Antibody Response to COVID-19 mRNA Vaccines in Oncologic and Hematologic Patients Undergoing Chemotherapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Groups
2.2. Antibody Evaluation
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robinson, A.G.; Gyawali, B.; Evans, G. COVID-19 and cancer: Do we really know what we think we know? Nat. Rev. Clin. Oncol. 2020, 17, 386–388. [Google Scholar] [CrossRef] [PubMed]
- Barry, A.; Apisarnthanarax, S.; O’Kane, G.M.; Sapisochin, G.; Beecroft, R.; Salem, R. Management of primary hepatic malignancies during the covid-19 pandemic: Recommendations for risk mitigation from a multidisciplinary perspective. Lancet Gastroenterol. Hepatol. 2020, 5, 765–775. [Google Scholar] [CrossRef]
- Vuagnat, P.; Frelaut, M.; Ramtohul, T.; Basse, C.; Diakite, S.; Noret, A. COVID-19 in breast cancer patients: A cohort at the institut Curie hospitals in the Paris area. Breast Cancer Res. 2020, 22, 55. [Google Scholar] [CrossRef] [PubMed]
- Basse, C.; Diakite, S.; Servois, V.; Frelaut, M.; Noret, A.; Bellesœur, A. Characteristics and outcome of SARS-CoV-2 infection in cancer patients. JNCL Cancer Spectrum. 2021, 5, pkaa090. [Google Scholar] [CrossRef]
- Brugel, M.; Carlier, C.; Essner, C.; Debreuve-Theresette, A.; Beck, M.F.; Merrouche, Y. Dramatic changes in oncology care pathways during the COVID-19 pandemic: The French ONCOCARE-COV study. Oncologist 2020, 26, e338–e341. [Google Scholar] [CrossRef]
- Alfano, G.; Morisi, N.; Frisina, M.; Ferrari, A.; Fontan, F.; Tonelli, R.; Franceschini, E.; Meschiari, M.; Donati, G.; Guaraldi, G. Awaiting a cure for COVID-19: Therapeutic approach in patients with different severity levels of COVID-19. Infez Med. 2022, 30, 11–21. [Google Scholar] [CrossRef]
- Drożdżal, S.; Rosik, J.; Lechowicz, K.; Machaj, F.; Szostak, B.; Przybyciński, J.; Lorzadeh, S.; Kotfis, K.; Ghavami, S.; Łos, M.J. An update on drugs with therapeutic potential for SARS-CoV-2 (COVID-19) treatment. Drug Resist. Updates 2021, 59, 100794. [Google Scholar] [CrossRef]
- Moreno, S.; Alcáza, B.; Dueñas, C.; González Del Castillo, J.; Olalla, J.; Antela, A. Use of Antivirals in SARS-CoV-2 Infection. Critical Review of the Role of Remdesivir. Drug Des. Devel. Ther. 2022, 16, 827–841. [Google Scholar] [CrossRef]
- Deng, J.; Heybati, K.; Ramaraju, H.B.; Zhou, F.; Rayner, D.; Heybati, S. Differential efficacy and safety of anti-SARS-CoV-2 antibody therapies for the management of COVID-19: A systematic review and network meta-analysis. Infection 2022, 19, 1–15. [Google Scholar] [CrossRef]
- Robinson, P.C.; Liew, D.F.L.; Tanner, H.L.; Grainger, J.R.; Dwek, R.A.; Reisler, R.B.; Steinman, L.; Feldmann, M.; Ho, L.P.; Hussell, T.; et al. COVID-19 therapeutics: Challenges and directions for the future. Proc. Natl. Acad. Sci. USA 2022, 119, e2119893119. [Google Scholar] [CrossRef]
