An Individualized Exercise Intervention for People with Multiple Myeloma—Study Protocol of a Randomized Waitlist-Controlled Trial
Abstract
:1. Introduction
2. Methodology
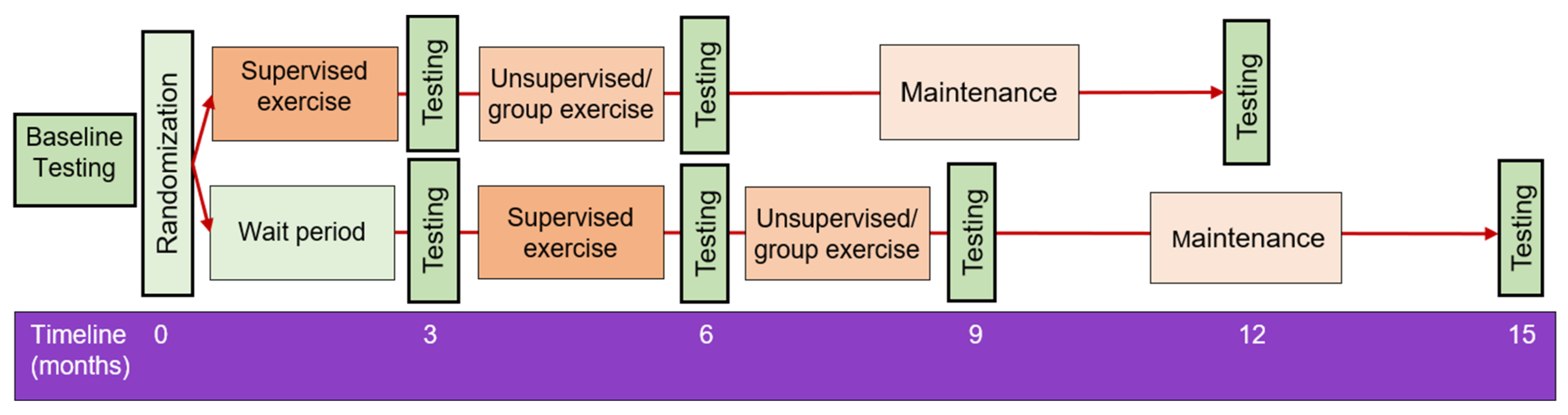
2.1. Trial Design and Setting
2.2. Participants
- i
- ≥18 years old;
- ii
- Diagnosis of MM;
- iii
- Free of any musculoskeletal, neurological, respiratory, metabolic, or cardiovascular conditions that may prevent safe completion of the exercise demands of the study;
- iv
- Able to give informed consent; and
- v
- Able to attend participating sites across southeast Queensland, Australia, to complete exercise training sessions and The University of Queensland for the testing sessions.
- i
- Abnormal resting electrocardiogram (ECG) with changes that suggest increased risk of exercise-induced cardiac event;
- ii
- Unstable angina;
- iii
- Cognitive impairment that impedes the ability to complete questionnaires; and
- iv
- Any intellectual or physical disability which would make participation in an exercise intervention unsafe for the individual.
2.3. Recruitment
2.4. Randomization
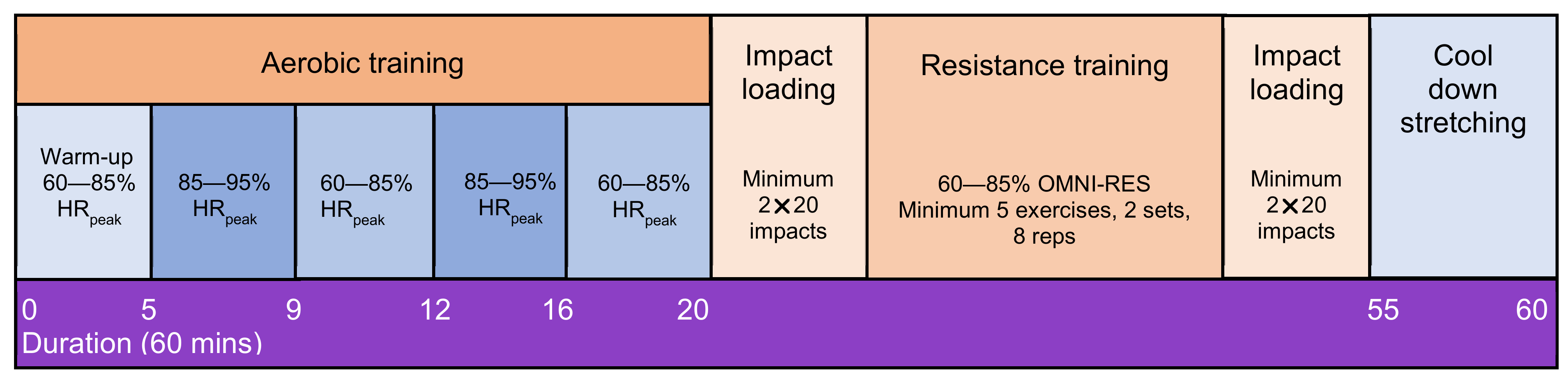
2.5. Exercise Intervention
Stepped Down Supervision Approach
3. Outcome Measures
3.1. Primary Outcome
Cancer-Specific Quality of Life (EORTC QLQ-C30 and QLQ-MY20)
3.2. Secondary Outcomes
3.2.1. Cardiorespiratory Fitness (V̇O2peak and Oxygen Efficiency Uptake Slope)
3.2.2. Body Composition, Bone Mineral Density, and Bone Architecture
3.2.3. Anthropometry
3.2.4. Neuromuscular Strength and Balance
Grip Strength
Leg Neuromuscular Power
Isometric Mid-Thigh Pull
Y-Balance Test
3.2.5. Pain and Bone Pain
3.2.6. Cancer-Related Fatigue (FACIT-F)
3.2.7. Functional Disability (Oswestry Low Back Pain Disability Questionnaire)
3.2.8. Myeloma-Specific Patient Reported Outcomes (MyPOS and FACT-MM)
3.2.9. Falls Self-Efficacy (Falls Efficacy Scale—International (FES-I))
3.2.10. Blood Biomarkers
Adipokines
Bone Health
Immune Function
Metabolomic and Lipidomic Analyses
3.2.11. Self-Reported and Objective Physical Activity
3.2.12. Enjoyment (Physical Activity Enjoyment Scale-8 (PACES-8)
3.3. Tertiary Outcomes
3.3.1. Qualitative Analysis
3.3.2. Feasibility and Adherence
3.3.3. Adverse and Serious Adverse Events
4. Data Collection, Management and Monitoring
5. Statistical Considerations and Data Analyses
Cost-Effectiveness Analysis
6. Ethics and Dissemination
7. Discussion
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kazandjian, D. Multiple myeloma epidemiology and survival: A unique malignancy. Semin. Oncol. 2016, 43, 676–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshy, G.; Thandrayen, J.; Koczwara, B.; Butow, P.; Laidsaar-Powell, R.; Rankin, N.; Canfell, K.; Stubbs, J.; Grogan, P.; Bailey, L.; et al. Disability, psychological distress and quality of life in relation to cancer diagnosis and cancer type: Population-based Australian study of 22,505 cancer survivors and 244,000 people without cancer. BMC Med. 2020, 18, 372. [Google Scholar] [CrossRef] [PubMed]
- Gulbrandsen, N.; Wisloff, F.; Brinch, L.; Carlson, K.; Dahl, I.M.; Gimsing, P.; Hippe, E.; Hjorth, M.; Knudsen, L.M.; Lamvik, J.; et al. Health-related quality of life in multiple myeloma patients receiving high-dose chemotherapy with autologous blood stem-cell support. Med. Oncol. 2001, 18, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Wisloff, F.; Hjorth, M.; Kaasa, S.; Westin, J. Effect of interferon on the health-related quality of life of multiple myeloma patients: Results of a Nordic randomized trial comparing melphalan-prednisone to melphalan-prednisone + alpha-interferon. The Nordic Myeloma Study Group. Br. J. Haematol. 1996, 94, 324–332. [Google Scholar] [CrossRef]
