The Health Economics of Metastatic Hormone-Sensitive and Non-Metastatic Castration-Resistant Prostate Cancer—A Systematic Literature Review with Application to the Canadian Context
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Literature Search
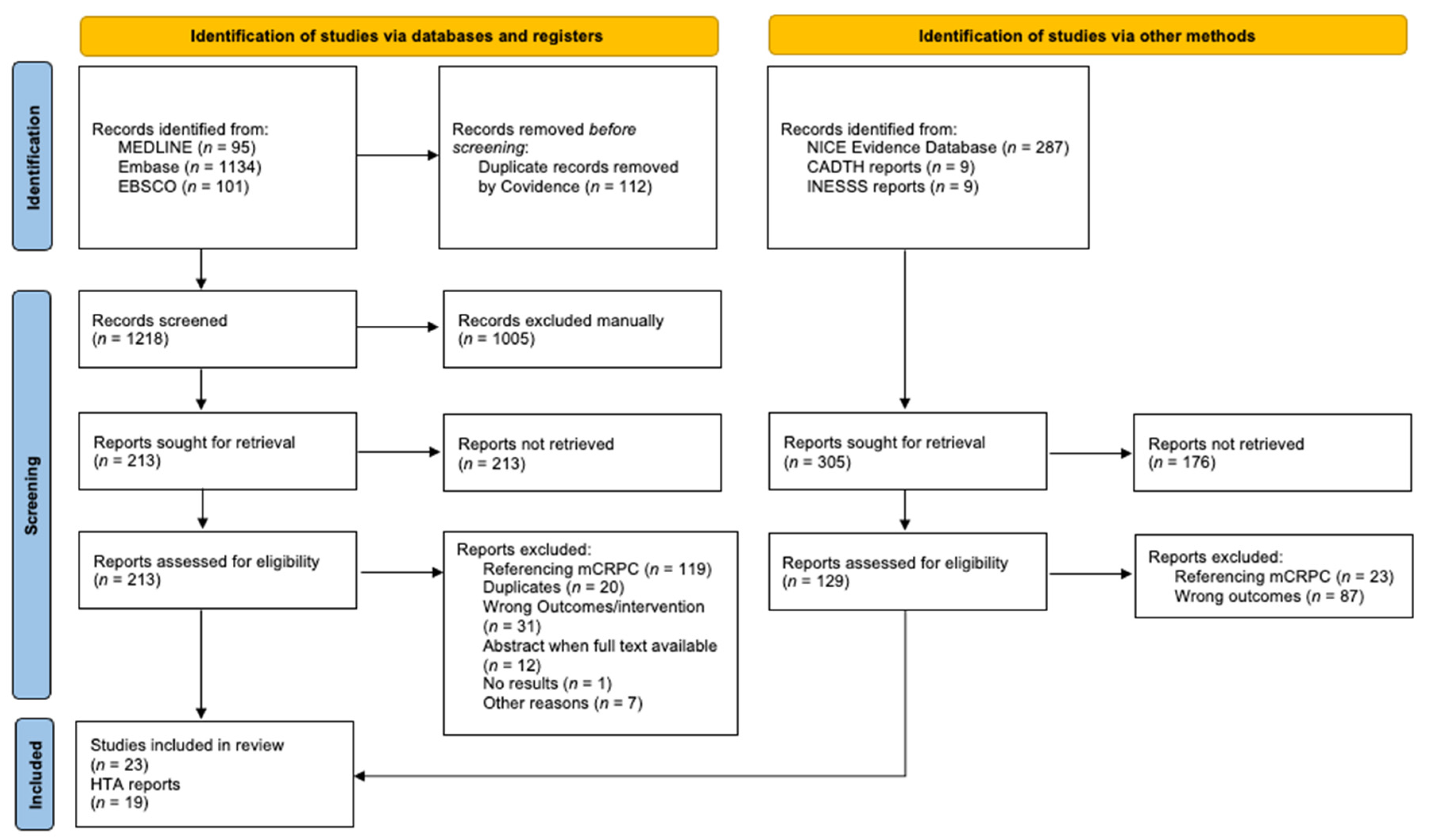
2.3. Study Selection
2.4. Data-Collection Process
2.5. Data Items
2.6. Assessments from HTA Agencies
2.7. Risk-of-Bias Assessment
2.8. Transferability Analysis
2.9. Effect Measures
2.10. Synthesis Methods
3. Results
3.1. Summary
3.1.1. Assessments from HTA Agencies
3.1.2. Economic Evaluations
3.1.3. Cost-Analysis Studies
3.1.4. Results from Real-World Data Studies
3.1.5. Risk-of-Bias Assessment
3.1.6. Transferability Assessment
4. Discussion
4.1. Summary of Results
4.2. mHSPC
4.3. nmCRPC
4.4. Real-World Data Studies
4.5. Risk-of-Bias Assessment
4.6. Strengths
4.7. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Embase <1996 to 2021 Week 28> | ||
|---|---|---|
| # | Query | Results |
| 1 | ((hormone or castrat *) adj (sensitive or naive) adj prostat * adj25 (metasta * or oligometasta * or oligo-metasta * or micrometasta * or micro-metasta *)).tw. | 944 |
| 2 | (mHSPC or m-HSPC or mHNPC or m-HNPC or mCSPC or m-CSPC or mCNPC or m-CNPC).tw. | 527 |
| 3 | 1 or 2 | 1042 |
| 4 | Animal/not (Animal/and Human/) | 699,130 |
| 5 | 3 not 4 | 1042 |
| 6 | Castration resistant prostate cancer/and (nonmetastatic or non-metastatic).tw. | 633 |
| 7 | (castrat * adj (resistant or independent) adj prostat * adj25 (nonmetastatic or non-metastatic)).tw. | 517 |
| 8 | ((androgen or hormone) adj (independent or insensitive or resistant or refractory) adj prostat * adj25 (nonmetastatic or non-metastatic)).tw. | 12 |
| 9 | (nmCRPC or nm-CRPC).tw. | 293 |
| 10 | 6 or 7 or 8 or 9 | 728 |
| 11 | Animal/not (Animal/and Human/) | 699,130 |
| 12 | 10 not 11 | 728 |
| 13 | Castration resistant prostate cancer/and exp metastasis/ | 5668 |
| 14 | Castration resistant prostate cancer/and (metasta* or oligometasta * or oligo-metasta * or micrometasta * or micro-metasta *).tw. | 9287 |
| 15 | Castration resistant prostate cancer/and ((cancer or tumor? or tumour? or neoplasm?) adj1 (spread * or disseminat * or migration? or seeding? or circulating)).tw. | 897 |
| 16 | (mCRPC or m-CRPC).tw. | 5538 |
| 17 | (castrat * adj (resistant or independent) adj prostat * adj25 (metasta * or oligometasta * or oligo-metasta * or micrometasta * or micro-metasta *)).tw. | 8775 |
| 18 | (castrat * adj (resistant or independent) adj prostat * adj25 ((cancer or tumor? or tumour? or neoplasm?) adj1 (spread* or disseminat * or migration? or seeding? or circulating))).tw. | 441 |
| 19 | ((androgen or hormone) adj (independent or insensitive or resistant or refractory) adj prostat * adj25 (metasta * or oligometasta * or oligo-metasta * or micrometasta * or micro-metasta*)).tw. | 1005 |
| 20 | ((androgen or hormone) adj (independent or insensitive or resistant or refractory) adj prostat * adj25 ((cancer or tumor? or tumour? or neoplasm?) adj1 (spread * or disseminat* or migration? or seeding? or circulating))).tw. | 11 |
| 21 | 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 | 13,816 |
| 22 | Animal/not (Animal/and Human/) | 699,130 |
| 23 | exp docetaxel/or (docetaxel or “RP-56976” or “RP 56976” or RP56976 or RP56976s or “NSC 628503” or “NSC-628503” or NSC628503 or docetaxol or Taxoltere or Taxotere or daxotel or dexotel or docefrez or “lit 976” or “lit-976” or lit976 or oncodocel or taxespira or taxoter or texot).tw,ot. | 64,427 |
| 24 | abiraterone acetate/or exp abiraterone/or (abiraterone or zytiga or “154229-18-2” or “cb 7630” or “cb-7630” or cb7630 or “CB 7598” or “CB-7598” or CB7598 or yonsa).tw,ot. | 8079 |
| 25 | exp enzalutamide/or (enzalutamide or “MDV-3100” or MDV3100 or xtandi).tw,ot. | 7708 |
| 26 | exp apalutamide/or (Apalutamide or erleada or “ARN-509” or “ARN 509” or ARN509).tw,ot. | 979 |
| 27 | exp darolutamide/or (Darolutamide or Nubeqa or “ORM-16497” or “ORM 16497” or ORM16497 or “ODM-201” or “ODM 201” or ODM201 or “ORM-16555” or “ORM 16555” or ORM16555 or “bay 1841788” or “bay-1841788” or bay1841788).tw,ot. | 435 |
| 28 | exp cabazitaxel/or (cabazitaxel or kabazitaxel or Jevtana or “rpr 116258 a” or “rpr-116258-a” or “rpr 116258a” or “rpr-116258a” or rpr116258a or “txd 258” or “txd-258” or txd258 or “xrp 6258” or “xrp-6258” or xrp6258).tw,ot. | 3408 |
| 29 | ZOLEDRONIC ACID/or (zoledronic * or zoledronat * or zometa * or zomera * or aclasta * or zoldron * or reclast * or aredia * or m05BA08 or “CGP-42446” or “CGP 42446” or CGP42446 * or “zol-446” or “zol 446” or zol446 or “158859-43-9” or 70hz18ph24 or orazol).tw,ot. | 18,442 |
| 30 | (Denosumab or Xgeva or “AMG 162” or “AMG-162” or AMG162 or Prolia or amgiva).tw,ot. | 6649 |
| 31 | exp radium chloride ra 223/or (Ra223 or “Ra 223” or “Ra-223” or Radium223 or “Radium 223” or “Radium-223” or 223radium or “223-radium” or “223 radium” or alpharadin or xofigo or “bay 88 8223” or “bay 88-8223” or “bay88 8223” or “bay88-8223”).tw,ot. | 2410 |
| 32 | (Olaparib or Lymparza or “AZD-2281” or “AZD 2281” or “MK-7339” or “MK 7339 OR KU0059436”).tw,ot. | 3936 |
| 33 | socioeconomics/or exp “Quality of Life”/or nottingham health profile/or sickness impact profile/or exp health status indicator/or patient satisfaction/or patient preference/or daily life activity/or personal autonomy/or self concept/or sickness impact profile/ | 948,945 |
| 34 | 21 not 22 | 13,813 |
| 35 | 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 | 97,781 |
| 36 | 33 and 34 and 35 | 917 |
| 37 | limit 36 to (human and english language and yr = “2010 -Current”) | 817 |
| 38 | Economics/or “cost benefit analysis”/or exp Health economics/or Budget/or exp statistical model/or Probability/or monte carlo method/or Decision Theory/or Decision Tree/or budget/or markov chain/or Cost minimization analysis/ | 1,250,421 |
| 39 | Economics/or exp “Costs and Cost Analysis”/or Economics, Nursing/or Economics, Medical/or Economics, Pharmaceutical/or exp Economics, Hospital/or Economics, Dental/or exp “Fees and Charges”/or exp Budgets/or exp models, economic/or markov chains/or monte carlo method/or exp Decision Theory/ | 945,271 |
| 40 | (budget * or economic * or cost or costs or costly or costing or price? or pricing or pharmacoeconomic * or pharmaco-economic * or expenditure? or expense? or financ * or (value? adj2 (money or monetary)) or Markov or monte carlo or (decision * adj2 (tree * or analy * or model *))).tw,kw. | 1,296,893 |
| 41 | 38 or 39 or 40 | 2,096,764 |
| 42 | 34 and 35 and 41 | 1194 |
| 43 | limit 42 to (human and english language and yr = “2010 -Current”) | 1134 |
| 44 | from 37 keep 1-817 | 817 |
| 45 | ((hormone or castrat *) adj (sensitive or naive) adj prostat * adj25 (metasta * or oligometasta * or oligo-metasta * or micrometasta * or micro-metasta *)).tw. | 944 |
| 46 | (mHSPC or m-HSPC or mHNPC or m-HNPC or mCSPC or m-CSPC or mCNPC or m-CNPC).tw. | 527 |
| 47 | 45 or 46 | 1042 |
| 48 | Animal/not (Animal/and Human/) | 699,130 |
| 49 | 47 not 48 | 1042 |
| 50 | Castration resistant prostate cancer/and (nonmetastatic or non-metastatic).tw. | 633 |
| 51 | (castrat * adj (resistant or independent) adj prostat * adj25 (nonmetastatic or non-metastatic)).tw. | 517 |
| 52 | ((androgen or hormone) adj (independent or insensitive or resistant or refractory) adj prostat * adj25 (nonmetastatic or non-metastatic)).tw. | 12 |
| 53 | (nmCRPC or nm-CRPC).tw. | 293 |
| 54 | 50 or 51 or 52 or 53 | 728 |
| 55 | Animal/not (Animal/and Human/) | 699,130 |
| 56 | 54 not 55 | 728 |
| 57 | Castration resistant prostate cancer/and exp metastasis/ | 5668 |
| 58 | Castration resistant prostate cancer/and (metasta * or oligometasta * or oligo-metasta * or micrometasta * or micro-metasta *).tw. | 9287 |
| 59 | Castration resistant prostate cancer/and ((cancer or tumor? or tumour? or neoplasm?) adj1 (spread * or disseminat * or migration? or seeding? or circulating)).tw. | 897 |
| 60 | (mCRPC or m-CRPC).tw. | 5538 |
| 61 | (castrat * adj (resistant or independent) adj prostat * adj25 (metasta * or oligometasta * or oligo-metasta* or micrometasta * or micro-metasta *)).tw. | 8775 |
| 62 | (castrat * adj (resistant or independent) adj prostat * adj25 ((cancer or tumor? or tumour? or neoplasm?) adj1 (spread * or disseminat * or migration? or seeding? or circulating))).tw. | 441 |
| 63 | ((androgen or hormone) adj (independent or insensitive or resistant or refractory) adj prostat * adj25 (metasta * or oligometasta * or oligo-metasta * or micrometasta * or micro-metasta *)).tw. | 1005 |
| 64 | ((androgen or hormone) adj (independent or insensitive or resistant or refractory) adj prostat * adj25 ((cancer or tumor? or tumour? or neoplasm?) adj1 (spread * or disseminat * or migration? or seeding? or circulating))).tw. | 11 |
| 65 | 57 or 58 or 59 or 60 or 61 or 62 or 63 or 64 | 13,816 |
| 66 | Animal/not (Animal/and Human/) | 699,130 |
| 67 | exp docetaxel/or (docetaxel or “RP-56976” or “RP 56976” or RP56976 or RP56976s or “NSC 628503” or “NSC-628503” or NSC628503 or docetaxol or Taxoltere or Taxotere or daxotel or dexotel or docefrez or “lit 976” or “lit-976” or lit976 or oncodocel or taxespira or taxoter or texot).tw,ot. | 64,427 |
| 68 | abiraterone acetate/or exp abiraterone/or (abiraterone or zytiga or “154229-18-2” or “cb 7630” or “cb-7630” or cb7630 or “CB 7598” or “CB-7598” or CB7598 or yonsa).tw,ot. | 8079 |
| 69 | exp enzalutamide/or (enzalutamide or “MDV-3100” or MDV3100 or xtandi).tw,ot. | 7708 |
