Osseous Union after Mandible Reconstruction with Fibula Free Flap Using Manually Bent Plates vs. Patient-Specific Implants: A Retrospective Analysis of 89 Patients
Abstract
:1. Introduction
2. Material and Methods
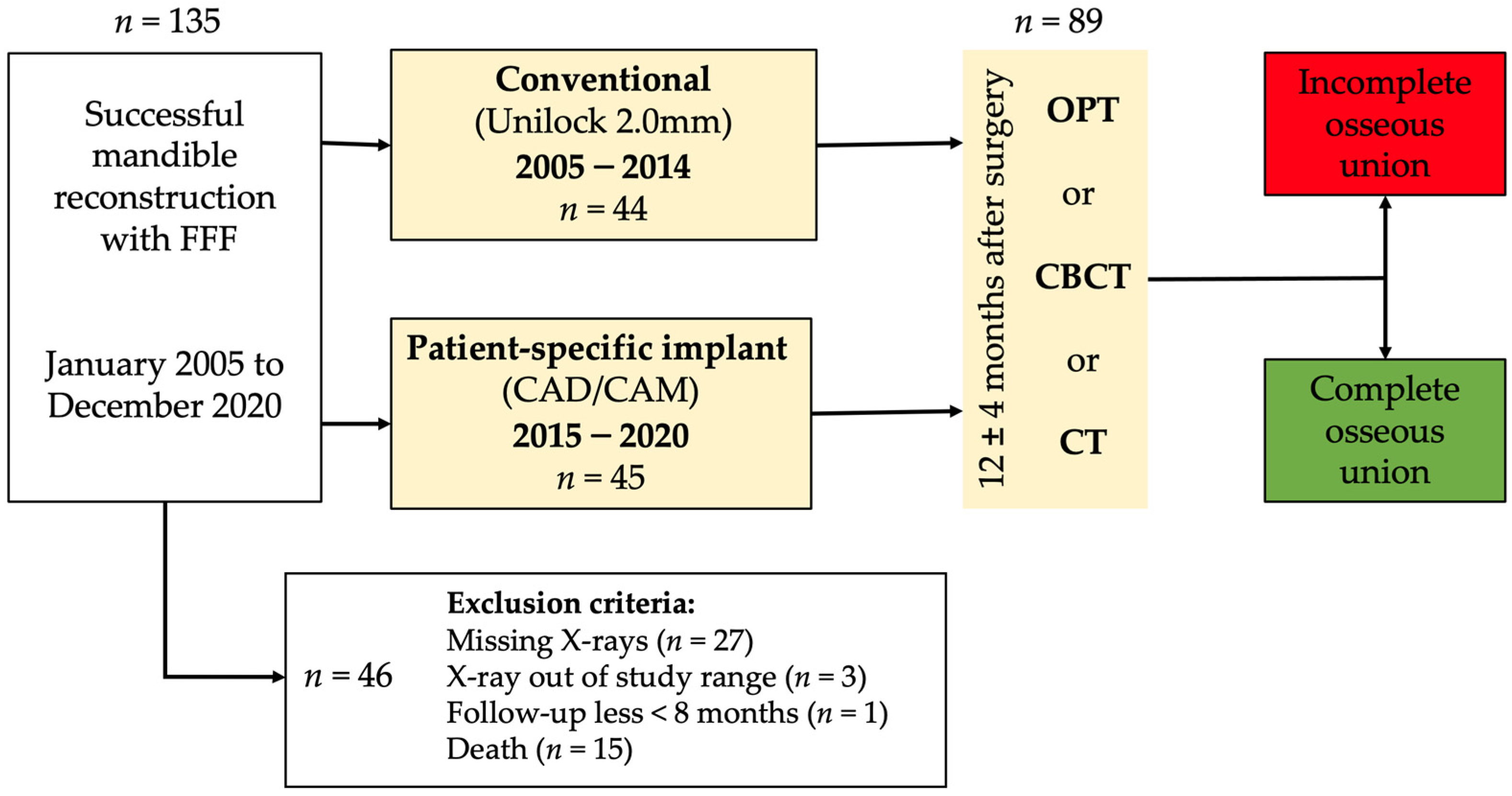
2.1. Study Design and Patient Population
2.2. Inclusion and Exclusion Criteria for Study Subjects
2.3. Study Parameters and Evaluator Calibration
2.4. Statistical Analyses
2.5. Ethics Statement/Confirmation of Patients’ Permission
3. Results
4. Discussion
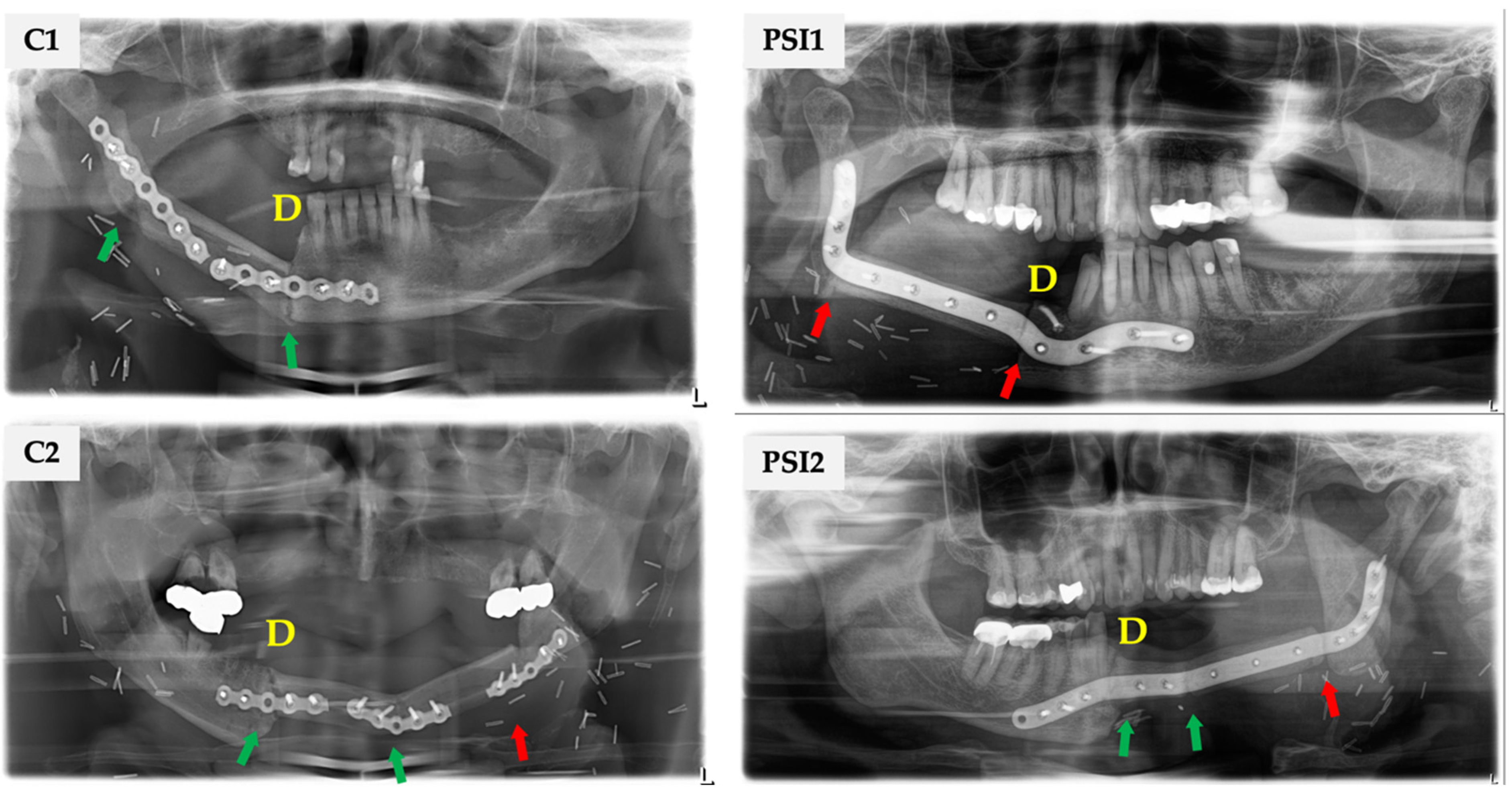
4.1. Are There Differences in Incomplete Osseous Union (IOU) Rates according to the Used Plate Type (Conventional vs. PSI)?
4.2. How Is the Distribution and Frequency of Complete and Incomplete Osseous Union regarding FFF’s Proximal or Distal End?
4.3. What Is the Frequency of Plate-Related Complications (Loosening of Osteosynthesis, Plate Exposure)?
5. Implications
6. Limitations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Taylor, G.I.; Miller, G.D.; Ham, F.J. The free vascularized bone graft. A clinical extension of microvascular techniques. Plast. Reconstr. Surg. 1975, 55, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.C.; Chen, H.C.; Chuang, C.C.; Noordhoff, M.S. Fibular osteoseptocutaneous flap: Anatomic study and clinical application. Plast. Reconstr. Surg. 1986, 78, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Kansy, K.; Mueller, A.A.; Mücke, T.; Kopp, J.B.; Koersgen, F.; Wolff, K.D.; Zeilhofer, H.F.; Hölzle, F.; Pradel, W.; Schneider, M.; et al. Microsurgical reconstruction of the head and neck—Current concepts of maxillofacial surgery in Europe. J. Cranio-Maxillofac. Surg. 2014, 42, 1610–1613. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, P.G.; Disa, J.J.; Hidalgo, D.A.; Hu, Q.Y. Reconstruction of the mandible with osseous free flaps: A 10-year experience with 150 consecutive patients. Plast. Reconstr. Surg. 1999, 104, 1314–1320. [Google Scholar] [CrossRef] [PubMed]
- Chana, J.S.; Chang, Y.M.; Wei, F.C.; Shen, Y.F.; Chan, C.P.; Lin, H.N.; Tsai, C.Y.; Jeng, S.F. Segmental mandibulectomy and immediate free fibula osteoseptocutaneous flap reconstruction with endosteal implants: An ideal treatment method for mandibular ameloblastoma. Plast. Reconstr. Surg. 2004, 113, 80–87. [Google Scholar] [CrossRef]
- Attia, S.; Wiltfang, J.; Streckbein, P.; Wilbrand, J.F.; El Khassawna, T.; Mausbach, K.; Howaldt, H.P.; Schaaf, H. Functional and aesthetic treatment outcomes after immediate jaw reconstruction using a fibula flap and dental implants. J. Cranio-Maxillofac. Surg. 2019, 47, 786–791. [Google Scholar] [CrossRef]
- Chen, X.F.; Chen, Y.M.; Gokavarapu, S.; Shen, Q.C.; Ji, T. Free flap reconstruction for patients aged 85 years and over with head and neck cancer: Clinical considerations for comprehensive care. Br. J. Oral Maxillofac. Surg. 2017, 55, 793–797. [Google Scholar] [CrossRef]
- Shroff, S.S.; Nair, S.C.; Shah, A.; Kumar, B. Versatility of fibula free flap in reconstruction of facial defects: A center study. J. Maxillofac. Oral Surg. 2017, 16, 101–107. [Google Scholar] [CrossRef] [Green Version]
- Barone, S.; Cosentini, G.; Bennardo, F.; Antonelli, A.; Giudice, A. Incidence and management of condylar resorption after orthognathic surgery: An overview. Korean J. Orthod. 2022, 52, 29–41. [Google Scholar] [CrossRef]
- Rustemeyer, J.; Busch, A.; Sari-Rieger, A. Application of computer-aided designed/computer-aided manufactured techniques in reconstructing maxillofacial bony structures. Oral Maxillofac. Surg. 2014, 18, 471–476. [Google Scholar] [CrossRef]
- Mascha, F.; Winter, K.; Pietzka, S.; Heufelder, M.; Schramm, A.; Wilde, F. Accuracy of computer-assisted mandibular reconstructions using patient-specific implants in combination with CAD/CAM fabricated transfer keys. J. Cranio-Maxillofac. Surg. 2017, 45, 1884–1897. [Google Scholar] [CrossRef]