- Strasser-Weippl, K.; Ludwig, H. Psychosocial QOL is an independent predictor of overall survival in newly diagnosed patients with multiple myeloma. Eur. J. Haematol. 2008, 81, 374–379. [Google Scholar] [CrossRef]
- Dubois, D.; Dhawan, R.; van de Velde, H.; Esseltine, D.; Gupta, S.; Viala, M.; de la Loge, C. Descriptive and prognostic value of patient-reported outcomes: The bortezomib experience in relapsed and refractory multiple myeloma. J. Clin. Oncol. 2006, 24, 976–982. [Google Scholar] [CrossRef]
- Husson, O.; de Rooij, B.H.; Kieffer, J.; Oerlemans, S.; Mols, F.; Aaronson, N.K.; van der Graaf, W.T.A.; van de Poll-Franse, L.V. The EORTC QLQ-C30 Summary Score as prognostic factor for survival of patients with cancer in the "real-world": Results from the population-based PROFILES registry. Oncologist 2020, 25, e722–e732. [Google Scholar] [CrossRef] [Green Version]
- Campbell, K.L.; Winters-Stone, K.M.; Wiskemann, J.; May, A.M.; Schwartz, A.L.; Courneya, K.S.; Zucker, D.S.; Matthews, C.E.; Ligibel, J.A.; Gerber, L.H.; et al. Exercise guidelines for cancer survivors: Consensus statement from international multidisciplinary roundtable. Med. Sci. Sports Exerc. 2019, 51, 2375–2390. [Google Scholar] [CrossRef] [Green Version]
- Groeneveldt, L.; Mein, G.; Garrod, R.; Jewell, A.P.; Someren, K.V.; Stephens, R.; D’Sa, S.P.; Yong, K.L. A mixed exercise training programme is feasible and safe and may improve quality of life and muscle strength in multiple myeloma survivors. BMC Cancer 2013, 13, 31. [Google Scholar] [CrossRef] [Green Version]
- Koutoukidis, D.A.; Land, J.; Hackshaw, A.; Heinrich, M.; McCourt, O.; Beeken, R.J.; Philpott, S.; DeSilva, D.; Rismani, A.; Rabin, N.; et al. Fatigue, quality of life and physical fitness following an exercise intervention in multiple myeloma survivors (MASCOT): An exploratory randomised Phase 2 trial utilising a modified Zelen design. Br. J. Cancer 2020, 123, 187–195. [Google Scholar] [CrossRef]
- Stout, N.L.; Baima, J.; Swisher, A.K.; Winters-Stone, K.M.; Welsh, J. A systematic review of exercise systematic reviews in the cancer literature (2005–2017). PM R 2017, 9, S347–S384. [Google Scholar] [CrossRef] [PubMed]
- Ramsenthaler, C.; Kane, P.; Gao, W.; Siegert, R.J.; Edmonds, P.M.; Schey, S.A.; Higginson, I.J. Prevalence of symptoms in patients with multiple myeloma: A systematic review and meta-analysis. Eur. J. Haematol. 2016, 97, 416–429. [Google Scholar] [CrossRef] [PubMed]
- Cramp, F.; Byron-Daniel, J. Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst. Rev. 2012, 11, Cd006145. [Google Scholar] [CrossRef]
- Coleman, A.E.; Coon, A.S.; Hall-Barrow, A.J.; Richards, A.K.; Gaylor, A.D.; Stewart, A.B. Feasibility of exercise during treatment for multiple myeloma. Cancer Nurs. 2003, 26, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Coleman, E.A.; Goodwin, J.A.; Kennedy, R.; Coon, S.K.; Richards, K.; Enderlin, C.; Stewart, C.B.; McNatt, P.; Lockhart, K.; Anaissie, E.J. Effects of exercise on fatigue, sleep, and performance: A randomized trial. Oncol. Nurs. Forum 2012, 39, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Weston, K.S.; Wisløff, U.; Coombes, J.S. High-intensity interval training in patients with lifestyle-induced cardiometabolic disease: A systematic review and meta-analysis. Br. J. Sports Med. 2014, 48, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- Milanović, Z.; Sporiš, G.; Weston, M. Effectiveness of high-intensity interval training (HIT) and continuous endurance training for VO2max improvements: A systematic review and meta-analysis of controlled trials. Sports Med. 2015, 45, 1469–1481. [Google Scholar] [CrossRef]