| 70 | exp apalutamide/or (Apalutamide or erleada or “ARN-509” or “ARN 509” or ARN509).tw,ot. | 979 |
| 71 | exp darolutamide/or (Darolutamide or Nubeqa or “ORM-16497” or “ORM 16497” or ORM16497 or “ODM-201” or “ODM 201” or ODM201 or “ORM-16555” or “ORM 16555” or ORM16555 or “bay 1841788” or “bay-1841788” or bay1841788).tw,ot. | 435 |
| 72 | exp cabazitaxel/or (cabazitaxel or kabazitaxel or Jevtana or “rpr 116258 a” or “rpr-116258-a” or “rpr 116258a” or “rpr-116258a” or rpr116258a or “txd 258” or “txd-258” or txd258 or “xrp 6258” or “xrp-6258” or xrp6258).tw,ot. | 3408 |
| 73 | ZOLEDRONIC ACID/or (zoledronic * or zoledronat * or zometa * or zomera * or aclasta * or zoldron * or reclast * or aredia * or m05BA08 or “CGP-42446” or “CGP 42446” or CGP42446 * or “zol-446” or “zol 446” or zol446 or “158859-43-9” or 70hz18ph24 or orazol).tw,ot. | 18,442 |
| 74 | (Denosumab or Xgeva or “AMG 162” or “AMG-162” or AMG162 or Prolia or amgiva).tw,ot. | 6649 |
| 75 | exp radium chloride ra 223/or (Ra223 or “Ra 223” or “Ra-223” or Radium223 or “Radium 223” or “Radium-223” or 223radium or “223-radium” or “223 radium” or alpharadin or xofigo or “bay 88 8223” or “bay 88-8223” or “bay88 8223” or “bay88-8223”).tw,ot. | 2410 |
| 76 | (Olaparib or Lymparza or “AZD-2281” or “AZD 2281” or “MK-7339” or “MK 7339 OR KU0059436”).tw,ot. | 3936 |
| 77 | socioeconomics/or exp “Quality of Life”/or nottingham health profile/or sickness impact profile/or exp health status indicator/or patient satisfaction/or patient preference/or daily life activity/or personal autonomy/or self concept/or sickness impact profile/ | 948,945 |
| 78 | 65 not 66 | 13,813 |
| 79 | 67 or 68 or 69 or 70 or 71 or 72 or 73 or 74 or 75 or 76 | 97,781 |
| 80 | 77 and 78 and 79 | 917 |
| 81 | limit 80 to (human and english language and yr = “2010 -Current”) | 817 |
| 82 | Economics/or “cost benefit analysis”/or exp Health economics/or Budget/or exp statistical model/or Probability/or monte carlo method/or Decision Theory/or Decision Tree/or budget/or markov chain/or Cost minimization analysis/ | 1,250,421 |
| 83 | Economics/or exp “Costs and Cost Analysis”/or Economics, Nursing/or Economics, Medical/or Economics, Pharmaceutical/or exp Economics, Hospital/or Economics, Dental/or exp “Fees and Charges”/or exp Budgets/or exp models, economic/or markov chains/or monte carlo method/or exp Decision Theory/ | 945,271 |
| 84 | (budget * or economic * or cost or costs or costly or costing or price? or pricing or pharmacoeconomic * or pharmaco-economic * or expenditure? or expense? or financ * or (value? adj2 (money or monetary)) or Markov or monte carlo or (decision * adj2 (tree * or analy * or model *))).tw,kw. | 1,296,893 |
| 85 | 82 or 83 or 84 | 2,096,764 |
| 86 | 78 and 79 and 85 | 1194 |
| 87 | limit 86 to (human and english language and yr = “2010–Current”) | 1134 |
| Extraction Performed by: | |
|---|---|
| ID | |
| Author | |
| Year | |
| Publication type | |
| Setting | |
| Health state | |
| N (sample size) | |
| Type of analysis | |
| Trial- or model- based EE | |
| Intervention | |
| Comparator | |
| Outcome measure(s) | |
| Perspective | |
| Data source | |
| Disc. Rate | |
| Sponsor | |
| Methods of measurement of costs | |
| Costs | |
| Methods of measurement of effects | |
| Effects | |
| RESULTS (ICER/ICUR) | |
| Sensitivity analysis | |
| Favorable strategy | |
| Conclusions | |
| Study ID | |
|---|---|
| Author | |
| 1 Is the study population clearly described? | 0/1 |
| 2 Are competing alternatives clearly described? | 0/1 |
| 3 Is a well-defined research question posed in answerable form? | 0/1 |
| 4 Is the economic study design appropriate to the stated objective? | 0/1 |
| 5 Are the structural assumptions and the validation methods of the model properly reported? | 0/1 |
| 6 Is the chosen time horizon appropriate in order to include relevant costs and consequences? | 0/1 |
| 7 Is the actual perspective chosen appropriate? | 0/1 |
| 8 Are all important and relevant costs for each alternative identified? | 0/1 |
| 9 Are all costs measured appropriately in physical units? | 0/1 |
| 10 Are costs valued appropriately? | 0/1 |
| 11 Are all important and relevant outcomes for each alternative identified? | 0/1 |
| 12 Are all outcomes measured appropriately? | 0/1 |
| 13 Are outcomes valued appropriately? | 0/1 |
| 14 Is an appropriate incremental analysis of costs and outcomes of alternatives performed? | 0/1 |
| 15 Are all future costs and outcomes discounted appropriately? | 0/1 |
| 16 Are all important variables, whose values are uncertain, appropriately subjected to sensitivity analysis? | 0/1 |
| 17 Do the conclusions follow from the data reported? | 0/1 |
| 18 Does the study discuss the generalizability of the results to other settings and patient/client groups? | 0/1 |
| 19 Does the article/report indicate that there is no potential conflict of interest of study researcher(s) and funder(s)? | 0/1 |
| 20 Are ethical and distributional issues discussed appropriately? | 0/1 |
| Total | /20 |
| US | Estimated Relevance | Correspondence between Study A and Decision Country B | ICER of Decision (Canada) Based on ICER of Study Country (US): |
|---|---|---|---|
| General knockout criteria | |||
| 1. The evaluated technology is not comparable to the one that shall be used in the decision country. | - | NA | Passed |
| 2. The comparator is not comparable to the one that is relevant to the decision country. | - | NA | Passed |
| 3. The study does not possess an acceptable quality. | - | NA | Passed |
| Methodological characteristics | |||
| Perspective | Very High | High (payer/societal) | Unbiased |
| Discount rate | Very High | Medium (1.5 vs. 3%) | Too low |
| Medical cost approach | Very High | High | Unbiased |
| Productivity cost approach | Low | Low (unreported) | Too low or too high |
| Healthcare-system characteristics | |||
| Absolute and relative prices in healthcare | Very High | Medium | Too high |
| Practice variation | High | High | Unbiased |
| Technology availability | High | High | Unbiased |
| Population characteristics | |||
| Disease incidence/prevalence | Very High | Medium | Too low or too high |
| Case-mix | High | Medium | Too low |
| Life expectancy | High | Medium (80.0 vs. 76.3) | Too low |
| Health-status preferences | High | High | Unbiased |
| Acceptance, compliance, and incentives to patients | Medium | High | Unbiased |
| Productivity and work-loss time | Low | Low (unreported) | Too low or too high |
| Disease spread | Not relevant (no infectious disease) | Unbiased | |
| CHINA | Estimated relevance | Correspondence between study A and decision country B | ICER of decision Canada based on ICER of study country (China): |
| General knockout criteria | |||
| 1. The evaluated technology is not comparable to the one that shall be used in the decision country. | - | NA | Passed |
| 2. The comparator is not comparable to the one that is relevant to the decision country. | - | NA | Passed |
| 3. The study does not possess an acceptable quality. | - | NA | Passed |
| Methodological characteristics | |||
| Perspective | Very High | Very high | Unbiased |
| Discount rate | Very High | Medium (1.5% vs. 3%) | Too low |
| Medical cost approach | Very High | High | Unbiased |
| Productivity cost approach | Low | High | Unbiased |
| Healthcare-system characteristics | |||
| Absolute and relative prices in healthcare | Very High | High | Unbiased |
| Practice variation | High | Medium | Too low or too high |
| Technology availability | High | High | Unbiased |
| Population characteristics | |||
| Disease incidence/prevalence | Very High | Low | Too low |
| Case-mix | High | Low | Too low or too high |
| Life expectancy | High | Medium (80 vs. 75) | Too low |
| Health-status preferences | High | Very high | Unbiased |
| Acceptance, compliance, and incentives to patients | Medium | Medium | Too low |
| Productivity and work-loss time | Low | Medium | Too low |
| Disease spread | Not relevant (no infectious disease) | Unbiased | |
| UK | Estimated relevance | Correspondence between study A and decision country B | ICER of decision Canada based on ICER of study country (UK): |
| General knockout criteria | |||
| 1. The evaluated technology is not comparable to the one that shall be used in the decision country. | - | NA | Passed |
| 2. The comparator is not comparable to the one that is relevant to the decision country. | - | NA | Passed |
| 3. The study does not possess an acceptable quality. | - | NA | Passed |
| Methodological characteristics | |||
| Perspective | Very High | High | Unbiased |
| Discount rate | Very High | Medium (1.5% vs. 3.5%) | Too low |
| Medical cost approach | Very High | High | Unbiased |
| Productivity cost approach | Low | Low (not evaluated) | Too low |
| Healthcare-system characteristics | |||
| Absolute and relative prices in healthcare | Very High | Medium | Too high |
| Practice variation | High | Medium | Too high |
| Technology availability | High | Very high | Unbiased |
| Population characteristics | |||
| Disease incidence/prevalence | Very High | Very high | Unbiased |
| Case-mix | High | High | Unbiased |
| Life expectancy | High | Very high | Unbiased |
| Health-status preferences | High | Very high | Unbiased |
| Acceptance, compliance, and incentives to patients | Medium | High | Unbiased |
| Productivity and work-loss time | Low | High | Unbiased |