- Zhou, H.; Ni, W.J.; Huang, W.; Wang, Z.; Cai, M.; Sun, Y.C. Advances in Pathogenesis, Progression, Potential Targets and Targeted Therapeutic Strategies in SARS-CoV-2-Induced COVID-19. Front. Immunol. 2022, 13, 834942. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; Chen, C.; Tang, J.; Wang, C.; Zhou, M.; Cheng, Y.; Zhou, X.; Wu, Q.; Zhang, X.; Feng, Z.; et al. Efficacy and safety of three new oral antiviral treatment (molnupiravir, fluvoxamine and Paxlovid) for COVID-19; a meta-analysis. Ann. Med. 2022, 54, 516–552. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, T.; Krammer, F.; Iwasaki, A. The first 12 months of COVID-19: A timeline of immunological insights. Nat. Rev. Immunol. 2021, 21, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Tougeron, D.; Hentzien, M.; Seitz-Polski, B.; Bani-Sadr, F.; Bourhis, J.; Ducreux, M.; Gaujoux, S.; Gorphe, P.; Guiu, B.; Hoang-Xuan, K.; et al. Severe acute respiratory syndrome coronavirus 2 vaccination for patients with solid cancer: Review and point of view of a French oncology intergroup (GCO, TNCD, UNICANCER). Eur. J. Cancer. 2021, 150, 232–239. [Google Scholar] [CrossRef]
- Amatu, A.; Pani, A.; Patelli, G.; Gagliardi, O.M.; Loparco, M.; Piscazzi, D.; Cassingena, A.; Tosi, F.; Ghezzi, S.; Campisi, D.; et al. Impaired seroconversion after SARS-CoV-2 mRNA vaccines in patients with solid tumours receiving anticancer treatment. Eur. J. Cancer. 2022, 163, 16–25. [Google Scholar] [CrossRef]
- Zeng, C.; Evans, J.P.; Reisinger, S.; Woyach, J.; Liscynesky, C.; Boghdadly, Z.E.; Rubinstein, M.P.; Chakravarthy, K.; Saif, L.; Oltz, E.M.; et al. Impaired neutralizing antibody response to COVID-19 mRNA vaccines in cancer patients. Cell. Biosci. 2021, 21, 197. [Google Scholar] [CrossRef]
- Gagelmann, N.; Passamonti, F.; Wolschke, C.; Massoud, R.; Niederwieser, C.; Adjallé, R.; Mora, B.; Ayuk, F.; Kröger, N. Antibody response after vaccination against SARS-CoV-2 in adults with haematological malignancies: A systematic review and meta-analysis. Haematologica 2021. ahead of print. [Google Scholar] [CrossRef]
- Grinshpun, A.; Rottenberg, Y.; Ben-Dov, I.Z.; Djian, E.; Wolf, D.G.; Kadouri, L. Serologic response to COVID-19 infection and/or vaccine in cancer patients on active treatment. ESMO Open 2021, 6, 100283. [Google Scholar] [CrossRef]
- Robinson, A.; Mazurek, A.; Xu, M.; Gong, Y. Quantitative Analysis of SARS-CoV-2 Antibody Status between Patients with Cancer and Healthy Individuals with Extended Vaccination Dosing Intervals in Canada. Curr. Oncol. 2021, 24, 68–76. [Google Scholar] [CrossRef]
- Naranbhai, V.; Pernat, C.A.; Gavralidis, A.; St Denis, K.J.; Lam, E.C.; Spring, L.M.; Isakoff, S.J.; Farmer, J.R.; Zubiri, L.; Hobbs, G.S.; et al. Immunogenicity and Reactogenicity of SARS-CoV-2 Vaccines in Patients with Cancer: The CANVAX Cohort Study. J. Clin. Oncol. 2022, 40, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Mair, M.J.; Berger, J.M.; Berghoff, A.S.; Starzer, A.M.; Ortmayr, G.; Puhr, H.C.; Steindl, A.; Perkmann, T.; Haslacher, H.; Strassl, R.; et al. Humoral Immune Response in Hematooncological Patients and Health Care Workers Who Received SARS-CoV-2 Vaccinations. JAMA Oncol. 