- Mugele, H.; Freitag, N.; Wilhelmi, J.; Yang, Y.; Cheng, S.; Bloch, W.; Schumann, M. High-intensity interval training in the therapy and aftercare of cancer patients: A systematic review with meta-analysis. J. Cancer Surviv. 2019, 13, 205–223. [Google Scholar] [CrossRef]
- Viana, R.B.; Naves, J.P.A.; Coswig, V.S.; de Lira, C.A.B.; Steele, J.; Fisher, J.P.; Gentil, P. Is interval training the magic bullet for fat loss? A systematic review and meta-analysis comparing moderate-intensity continuous training with high-intensity interval training (HIIT). Br. J. Sports Med. 2019, 53, 655–664. [Google Scholar] [CrossRef]
- Kampshoff, C.S.; Chinapaw, M.J.; Brug, J.; Twisk, J.W.; Schep, G.; Nijziel, M.R.; van Mechelen, W.; Buffart, L.M. Randomized controlled trial of the effects of high intensity and low-to-moderate intensity exercise on physical fitness and fatigue in cancer survivors: Results of the Resistance and Endurance exercise After ChemoTherapy (REACT) study. BMC Med. 2015, 13, 275. [Google Scholar] [CrossRef] [Green Version]
- Martin, E.; Battaglini, C.; Hands, B.; Naumann, F.L. Higher-intensity exercise helps cancer survivors remain motivated. J. Cancer Surviv. 2016, 10, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Knols, R.H.; de Bruin, E.D.; Uebelhart, D.; Aufdemkampe, G.; Schanz, U.; Stenner-Liewen, F.; Hitz, F.; Taverna, C.; Aaronson, N.K. Effects of an outpatient physical exercise program on hematopoietic stem-cell transplantation recipients: A randomized clinical trial. Bone Marrow Transplant. 2011, 46, 1245–1255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Persoon, S.; Chinapaw, M.J.M.; Buffart, L.M.; Liu, R.D.K.; Wijermans, P.; Koene, H.R.; Minnema, M.C.; Lugtenburg, P.J.; Marijt, E.W.A.; Brug, J.; et al. Randomized controlled trial on the effects of a supervised high intensity exercise program in patients with a hematologic malignancy treated with autologous stem cell transplantation: Results from the EXIST study. PLoS ONE 2017, 12, e0181313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicol, J.L.; Hill, M.M.; Burton, N.W.; Skinner, T.L. Promoting exercise for patients with multiple myeloma: Attitudes and practices of clinical haematologists. J. Cancer Surviv. 2021, 1–8. [Google Scholar] [CrossRef]
- Nicol, J.L.; Woodrow, C.; Burton, N.W.; Mollee, P.; Nicol, A.J.; Hill, M.M.; Skinner, T.L. Physical activity in people with multiple myeloma: Associated factors and exercise program preferences. J. Clin. Med. 2020, 9, 3277. [Google Scholar] [CrossRef] [PubMed]
- Roodman, G.D. Pathogenesis of myeloma bone disease. Leukemia 2009, 23, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Roodman, G.D. Pathogenesis of myeloma bone disease. J. Cell. Biochem. 2010, 109, 283–291. [Google Scholar] [CrossRef]
- Quach, J.M.; Askmyr, M.; Jovic, T.; Baker, E.K.; Walsh, N.C.; Harrison, S.J.; Neeson, P.; Ritchie, D.; Ebeling, P.R.; Purton, L.E. Myelosuppressive therapies significantly increase pro-inflammatory cytokines and directly cause bone loss. J. Bone Miner. Res. 2015, 30, 886–897. [Google Scholar] [CrossRef]
