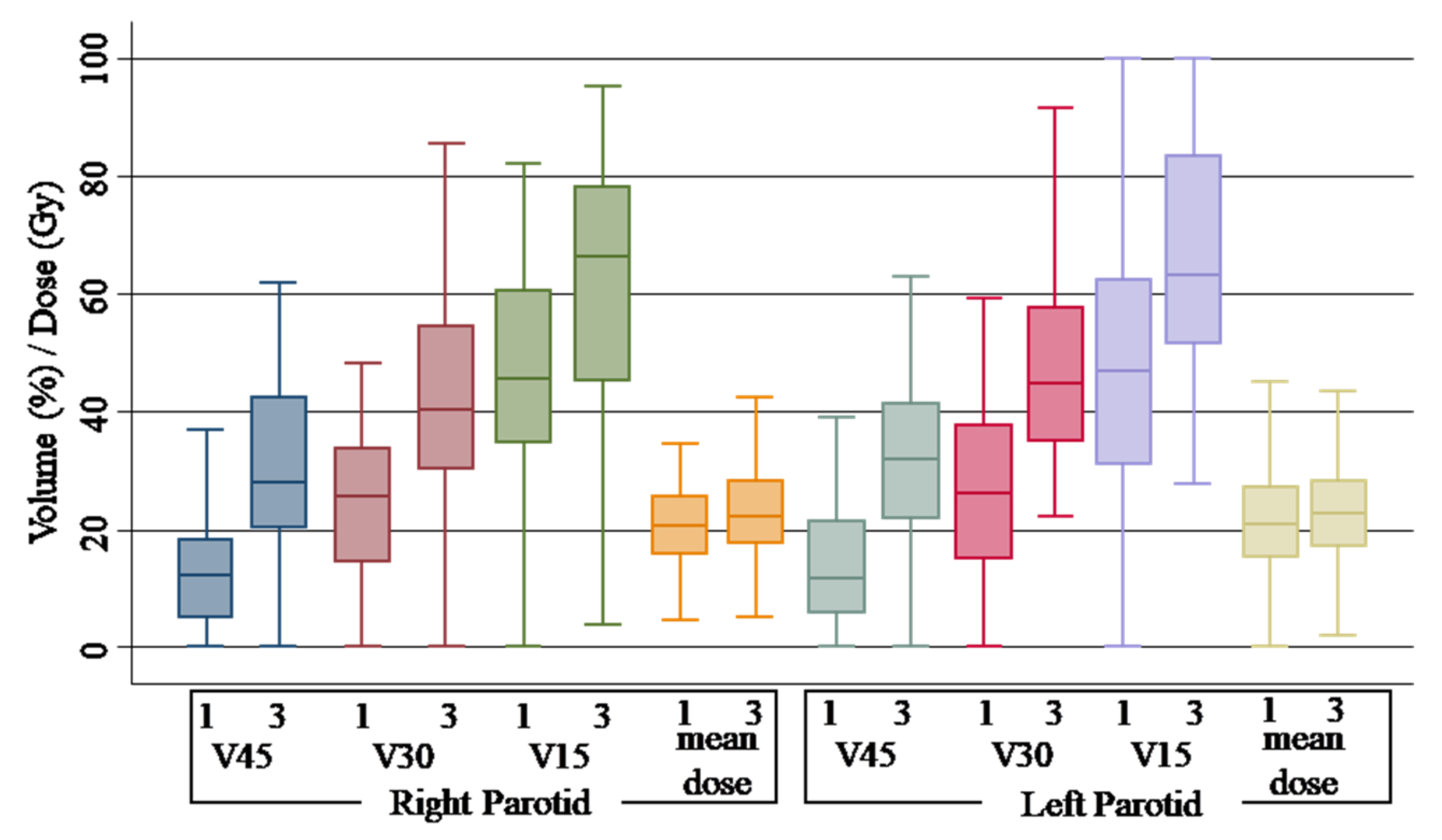
| Parotid right | Dmean | 1 | 20.54 Gy (15.86–25.52) | |||
| 2 | 20.82 Gy (17.56–25.67) | 0.28 Gy | 1.36% | 0.68 | ||
| 3 | 22.14 Gy (17.80–28.20) | 1.6 Gy | 7.78% | 0.30 | ||
| V15Gy | 1 | 45.70% (34.70–60.60) | ||||
| 2 | 47.50% (36.30–57.15) | 1.8% | 3.93% | 0.74 | ||
| 3 | 66.30% (45.10–78.10) | 20.6% | 45.07% | 0.00 | ||
| V30Gy | 1 | 25.50% (14.70–33.90) | ||||
| 2 | 26.20% (17.30–34.00) | 1.2% | 4.70% | 0.84 | ||
| 3 | 40.28% (30.30–54.60) | 14.78% | 57.96% | 0.00 | ||
| V45Gy | 1 | 12.40% (05.00–18.30) | ||||
| 2 | 14.80% (06.70–19.90) | 2.4% | 19.35% | 0.63 | ||
| 3 | 27.95% (20.20–42.40) | 15.55% | 124.40% | 0.00 | ||
| Parotid left | Dmean | 1 | 20.80 Gy (15.25–27.24) | |||
| 2 | 20.68 Gy (14.73–26.00) | −0.12 Gy% | −0.57% | 0.84 | ||
| 3 | 22.77 Gy (17.22–28.29) | 1.97 Gy% | 9.47% | 0.26 | ||
| V15Gy | 1 | 47.00% (30.90–62.40) | ||||
| 2 | 45.60% (34.40–58.30) | −1.4% | −2.97% | 0.51 | ||
| 3 | 63.25% (51.60–83.30) | 16.25% | 34.57% | 0.00 | ||
| V30Gy | 1 | 26.10% (15.20–37.70) | ||||
| 2 | 27.90% (14.90–36.30) | 1.8% | 6.89% | 0.93 | ||
| 3 | 44.80% (34.90–57.70) | 18.7% | 71.64% | 0.00 | ||
| V45Gy | 1 | 11.70% (05.70–21.40) | ||||
| 2 | 12.60% (05.50–22.90) | 0.9% | 7.69% | 0.88 | ||
| 3 | 31.89% (22.00–41.40) | 20.19% | 172.56% | 0.00 | ||
| Larynx | Dmean | 1 | 33.47 Gy (25.33–39.81) | |||
| 2 | 33.96 Gy (27.24–42.49) | 0.49 Gy | 1.46% | 0.56 | ||
| 3 | 39.00 Gy (32.85–42.50) | 5.53 Gy | 16.04% | 0.02 | ||
| V40Gy | 1 | 24.30% (00.00–40.84) | ||||
| 2 | 27.60% (00.00–51.50) | 3.3% | 13.58% | 0.41 | ||
| 3 | 72.95% (57.25–98.05) | 48.65% | 200.20% | 0.00 | ||
| Oral cavity | V40Gy | 1 | 33.45% (08.45–47.70) | |||
| 2 | 37.05% (14.50–52.65) | 3.6% | 10.76% | 0.53 | ||
| 3 | 70.40% (45.10–89.20) | 36.95% | 110.46% | 0.00 |
| Organs at Risk | DVH Metric | S1 * % | S2 * % | S3 * % | p ** |
|---|---|---|---|---|---|
| Spinal cord | D2% | 00.00 | 00.00 | 06.25 | 0.060 |
| Parotids, either left or right | Dmean | 42.11 | 44.64 | 53.06 | 0.260 |
| V45Gy | 31.58 | 35.09 | 87.76 | 0.000 | |
| V30Gy | 21.05 | 15.79 | 69.39 | 0.001 | |
| V15Gy | 24.56 | 21.05 | 55.10 | 0.001 | |
| Larynx | V40Gy | 12.96 | 25.45 | 80.56 | 0.000 |
| Dmean | 43.75 | 46.94 | 69.44 | 0.019 | |