- Knitschke, M.; Sonnabend, S.; Bäcker, C.; Schmermund, D.; Böttger, S.; Howaldt, H.P.; Attia, S. Partial and total flap failure after fibula free flap in head and neck reconstructive surgery: Retrospective analysis of 180 flaps over 19 years. Cancers 2021, 13, 865. [Google Scholar] [CrossRef]
- Zavattero, E.; Fasolis, M.; Garzino-Demo, P.; Berrone, S.; Ramieri, G.A. Evaluation of plate-related complications and efficacy in fibula free flap mandibular reconstruction. J. Craniofac. Surg. 2014, 25, 397–399. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.; Sambrook, P.; Munn, Z.; Boase, S. Effectiveness of computer-assisted virtual planning, cutting guides and pre-engineered plates on outcomes in mandible fibular free flap reconstructions over traditional freehand techniques: A systematic review protocol. JBI Database Syst. Rev. Implement. Rep. 2019, 17, 2136–2151. [Google Scholar] [CrossRef]
- Möllmann, H.L.; Apeltrath, L.; Karnatz, N.; Wilkat, M.; Riedel, E.; Singh, D.D.; Rana, M. Comparison of the accuracy and clinical parameters of patient-specific and conventionally bended plates for mandibular reconstruction. Front. Oncol. 2021, 11, 719028. [Google Scholar] [CrossRef]
- Brown, J.S.; Barry, C.; Ho, M.; Shaw, R. A new classification for mandibular defects after oncological resection. Lancet Oncol. 2016, 17, e23–e30. [Google Scholar] [CrossRef]
- Bartier, S.; Mazzaschi, O.; Benichou, L.; Sauvaget, E. Computer-assisted versus traditional technique in fibular free-flap mandibular reconstruction: A CT symmetry study. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2021, 138, 23–27. [Google Scholar] [CrossRef] [PubMed]
- De Maesschalck, T.; Courvoisier, D.S.; Scolozzi, P. Computer-assisted versus traditional freehand technique in fibular free flap mandibular reconstruction: A morphological comparative study. Eur. Arch. Otorhinolaryngol. 2017, 274, 517–526. [Google Scholar] [CrossRef]
- Ren, W.; Gao, L.; Li, S.; Chen, C.; Li, F.; Wang, Q.; Zhi, Y.; Song, J.; Dou, Z.; Xue, L.; et al. Virtual planning and 3D printing modeling for mandibular reconstruction with fibula free flap. Med. Oral Patol. Oral Cir. Bucal 2018, 23, e359–e366. [Google Scholar] [CrossRef]
- Egger, J.; Wallner, J.; Gall, M.; Chen, X.; Schwenzer-Zimmerer, K.; Reinbacher, K.; Schmalstieg, D. Computer-aided position planning of miniplates to treat facial bone defects. PLoS ONE 2017, 12, e0182839. [Google Scholar] [CrossRef] [Green Version]
- Wilde, F.; Cornelius, C.P.; Schramm, A. Computer-assisted mandibular reconstruction using a patient-specific reconstruction plate fabricated with computer-aided design and manufacturing techniques. Craniomaxillofac. Trauma Reconstr. 2014, 7, 158–166. [Google Scholar] [CrossRef] [Green Version]
- Wilde, F.; Hanken, H.; Probst, F.; Schramm, A.; Heiland, M.; Cornelius, C.P. Multicenter study on the use of patient-specific CAD/CAM reconstruction plates for mandibular reconstruction. Int. J. Comput. Assist. Radiol. Surg. 2015, 10, 2035–2051. [Google Scholar] [CrossRef] [PubMed]
- Geusens, J.; Sun, Y.; Luebbers, H.T.; Bila, M.; Darche, V.; Politis, C. Accuracy of computer-aided design/computer-aided manufacturing-assisted mandibular reconstruction with a fibula free flap. J. Craniofac. Surg. 2019, 30, 2319–2323. [Google Scholar] [CrossRef] [PubMed]
- Han, H.H.; Kim, H.Y.; Lee, J.Y. The pros and cons of computer-aided surgery for segmental mandibular reconstruction after oncological surgery. Arch. Craniofac. Surg. 2017, 18, 149–154. [Google Scholar] [CrossRef] [Green Version]
- Alassaf, M.H.; Li, W.; Joshi, A.S.; Hahn, J.K. Computer-based planning system for mandibular reconstruction. Stud. Health Technol. Inform. 2014, 196, 6–10. [Google Scholar] [PubMed]