- Zaleta, A.K.; Miller, M.F.; Olson, J.S.; Yuen, E.Y.N.; LeBlanc, T.W.; Cole, C.E.; McManus, S.; Buzaglo, J.S. Symptom burden, perceived control, and quality of life among patients living with multiple myeloma. J. Natl. Compr. Canc. Netw. 2020, 18, 1087–1095. [Google Scholar] [CrossRef]
- Burwick, N.; Sharma, S. Glucocorticoids in multiple myeloma: Past, present, and future. Ann. Hematol. 2019, 98, 19–28. [Google Scholar] [CrossRef]
- Williams, A.; Baruah, D.; Patel, J.; Szabo, A.; Chhabra, S.; Dhakal, B.; Hari, P.; Janz, S.; Stolley, M.; D’Souza, A. Prevalence and significance of sarcopenia in multiple myeloma patients undergoing autologous hematopoietic cell transplantation. Bone Marrow Transplant. 2021, 56, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Hillsdon, M.; Foster, C. What are the health benefits of muscle and bone strengthening and balance activities across life stages and specific health outcomes? J. Frailty Sarcopenia Falls 2018, 3, 66–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foster, C.; Armstrong, M.E.G. What types of physical activities are effective in developing muscle and bone strength and balance? J. Frailty Sarcopenia Falls 2018, 3, 58–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marques, E.A.; Mota, J.; Viana, J.L.; Tuna, D.; Figueiredo, P.; Guimarães, J.T.; Carvalho, J. Response of bone mineral density, inflammatory cytokines, and biochemical bone markers to a 32-week combined loading exercise programme in older men and women. Arch. Gerontol. Geriatr. 2013, 57, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Buffart, L.M.; Kalter, J.; Sweegers, M.G.; Courneya, K.S.; Newton, R.U.; Aaronson, N.K.; Jacobsen, P.B.; May, A.M.; Galvão, D.A.; Chinapaw, M.J.; et al. Effects and moderators of exercise on quality of life and physical function in patients with cancer: An individual patient data meta-analysis of 34 RCTs. Cancer Treat. Rev. 2017, 52, 91–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sansano-Nadal, O.; Giné-Garriga, M.; Brach, J.S.; Wert, D.M.; Jerez-Roig, J.; Guerra-Balic, M.; Oviedo, G.; Fortuño, J.; Gómara-Toldrà, N.; Soto-Bagaria, L.; et al. Exercise-based interventions to enhance long-term sustainability of physical activity in older adults: A systematic review and meta-analysis of randomized clinical trials. Int. J. Environ. Res. Public Health 2019, 16, 2527. [Google Scholar] [CrossRef] [Green Version]
- Juvet, L.K.; Thune, I.; Elvsaas, I.; Fors, E.A.; Lundgren, S.; Bertheussen, G.; Leivseth, G.; Oldervoll, L.M. The effect of exercise on fatigue and physical functioning in breast cancer patients during and after treatment and at 6 months follow-up: A meta-analysis. Breast 2017, 33, 166–177. [Google Scholar] [CrossRef]
- Chan, A.W.; Tetzlaff, J.M.; Altman, D.G.; Laupacis, A.; Gøtzsche, P.C.; Krleža-Jerić, K.; Hróbjartsson, A.; Mann, H.; Dickersin, K.; Berlin, J.A.; et al. SPIRIT 2013 statement: Defining standard protocol items for clinical trials. Ann. Intern. Med. 2013, 158, 200–207. [Google Scholar] [CrossRef] [Green Version]
- Steins Bisschop, C.N.; Courneya, K.S.; Velthuis, M.J.; Monninkhof, E.M.; Jones, L.W.; Friedenreich, C.; van der Wall, E.; Peeters, P.H.; May, A.M. Control group design, contamination and drop-out in exercise oncology trials: A systematic review. PLoS ONE 2015, 10, e0120996. [Google Scholar] [CrossRef] [Green Version]
- American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2014. [Google Scholar]
- Tanaka, H.; Monahan, K.D.; Seals, D.R. Age-predicted maximal heart rate revisited. J. Am. Coll. Cardiol. 2001, 37, 153–156. [Google Scholar] [CrossRef] [Green Version]