| Disease spread | Not relevant (no infectious disease) | Unbiased | |
| Brazil | Estimated relevance | Correspondence between study A and decision country B | ICER of decision (Canada) based on ICER of study country (Brazil): |
| General knockout criteria | |||
| 1. The evaluated technology is not comparable to the one that shall be used in the decision country. | - | NA | Passed |
| 2. The comparator is not comparable to the one that is relevant to the decision country. | - | NA | Passed |
| 3. The study does not possess an acceptable quality. | - | NA | Passed |
| Methodological characteristics | |||
| Perspective | Very High | Medium (societal vs. public payer) | Too low |
| Discount rate | Very High | Low (not reported) | Too low |
| Medical cost approach | Very High | Low (AE not considered) | Too high |
| Productivity cost approach | Low | Low (not considered) | Too high |
| Healthcare-system characteristics | |||
| Absolute and relative prices in healthcare | Very High | Medium | Too high |
| Practice variation | High | Medium | Too low or too high |
| Technology availability | High | High | Unbiased |
| Population characteristics | |||
| Disease incidence/prevalence | Very High | Medium | Too low or too high |
| Case-mix | High | Medium | Too low or too high |
| Life expectancy | High | Medium | Too low or too high |
| Health-status preferences | High | High | Unbiased |
| Acceptance, compliance, and incentives to patients | Medium | Medium | Too low or too high |
| Productivity and work-loss time | Low | Low (not considered) | Too high |
| Disease spread | Not relevant (no infectious disease) | Unbiased | |
| France | Estimated relevance | Correspondence between study A and decision country B | ICER of decision Canada based on ICER of study country (France): |
| General knockout criteria | |||
| 1. The evaluated technology is not comparable to the one that shall be used in the decision country. | - | NA | Passed |
| 2. The comparator is not comparable to the one that is relevant to the decision country. | - | NA | Passed |
| 3. The study does not possess an acceptable quality. | - | NA | Passed |
| Methodological characteristics | |||
| Perspective | Very High | High | Unbiased |
| Discount rate | Very High | High (1.5% vs. 2.5%) | Too low |
| Medical cost approach | Very High | High | Unbiased |
| Productivity cost approach | Low | Low | Too low |
| Healthcare-system characteristics | |||
| Absolute and relative prices in healthcare | Very High | Medium | Too low |
| Practice variation | High | Medium | Too low or too high |
| Technology availability | High | Very high | Unbiased |
| Population characteristics | |||
| Disease incidence/prevalence | Very High | Very high | Unbiased |
| Case-mix | High | High | Unbiased |
| Life expectancy | High | Very high | Unbiased |
| Health-status preferences | High | Very high | Unbiased |
| Acceptance, compliance, and incentives to patients | Medium | High | Unbiased |
| Productivity and work-loss time | Low | High | Unbiased |
| Disease spread | Not relevant (no infectious disease) | Unbiased | |
| Greece | Estimated relevance | Correspondence between study A and decision country B | ICER of decision Canada based on ICER of study country (Greece): |
| General knockout criteria | |||
| 1. The evaluated technology is not comparable to the one that shall be used in the decision country. | - | NA | Passed |
| 2. The comparator is not comparable to the one that is relevant to the decision country. | - | NA | Passed |
| 3. The study does not possess an acceptable quality. | - | NA | Passed |
| Methodological characteristics | |||
| Perspective | Very High | Medium | Too low |
| Discount rate | Very High | Low (not reported) | Too low |
| Medical cost approach | Very High | Low (not described) | Too high |
| Productivity cost approach | Low | Low (not considered) | Too high |
| Healthcare-system characteristics | |||
| Absolute and relative prices in healthcare | Very High | Medium | Too low |
| Practice variation | High | Medium | Too low or too high |
| Technology availability | High | High | Unbiased |
| Population characteristics | |||
| Disease incidence/prevalence | Very High | High | Unbiased |
| Case-mix | High | High | Unbiased |
| Life expectancy | High | High | Unbiased |
| Health-status preferences | High | Medium | Too low or too high |
| Acceptance, compliance, and incentives to patients | Medium | Medium | Too low or too high |
| Productivity and work-loss time | Low | Low (not considered) | Too high |
| Disease spread | Not relevant (no infectious disease) | Unbiased | |
| Sweden | Estimated relevance | Correspondence between study A and decision country B | ICER of decision Canada based on ICER of study country (Sweden): |
| General knockout criteria | |||
| 1. The evaluated technology is not comparable to the one that shall be used in the decision country. | - | NA | Passed |
| 2. The comparator is not comparable to the one that is relevant to the decision country. | - | NA | Passed |
| 3. The study does not possess an acceptable quality. | - | NA | Passed |
| Methodological characteristics | |||
| Perspective | Very High | High | Unbiased |
| Discount rate | Very High | Low | Too high |
| Medical cost approach | Very High | High | Unbiased |
| Productivity cost approach | Low | Low (not measured) | Too low |
| Healthcare-system characteristics | |||
| Absolute and relative prices in healthcare | Very High | Medium | Too high |
| Practice variation | High | High | Unbiased |
| Technology availability | High | High | Unbiased |
| Population characteristics | |||
| Disease incidence/prevalence | Very High | High | Unbiased |
| Case-mix | High | High | Unbiased |
| Life expectancy | High | High | Unbiased |
| Health-status preferences | High | High | Unbiased |
| Acceptance, compliance, and incentives to patients | Medium | High | Unbiased |
| Productivity and work-loss time | Low | Low (not measured) | Too low |
| Disease spread | Not relevant (no infectious disease) | Unbiased | |
| Mexico | Estimated relevance | Correspondence between study A and decision country B | ICER of decision Canada based on ICER of study country (Mexico): |
| General knockout criteria | |||
| 1. The evaluated technology is not comparable to the one that shall be used in the decision country. | - | NA | Passed |
| 2. The comparator is not comparable to the one that is relevant to the decision country. | - | NA | Passed |
| 3. The study does not possess an acceptable quality. | - | NA | Passed |
| Methodological characteristics | |||
| Perspective | Very High | High | Unbiased |
| Discount rate | Very High | Low (1.5% vs. 5%) | Low |
| Medical cost approach | Very High | High | Unbiased |
| Productivity cost approach | Low | Low (not considered) | Too low |
| Healthcare-system characteristics | |||
| Absolute and relative prices in healthcare | Very High | Medium | Too low or too high |
| Practice variation | High | Medium | Too low or too high |
| Technology availability | High | High | Unbiased |
| Population characteristics | |||
| Disease incidence/prevalence | Very High | High | Unbiased |
| Case-mix | High | Medium | Too low or too high |
| Life expectancy | High | Medium | Too low |
| Health-status preferences | High | Medium | Too low or too high |
| Acceptance, compliance, and incentives to patients | Medium | Medium | Too low or too high |
| Productivity and work-loss time | Low | Low (not considered) | Too low |
| Disease spread | Not relevant (no infectious disease) | Unbiased | |
| Columbia | Estimated relevance | Correspondence between study A and decision country B | ICER of decision Canada based on ICER of study country (Columbia) |
| General knockout criteria | |||
| 1. The evaluated technology is not comparable to the one that shall be used in the decision country. | - | NA | Passed |
| 2. The comparator is not comparable to the one that is relevant to the decision country. | - | NA | Passed |
| 3. The study does not possess an acceptable quality. | - | NA | Passed |
| Methodological characteristics | |||
| Perspective | Very High | Medium | Too low |
| Discount rate | Very High | Low (not reported) | Too low |
| Medical cost approach | Very High | Medium | Too low |
| Productivity cost approach | Low | Low (not reported) | Too low |
| Healthcare-system characteristics | |||
| Absolute and relative prices in healthcare | Very High | Medium | Too high |
| Practice variation | High | Medium | Too low or too high |
| Technology availability | High | High | Unbiased |
| Population characteristics | |||
| Disease incidence/prevalence | Very High | Medium | Too low |
| Case-mix | High | Medium | Too low |
| Life expectancy | High | Medium | Too low |
| Health-status preferences | High | High | Unbiased |
| Acceptance, compliance, and incentives to patients | Medium | High | Unbiased |
| Productivity and work-loss time | Low | Low (not reported) | Too low |
| Disease spread | Not relevant (no infectious disease) | Unbiased |
References
- Cattrini, C.; Castro, E.; Lozano, R.; Zanardi, E.; Rubagotti, A.; Boccardo, F.; Olmos, D. Current Treatment Options for Metastatic Hormone-Sensitive Prostate Cancer. Cancers 2019, 11, 1355. [Google Scholar] [CrossRef] [PubMed]
- Canadian Cancer Statistics Advisory Committee. Canadian Cancer Statistics 2019; Canadian Cancer Society: Toronto, ON, Canada, 2019; Available online: Cancer.ca/Canadian-Cancer-Statistics-2019-EN (accessed on 9 March 2022).