- Ciocca, L.; Marchetti, C.; Mazzoni, S.; Baldissara, P.; Gatto, M.R.; Cipriani, R.; Scotti, R.; Tarsitano, A. Accuracy of fibular sectioning and insertion into a rapid-prototyped bone plate, for mandibular reconstruction using CAD-CAM technology. J. Cranio-Maxillofac. Surg. 2015, 43, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Toto, J.M.; Chang, E.I.; Agag, R.; Devarajan, K.; Patel, S.A.; Topham, N.S. Improved operative efficiency of free fibula flap mandible reconstruction with patient-specific, computer-guided preoperative planning. Head Neck 2015, 37, 1660–1664. [Google Scholar] [CrossRef] [PubMed]
- Culie, D.; Dassonville, O.; Poissonnet, G.; Riss, J.C.; Fernandez, J.; Bozec, A. Virtual planning and guided surgery in fibular free-flap mandibular reconstruction: A 29-case series. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2016, 133, 175–178. [Google Scholar] [CrossRef]
- Hanken, H.; Schablowsky, C.; Smeets, R.; Heiland, M.; Sehner, S.; Riecke, B.; Nourwali, I.; Vorwig, O.; Grobe, A.; Al-Dam, A. Virtual planning of complex head and neck reconstruction results in satisfactory match between real outcomes and virtual models. Clin. Oral Investig. 2015, 19, 647–656. [Google Scholar] [CrossRef]
- Knitschke, M.; Bäcker, C.; Schmermund, D.; Böttger, S.; Streckbein, P.; Howaldt, H.P.; Attia, S. Impact of planning method (conventional versus virtual) on time to therapy initiation and resection margins: A retrospective analysis of 104 immediate jaw reconstructions. Cancers 2021, 13, 3013. [Google Scholar] [CrossRef]
- Yang, W.F.; Choi, W.S.; Wong, M.C.; Powcharoen, W.; Zhu, W.Y.; Tsoi, J.K.; Chow, M.; Kwok, K.W.; Su, Y.X. Three-dimensionally printed patient-specific surgical plates increase accuracy of oncologic head and neck reconstruction versus conventional surgical plates: A comparative study. Ann. Surg. Oncol. 2021, 28, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Rendenbach, C.; Steffen, C.; Hanken, H.; Schluermann, K.; Henningsen, A.; Beck-Broichsitter, B.; Kreutzer, K.; Heiland, M.; Precht, C. Complication rates and clinical outcomes of osseous free flaps: A retrospective comparison of CAD/CAM versus conventional fixation in 128 patients. Int. J. Oral Maxillofac. Surg. 2019, 48, 1156–1162. [Google Scholar] [CrossRef] [PubMed]
- Fichter, A.M.; Ritschl, L.M.; Georg, R.; Kolk, A.; Kesting, M.R.; Wolff, K.D.; Mücke, T. Effect of segment length and number of osteotomy sites on cancellous bone perfusion in free fibula flaps. J. Reconstr. Microsurg. 2019, 35, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Mücke, T.; Ritschl, L.M.; Roth, M.; Güll, F.D.; Rau, A.; Grill, S.; Kesting, M.R.; Wolff, K.D.; Loeffelbein, D.J. Predictors of free flap loss in the head and neck region: A four-year retrospective study with 451 microvascular transplants at a single centre. J. Cranio-Maxillofac. Surg. 2016, 44, 1292–1298. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.I.; Jenkins, M.P.; Patel, S.A.; Topham, N.S. Long-term operative outcomes of preoperative computed tomography-guided virtual surgical planning for osteocutaneous free flap mandible reconstruction. Plast. Reconstr. Surg. 2016, 137, 619–623. [Google Scholar] [CrossRef]
- Foster, R.D.; Anthony, J.P.; Sharma, A.; Pogrel, M.A. Vascularized bone flaps versus nonvascularized bone grafts for mandibular reconstruction: An outcome analysis of primary bony union and endosseous implant success. Head Neck 1999, 21, 66–71. [Google Scholar] [CrossRef]
- Mehra, P.; Murad, H. Internal fixation of mandibular angle fractures: A comparison of 2 techniques. J. Oral Maxillofac. Surg. 2008, 66, 2254–2260. [Google Scholar] [CrossRef]
- Swendseid, B.; Kumar, A.; Sweeny, L.; Zhan, T.; Goldman, R.A.; Krein, H.; Heffelfinger, R.N.; Luginbuhl, A.J.; Curry, J.M. Natural history and consequences of nonunion in mandibular and maxillary free flaps. Otolaryngol. Head Neck Surg. 2020, 163, 956–962. [Google Scholar] [CrossRef]