2022, 8, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, A.; Gonzalez-Lugo, J.D.; Goradia, N.; Gali, R.; Shapiro, L.C.; Pradhan, K.; Rahman, S.; Kim, S.Y.; Ko, B.; Sica, R.A.; et al. Seroconversion rates following COVID-19 vaccination among patients with cancer. Cancer Cell. 2021, 39, 1081–1090.e2. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, S.L.; Yasawardene, P.; Wijerathne, W.; Somawardana, B. COVID-19 vaccination in cancer patients: A narrative review. J. Int. Med. Res. 2022, 50, 3000605221086155. [Google Scholar] [CrossRef] [PubMed]
- McNutt, L.A.; Wu, C.; Xue, X.; Hafner, J.P. Estimating the Relative Risk in Cohort Studies and Clinical Trials of Common Outcomes. Am. J. Epidemiol. 2003, 157, 940–943. [Google Scholar] [CrossRef] [PubMed]
- Hilbe, J.M. Negative Binomial Regression; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Vijenthira, A.; Gong, I.Y.; Fox, T.A.; Booth, S.; Cook, G.; Fattizzo, B.; Martín-Moro, F.; Razanamahery, J.; Riches, J.C.; Zwicker, J.; et al. Outcomes of patients with hematologic malignancies and COVID-19: A systematic review and meta-analysis of 3377 patients. Blood 2020, 136, 2881–2892. [Google Scholar] [CrossRef]
- Mandal, A.; Singh, P.; Samaddar, A.; Singh, D.; Verma, M.; Rakesh, A.; Ranjan, R. Vaccination of cancer patients against COVID-19: Towards the end of a dilemma. Med. Oncol. 2021, 38, 92. [Google Scholar] [CrossRef]
- Dai, M.; Liu, D.; Liu, M.; Zhou, F.; Li, G.; Chen, Z.; Zhang, Z.; You, H.; Wu, M.; Zheng, Q.; et al. Patients with Cancer Appear More Vulnerable to SARS-CoV-2: A Multicenter Study during the COVID-19 Outbreak. Cancer Discov. 2020, 10, 783–791. [Google Scholar] [CrossRef]
- Goshen-Lago, T.; Waldhorn, I.; Holland, R.; Szwarcwort-Cohen, M.; Reiner-Benaim, A.; Shachor-Meyouhas, Y.; Hussein, K.; Fahoum, L.; Baru, M.; Peer, A.; et al. Serologic Status and Toxic Effects of the SARS-CoV-2 BNT162b2 Vaccine in Patients Undergoing Treatment for Cancer. JAMA Oncol. 2021, 7, 507–513. [Google Scholar] [CrossRef]
- Semenzato, L.; Botton, J.; Drouin, J.; Cuenot, F.; Dray-Spira, R.; Weill, A.; Zureik, M. Chronic diseases, health conditions and risk of COVID-19-related hospitalization and in-hospital mortality during the first wave of the epidemic in France: A cohort study of 66 million people. Lancet Reg. Health Eur. 2021, 8, 100158. [Google Scholar] [CrossRef]
- Habibzadeh, P.; Stoneman, E.K. The Novel Coronavirus: A Bird’s Eye View. Int. J. Occup. Environ. Med. 2020, 11, 65–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skowronski, D.M.; De Serres, G. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2021, 384, 1576–1578. [Google Scholar] [PubMed]
- Moderna Vaccines and Related Biological Products Advisory Committee Meeting, 17 December 2020. Available online: https://www.fda.gov/media/144434/download (accessed on 1 January 2021).
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S. Experts Recommend People with Cancer Be Prioritized for a COVID-19 Vaccine. Available online: https://connect.uclahealth.org/2021/01/25/experts-recommend-people-with-cancer-be-prioritized-for-the-covid-19-vaccine/ (accessed on 5 February 2021).