- Saad, F.; Aprikian, A.; Finelli, A.; Fleshner, N.E.; Gleave, M.; Kapoor, A.; Niazi, T.; North, S.A.; Pouliot, F.; Rendon, R.A.; et al. 2021 Canadian Urological Association (CUA)—Canadian Uro Oncology Group (CUOG) Guideline: Management of Castration-Resistant Prostate Cancer (CRPC). Can. Urol. Assoc. J. 2021, 15, E81–E90. [Google Scholar] [CrossRef] [PubMed]
- So, A.I.; Chi, K.N.; Danielson, B.; Fleshner, N.E.; Kapoor, A.; Niazi, T.; Pouliot, F.; Rendon, R.A.; Shayegan, B.; Sridhar, S.; et al. Canadian Urological Association—Canadian Urologic Oncology Group guideline on metastatic castration-naive and castration-sensitive prostate cancer. Can. Urol. Assoc. J. 2019, 14, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Grover, S.A. Economics of prostate cancer: A computer model. Can. J. Urol. 1997, 4, 88–89. [Google Scholar] [PubMed]
- Mauskopf, J.A.; Sullivan, S.D.; Annemans, L.; Caro, J.; Mullins, C.D.; Nuijten, M.; Orlewska, E.; Watkins, J.; Trueman, P. Principles of good practice for budget impact analysis: Report of the ISPOR Task Force on good research practices—Budget impact analysis. Value Health 2007, 10, 336–347. [Google Scholar] [CrossRef]
- Budget Impact Analysis. Available online: https://www.herc.research.va.gov/include/page.asp?id=budget-impact-analysis (accessed on 7 April 2022).
- Institut National D’excellence en Santé et en Services Sociaux. Guide de Soumission d’une Demande à l’INESSS. INESSS (QC). 2018. Available online: https://www.inesss.qc.ca/fileadmin/doc/INESSS/Inscription_medicaments/Fiches_inscription/Guide_soumission.pdf (accessed on 9 March 2022).
- CADTH Database Search Filters. Ottawa: CADTH. 2021. Available online: https://www.cadth.ca/strings-attached-cadths-database-search-filters (accessed on 28 October 2021).
- Covidence Systematic Review Software. Veritas Health Innovation, Melbourne, Australia. Available online: www.covidence.org (accessed on 29 June 2021).
- Wijnen, B.; Van Mastrigt, G.; Redekop, W.K.; Majoie, H.; De Kinderen, R.; Evers, S. How to prepare a systematic review of economic evaluations for informing evidence-based healthcare decisions: Data extraction, risk of bias, and transferability (part 3/3). Expert Rev. Pharm. Outcomes Res. 2016, 16, 723–732. [Google Scholar] [CrossRef]
- Buckley, L.F.; Dixon, D.L.; Wohlford, G.F.; Wijesinghe, D.S.; Baker, W.L.; Van Tassell, B.W. Effect of intensive blood pressure control in patients with type 2 diabetes mellitus over 9 years of follow-up: A subgroup analysis of high-risk ACCORDION trial participants. Diabetes Obes. Metab. 2018, 20, 1499–1502. [Google Scholar] [CrossRef]
- Evers, S.; Goossens, M.; de Vet, H.; van Tulder, M.; Ament, A. Criteria list for assessment of methodological quality of economic evaluations: Consensus on Health Economic Criteria. Int. J. Technol. Assess Health Care 2005, 21, 240–245. [Google Scholar] [CrossRef]
- Knies, S.; Ament, A.J.; Evers, S.M.; Severens, J.L. The transferability of economic evaluations:testing the model of Welte. Value Health 2009, 12, 730–738. [Google Scholar] [CrossRef]
- Welte, R.; Feenstra, T.; Jager, H.; Leidl, R. A decision chart for assessing and improving the transferability of economic evaluation results between countries. Pharmacoeconomics 2004, 22, 857–876. [Google Scholar] [CrossRef]
- The Professional Society for Health Economics and Outcomes Research: Pharmacoeconomic Guidelines Around The World. ISPOR. 2022. Available online: https://tools.ispor.org/peguidelines/ (accessed on 9 March 2022).
- Candian Cancer Society: Prostate Cancer Statistics. 2021. Available online: https://cancer.ca/en/cancer-information/cancer-types/prostate/statistics (accessed on 9 March 2022).
- The World Bank: Life Expectancy at Birth, Total (Years). 2022. Available online: https://data.worldbank.org/indicator/SP.DYN.LE00.IN?locations=CN (accessed on 9 March 2022).
- International Agency for Cancer Research: Population Fact Sheets. World Health Organization. 2020. Available online: https://gco.iarc.fr/today/fact-sheets-populations (accessed on 9 March 2022).
- OFX. Yearly Average Rates. Available online: https://www.ofx.com/en-ca/forex-news/historical-exchange-rates/yearly-average-rates/ (accessed on 7 February 2022).
- Statistics Canada. Consumer Price Index, Annual Average, Not Seasonally Adjusted; Table 18-10-0005-01; Statistics Canada: Ottawa, ON, Canada, 2020.
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, n71. [Google Scholar] [CrossRef] [PubMed]
- Pharmacoeconomic Report Apalutamide (Erleada) for Metastatic Castration-Sensitive Prostate Cancer. pERC Meeting: 20 March 2020; Early Conversion 22 April 2020; Toronto (ON) 2020. Available online: https://www.cadth.ca/sites/default/files/pcodr/Reviews2020/10200ApalutamidemCSPC_fnEGR_REDACT-ABBREV_EC_22Apr2020_final.pdf (accessed on 5 November 2021).
- Cadth Drug Reimbursement Review. Pharmacoeconomic Report for Enzalutamide (XtandI) (Astellas Pharma Canada, Inc.) Indication: In Combination with Androgen-Deprivation Therapy for the Treatment of Patients with Metastatic Castration Sensitive Prostate Cancer. Toronto (ON): CADTH. 2020. Available online: https://www.cadth.ca/sites/default/files/pcodr/Reviews2020/10209EnzalutamidemCSPC_fnEGR_REDACT-ABBREV_Post_23Sep2020_final.pdf (accessed on 5 November 2021).
- Institut National D’excellence en Santé et en Services Sociaux. ERLEADA MC—Cancer de la Prostate. Quebec (QC) INESSS. 2020. Available online: https://www.inesss.qc.ca/fileadmin/doc/INESSS/Inscription_medicaments/Avis_au_ministre/Juin_2020/Erleada_2020_05.pdf (accessed on 5 November 2021).
- Institut National D’excellence en Santé et en Services Sociaux. XTANDI MC—Cancer de la Prostate Métastatique Sensible à la Castration. Quebec (QC) INESSS. 2020. Available online: https://www.inesss.qc.ca/fileadmin/doc/INESSS/Inscription_medicaments/Avis_au_ministre/Septembre_2020/Xtandi_CPSCm_2020_08.pdf (accessed on 5 November 2021).
- Scottish Medicines Consortium—SMC, Abiraterone Acetate (Zytiga). 2020. Available online: https://www.scottishmedicines.org.uk/medicines-advice/abiraterone-acetate-zytiga-full-smc2215/ (accessed on 5 November 2021).
- National Institute for Health and Care Excellence. Enzalutamide for Treating Hormone-Sensitive Metastatic Prostate Cancer—Guidance (TA712). NICE (UK) 2021. Available online: https://www.nice.org.uk/guidance/ta712 (accessed on 5 November 2021).
- National Institute for Health and Care Excellence. Abiraterone for Treating Newly Diagnosed High-Risk Hormone-Sensitive Metastatic Prostate Cancer—Guidance (TA721). NICE (UK) 2021. Available online: https://www.nice.org.uk/guidance/ta721 (accessed on 5 November 2021).
- National Institute for Health and Care Excellence. Apalutamide with Androgen Deprivation Therapy for Treating Hormone-Sensitive Metastatic Prostate Cancer—Guidance (TA741). NICE (UK) 2021. Available online: https://www.nice.org.uk/guidance/ta741 (accessed on 5 November 2021).