- Yeh, D.H.; Lee, D.J.; Sahovaler, A.; Fung, K.; MacNeil, D.; Nichols, A.C.; Yoo, J. Shouldering the load of mandible reconstruction: 81 cases of oromandibular reconstruction with the scapular tip free flap. Head Neck 2019, 41, 30–36. [Google Scholar] [CrossRef] [Green Version]
- Yla-Kotola, T.M.; Bartlett, E.; Goldstein, D.P.; Armstrong, K.; Gilbert, R.W.; Hofer, S.O. Union and bone resorption of free fibular flaps in mandibular reconstruction. J. Reconstr. Microsurg. 2013, 29, 427–432. [Google Scholar] [CrossRef]
- Claes, L.E.; Heigele, C.A.; Neidlinger-Wilke, C.; Kaspar, D.; Seidl, W.; Margevicius, K.J.; Augat, P. Effects of mechanical factors on the fracture healing process. Clin. Orthop. Relat. Res. 1998, 355, S132–S147. [Google Scholar] [CrossRef] [PubMed]
- Kennady, M.C.; Tucker, M.R.; Lester, G.E.; Buckley, M.J. Stress shielding effect of rigid internal fixation plates on mandibular bone grafts. A photon absorption densitometry and quantitative computerized tomographic evaluation. Int. J. Oral Maxillofac. Surg. 1989, 18, 307–310. [Google Scholar] [CrossRef]
- Robey, A.B.; Spann, M.L.; McAuliff, T.M.; Meza, J.L.; Hollins, R.R.; Johnson, P.J. Comparison of miniplates and reconstruction plates in fibular flap reconstruction of the mandible. Plast. Reconstr. Surg. 2008, 122, 1733–1738. [Google Scholar] [CrossRef] [PubMed]
- Zoumalan, R.A.; Hirsch, D.L.; Levine, J.P.; Saadeh, P.B. Plating in microvascular reconstruction of the mandible: Can fixation be too rigid? J. Craniofac. Surg. 2009, 20, 1451–1454. [Google Scholar] [CrossRef] [Green Version]
- Ghiasi, M.S.; Chen, J.; Vaziri, A.; Rodriguez, E.K.; Nazarian, A. Bone fracture healing in mechanobiological modeling: A review of principles and methods. Bone Rep. 2017, 6, 87–100. [Google Scholar] [CrossRef]
- Rendenbach, C.; Sellenschloh, K.; Gerbig, L.; Morlock, M.M.; Beck-Broichsitter, B.; Smeets, R.; Heiland, M.; Huber, G.; Hanken, H. CAD-CAM plates versus conventional fixation plates for primary mandibular reconstruction: A biomechanical in vitro analysis. J. Cranio-Maxillofac. Surg. 2017, 45, 1878–1883. [Google Scholar] [CrossRef]
- Kreutzer, K.; Steffen, C.; Nahles, S.; Koerdt, S.; Heiland, M.; Rendenbach, C.; Beck-Broichsitter, B. Removal of patient-specific reconstruction plates after mandible reconstruction with a fibula free flap: Is the plate the problem? Int. J. Oral Maxillofac. Surg. 2021, 51, 182–190. [Google Scholar] [CrossRef]
- Kreutzer, K.; Steffen, C.; Koerdt, S.; Doll, C.; Ebker, T.; Nahles, S.; Flügge, T.; Heiland, M.; Beck-Broichsitter, B.; Rendenbach, C. Patient-specific 3D-printed miniplates for free flap fixation at the mandible: A feasibility study. Front. Surg. 2022, 9. [Google Scholar] [CrossRef]
- Zhong, S.; Shi, Q.; Sun, Y.; Yang, S.; van Dessel, J.; Gu, Y.; Chen, X.; Lubbers, H.T.; Politis, C. Biomechanical comparison of locking and non-locking patient-specific mandibular reconstruction plate using finite element analysis. J. Mech. Behav. Biomed. Mater. 2021, 124, 104849. [Google Scholar] [CrossRef]
- Kristina, H. Finite Element Analysis of Orthopaedic Plates and Screws to Reduce the Effects of Stress Shielding. Ph.D. Thesis, University of Ottawa, Ottawa, ON, Canada, 2009. [Google Scholar]
- Koo, H.; Hupel, T.; Zdero, R.; Tov, A.; Schemitsch, E.H. The effect of muscle contusion on cortical bone and muscle perfusion following reamed, intramedullary nailing: A novel canine tibia fracture model. J. Orthop. Surg. Res. 2010, 5, 89. [Google Scholar] [CrossRef] [Green Version]
- Coletti, D.P.; Ord, R.; Liu, X. Mandibular reconstruction and second generation locking reconstruction plates: Outcome of 110 patients. Int. J. Oral Maxillofac. Surg. 2009, 38, 960–963. [Google Scholar] [CrossRef] [PubMed]