- Massarweh, A.; Eliakim-Raz, N.; Stemmer, A.; Levy-Barda, A.; Yust-Katz, S.; Zer, A.; Benouaich-Amiel, A.; Ben-Zvi, H.; Moskovits, N.; Brenner, B. Evaluation of Seropositivity Following BNT162b2 Messenger RNA Vaccination for SARS-CoV-2 in Patients Undergoing Treatment for Cancer. JAMA Oncol. 2021, 7, 1133–1140. [Google Scholar] [CrossRef]
- Barrière, J.; Re, D.; Peyrade, F.; Carles, M. Current perspectives for SARS-CoV-2 vaccination efficacy improvement in patients with active treatment against cancer. Eur. J. Cancer. 2021, 154, 66–72. [Google Scholar] [CrossRef]
- Monin, L.; Laing, A.G.; Muñoz-Ruiz, M.; McKenzie, D.R.; Del Molino, I.; Del Barrio, I.; Alaguthurai, T.; Domingo-Vila, C.; Hayday, T.S.; Graham, C.; et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: Interim analysis of a prospective observational study. Lancet Oncol. 2021, 22, 765–778. [Google Scholar] [CrossRef]
- Cavanna, L.; Proietto, M.; Citterio, C.; Anselmi, E.; Zaffignani, E.; Stroppa, E.M.; Borsotti, M.T.; Contini, A.; Di Girolamo, G.; Quitadamo, V.M. COVID-19 Vaccination in Cancer Patients Older Than 70 Years Undergoing Active Treatment. Seroconversion Rate and Safety. Vaccines 2022, 10, 164. [Google Scholar] [CrossRef]
- Ferrara, P.; Ponticelli, D.; Agüero, F.; Caci, G.; Vitale, A.; Borrelli, M.; Schiavone, B.; Antonazzo, I.C.; Mantovani, L.G.; Tomaselli, V.; et al. Does smoking have an impact on the immunological response to COVID-19 vaccines? Evidence from the VASCO study and need for further studies. Public Health 2022, 203, 97–99. [Google Scholar] [CrossRef]
- Herishanu, Y.; Avivi, I.; Aharon, A.; Shefer, G.; Levi, S.; Bronstein, Y.; Morales, M.; Ziv, T.; Shorer Arbel, Y.; Scarfò, L. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood 2021, 137, 3165–3173. [Google Scholar] [CrossRef]
- Vassilaki, N.; Gargalionis, A.N.; Bletsa, A.; Papamichalopoulos, N.; Kontou, E.; Gkika, M.; Patas, K.; Theodoridis, D.; Manolis, I.; Ioannidis, A.; et al. Impact of Age and Sex on Antibody Response Following the Second Dose of COVID-19 BNT162b2 mRNA Vaccine in Greek Healthcare Workers. Microorganisms 2021, 9, 1725. [Google Scholar] [CrossRef]