- Aguiar, P.N., Jr.; Tan, P.S.; Simko, S.; Barreto, C.M.N.; Gutierres, B.S.; Giglio, A.D.; Lopes, G.L., Jr. Cost-effectiveness analysis of abiraterone, docetaxel or placebo plus androgen deprivation therapy for hormone-sensitive advanced prostate cancer. Einstein 2019, 17, eGS4414. [Google Scholar] [CrossRef] [PubMed]
- Beca, J.; Majeed, H.; Chan, K.K.W.; Hotte, S.J.; Loblaw, A.; Hoch, J.S. Cost-effectiveness of docetaxel in high-volume hormone-sensitive metastatic prostate cancer. Can. Urol. Assoc. J. 2019, 13, 396–403. [Google Scholar] [CrossRef]
- Parikh, N.R.; Chang, E.M.; Nickols, N.G.; Rettig, M.B.; Raldow, A.C.; Steinberg, M.L.; Koontz, B.F.; Vapiwala, N.; Deville, C.; Feng, F.Y.; et al. Cost-Effectiveness of Metastasis-Directed Therapy in Oligorecurrent Hormone-Sensitive Prostate Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Pelloux-Prayer, R.; Schiele, P.; Oudard, S.; Gravis, G.; Kleinclauss, F.; Crehange, G.; Hennequin, C.; Morgans, A.; Geoffrois, L.; Limat, S.; et al. Cost-effectiveness Analysis of Innovative Therapy for Patients with Newly Diagnosed Hormone-Sensitive Metastatic Prostate Cancer. Clin. Genitourin. Cancer 2021, 19, e326–e333. [Google Scholar] [CrossRef] [PubMed]
- Ramamurthy, C.; Handorf, E.; Correa, A.; Beck, J.; Geynisman, D. Cost-effectiveness of abiraterone versus docetaxel in the treatment of metastatic hormone naive prostate cancer. Urol. Oncol. 2019, 37, 688–695. [Google Scholar] [CrossRef]
- Sathianathen, N.; Alarid-Escudero, F.; Kuntz, K.; Lawrentschuk, N.; Bolton, D.; Murphy, D.; Kim, S.; Konety, B. A Cost-effectiveness Analysis of Systemic Therapy for Metastatic Hormone-sensitive Prostate Cancer. Eur. Urol. Oncol. 2019, 2, 649–655. [Google Scholar] [CrossRef]
- Woods, B.; Sideris, E.; Sydes, M.; Gannon, M.; Parmar, M.; Alzouebi, M.; Attard, G.; Birtle, A.; Brock, S.; Cathomas, R.; et al. Addition of Docetaxel to First-line Long-term Hormone Therapy in Prostate Cancer (STAMPEDE): Modelling to Estimate Long-term Survival, Quality-adjusted Survival, and Cost-effectiveness. Eur. Urol. Oncol. 2018, 1, 449–458. [Google Scholar] [CrossRef]
- Zhang, P.; Wen, F.; Fu, P.; Yang, Y.; Li, Q. Addition of docetaxel and/or zoledronic acid to standard of care for hormone-naive prostate cancer: A cost-effectiveness analysis. Tumori 2017, 103, 380–386. [Google Scholar] [CrossRef]
- Zhang, P.; Xie, D.; Li, Q. Adding Enzalutamide to First-Line Treatment for Metastatic Hormone-Sensitive Prostate Cancer: A Cost-Effectiveness Analysis. Front. Public Health 2021, 9, 608375. [Google Scholar] [CrossRef]
- Zheng, H.R.; Wen, F.; Wu, Y.F.; Wheeler, J.R.C.; Li, Q. Cost-effectiveness analysis of additional docetaxel for metastatic hormone-sensitive prostate cancer treated with androgen-deprivation therapy from a Chinese perspective. Eur. J. Cancer Care 2017, 26, e12505. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, P., Jr.; Barreto, C.; Gutierres, B.; Tadokoro, H.; Lopes, G., Jr. Cost effectiveness of chemohormonal therapy in patients with metastatic hormone-sensitive and non-metastatic high-risk prostate cancer. Einstein 2017, 15, 349–354. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence. Hormone-Sensitive Metastatic Prostate Cancer: Docetaxel—Evidence Summary (ESUOM50). NICE (UK) 2016. Available online: https://www.nice.org.uk/advice/esuom50 (accessed on 5 November 2021).
- pCODR Final Economic Guidance Report—Darolutamide (Nubeqa) for Non-Metastatic Castration Resistant Prostate Cancer. pERC Meeting: 19 March 2020; Early Conversion: 22 April 2020; Unredacted: 5 February 2021; Toronto (ON) 2021. Available online: https://www.cadth.ca/sites/default/files/pcodr/Reviews2020/10196DarolutamidenmCRPC_fnEGR_REDACT-ABBREV_EC_22Apr2020_final.pdf (accessed on 5 November 2021).
- pCODR Final Economic Guidance Report—Apalutamide (Erleada) for Castration-Resistant Prostate Cancer. pERC Meeting: 16 August 2018; pERC Reconsideration Meeting: 18 October 2018. Toronto (ON) 2018. Available online: https://www.cadth.ca/sites/default/files/pcodr/pcodr_apalutamide_erleada_crpc_fn_egr.pdf (accessed on 5 November 2021).
- pan-Canadian Oncology Drug Review Final Economic Guidance Report, Enzalutamide (Xtandi) for Non-Metastatic Castration-Resistant Prostate Cancer. Toronto (ON): CADTH. 2019. Available online: https://cadth.ca/sites/default/files/pcodr/Reviews2019/10149Enzalutamidenm-CRPC_fnEGR_EC_NOREDACT-ABBREV_Post_26Mar2019_final.pdf (accessed on 5 November 2021).
- Institut National D’excellence en Santé et en Services Sociaux. Nubequa MC—Cancer de la Prostate. Quebec (QC) INESSS. 2020. Available online: https://www.inesss.qc.ca/fileadmin/doc/INESSS/Inscription_medicaments/Avis_au_ministre/Avril_2020/Nubeqa_2020_03.pdf (accessed on 5 November 2021).
- Institut National D’excellence en Santé et en Services Sociaux. Erleada MC—Cancer de la Prostate. Quebec (QC) INESSS. 2018. Available online: https://www.inesss.qc.ca/fileadmin/doc/INESSS/Inscription_medicaments/Avis_au_ministre/Octobre_2018/Erleada_2018_09.pdf (accessed on 5 November 2021).
- National Institute for Health and Care Excellence. Darolutamide with Androgen Deprivation Therapy for Treating Hormone-relapsed Non-Metastatic Prostate Cancer—Guidance (TA660). NICE (UK) 2020. Available online: https://www.nice.org.uk/guidance/ta660 (accessed on 5 November 2021).
- National Institute for Health and Care Excellence. Enzalutamide for Hormone-Relapsed Non-Metastatic Prostate Cancer—Guidance (TA580). NICE (UK) 2019. Available online: https://www.nice.org.uk/guidance/ta580 (accessed on 5 November 2021).
- Scottish Medicines Consortium. Darolutamide (Nubeqa) is Accepted for Use within NHS Scotland. SMC (SC) 2020. Available online: https://www.scottishmedicines.org.uk/medicines-advice/darolutamide-nubeqa-full-smc2297/ (accessed on 5 November 2021).
- Scottish Medicines Consortium. Enzalutamide 40 mg Soft Capsules (Xtandi®). SMC (UK) 2019. Available online: https://www.scottishmedicines.org.uk/medicines-advice/enzalutamide-xtandi-full-smc2195/ (accessed on 5 November 2021).
- National Institute for Health and Care Excellence. Apalutamide with Androgen Deprivation Therapy for Treating High-Risk Hormone-Relapsed Non-Metastatic Prostate Cancer—Guidance (TA740). NICE (UK) 2021. Available online: https://www.nice.org.uk/guidance/ta740 (accessed on 5 November 2021).
- Toro, W.; Braun, S.; Sanchez, L.; Anaya, P. Pcn129 a Cost-Utility and Budget Impact Analysis of Enzalutamide for the Treatment of Nonmetastatic Castration-Resistant Prostate Cancer (Nmcrpc) in Mexico. Value Health 2020, 23, S45–S46. [Google Scholar] [CrossRef]
- Tsiatas, M.; Van Oostrum, I.; Tritaki, G.; Sermon, J.; Chatzimouratidis, K. Pcn218 Cost-Effectiveness of Apalutamide + Adt Versus Enzalutamide + Adt in Non-Metastatic Castration Resistant Prostate Cancer in Greece. Value Health 2019, 22, S478. [Google Scholar] [CrossRef]
- Zhou, Z.; Hu, X. Cost-Effectiveness Analysis of Apalutamide for Treatment in Non- Metastasis Castration-Resistant Prostate Cancer. Value Health 2018, 21, S40–S41. [Google Scholar] [CrossRef]
- Hu, X.; Qu, S.; Yao, X.; Li, C.; Liu, Y.; Wang, J. Abiraterone acetate and docetaxel with androgen deprivation therapy in high-volume metastatic hormone-sensitive prostate cancer in China: An indirect treatment comparison and cost analysis. Cost Eff. Resour. Alloc. 2019, 17, 27. [Google Scholar] [CrossRef]
- Ke, X.; Lafeuille, M.; Romdhani, H.; Kinkead, F.; Pilon, D.; Lefebvre, P.; Francis, P.; D’Andrea, D.; Ryan, C.; Freedland, S. Healthcare resource use and costs associated with metastatic castration-sensitive prostate cancer in medicare advantage and commercially insured patients in The United States. J. Manag. Care Spec. Pharm. 2019, 25. [Google Scholar] [CrossRef]
- Wong, S.; Everest, L.; Jiang, D.; Saluja, R.; Chan, K.; Sridhar, S. Application of the ASCO Value framework and ESMO magnitude of clinical benefit scale to assess the value of abiraterone and enzalutamide in advanced prostate cancer. JCO Oncol. Pract. 2020, 16, E201–E210. [Google Scholar] [CrossRef]
- Svensson, J.; Lissbrant, I.; Gauffin, O.; Hjalm-Eriksson, M.; Kilany, S.; Fagerlund, K.; Stattin, P. Time spent in hormone-sensitive and castration-resistant disease states in men with advanced prostate cancer, and its health economic impact: Registry-based study in Sweden. Scand. J. Urol. 2021, 55, 1–8. [Google Scholar] [CrossRef]
- Freedland, S.; Pilon, D.; Bhak, R.; Lefebvre, P.; Li, S.; Young-Xu, Y. Predictors of survival, healthcare resource utilization, and healthcare costs in veterans with non-metastatic castration-resistant prostate cancer. Urol. Oncol. Semin. Orig. Investig. 2020, 38, 930.e13–930.e21. [Google Scholar] [CrossRef]
- George, D.; Schultz, N.; Huang, A.; Wang, L.; Baser, O.; Ramaswamy, K.; Mardekian, J. Increased costs associated with progression to metastatic castrate-resistant prostate cancer. J. Manag. Care Spec. Pharm. 2018, 24, S26–S27. [Google Scholar] [CrossRef]
- Seal, B.; Sullivan, S.; Ramsey, S.; Asche, C.; Shermock, K.; Sarma, S.; Zagadailov, E.; Farrelly, E.; Eaddy, M. Comparing hospital-based resource utilization and costs for prostate cancer patients with and without bone metastases. Appl. Health Econ. Health Policy 2014, 12, 547–557. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shah, A.; Shah, R.; Kebede, N.; Mohamed, A.; Botteman, M.; Waldeck, R.; Hussain, A. Real-world incidence and burden of adverse events among non-metastatic prostate cancer patients treated with secondary hormonal therapies following androgen deprivation therapy. J. Med. Econ. 2020, 23, 330–346. [Google Scholar] [CrossRef]
- Wu, B.; Li, S.; Song, J.; Pericone, C.; Behl, A.; Dawson, N. Total cost of care for castration-resistant prostate cancer in a commercially insured population and a medicare supplemental insured population. J. Med. Econ. 2020, 23, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Xie, D.; Li, Q. Cost-effectiveness analysis of cabazitaxel for metastatic castration resistant prostate cancer after docetaxel and androgen-signaling-targeted inhibitor resistance. BMC Cancer 2021, 21, 7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.Y.; Fu, A.Z. Cost-effectiveness of a behavioral intervention for persistent urinary incontinence in prostate cancer patients. Psycho-Oncology 2016, 25, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Medicare Advantage Plans. Available online: https://www.medicare.gov/sign-up-change-plans/types-of-medicare-health-plans/medicare-advantage-plans (accessed on 12 April 2022).