- Lippuner, K.; Vogel, R.; Tepic, S.; Rahn, B.A.; Cordey, J.; Perren, S.M. Effect of animal species and age on plate-induced vascular damage in cortical bone. Arch. Orthop. Trauma Surg. 1992, 111, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, L.A.; Ogata, K.; Lesker, P.; Reynolds, F.C. The acute effects of periosteal stripping and medullary reaming on regional bone blood flow. Clin. Orthop. Relat. Res. 1978, 131, 266–272. [Google Scholar] [CrossRef]
- Leiggener, C.; Messo, E.; Thor, A.; Zeilhofer, H.F.; Hirsch, J.M. A selective laser sintering guide for transferring a virtual plan to real time surgery in composite mandibular reconstruction with free fibula osseous flaps. Int. J. Oral Maxillofac. Surg. 2009, 38, 187–192. [Google Scholar] [CrossRef]
- Weitz, J.; Wolff, K.D.; Kesting, M.R.; Nobis, C.P. Development of a novel resection and cutting guide for mandibular reconstruction using free fibula flap. J. Cranio-Maxillofac. Surg. 2018, 46, 1975–1978. [Google Scholar] [CrossRef]
- Pitak-Arnnop, P.; Hemprich, A.; Dhanuthai, K.; Pausch, N.C. Fibular flap for mandibular reconstruction: Are there old tricks for an old dog? Rev. Stomatol. Chir. Maxillo-Fac. Chir. Orale 2013, 114, 15–18. [Google Scholar] [CrossRef]
- Menck, J.; Sander, A. Periosteal and endosteal blood supply of the human fibula and its clinical importance. Acta Anat. 1992, 145, 400–405. [Google Scholar] [CrossRef]
- Strachan, R.K.; Mccarthy, I.; Fleming, R.; Hughes, S.P.F. The role of the tibial nutrient artery—Microsphere estimation of blood-flow in the osteotomised canine tibia. J. Bone Jt. Surg.-Br. Vol. 1990, 72, 391–394. [Google Scholar] [CrossRef]
- Kregor, P.J.; Senft, D.; Parvin, D.; Campbell, C.; Toomey, S.; Parker, C.; Gillespy, T.; Swiontkowski, M.F. Cortical bone perfusion in plated fractured sheep tibiae. J. Orthop. Res. 1995, 13, 715–724. [Google Scholar] [CrossRef]
- Seibold, R.; Schlegel, U.; Kessler, S.B.; Cordey, J.; Perren, S.M.; Schweiberer, L. Healing of spiral fractures in the sheep tibia comparing different methods—Osteosynthesis with internal fixation, interlocking nailing and dynamic compression plate. Unfallchirurg 1995, 98, 620–626. [Google Scholar]
- Bartnikowski, N.; Claes, L.E.; Koval, L.; Glatt, V.; Bindl, R.; Steck, R.; Ignatius, A.; Schuetz, M.A.; Epari, D.R. Modulation of fixation stiffness from flexible to stiff in a rat model of bone healing. Acta Orthop. 2017, 88, 217–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoda, N.; Zheng, K.; Chen, J.; Liao, Z.; Koyama, S.; Peck, C.; Swain, M.; Sasaki, K.; Li, Q. Biomechanical analysis of bone remodeling following mandibular reconstruction using fibula free flap. Med. Eng. Phys. 2018, 56, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greksa, F.; Butt, E.; Csonka, E.; Javor, P.; Tuboly, E.; Torok, L.; Szabo, A.; Varga, E.; Hartmann, P. Periosteal and endosteal microcirculatory injury following excessive osteosynthesis. Injury 2021, 52 (Suppl. S1), S3–S6. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, M.J.; Schemitsch, E.H.; Kregor, P.J.; Senft, D.; Swiontkowski, M.F. Effect of periosteal stripping on cortical bone perfusion: A laser doppler study in sheep. Calcif. Tissue Int. 1996, 59, 24–26. [Google Scholar] [CrossRef]
- Schemitsch, E.H.; Kowalski, M.J.; Swiontkowski, M.F.; Harrington, R.M. Comparison of the effect of reamed and unreamed locked intramedullary nailing on blood flow in the callus and strength of union following fracture of the sheep tibia. J. Orthop. Res. 1995, 13, 382–389. [Google Scholar] [CrossRef]
- Shapiro, F. Bone development and its relation to fracture repair. The role of mesenchymal osteoblasts and surface osteoblasts. Eur. Cells Mater. 2008, 15, 53–76. [Google Scholar] [CrossRef]