- Addeo, A.; Shah, P.K.; Bodry, N.; Hudson, R.D.; Albracht, B.; Marco, M.D.; Kaklamani, V.; Dietrich, P.-Y.; Taylor, B.S.; Simand, P.-F. Immunogeniclty of SARS-CoV-2 messenger RNA Vaccines in Patients with Cancer. Cancer Cell. 2021, 39, 1091–1098.e2. [Google Scholar] [CrossRef] [PubMed]
- Coggins, S.A.A.; Laing, E.D.; Olsen, C.H.; Goguet, E.; Moser, M.; Jackson-Thompson, B.M.; Samuels, E.C.; Pollett, S.D.; Tribble, D.R.; Davies, J.; et al. Adverse effects and antibody titers in response to the BNT162b2 mRNA COVID-19 vaccine in a prospective study of healthcare workers. medRxiv 2021. [Google Scholar] [CrossRef] [PubMed]
- Collier, D.A.; Ferreira, I.A.T.M.; Kotagiri, P.; Datir, R.P.; Lim, E.Y.; Touizer, E.; Meng, B.; Abdullahi, A.; CLTIID-NIHR BioResource COVID-19 Collaboration; Elmer, A.; et al. Age-related immune response heterogeneity to SARS-CoV-2 vacclne BNT162b2. Nature 2021, 596, 417–422. [Google Scholar] [CrossRef]
- Subbarao, S.; Warrener, L.; Hoschler, K.; Perry, K.; Shute, J.; Whitaker, H.; O’Brien, M.; Baawuah, F.; Moss, P.; Parry, H.; et al. Robust Antibody Responses in 70-80 Year Olds following 1 or 2 Doses of Pfizer COVID-19 Vaccine. Eurosurveillance 2021, 26, 2100329. [Google Scholar] [CrossRef]
| Characteristics | Cases (Cancer Patients) | Controls (Healthcare Workers) | ||
|---|---|---|---|---|
| No | % | No | % | |
| Age | ||||
| 20–50 | 14 | 7.2 | 145 | 36.3 |
| 51–55 | 13 | 6.7 | 93 | 23.3 |
| 56–60 | 20 | 10.3 | 103 | 25.8 |
| 61–94 | 148 | 75.9 | 59 | 14.8 |
| Gender | ||||
| Male | 82 | 42.1 | 103 | 25.8 |
| Female | 113 | 57.9 | 297 | 74.3 |
| Cancer site | ||||
| HLP | 26 | 13.3 | - | - |
| Colon | 34 | 17.4 | - | - |
| Breast | 50 | 25.6 | - | - |
| Lung | 22 | 11.3 | - | - |
| Prostate | 10 | 5.1 | - | - |
| Other | 53 | 27.2 | - | - |
| Chemotherapy | ||||
| Adjuvant | 54 | 27.7 | - | - |
| Advanced | 140 | 71.8 | - | - |
| Missing | 1 | 0.5 | - | - |
| Whole sample | 195 | 100.0 | 400 | 100.0 |
| Subjects’ Characteristics | No. | % | GM (BAU/mL) | 95% CL (BAU/mL) |