- Hart, N.H.; Galvao, D.A.; Saunders, C.; Taaffe, D.R.; Feeney, K.T.; Spry, N.A.; Tsoi, D.; Martin, H.; Chee, R.; Clay, T.; et al. Mechanical suppression of osteolytic bone metastases in advanced breast cancer patients: A randomised controlled study protocol evaluating safety, feasibility and preliminary efficacy of exercise as a targeted medicine. Trials 2018, 19, 695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rief, H.; Petersen, L.C.; Omlor, G.; Akbar, M.; Bruckner, T.; Rieken, S.; Haefner, M.F.; Schlampp, I.; Förster, R.; Debus, J.; et al. The effect of resistance training during radiotherapy on spinal bone metastases in cancer patients—A randomized trial. Radiother. Oncol. 2014, 112, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Colado, J.C.; Pedrosa, F.M.; Juesas, A.; Gargallo, P.; Carrasco, J.J.; Flandez, J.; Chupel, M.U.; Teixeira, A.M.; Naclerio, F. Concurrent validation of the OMNI-Resistance Exercise Scale of perceived exertion with elastic bands in the elderly. Exp. Gerontol. 2018, 103, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Bolam, K.A.; Skinner, T.L.; Jenkins, D.G.; Galvão, D.A.; Taaffe, D.R. The osteogenic effect of impact-loading and resistance exercise on bone mineral density in middle-aged and older men: A pilot study. Gerontology 2016, 62, 22–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giesinger, J.M.; Kieffer, J.M.; Fayers, P.M.; Groenvold, M.; Petersen, M.A.; Scott, N.W.; Sprangers, M.A.; Velikova, G.; Aaronson, N.K. Replication and validation of higher order models demonstrated that a summary score for the EORTC QLQ-C30 is robust. J. Clin. Epidemiol. 2016, 69, 79–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; de Haes, J.C.; et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef]
- Osoba, D.; Zee, B.; Pater, J.; Warr, D.; Kaizer, L.; Latreille, J. Psychometric properties and responsiveness of the EORTC quality of Life Questionnaire (QLQ-C30) in patients with breast, ovarian and lung cancer. Qual. Life Res. 1994, 3, 353–364. [Google Scholar] [CrossRef]
- Wisloff, F.; Eika, S.; Hippe, E.; Hjorth, M.; Holmberg, E.; Kaasa, S.; Palva, I.; Westin, J. Measurement of health-related quality of life in multiple myeloma. Nordic Myeloma Study Group. Br. J. Haematol. 1996, 92, 604–613. [Google Scholar] [CrossRef]
- Blade, J.; Calleja, M.; Lahuerta, J.J.; Poveda, J.L.; de Paz, H.D.; Lizán, L. Defining a set of standardised outcome measures for newly diagnosed patients with multiple myeloma using the Delphi consensus method: The IMPORTA project. BMJ Open 2018, 8, e018850. [Google Scholar] [CrossRef] [Green Version]
- Kvam, A.K.; Fayers, P.; Wisloff, F. What changes in health-related quality of life matter to multiple myeloma patients? A prospective study. Eur. J. Haematol. 2010, 84, 345–353. [Google Scholar] [CrossRef]
- Cocks, K.; Cohen, D.; Wisloff, F.; Sezer, O.; Lee, S.; Hippe, E.; Gimsing, P.; Turesson, I.; Hajek, R.; Smith, A.; et al. An international field study of the reliability and validity of a disease-specific questionnaire module (the QLQ-MY20) in assessing the quality of life of patients with multiple myeloma. Eur. J. Cancer 2007, 43, 1670–1678. [Google Scholar] [CrossRef] [PubMed]