- Augat, P.; Margevicius, K.; Simon, J.; Wolf, S.; Suger, G.; Claes, L. Local tissue properties in bone healing: Influence of size and stability of the osteotomy gap. J. Orthop. Res. 1998, 16, 475–481. [Google Scholar] [CrossRef]
- Claes, L.; Augat, P.; Suger, G.; Wilke, H.J. Influence of size and stability of the osteotomy gap on the success of fracture healing. J. Orthop. Res. 1997, 15, 577–584. [Google Scholar] [CrossRef]
- Hidalgo, D.A. Titanium miniplate fixation in free flap mandible reconstruction. Ann. Plast. Surg. 1989, 23, 498–507. [Google Scholar] [CrossRef]
- Likhterov, I.; Roche, A.M.; Urken, M.L. Contemporary osseous reconstruction of the mandible and the maxilla. Oral Maxillofac. Surg. Clin. North Am. 2019, 31, 101–116. [Google Scholar] [CrossRef]
- Shimamoto, H.; Sumida, I.; Kakimoto, N.; Marutani, K.; Okahata, R.; Usami, A.; Tsujimoto, T.; Murakami, S.; Furukawa, S.; Tetradis, S. Evaluation of the scatter doses in the direction of the buccal mucosa from dental metals. J. Appl. Clin. Med. Phys. 2015, 16, 5374. [Google Scholar] [CrossRef] [PubMed]
- Chiodo, A.A.; Gur, E.; Pang, C.Y.; Neligan, P.C.; Boyd, J.B.; Binhammer, P.M.; Forrest, C.R. The vascularized pig fibula bone flap model: Effect of segmental osteotomies and internal fixation on blood flow. Plast. Reconstr. Surg. 2000, 105, 1004–1012. [Google Scholar] [CrossRef] [PubMed]
- Gur, E.; Chiodo, A.; Pang, C.Y.; Mendes, M.; Pritzker, K.P.; Neligan, P.C.; Shpitzer, T.; Forrest, C.R. The vascularized pig fibula bone flap model: Effects of multiple segmental osteotomies on growth and viability. Plast. Reconstr. Surg. 1999, 103, 1436–1442. [Google Scholar] [CrossRef] [PubMed]
- Strackee, S.D.; Kroon, F.H.; Jaspers, J.E.; Bos, K.E. Modeling a fibula transplant in mandibular reconstructions: Evaluation of the effects of a minimal number of osteotomies on the contour of the jaw. Plast. Reconstr. Surg. 2001, 108, 1915–1921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, F.C.; Santamaria, E.; Chang, Y.M.; Chen, H.C. Mandibular reconstruction with fibular osteoseptocutaneous free flap and simultaneous placement of osseointegrated dental implants. J. Craniofac. Surg. 1997, 8, 512–521. [Google Scholar] [CrossRef]
- Bähr, W. Blood supply of small fibula segments: An experimental study on human cadavers. J. Cranio-Maxillofac. Surg. 1998, 26, 148–152. [Google Scholar] [CrossRef]
- Knitschke, M.; Baumgart, A.K.; Bäcker, C.; Adelung, C.; Roller, F.; Schmermund, D.; Böttger, S.; Streckbein, P.; Howaldt, H.P.; Attia, S. Impact of periosteal branches and septo-cutaneous perforators on free fibula flap outcome: A retrospective analysis of computed tomography angiography scans in virtual surgical planning. Front. Oncol. 2021, 11, 821851. [Google Scholar] [CrossRef]
- Trignano, E.; Fallico, N.; Faenza, M.; Rubino, C.; Chen, H.C. Free fibular flap with periosteal excess for mandibular reconstruction. Microsurgery 2013, 33, 527–533. [Google Scholar] [CrossRef]
- Smeele, L.E.; Slotman, B.J.; Mens, J.W.; Tiwari, R. Local radiation dose, fixation, and non-union of mandibulotomies. Head Neck 1999, 21, 315–318. [Google Scholar] [CrossRef]
- Yao, C.M.; Ziai, H.; Tsang, G.; Copeland, A.; Brown, D.; Irish, J.C.; Gilbert, R.W.; Goldstein, D.P.; Gullane, P.J.; de Almeida, J.R. Surgical site infections following oral cavity cancer resection and reconstruction is a risk factor for plate exposure. J. Otolaryngol. Head Neck Surg. 2017, 46, 30. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.L.; Wang, S.; Sun, C.F.; Xu, Z.F. Miniplates versus reconstruction plates in vascularized osteocutaneous flap reconstruction of the mandible. J. Craniofac. Surg. 2019, 30, e119–e125. [Google Scholar] [CrossRef] [PubMed]