- Medicare Advantage vs. Medigap. Available online: https://www.investopedia.com/articles/personal-finance/071014/medigap-vs-medicare-advantage-which-better.asp (accessed on 12 April 2022).
- Guidelines for the Economic Evaluation of Health Technologies: Canada, 4th ed.; CADTH: Ottawa, ON, Canada, 2017.
- The pan-Canadian Pharmaceutical Alliance. Available online: https://www.pcpacanada.ca/node/30 (accessed on 8 February 2022).
- The pan-Canadian Pharmaceutical Alliance. Brand Name Drug Negotiations Status. Available online: https://www.pcpacanada.ca/negotiations (accessed on 8 February 2022).
- Régie de L’assurance Maladie Quebec. Liste des Médicaments. RAQM (QC) 2 March 2022. Available online: https://www.ramq.gouv.qc.ca/sites/default/files/documents/liste_med_2022-03-02_fr.pdf (accessed on 9 March 2022).
- Fizazi, K.; Shore, N.; Tammela, T.L.; Ulys, A.; Vjaters, E.; Polyakov, S.; Jievaltas, M.; Luz, M.; Alekseev, B.; Kuss, I.; et al. Nonmetastatic, Castration-Resistant Prostate Cancer and Survival with Darolutamide. N. Engl. J. Med. 2020, 383, 1040–1049. [Google Scholar] [CrossRef]
- Small, E.J.; Saad, F.; Chowdhury, S.; Oudard, S.; Hadaschik, B.A.; Graff, J.N.; Olmos, D.; Mainwaring, P.N.; Lee, J.Y.; Uemura, H.; et al. Apalutamide and overall survival in non-metastatic castration-resistant prostate cancer. Ann. Oncol. 2019, 30, 1813–1820. [Google Scholar] [CrossRef]
- Sternberg, C.N.; Fizazi, K.; Saad, F.; Shore, N.D.; De Giorgi, U.; Penson, D.F.; Ferreira, U.; Efstathiou, E.; Madziarska, K.; Kolinsky, M.P.; et al. Enzalutamide and Survival in Nonmetastatic, Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2020, 382, 2197–2206. [Google Scholar] [CrossRef]
- Régie de L’assurance Maladie du Québec. Liste des Médicaments. Québec (QC); 13 December 2021. Available online: https://www.ramq.gouv.qc.ca/sites/default/files/documents/liste_med_2021-12-15_fr.pdf (accessed on 5 November 2021).
- Fujiwara, M.; Yuasa, T.; Komai, Y.; Numao, N.; Yamamoto, S.; Fukui, I.; Yonese, J. Efficacy, Prognostic Factors, and Safety Profile of Enzalutamide for Non-metastatic and Metastatic Castration-Resistant Prostate Cancer: A Retrospective Single-Center Analysis in Japan. Target Oncol. 2020, 15, 635–643. [Google Scholar] [CrossRef]
- Jaime Caro, J.; Eddy, D.M.; Kan, H.; Kaltz, C.; Patel, B.; Eldessouki, R.; Briggs, A.H. Questionnaire to Assess Relevance and Credibility of Modeling Studies for Informing Health Care Decision Making: An ISPOR-AMCP-NPC Good Practice Task Force Report. Value Health 2014, 17, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Philips, Z.; Bojke, L.; Sculpher, M.; Claxton, K.; Golder, S. Good Practice Guidelines for Decision-Analytic Modelling in Health Technology Assessment. PharmacoEconomics 2006, 24, 355–371. [Google Scholar] [CrossRef] [PubMed]
- Grochtdreis, T.; König, H.-H.; Dobruschkin, A.; Von Amsberg, G.; Dams, J. Cost-effectiveness analyses and cost analyses in castration-resistant prostate cancer: A systematic review. PLoS ONE 2018, 13, e0208063. [Google Scholar] [CrossRef] [PubMed]
- Grover, S.A.; Zowall, H.; Coupal, L.; Krahn, M. Prostate cancer: 12. The economic burden. CMAJ Can. Med. Assoc. J. 1999, 685–690. [Google Scholar]
| Type of Evaluation | Country | Year | First Author | Health State | Treatment and Comparator | Data Source | Model Type | Perspective | Year of Value |
|---|---|---|---|---|---|---|---|---|---|
| CA | Canada | 2020 | Wong [58] | mHSPC | ABI vs. ENZA | ATITUDE, STAMPEDE, ENZAMET, and ARCHES | - | NR | 2017 |
| CA | China | 2019 | Hu [56] | mHSPC | ABI vs. DOCE vs. ADT | CHAARTED, LATITUDE and GETUG-AFU-15 | - | Healthcare system and patient | 2017 |
| CA | Sweden | 2021 | Svenson [59] | mHSPC, nmCRPC | - | Real-world data PCa data Base Sweden | - | Healthcare system | 2018 |
| CA | US | 2014 | Seal [62] | nmCRPC | - | Real-world data Patients in the Premier Perspective Database | - | Institutional | 2010 |
| CA | US | 2018 | George [61] | nmCRPC | - | Real-world data Veterans’ Health Administration (VHA) database | - | Healthcare system | NR |
| CA | US | 2019 | Ke [57] | mHSPC | - | Real-world data (Optum Clinformatics Extended DataMart) | - | Public and private payer | 2018 |
| CA | US | 2020 | Shah [63] | nmCRPC | ENZA vs. ABI vs. bicalutamide | Real-world data MarketScan database | - | Private payer | 2017 |
| CA | US | 2020 | Wu [64] | nmCRPC | - | Real-world data Truven Health MarketScan Commercial and Medicare Supplemental (Medigap) databases | - | Public and private payer | 2016 |
| CA | US | 2020 | Freedland [60] | nmCRPC | - | Real-world data Veterans Health Administration (VHA) database | - | Healthcare system | 2016 |
| CE | Brazil | 2017 | Aguiar [41] | mHSPC, nmCRPC | ABI vs. DOCE vs. ADT | GETUG-AFU 15 and CHAARTED | Analytical model | Public payer | 2016 |
| CE | Brazil | 2019 | Aguiar [31] | mHSPC | ABI vs. DOCE vs. ADT | STAMPEDE | Descriptive analytic model | Public payer | 2017 |
| CE | Canada | 2018 | CADTH 4 [44] | nmCRPC | APA vs. ADT | SPARTAN | Partitioned-survival model | Government | 2018 |
| CE | Canada | 2018 | INESSS 3 [47] | nmCRPC | APA vs. ADT DOCE | SPARTAN | Partitioned-survival model | Healthcare system/Societal | 2018 |
| CE | Canada | 2019 | Beca [32] | mHSPC | DOCE vs. ADT | CHAARTED | Partitioned-survival model | Public payer | 2017 |
| CE | Canada | 2019 | CADTH 3 [45] | nmCRPC | ENZA vs. ADT APA | PROSPER, SPARTAN | Markov model | Healthcare payer | 2018 |
| CE | Canada | 2020 | CADTH 1 [23] | mHSPC | APA vs. ADT vs. DOCE vs. ABI | TITAN | Partitioned-survival model | Public payer | 2020 |
| CE | Canada | 2020 | CADTH 2 [24] | mHSPC | ENZA vs. ADT vs. DOCE vs. APA vs. ABI | ARCHES and ENZAMET | Markov model | Public payer | 2020 |
| CE | Canada | 2020 | INESSS 1 [26] | mHSPC | ENZA vs. ADT vs. DOCE | ARCHES, ENZAMET, and MAenR | Markov model | Societal | 2020 |
| CE | Canada | 2020 | INESSS 2 [25] | mHSPC | APA vs. ADT | SPARTAN | Partitioned-survival model | Societal | 2020 |
| CE | Canada | 2020 | CADTH 5 [43] | nmCRPC | DARO vs. ADT | ARAMIS | Partitioned-survival model | Public payer | 2018 |
| CE | China | 2017 | Zheng [40] | mHSPC | DOCE vs. ADT | CHAARTED | Markov model | Societal | 2015 |
| CE | China | 2017 | Zhang [38] | mHSPC | Za vs. DOCE vs. DOCE+Za vs. ADT | Clinical trials | Markov model | Societal | 2016 |
| CE | France | 2021 | Pelloux-Prayer [34] | mHSPC | DOCE vs. ABI vs. ENZA vs. caba sequencing | CHAARTED, LATITUDE, COU-AA-302, PREVAIL, FIRSTANA | Markov model | Healthcare system | 2020 |
| CE | Greece | 2019 | Tsiatas [54] | nmCRPC | APA vs. ENZA | SPARTAN and PROSPER | Partitioned-survival model | Healthcare system | NR |
| CE | Mexico | 2020 | Toro [53] | nmCRPC | ENZA vs. APA vs. ADT | Clinical Trials | Semi-Markov model | Public payer | 2018 |
| CE | UK | 2016 | NICE 2 [42] | mHSPC | DOCE vs. ADT | STAMPEDE, CHAARETED, GETUG-AFU 15 | - | Healthcare system | 2015 |
| CE | UK | 2018 | Woods [37] | mHSPC | DOCE vs. ADT | STAMPEDE | Markov model | Healthcare system | 2014 |
| CE | UK | 2019 | NICE 5 [49] | nmCRPC | ENZA vs. ADT | PROSPER | Semi-Markov partitioned-survival model | Healthcare system | 2018 |
| CE | UK | 2019 | Scottish Medicines 2 [51] | nmCRPC | ENZA vs. ABI | PROSPER | Semi-Markov model | Healthcare system | 2019 |
| CE | UK | 2020 | Scottish Medicines 1 [27] | mHSPC | ABI vs. ADT DOCE | LATITUDE | Semi-Markov/Partitioned-survival | Healthcare system | 2019 |
| CE | UK | 2020 | NICE 7 [48] | nmCRPC | DARO vs. ADT | ARAMIS | Partitioned-survival model | Healthcare system | 2020 |
| CE | UK | 2020 | Scottish Medicines 3 [50] | nmCRPC | DARO vs. ADT | ARAMIS | Partitioned-survival model | Healthcare system | 2020 |
| CE | UK | 2021 | NICE 1 [28] | mHSPC | ENZA vs. ADT | ARCHES | Partitioned-survival model | Healthcare system | 2020 |
| CE | UK | 2021 | NICE 3 [29] | mHSPC | ABI vs. ADT vs DOCE | LATITUDE, STAMPEDE | Partitioned-survival model | Healthcare system | 2021 |
| CE | UK | 2021 | NICE 4 [30] | mHSPC | APA vs. ADT | TITAN | Partitioned-survival model | Healthcare system | 2021 |