- Schmid, D.; Leitzmann, M.F. Cardiorespiratory fitness as predictor of cancer mortality: A systematic review and meta-analysis. Ann. Oncol. 2015, 26, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Tuchman, S.A.; Lane, A.; Hornsby, W.E.; Bishop, C.; Thomas, S.; Herndon, J.E.; Long, G.; Gasparetto, C.; Jones, L.W. Quantitative measures of physical functioning after autologous hematopoietic stem cell transplantation in multiple myeloma: A feasibility study. Clin. Lymphoma Myeloma Leuk. 2015, 15, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Baba, R.; Nagashima, M.; Goto, M.; Nagano, Y.; Yokota, M.; Tauchi, N.; Nishibata, K. Oxygen uptake efficiency slope: A new index of cardiorespiratory functional reserve derived from the relation between oxygen uptake and minute ventilation during incremental exercise. J. Am. Coll. Cardiol. 1996, 28, 1567–1572. [Google Scholar] [CrossRef]
- Bongers, B.C.; Berkel, A.E.; Klaase, J.M.; van Meeteren, N.L. An evaluation of the validity of the pre-operative oxygen uptake efficiency slope as an indicator of cardiorespiratory fitness in elderly patients scheduled for major colorectal surgery. Anaesthesia 2017, 72, 1206–1216. [Google Scholar] [CrossRef] [Green Version]
- Hollenberg, M.; Tager, I.B. Oxygen uptake efficiency slope: An index of exercise performance and cardiopulmonary reserve requiring only submaximal exercise. J. Am. Coll. Cardiol. 2000, 36, 194–201. [Google Scholar] [CrossRef] [Green Version]
- Wasserman, K.; Hansen, J.E.; Sue, D.Y.; Stringer, W.W.; Sietsema, K.E.; Sun, X.-G.; Whipp, B.J. Principles of Exercise Testing and Interpretation: Including Pathophysiology and Clinical Applications; Wolters Kluwer Health/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012. [Google Scholar]
- Baim, S.; Leslie, W.D. Assessment of fracture risk. Curr. Osteoporos. Rep. 2012, 10, 28–41. [Google Scholar] [CrossRef]
- Sievänen, H.; Koskue, V.; Rauhio, A.; Kannus, P.; Heinonen, A.; Vuori, I. Peripheral quantitative computed tomography in human long bones: Evaluation of in vitro and in vivo precision. J. Bone Miner. Res. 1998, 13, 871–882. [Google Scholar] [CrossRef]
- Stewart, A.; Marfell-Jones, M.; Olds, T.; De Ridder, J. International Standards for Anthropometric Assessment, 3rd ed.; International Society for the Advancement of Kinanthropometry: Lower Hutt, New Zealand, 2011. [Google Scholar]
- Coombes, J.; Skinner, T.L. ESSA’s Student Manual for Health, Exercise & Sport Assessment; Elsevier: Chatswood, NSW, Australia, 2014. [Google Scholar]
- Curb, J.D.; Ceria-Ulep, C.D.; Rodriguez, B.L.; Grove, J.; Guralnik, J.; Willcox, B.J.; Donlon, T.A.; Masaki, K.H.; Chen, R. Performance-based measures of physical function for high-function populations. J. Am. Geriatr. Soc. 2006, 54, 737–742. [Google Scholar] [CrossRef]
- Wretenberg, P.; Arborelius, U.P. Power and work produced in different leg muscle groups when rising from a chair. Eur. J. Appl. Physiol. Occup. Physiol. 1994, 68, 413–417. [Google Scholar] [CrossRef]
- Cesari, M.; Kritchevsky, S.B.; Newman, A.B.; Simonsick, E.M.; Harris, T.B.; Penninx, B.W.; Brach, J.S.; Tylavsky, F.A.; Satterfield, S.; Bauer, D.C. Added value of physical performance measures in predicting adverse health-related events: Results from the Health, Aging and Body Composition Study. J. Am. Geriatr. Soc. 2009, 57, 251–259. [Google Scholar] [CrossRef] [PubMed]
- McGuigan, M.R.; Newton, M.J.; Winchester, J.B.; Nelson, A.G. Relationship between isometric and dynamic strength in recreationally trained men. J. Strength Cond. Res. 2010, 24, 2570–2573. [Google Scholar] [CrossRef] [PubMed]