| Oral cavity | V40Gy | 46.43 | 51.79 | 77.55 | 0.001 |
| Primary Tumor. Target | DVH Metric | S * | Median (P25–P75) % | Difference with S1 * | % | p ** |
|---|---|---|---|---|---|---|
| Planning target volume (PTV) | V95% | 1 | 98.72 (97.96–99.34) | |||
| 2 | 98.64 (97.25–99.37) | −0.08 | −0.08% | 0.35 | ||
| 3 | 94.70 (87.10–97.60) | −4.02 | −4.07% | 0.00 | ||
| Clinical target volume (CTV) | V95% | 1 | 99.96 (99.77–99.99) | |||
| 2 | 99.91 (99.31–99.99) | −0.05 | −0.05 | 0.30 | ||
| 3 | 97.90 (92.33–99.58) | −2.06 | −2.06 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franzese, C.; Tomatis, S.; Bianchi, S.P.; Pelizzoli, M.; Teriaca, M.A.; Badalamenti, M.; Comito, T.; Clerici, E.; Franceschini, D.; Navarria, P.; et al. Adaptive Volumetric-Modulated Arc Radiation Therapy for Head and Neck Cancer: Evaluation of Benefit on Target Coverage and Sparing of Organs at Risk. Curr. Oncol. 2023, 30, 3344-3354. https://doi.org/10.3390/curroncol30030254
Franzese C, Tomatis S, Bianchi SP, Pelizzoli M, Teriaca MA, Badalamenti M, Comito T, Clerici E, Franceschini D, Navarria P, et al. Adaptive Volumetric-Modulated Arc Radiation Therapy for Head and Neck Cancer: Evaluation of Benefit on Target Coverage and Sparing of Organs at Risk. Current Oncology. 2023; 30(3):3344-3354. https://doi.org/10.3390/curroncol30030254
Chicago/Turabian StyleFranzese, Ciro, Stefano Tomatis, Sofia Paola Bianchi, Marco Pelizzoli, Maria Ausilia Teriaca, Marco Badalamenti, Tiziana Comito, Elena Clerici, Davide Franceschini, Pierina Navarria, and et al. 2023. "Adaptive Volumetric-Modulated Arc Radiation Therapy for Head and Neck Cancer: Evaluation of Benefit on Target Coverage and Sparing of Organs at Risk" Current Oncology 30, no. 3: 3344-3354. https://doi.org/10.3390/curroncol30030254
APA StyleFranzese, C., Tomatis, S., Bianchi, S. P., Pelizzoli, M., Teriaca, M. A., Badalamenti, M., Comito, T., Clerici, E., Franceschini, D., Navarria, P., Di Cristina, L., Dei, D., Galdieri, C., Reggiori, G., Mancosu, P., & Scorsetti, M. (2023). Adaptive Volumetric-Modulated Arc Radiation Therapy for Head and Neck Cancer: Evaluation of Benefit on Target Coverage and Sparing of Organs at Risk. Current Oncology, 30(3), 3344-3354. https://doi.org/10.3390/curroncol30030254