| CE | UK | 2021 | NICE 6 [52] | nmCRPC | APA vs. ADT | SPARTAN | Partitioned-survival model | Healthcare system | 2021 |
| CE | US | 2018 | Zhou [55] | nmCRPC | APA vs. ADT | SPARTAN | Markov model | Societal | NR |
| CE | US | 2019 | Ramamurthy [35] | mHSPC | ABI vs. DOCE vs. ADT | CHAARTED, LATITUDE | Markov model | Public payer | 2018 |
| CE | US | 2019 | Sathianathen [36] | mHSPC | ABI vs. DOCE vs. ADT | GETUG-AFU15, CHAARTED, LATITUDE | Markov model | Private payer | 2017 |
| CE | US | 2020 | Parikh [33] | mHSPC | MDT vs. ABI followed by DOCE vs. DOCE followed ABI | STOMP, STAMPEDE, TAX-327, COU-AA-301 | Markov model | Public payer | 2020 |
| CE | US/China | 2021 | Zhang [65] | mHSPC | ENZA vs. ADT | Clinical Trials | Markov model | Public payer | NR |
| CE/cost-minimization | Canada | 2020 | INESSS 4 [46] | nmCRPC | DARO vs. APA | ARAMIS | - | Healthcare system | 2020 |
| First Author | Disc. Rate | Effectiveness | Cost | Cost Effectiveness (ICER) | Sensitivity Analysis | Cost-Effective Strategy Based on Specific Local WTP Thresholds |
|---|---|---|---|---|---|---|
| mHSPC | ||||||
| Zheng [40] | 3% | DOCE: 1.85 QALY ADT: 1.26 QALY | DOCE: CAD 38,520 ADT: CAD 20,293 | 37,973 CAD/QALY | PA demonstrated that when WTP threshold was lower than CAD 57,740 ADT alone was cost-effective. | ADT |
| Ramamurthy [35] | None | ADT: 1.21 PF-QALY DOCE: 1.53 PF-QALY ABI: 1.73 PF-QALY | ADT: CAD 14,444 DOCE: CAD 36,912 ABI: CAD 315,648 | DOCE: 70,459 CAD/QALY ABI: 1,409,461 CAD/QALY | PA: In 99.5% of scenarios, DOCE is cost-effective with a WTP of 209,331 CAD/PF-QALY. | DOCE |
| Parikh [33] | 3% | MDT: 4.63 QALY ABI: 4.89 QALY ADT: 4.00 QALY | MDT: CAD 197,394 ABI->DOCE: CAD 233,278 DOCE+ABI: CAD 190,410 | MDT: CAD 450,649 NMB ABI->DOCE: CAD 450,339 NMB DOCE->ABI: CAD 368,372 NMB | PA: 53.6% of simulations MDT was the cost-effective strategy | MDT |
| Beca [32] | 1.5% | DOCE: 3.915QALY ADT: 2.852 QALY | DOCE: CAD 147,427 ADT: CAD 119,287 | 25,478 CAD/QALY | 1WSA yield ICERs below 36,809 CAD/QALY | DOCE |
| Zhang 2021 [39] | China: 3% US: 3.% | US: ADT: 4.09 QALY ENZA: 6.21 QALY China: ADT: 3.78 QALY ENZA: 5.70 QALY | US: ADT: CAD 604,365 ENZA:CAD 1,746,917 China: ADT: CAD 104,624 ENZA: CAD 645,965 | US: 538,940 CAD/QALY China: 281,948 CAD/QALY | 1WSA demonstrated the utility for the PFS state and the cost of ENZA were the most influential | ADT |
| Woods [37] | 3.5% | ADT: 4.90 QALY DOCE: 5.79 QALY | nm: ADT: CAD 90,409 DOCE: CAD 89,998 mets: ADT: CAD 86,066 DOCE: CAD 90,637 | nm: DOCE: Dominant mets: DOCE: 9,045 CAD/QALY | Price of DOCE was sensitive to increase ICER above the 21,325 CAD/QALY threshold. | DOC |
| Zhang 2017 [38] | 3% | ADT: 2.65 QALY Za+ADT: 2.69 QALY DOCE: 2.85 QALY DOCE+Za: 2.78 QALY | ADT: CAD 29,820 Za+ADT: CAD 35,554 DOCE: CAD 40,905 DOCE+Za: CAD 46,417 | ADT: CAD 29,820; 2.65 QALY Za+ADT: CAD 35,554; 2.69 QALY; 143,351 CAD/QALY DOCE+ADT: CAD 40,905; 2.85 QALY; 55,429 CAD/QALY DOCE+Za+ADT: CAD 46,417; 2.78QALY; 127,679 CAD/QALY | 1WSA: The most impactful parameter were failure-free survival (FFS) state, cost of ADT, and utility of FFS state. PA confirmed conclusions, however SOC alone was the cost-effective option at a WTP threshold of CAD 28,870. | DOCE |
| Sathianathen [36] | 3% | ADT: 2.435 QALY DOCE: 2.737 QALY ABI: 4.272 QALY | ADT: CAD 286,885 DOCE: CAD 301,516 ABI: CAD 933,864 | DOCE: 48,457 CAD/QALY ABI: 411,980 CAD/QALY | ABI represented value high-health care only one threshold exceeded CAD 488,439. | DOCE |
| Aguiar 2019 [31] | NR | ABI vs. ADT: 0.999 QALY gain DOCE vs. ADT: 0.492 QALY gain | ABI vs. ADT: CAD 164,826 DOCE vs. ADT: CAD 62,517 | With an incremental investment of CAD 49,522 DOCE is cost-effective treatment in 91% of cases. | ADT at Brazilian threshold DOCE at WHO threshold | |
| Aguiar 2017 [41] | NR | HR nm: 0.12 QALY benefit of DOCE Metastatic: 0.52 QALY benefit of DOCE | DOCE: CAD 28,149 ADT: CAD 19,554 | Metastatic: 15,968 CAD/QALY HV metastatic disease: 11,970 CAD/QALY | Metastatic: 80% of scenarios DOCE cost-effective HV metastatic disease: 73% of scenarios DOCE cost-effective | DOCE |
| Pelloux-Prayer [34] | 2.5% | Asymptomatic/mildly symptomatic: DOCE->ABI: 4.24 LY DOCE->ENZA: 4.25 LY ABI->DOCE: 3.97 LY ABI->ENZA: 4.15 LY Symptomatic: DOCE->DOCE: 4.05 LY DOCE->Caba: 4.07 LY ABI->DOCE: 3.97 LY | Asymptomatic/mildly symptomatic: DOCE->ABI: CAD 144,133 DOCE->ENZA: CAD 285,649 ABI->DOCE: CAD 222,858 ABI->ENZA: CAD 250,395 Symptomatic: DOCE->DOCE: CAD 121,140 DOCE->Caba: CAD 157,253 ABI->DOCE: CAD 222,858 | Asymptomatic/mildly symptomatic: DOCE-> ENZA vs. DOCE->ABI = 708,983 CAD/QALY (ABI->DOCE, ABI->ENZA is dominated) Symptomatic: DOCE->Caba vs. DOCE->DOCE= 1,869,295 CAD/QALY (ABI->DOCE is dominated) | Asymptomatic/mildly symptomatic: Cost reduction of 70% of ABI or ENZA led to ABI->ENZA to become efficient at the 74,353 CAD/LY threshold. Symptomatic: Cost reduction of 70% of ABI and Caba leads to ABI->DOCE to be least costly and effective but ICER for the two other options exceeds the cost-effectiveness threshold. | DOCE |
| CADTH 1 [23] | 1.5% | NR | NR | ADT<980 CAD/QALY DOCE between 980 and 294,494 CAD/QALY; ABI is the preferred option if the WTP is more than 294,494 CAD/QALY. | NR | DOCE |
| CADTH 2 [24] | 1.5% | ENZA vs. DOCE 0.24 QALY | ENZA vs. DOCE: CAD 75,566 | ENZA vs. DOCE: 307,776 CAD/QALY | <=52,200 CAD/QALY = 0% need 75% price reduction | DOCE |
| INESSS 1 [26] | 1.5% | ENZA: 1.24 QALY ADT:0.13 QALY | ENZA vs. ADT CAD 152,469 (CAD 152,571–172,193) ENZA vs. DOCE CAD 122,906 (CAD 123,015–128,428) | vs ADT 122,755 CAD/QALY vs. DOCE 924,765 CAD/QALY | ENZA vs. ADT 107,253–138,837 CAD/QALY ENZA vs. DOCE 662,362–1,438,466 CAD/QALY | DOCE |
| INESSS 2 [25] | 1.5% | APA vs. ADT: 1.45QALY | APA vs. ADT: CAD 138,070.00 | APA vs. ADT: 95,484 CAD/QALY | 86,471–113,580 CAD/QALY <=52,200 CAD/QALY = 4% <=104,400 CAD/QALY = 57% | APA |
| NICE 1 [28] | 3.5% | NR | NR | NR | NR | ENZA |
| NICE 2 [42] | 3.5% | OS benefit of 10–15 months | Cost of 6 cycles of DOCE: CAD 10,018 | NR | NR | NR |
| NICE 3 [29] | 3.5% | NR | NR | >148,706 CAD/QALY gained vs. DOCE >44,612 CAD/QALY vs. ADT | NR | ABI is not recommended |
| NICE 4 [30] | 3.5% | NR | NR | Acceptable ICER would be lower than the middle of the range 29,741 to 44,227 CAD/QALY | NR | APA is recommended only if: DOCE is not suitable and the price of APA is rebated |
| Scottish Medicines 1 [27] | 3.5% | ABI vs. ADT: 0.987 ABI vs. DOCE: 0.401 | ABI vs. ADT: CAD 144,442 ABI vs. DOCE: CAD 321,706 | ABI vs. ADT: CAD 103,527–167,146 ABI vs. DOCE: CAD 254,536–515,315 | NR | |
| nmCRPC | ||||||
| Aguiar 2017 [41] | DOCE vs. ADT: 0.12 QALY | DOCE vs. ADT: CAD 4424 | DOCE vs. ADT: 36,875 CAD/QALY | In PA, 53% of the scenarios evaluated were cost-effective based on the three-fold gross domestic product (GDP) per capita 46,929 CAD/QALY. | DOCE | |
| Zhou [55] | NR | APA:NR ADT: NR | APA:NR ADT: NR | Apa vs. ADT ACER: 223,720 CAD/QALY ICER: 944,906 CAD/QALY | 1WSA demonstrated that OS and costs have the greatest impact on the results. | ADT |
| Tsiatas [54] | Yes | APA: 4.3 QALY ENZA: 3.8 QALY | APA: CAD 205,951 to 228,558 ENZA: CAD 200,263 | CAD 10,938 to 54,417 | APA cost-effective in 56% to 68% of scenarios at WTP threshold of CAD 78,154 | APA |
| Toro [53] | 5% | ENZA: 3.75 QALY APA: 3.27 QALY ADT: 3.00 QALY | ENZA: CAD 78,348 APA: CAD 91,406 ADT: CAD 765 | ENZA vs. ADT: 97,934.84 CAD/QALY Enza vs. APA: dominating | None | ENZA |
| CADTH 3 [45] | 1.5% | ENZA vs. ADT:0.44 ENZA vs. Apa+ADT: −0.28 | ADT: CAD 106,081 APA: CAD −6158 | ENZA vs. ADT: 243,679 CAD/QALY APA: 25,666 CAD/QALY * | NR | ENZA |
| CADTH 4 [44] | 1.5% | APA vs. ADT: 0.57 QALY | APA vs. ADT: CAD 12,1193 | 213,176 CAD/QALY | NR | APA |
| CADTH 5 [43] | 1.5% | DARO vs. ADT: 0.78 QALY | DARO vs. ADT: CAD 144,504 | DARO vs. ADT: 184,879 CAD/QALY | NR | DARO |
| INESSS 3 [47] | 1.5% | APA vs. ADT: 0.05 | APA vs. ADT: CAD 67,692 | APA vs. ADT: 1,237,896 CAD/QALY | 146,975–10,032,238 CAD/QALY | APA |