- Mijwel, S.; Backman, M.; Bolam, K.A.; Olofsson, E.; Norrbom, J.; Bergh, J.; Sundberg, C.J.; Wengström, Y.; Rundqvist, H. Highly favorable physiological responses to concurrent resistance and high-intensity interval training during chemotherapy: The OptiTrain breast cancer trial. Breast Cancer Res. Treat. 2018, 169, 93–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haff, G.G.; Ruben, R.P.; Lider, J.; Twine, C.; Cormie, P. A comparison of methods for determining the rate of force development during isometric midthigh clean pulls. J. Strength Cond. Res. 2015, 29, 386–395. [Google Scholar] [CrossRef]
- Lee, D.K.; Kang, M.H.; Lee, T.S.; Oh, J.S. Relationships among the Y balance test, Berg Balance Scale, and lower limb strength in middle-aged and older females. Braz. J. Phys. Ther. 2015, 19, 227–234. [Google Scholar] [CrossRef] [Green Version]
- Sipe, C.L.; Ramey, K.D.; Plisky, P.P.; Taylor, J.D. Y-balance test: A valid and reliable assessment in older adults. J. Aging Phys. Act. 2019, 27, 663–669. [Google Scholar] [CrossRef]
- Pelayo-Alvarez, M.; Perez-Hoyos, S.; Agra-Varela, Y. Reliability and concurrent validity of the Palliative Outcome Scale, the Rotterdam Symptom Checklist, and the Brief Pain Inventory. J. Palliat. Med. 2013, 16, 867–874. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.S.; Beaton, D.; Smith, P.M.; Hagen, N.A. Patterns of pain and interference in patients with painful bone metastases: A brief pain inventory validation study. J. Pain Symptom Manag. 2010, 39, 230–240. [Google Scholar] [CrossRef]
- Tan, G.; Jensen, M.P.; Thornby, J.I.; Shanti, B.F. Validation of the Brief Pain Inventory for chronic nonmalignant pain. J. Pain 2004, 5, 133–137. [Google Scholar] [CrossRef]
- Broom, R.; Du, H.; Clemons, M.; Eton, D.; Dranitsaris, G.; Simmons, C.; Ooi, W.; Cella, D. Switching breast cancer patients with progressive bone metastases to third-generation bisphosphonates: Measuring impact using the Functional Assessment of Cancer Therapy-Bone Pain. J. Pain Symptom Manag. 2009, 38, 244–257. [Google Scholar] [CrossRef]
- Cella, D.; Eton, D.T.; Lai, J.S.; Peterman, A.H.; Merkel, D.E. Combining anchor and distribution-based methods to derive minimal clinically important differences on the Functional Assessment of Cancer Therapy (FACT) anemia and fatigue scales. J. Pain Symptom Manag. 2002, 24, 547–561. [Google Scholar] [CrossRef]
- Ahlberg, K.; Ekman, T.; Gaston-Johansson, F.; Mock, V. Assessment and management of cancer-related fatigue in adults. Lancet 2003, 362, 640–650. [Google Scholar] [CrossRef]
- Saltychev, M.; Mattie, R.; McCormick, Z.; Barlund, E.; Laimi, K. Psychometric properties of the Oswestry Disability Index. Int. J. Rehabil. Res. 2017, 40, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Osborne, T.R.; Ramsenthaler, C.; Schey, S.A.; Siegert, R.J.; Edmonds, P.M.; Higginson, I.J. Improving the assessment of quality of life in the clinical care of myeloma patients: The development and validation of the Myeloma Patient Outcome Scale (MyPOS). BMC Cancer 2015, 15, 280. [Google Scholar] [CrossRef] [Green Version]
- Wagner, L.I.; Robinson, D., Jr.; Weiss, M.; Katz, M.; Greipp, P.; Fonseca, R.; Cella, D. Content development for the Functional Assessment of Cancer Therapy-Multiple Myeloma (FACT-MM): Use of qualitative and quantitative methods for scale construction. J. Pain Symptom Manag. 2012, 43, 1094–1104. [Google Scholar] [CrossRef]
- Yardley, L.; Beyer, N.; Hauer, K.; Kempen, G.; Piot-Ziegler, C.; Todd, C. Development and initial validation of the Falls Efficacy Scale-International (FES-I). Age Ageing 2005, 34, 614–619. [Google Scholar] [CrossRef] [Green Version]