|---|---|---|---|---|
| Age | ||||
| 20–50 | 159 | 26.7 | 323.1 | 273.0–382.4 |
| 51–55 | 106 | 17.8 | 333.8 | 271.4–410.6 |
| 56–60 | 123 | 20.7 | 299.5 | 235.8–380.3 |
| 61–94 | 207 | 34.8 | 194.7 | 150.5–252.0 |
| Gender | ||||
| Male | 185 | 31.1 | 139.5 | 189.5–302.7 |
| Female | 410 | 68.9 | 282.3 | 246.4–323.4 |
| Disease status | ||||
| Controls | 400 | 67.2 | 319.3 | 283.6–359.4 |
| Cases | 195 | 32.8 | 187.6 | 144.3–243.9 |
| Cancer site | ||||
| Controls | 400 | 67.2 | 319.3 | 283.6–359.4 |
| HLP | 26 | 4.4 | 12.7 | 4.4–37.2 |
| Colon | 34 | 5.7 | 311.6 | 202.7–479.2 |
| Breast | 50 | 8.4 | 274.1 | 186.6–402.3 |
| Lung | 22 | 3.7 | 257.7 | 177.9–373.2 |
| Prostate | 10 | 1.7 | 267.9 | 82.9–865.8 |
| Other | 53 | 8.9 | 290.6 | 199.6–423.1 |
| Chemotherapy | ||||
| Controls | 400 | 67.2 | 319.3 | 283.6–359.4 |
| Adjuvant | 54 | 9.1 | 221.9 | 145.9–337.6 |
| Advanced | 140 | 23.5 | 174.8 | 125.7–243.0 |
| Whole sample | 595 | 100.0 | 268.2 | 238.3–302.0 |
| Model | Factor and Levels | Positive Response | Highly Positive Response | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | RR | 95% CL | n | % | RR | 95% CL | ||
| 1 | Age at vaccination | ||||||||
| 20–50 | 155 | 97.5 | 1.00 | (Ref.) | 128 | 80.5 | 1.00 | (Ref.) | |
| 51–55 | 103 | 97.2 | 0.99 | 0.96–1.04 | 93 | 87.7 | 1.10 | 0.99–1.22 | |
| 56–60 | 116 | 94.3 | 0.97 | 0.93–1.02 | 108 | 87.8 | 1.11 | 1.01–1.23 | |
| 61–94 | 188 | 90.8 | 0.95 | 0.89–1.00 | 144 | 69.6 | 0.99 | 0.87–1.13 | |
| Gender | |||||||||
| Male | 172 | 93.0 | 1.00 | (Ref.) | 142 | 76.8 | 1.00 | (Ref.) | |
| Female | 390 | 95.1 | 1.01 | 0.96–1.06 | 331 | 80.7 | 1.00 | 0.87–1.13 | |
| Disease status | |||||||||
| Control | 385 | 96.3 | 1.00 | (Ref.) | 345 | 86.3 | 1.00 | (Ref.) | |
| Case | 177 | 90.8 | 0.95 | 0.89–1.02 | 128 | 95.6 | 0.76 | 0.66–0.87 | |
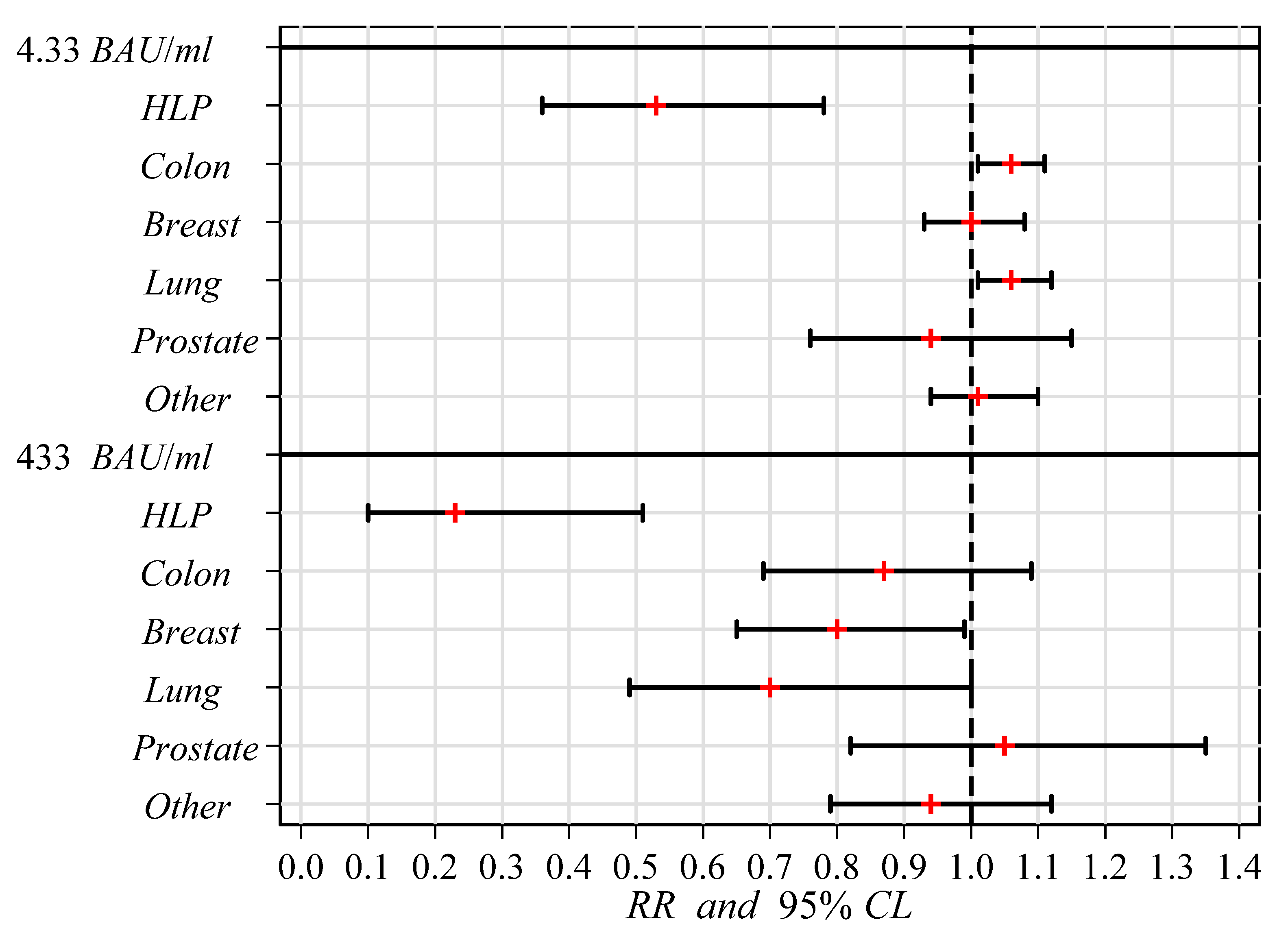
| 2 | Cancer site | ||||||||
| Control | 385 | 93.3 | 1.00 | (Ref.) | 345 | 86.3 | 1.00 | (Ref.) | |
| HLP | 13 | 50.0 | 0.53 | 0.36–0.78 | 5 | 19.3 | 0.23 | 0.10–0.51 | |