| INESSS 4 [46] * | 1.5% | NR | DARO vs. ADT: CAD 3551 (same as APA) | NR | NR | DARO |
| NICE 5 [49] | 3.5% | NR | NR | ENZA vs. ADT: 92,138 CAD/QALY | NR | ENZA is not cost-efficient vs. ADT |
| NICE 6 [52] | 3.5% | NR | NR | NR | Middle of the range normally considered a cost-effective use of NHS resources | APA |
| NICE 7 [48] | 3.5% | Survival in mCRPC 3–4 shorter after DARO than ADT | NR | NR | 31,927–47,890 CAD/QALY | DARO |
| Scottish Medicines 2 [51] | 3.5% | ADT: 3.18 ENZA: 4.17 | ADT: CAD 122,016 ENZA: CAD 271,587 | ENZA vs. ADT: 150,857 CAD/QALY with PAS | 109,921–431,601 CAD/QALY | ENZA is not cost-efficient |
| Scottish Medicines 3 [50] | 3.5% | NR | NR | NR | NR | DARO |
| First Author | Time Period of Reported Costs | Costing Methods | Inpatient Costs | Outpatient Cost | Medical Costs | Pharmaceutical Costs | Cancer Specific Costs | Total Costs |
|---|---|---|---|---|---|---|---|---|
| mHSPC | ||||||||
| Hu [56] | Lifetime | Decision-analytic model | ||||||
| Healthcare perspective | - | - | - | - | DOCE: CAD 5877 ABI: CAD 6329 | DOCE: CAD 26,432 ABI CAD 248,609 | DOCE: CAD 80,754 ABI: CAD 259,909 | |
| Patient perspective | - | - | - | - | DOCE: CAD 1304 ABI: CAD 1582 | DOCE: CAD 3802 ABI: CAD 13,029 | DOCE: CAD 18,823 ABI: CAD 64,510 | |
| Wong [58] | Total prices of treatment under the trial’s experimental and control arms | |||||||
| ABI (AWP) | 33 to 42 months | - | - | - | - | - | CAD 540,299 to CAD 707,544 | |
| ENZA (AWP) | 13 to 36 months | - | - | - | - | - | CAD 225,387 to CAD 602,822 | |
| Svensson [59] | 12 months | Bottom-up | - | - | - | - | - | CAD 11,893.00 |
| Ke [57] | 1 year | Top-down | ||||||
| U.S. Medicare Advantage | - | CAD 188,676 | - | - | - | - | - | |
| Commercially-insured | - | CAD 174,525 | - | - | - | - | - | |
| nmCRPC | ||||||||
| Shaha [63] | 1 year | Bottom-up | ||||||
| CNS AEs | - | - | - | - | - | AEs: CAD 71,485 No AE: CAD 45,582 | ||
| Any AEs | - | - | - | - | - | AEs: CAD 63,619 No AE: CAD 47,212 | ||
| Seal [62] | Mean cost per patient | Top-down | ||||||
| nmCRPC | CAD 15,062 | CAD 5576 | - | - | - | CAD 9338 | ||
| mCRPC | CAD 17,837 | CAD 8680 | - | - | - | CAD 12,267 | ||
| Wu [64] | Top-down | |||||||
| Commercial | nmCRPC: 12.0 months mCRPC: 13.9 months | - | - | nmCRPC: CAD 36,452 mCRPC: CAD 108,741 | nmCRPC: CAD 4373 mCRPC: CAD 8180 | - | nmCRPC: CAD 40,825 mCRPC: CAD 254,743 | |
| Medigap | nmCRPC: 12.0 months mCRPC: 14.6 months | - | - | nmCRPC: CAD 31,976 mCRPC: CAD 72,686 | nmCRPC: CAD 6,551 mCRPC: CAD 101,651 | - | nmCRPC: CAD 38,527 mCRPC: CAD 195,547 | |
| Svensson [59] | 12 months | Bottom-up | - | - | - | - | - | CAD 6024 |
| George [61] | 4 years until death, health plan disenrollment or the study end date | Top-down | ||||||
| nmCRPC | - | - | CAD 1883 | CAD 556 | - | |||
| mCRPC | - | - | CAD 5460 | CAD 3675 | - | |||
| Freedland [60] * | 1 year | Top-down | ||||||
| nmCRPC | CAD 5121 | CAD 13,803 | - | CAD 2900 | - | - | ||
| mCRPC | CAD 16,014 | CAD 19,559 | - | CAD 9564 | - | - | ||
| Questionnaire Item | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study ID | Population | Competing alternatives | Research question | Design | Assumptions/validation | Time horizon | Perspective | Costs identification | Costs measure | Costs valuation | Outcome identification | Outcome measure | Outcome valuation | Incremental analysis | Discounting | Sensitivity analysis | Conclusions | Generalizability | Conflict of interest | Ethical/distributional | Total |
| mHSPC | |||||||||||||||||||||
| Pelloux-Prayer [34] | 19 | ||||||||||||||||||||
| Sathianathen [36] | 19 | ||||||||||||||||||||
| Zhang 2017 [38] | 19 | ||||||||||||||||||||
| Zhang 2021 [39] | 19 | ||||||||||||||||||||
| Woods [37] | 19 | ||||||||||||||||||||
| Parikh [33] | 18 | ||||||||||||||||||||
| Zheng [40] | 18 | ||||||||||||||||||||
| Beca [32] | 17 | ||||||||||||||||||||
| Aguiar 2019 [31] | 17 | ||||||||||||||||||||
| Hu [56] | 16 | ||||||||||||||||||||
| Ramamurthy [35] | 16 | ||||||||||||||||||||
| Svensson [59] | 15 | ||||||||||||||||||||
| Ke [57] | 11 | ||||||||||||||||||||
| Wong [58] | 11 | ||||||||||||||||||||
| Aguiar 2017 [41] * | 18 | ||||||||||||||||||||
| nmCRPC | |||||||||||||||||||||
| Toro [53] | 17 | ||||||||||||||||||||
| Freedland [60] | 17 | ||||||||||||||||||||
| Shah [63] | 16 | ||||||||||||||||||||
| Zhou [55] | 16 | ||||||||||||||||||||
| Wu [64] | 16 | ||||||||||||||||||||
| Seal [62] | 14 | ||||||||||||||||||||
| Tsiatas [54] | 14 | ||||||||||||||||||||
| George [61] | 11 | ||||||||||||||||||||
| Brazil | China | Columbia | France | Greece | Mexico | Sweden | UK | US | |
|---|---|---|---|---|---|---|---|---|---|
| Methodological Characteristics | |||||||||
| Perspective | Medium (societal vs. public payer) | Very high | Medium | High | Medium (societal vs. healthcare) | High | High | High | High (payer/societal) |
| Discount rate | Low (not reported) | Medium (1.5% vs. 3%) | Low (not reported) | High (1.5% vs. 2.5%) | Low (not reported) | Low (1.5% vs. 5%) | Low | Medium (1.5% vs. 3.5%) | Medium (1.5 vs. 3%) |
| Medical cost approach | Low (AE not considered) | High | Medium | High | Low (not described) | High | High | High | High |
| Productivity cost approach | Low (not considered) | High | Low (not reported) | Low | Low (not considered) | Low (not considered) | Low (not measured) | Low (not evaluated) | Low (not evaluated) |
| Healthcare-System Characteristics | |||||||||
| Absolute and relative prices in health care | Medium | High | Medium | Medium | Medium | Medium | Medium | Medium | Medium |
| Practice variation | Medium | Medium | Medium | Medium | Medium | Medium | High | Medium | High |
| Technology availability | High | High | High | Very high | High | High | High | Very high | High |
| Population characteristics | |||||||||
| Disease incidence/prevalence | Medium | Low | Medium | Very high | High | High | High | Very high | Medium |
| Case-mix | Medium | Low | Medium | High | High | Medium | High | High | Medium |
| Life expectancy | Medium | Medium (80 vs. 75) | Medium | Very high | High | Medium | High | Very high | Medium (80.0 vs. 76.3) |
| Health-status preferences | High | Very high | High | Very high | Medium | Medium | High | Very high | High |
| Acceptance, compliance, and incentives to patients | Medium | Medium | High | High | Medium | Medium | High | High | High |
| Productivity and work-loss time | Low (not considered) | Medium | Low (not reported) | High | Low (not considered) | Low (not considered) | Low (not measured) | High | Low (not measured) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yanev, I.; Gatete, J., Jr.; Aprikian, A.G.; Guertin, J.R.; Dragomir, A. The Health Economics of Metastatic Hormone-Sensitive and Non-Metastatic Castration-Resistant Prostate Cancer—A Systematic Literature Review with Application to the Canadian Context. Curr. Oncol. 2022, 29, 3393-3424. https://doi.org/10.3390/curroncol29050275
Yanev I, Gatete J Jr., Aprikian AG, Guertin JR, Dragomir A. The Health Economics of Metastatic Hormone-Sensitive and Non-Metastatic Castration-Resistant Prostate Cancer—A Systematic Literature Review with Application to the Canadian Context. Current Oncology. 2022; 29(5):3393-3424. https://doi.org/10.3390/curroncol29050275
Chicago/Turabian StyleYanev, Ivan, Jessy Gatete, Jr., Armen G. Aprikian, Jason Robert Guertin, and Alice Dragomir. 2022. "The Health Economics of Metastatic Hormone-Sensitive and Non-Metastatic Castration-Resistant Prostate Cancer—A Systematic Literature Review with Application to the Canadian Context" Current Oncology 29, no. 5: 3393-3424. https://doi.org/10.3390/curroncol29050275
APA StyleYanev, I., Gatete, J., Jr., Aprikian, A. G., Guertin, J. R., & Dragomir, A. (2022). The Health Economics of Metastatic Hormone-Sensitive and Non-Metastatic Castration-Resistant Prostate Cancer—A Systematic Literature Review with Application to the Canadian Context. Current Oncology, 29(5), 3393-3424. https://doi.org/10.3390/curroncol29050275