- Militsakh, O.N.; Wallace, D.I.; Kriet, J.D.; Girod, D.A.; Olvera, M.S.; Tsue, T.T. Use of the 2.0-mm locking reconstruction plate in primary oromandibular reconstruction after composite resection. Otolaryngol. Head Neck Surg. 2004, 131, 660–665. [Google Scholar] [CrossRef] [PubMed]
| Conventional (Unilock 2.0) (n = 44) | CAD/CAM (PSI) (n = 45) | p-Value | |
|---|---|---|---|
| Age (years), mean ± SD | 58.54 ± 10.46 | 59.23 ± 12.23 | 0.777 |
| Image osseous union (months), mean ± SD | 11.25 ± 2.52 | 11.0 ± 2.90 | 0.665 |
| Follow-up (months), median; IQI (Q1, Q3) | 88.0 (35.75, 125.5) | 19.0 (12.5, 34.5) | 0.001 |
| Gender, n (%) | |||
| Male | 31 (70.4) | 30 (66.7) | |
| Female | 13 (29.6) | 15 (33.3) | 0.699 |
| Image type | |||
| OPT | 29 (65.9) | 23 (51.1) | |
| CBCT | 2 (4.5) | 2 (4.4) | |
| CT | 13 (29.5) | 20 (44.4) | 0.321 |
| Pathology, n (%) | |||
| Benign tumor | 1 (2.3) | 5 (11.1) | |
| Malignant tumor | 41 (93.1) | 36 (80.0) | |
| MRONJ | - | 1 (2.2) | |
| Osteoradionecrosis | 1 (2.3) | 1 (2.2) | |
| Osteomyelitis | 1 (2.3) | 2 (4.4) | 0.366 |
| ASA, n (%) | |||
| 1 | 4 (9.1) | 1 (2.2) | |
| 2 | 22 (50.0) | 22 (48.9) | |
| 3 | 18 (40.9) | 21 (46.7) | |
| 4 | - | 1 (2.2) | 0.389 |
| BMI (kg/m2), n (%) | |||
| <18 | 3 (6.8) | 3 (6.7) | |
| 18 ≥ 25 | 24 (54.5) | 22 (48.9) | |
| 25 ≥ 30 | 12 (27.3) | 14 (31.1) | |
| 30 ≥ 35 | 3 (6.8) | 5 (11.1) | |
| >35 | 2 (4.5) | 1 (2.2) | 0.901 |
| Tobacco abuses, n (%) | 32 (72.7) | 29 (64.4) | 0.495 |
| Alcohol abuses, n (%) | 16 (36.4) | 21 (46.7) | 0.392 |
| Time of reconstruction, n (%) | |||
| Immediate | 42 (95.5) | 39 (86.7) | |
| Delayed | 2 (4.5) | 6 (13.3) | 0.266 |
| Brown Classification, n (%) | |||
| I(c) | 16 (36.4) | 10 (22.2) | |
| II(c) | 12 (27.3) | 16 (35.6) | |
| III | 16 (36.4) | 16 (35.6) | |
| IV(c) | - | 3 (6.7) | 0.175 |
| Number of segments | |||
| 1 | 19 (43.2) | 8 (17.8) | |
| 2 | 19 (43.2) | 21 (46.7) | |
| 3 | 6 (13.6) | 16 (35.6) | 0.010 |
| Conventional (Unilock 2.0) (n = 44) | CAD/CAM (PSI) (n = 45) | p-Value | |
|---|---|---|---|
| Plate related fixation failures, n (%) | 4 (9.1) | 7 (15.6) | 0.522 |
| Plate exposure, n (%) | 10 (22.7) | 11 (24.4) | 1.000 |
| Radiotherapy, n (%) | |||
| Preoperative | 5 (11.4) | 6 (13.3) | |
| Postoperative | 15 (34.1) | 24 (53.3) | |
| None | 24 (54.5) | 15 (33.3) | 0.121 |
| OU: M ↔ F and F ↔ F, n (%) | 44 | 45 | |
| COU | 38 (86.4) | 29 (64.6) | |
| IOU | 6 (13.6) | 16 (35.6) | 0.017 |
| OU: M ↔ F and F ↔ F, n = all junctions (%) | 120 | 129 | |
| COU | 114 (95.0) | 104 (80.6) | |
| IOU | 6 (5.0) | 25 (19.4) | <0.001 |
| OU: M ↔ F, n = only proximal and distal junctions, (%) | 88 | 79 | |
| COU | 82 (93.2) | 62 (78.5) | |
| IOU | 6 (6.8) | 17 (21.5) | 0.006 |
| OU: F ↔ F, n = only intersegmental junctions, (%) | 32 | 50 | |
| COU | 32 (100.0) | 42 (84.0) | |
| IOU | 0 | 8 (16.0) | 0.015 |
| OU uni-segmental reconstruction, n (%) | 19 | 8 | |
| COU | 16 (84.2) | 5 (62.5) | |
| IOU | 3 (15.8) | 3 (37.5) | 0.215 |
| OU poly-segmental reconstruction, n (%) | 25 | 37 | |
| COU | 22 (88.0) | 24 (64.9) | |
| IOU | 3 (12.0) | 13 (35.1) | 0.041 |
| OU lateral reconstruction, n (%) | 28 | 25 | |
| COU | 24 (85.7) | 14 (56.0) | |
| IOU | 4 (14.3) | 11 (44.0) | 0.016 |
| OU anterior reconstruction, n (%) | 16 | 20 | |
| COU | 14 (87.5) | 15 (75.0) | |
| IOU | 2 (12.5) | 5 (25.0) | 0.346 |
| Co | Sco | A | Pc | Ac | C | All | IOU-Rate | p-Value | |
|---|---|---|---|---|---|---|---|---|---|
| Distal junction | (89) 80 * | 16.3% * | |||||||
| COU, conventional | - | 5 | 4 | 12 | 10 | 8 | 39 | - | |
| IOU, conventional | - | 1 | 1 | - | 1 | 2 | 5 | 11.4% | |
| COU, PSI | (9) 0 * | 10 | 3 | 9 | 2 | 4 | (37) 28 * | - | |
| IOU, PSI | - | 2 | 1 | 1 | 2 | 1 | 8 | 22.2% * | 0.190 |
| Proximal junction | (87) 85 ‡ | 10.6% ‡ | |||||||
| COU, conventional | - | 5 | 9 | 15 | 6 | 8 | 43 | - | |
| IOU, conventional | - | 1 | - | - | - | - | 1 | 2.2% | |