| Colon | 34 | 100.0 | 1.06 | 1.01–1.11 | 25 | 73.5 | 0.87 | 0.69–1.09 | |
| Breast | 48 | 96.0 | 1.00 | 0.93–1.08 | 34 | 68.0 | 0.80 | 0.65–0.99 | |
| Lung | 22 | 100.0 | 1.06 | 1.01–1.12 | 13 | 59.1 | 0.70 | 0.49–1.00 | |
| Prostate | 9 | 90.0 | 0.94 | 0.76–1.15 | 9 | 90.0 | 1.05 | 0.82–1.35 | |
| Other | 51 | 96.2 | 1.01 | 0.94–1.10 | 42 | 79.3 | 0.94 | 0.79–1.12 | |
| 3 | Chemotherapy | ||||||||
| Control | 385 | 93.3 | 1.00 | (Ref.) | 345 | 86.3 | 1.00 | (Ref.) | |
| Adjuvant | 51 | 94.4 | 0.97 | 0.89–1.06 | 35 | 64.8 | 0.73 | 0.59–0.91 | |
| Advanced | 125 | 89.3 | 0.94 | 0.87–1.01 | 92 | 65.7 | 0.77 | 0.66–0.89 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mencoboni, M.; Fontana, V.; Damiani, A.; Spitaleri, A.; Raso, A.; Bottaro, L.C.; Rossi, G.; Canobbio, L.; La Camera, A.; Filiberti, R.A.; et al. Antibody Response to COVID-19 mRNA Vaccines in Oncologic and Hematologic Patients Undergoing Chemotherapy. Curr. Oncol. 2022, 29, 3364-3374. https://doi.org/10.3390/curroncol29050273
Mencoboni M, Fontana V, Damiani A, Spitaleri A, Raso A, Bottaro LC, Rossi G, Canobbio L, La Camera A, Filiberti RA, et al. Antibody Response to COVID-19 mRNA Vaccines in Oncologic and Hematologic Patients Undergoing Chemotherapy. Current Oncology. 2022; 29(5):3364-3374. https://doi.org/10.3390/curroncol29050273
Chicago/Turabian StyleMencoboni, Manlio, Vincenzo Fontana, Azzurra Damiani, Antonino Spitaleri, Alessandro Raso, Luigi Carlo Bottaro, Giovanni Rossi, Luciano Canobbio, Antonella La Camera, Rosa Angela Filiberti, and et al. 2022. "Antibody Response to COVID-19 mRNA Vaccines in Oncologic and Hematologic Patients Undergoing Chemotherapy" Current Oncology 29, no. 5: 3364-3374. https://doi.org/10.3390/curroncol29050273
APA StyleMencoboni, M., Fontana, V., Damiani, A., Spitaleri, A., Raso, A., Bottaro, L. C., Rossi, G., Canobbio, L., La Camera, A., Filiberti, R. A., Taveggia, P., & Cavo, A. (2022). Antibody Response to COVID-19 mRNA Vaccines in Oncologic and Hematologic Patients Undergoing Chemotherapy. Current Oncology, 29(5), 3364-3374. https://doi.org/10.3390/curroncol29050273