| COU, PSI | - | (2) 1 ‡ | 5 | 9 | 7 | 11 | (34) 33 ‡ | - | |
| IOU, PSI | - | 1 | 2 | (2) 1 ‡ | 2 | 2 | (9) 8 ‡ | 19.5% | 0.009 |
| Incomplete Osseous Union | |||||
|---|---|---|---|---|---|
| Yes, n (%) | No, n (%) | p-Value | OR [95%-CI] | ||
| Patient-related parameter | |||||
| Age, years (Mean ± SD) | 58.91 ± 12.70 | 58.79 ± 10.92 | 0.967 | 1.001 [0.959; 1.045] | |
| Gender | Male | 14 (63.6) | 47 (70.1) | 0.568 | 0.745 [0.270; 2.053] |
| Female | 8 (36.4) | 20 (29.9) | |||
| Tobacco | Yes | 16 (72.7 | 45 (67.2) | 0.626 | 1.304 [0.448; 3.793] |
| No | 6 (27.3) | 22 (32.8) | |||
| Alcohol | Yes | 9 (40.9) | 28 (41.8) | 0.942 | 0.964 [0.362; 2.566] |
| No | 13 (59.1) | 39 (58.2) | |||
| ASA-Score ≥ 3 | Yes | 8 (36.4) | 31 (46.3) | 0.417 | 1.958 [0.428; 8.959] |
| No | 14 (63.6) | 36 (53.7) | |||
| BMI (Mean ± SD) | 24.81 ± 3.71 | 24.54 ± 4.90 | 0.811 | 1.013 [0.913; 1.124] | |
| Surgery related parameter | |||||
| Operation duration, minutes (Mean ± SD) | 533 ± 132 | 512 ± 91 | 0.395 | 1.002 [0.997; 1.007] | |
| Reconstruction | Immediate | 19 (86.4) | 62 (92.5) | 0.380 | 1.958 [0.428; 8.959] |
| Delayed | 3 (13.6) | 5 (7.5) | |||
| Plate system | Conventional | 6 (27.3) | 38 (56.7) | 0.017 | 3.494 [1.216; 10.040] |
| PSI | 16 (72.7) | 29 (43.3) | |||
| Fibular segments | 1 | 6 (27.3) | 21 (31.3) | 0.800 | 0.919 [0.479; 1.765] |
| 2 | 15 (54.5) | 28 (41.8) | |||
| 3 | 4 (18.2) | 18 (26.9) | |||
| Reconstruction site | Unilateral | 15 (68.2) | 39 (58.2) | 0.406 | 0.650 [0234; 1.803] |
| Bilateral | 7 (31.8) | 28 (41.8) | |||
| Radiotherapy | None | 8 (36.4) | 31 (46.3) | 0.432 | 1.231 [0.733; 2.068] |
| Preoperative | 3 (13.6) | 8 (11.9) | |||
| Postoperative | 11 (50.0) | 28 (41.8) | |||
| Complication | |||||
| Plate exposure | Yes | 9 (40.9) | 12 (17.9) | 0.027 | 3.173 [1.105; 9.110] |
| No | 13 (59.1) | 55 (82.1) | |||
| Plate related complication (screw loosening) | Yes | 6 (27.3) | 5 (7.5) | 0.014 | 4.650 [1.257; 17.197] |
| No | 16 (72.7) | 62 (92.5) | |||
| Parameter | p-Value | OR | 95% CI | |
|---|---|---|---|---|
| Plate system | 0.019 | 3.682 | 1.236 | 10.966 |
| Plate exposure | 0.031 | 3.389 | 1.118 | 10.275 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knitschke, M.; Sonnabend, S.; Roller, F.C.; Pons-Kühnemann, J.; Schmermund, D.; Attia, S.; Streckbein, P.; Howaldt, H.-P.; Böttger, S. Osseous Union after Mandible Reconstruction with Fibula Free Flap Using Manually Bent Plates vs. Patient-Specific Implants: A Retrospective Analysis of 89 Patients. Curr. Oncol. 2022, 29, 3375-3392. https://doi.org/10.3390/curroncol29050274
Knitschke M, Sonnabend S, Roller FC, Pons-Kühnemann J, Schmermund D, Attia S, Streckbein P, Howaldt H-P, Böttger S. Osseous Union after Mandible Reconstruction with Fibula Free Flap Using Manually Bent Plates vs. Patient-Specific Implants: A Retrospective Analysis of 89 Patients. Current Oncology. 2022; 29(5):3375-3392. https://doi.org/10.3390/curroncol29050274
Chicago/Turabian StyleKnitschke, Michael, Sophia Sonnabend, Fritz Christian Roller, Jörn Pons-Kühnemann, Daniel Schmermund, Sameh Attia, Philipp Streckbein, Hans-Peter Howaldt, and Sebastian Böttger. 2022. "Osseous Union after Mandible Reconstruction with Fibula Free Flap Using Manually Bent Plates vs. Patient-Specific Implants: A Retrospective Analysis of 89 Patients" Current Oncology 29, no. 5: 3375-3392. https://doi.org/10.3390/curroncol29050274
APA StyleKnitschke, M., Sonnabend, S., Roller, F. C., Pons-Kühnemann, J., Schmermund, D., Attia, S., Streckbein, P., Howaldt, H.-P., & Böttger, S. (2022). Osseous Union after Mandible Reconstruction with Fibula Free Flap Using Manually Bent Plates vs. Patient-Specific Implants: A Retrospective Analysis of 89 Patients. Current Oncology, 29(5), 3375-3392. https://doi.org/10.3390/curroncol29050274