- Delbaere, K.; Close, J.C.T.; Mikolaizak, A.S.; Sachdev, P.S.; Brodaty, H.; Lord, S.R. The Falls Efficacy Scale International (FES-I). A comprehensive longitudinal validation study. Age Ageing 2010, 39, 210–216. [Google Scholar] [CrossRef] [Green Version]
- Trotter, T.N.; Gibson, J.T.; Sherpa, T.L.; Gowda, P.S.; Peker, D.; Yang, Y. Adipocyte-lineage cells support growth and dissemination of multiple myeloma in bone. Am. J. Pathol. 2016, 186, 3054–3063. [Google Scholar] [CrossRef] [Green Version]
- Bullwinkle, E.M.; Parker, M.D.; Bonan, N.F.; Falkenberg, L.G.; Davison, S.P.; DeCicco-Skinner, K.L. Adipocytes contribute to the growth and progression of multiple myeloma: Unraveling obesity related differences in adipocyte signaling. Cancer Lett. 2016, 380, 114–121. [Google Scholar] [CrossRef]
- Yu, W.; Cao, D.D.; Li, Q.B.; Mei, H.L.; Hu, Y.; Guo, T. Adipocytes secreted leptin is a pro-tumor factor for survival of multiple myeloma under chemotherapy. Oncotarget 2016, 7, 86075–86086. [Google Scholar] [CrossRef]
- Caers, J.; Deleu, S.; Belaid, Z.; De Raeve, H.; van Valckenborgh, E.; De Bruyne, E.; Defresne, M.P.; van Riet, I.; van Camp, B.; Vanderkerken, K. Neighboring adipocytes participate in the bone marrow microenvironment of multiple myeloma cells. Leukemia 2007, 21, 1580–1584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allegra, A.; Innao, V.; Gerace, D.; Allegra, A.G.; Vaddinelli, D.; Bianco, O.; Musolino, C. The adipose organ and multiple myeloma: Impact of adipokines on tumor growth and potential sites for therapeutic intervention. Eur. J. Intern. Med. 2018, 53, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Styner, M.; Thompson, W.R.; Galior, K.; Uzer, G.; Wu, X.; Kadari, S.; Case, N.; Xie, Z.; Sen, B.; Romaine, A.; et al. Bone marrow fat accumulation accelerated by high fat diet is suppressed by exercise. Bone 2014, 64, 39–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez-Gil, A.M.; Elizondo-Montemayor, L. The role of exercise in the interplay between myokines, hepatokines, osteokines, adipokines, and modulation of inflammation for energy substrate redistribution and fat mass loss: A review. Nutrients 2020, 12, 1899. [Google Scholar] [CrossRef]
- Koenig, A.M.; Karabatsiakis, A.; Stoll, T.; Wilker, S.; Hennessy, T.; Hill, M.M.; Kolassa, I.T. Serum profile changes in postpartum women with a history of childhood maltreatment: A combined metabolite and lipid fingerprinting study. Sci. Rep. 2018, 8, 3468. [Google Scholar] [CrossRef] [Green Version]
- Mundra, P.A.; Barlow, C.K.; Nestel, P.J.; Barnes, E.H.; Kirby, A.; Thompson, P.; Sullivan, D.R.; Alshehry, Z.H.; Mellett, N.A.; Huynh, K.; et al. Large-scale plasma lipidomic profiling identifies lipids that predict cardiovascular events in secondary prevention. JCI Insight 2018, 3, e121326. [Google Scholar] [CrossRef]
- Wang, X.; Wilkinson, R.; Kildey, K.; Ungerer, J.P.J.; Hill, M.M.; Shah, A.K.; Mohamed, A.; Dutt, M.; Molendijk, J.; Healy, H.; et al. Molecular and functional profiling of apical versus basolateral small extracellular vesicles derived from primary human proximal tubular epithelial cells under inflammatory conditions. J. Extracell. Vesicles 2021, 10, e12064. [Google Scholar] [CrossRef]
- Puchades-Carrasco, L.; Lecumberri, R.; Martinez-Lopez, J.; Lahuerta, J.J.; Mateos, M.V.; Prosper, F.; San-Miguel, J.F.; Pineda-Lucena, A. Multiple myeloma patients have a specific serum metabolomic profile that changes after achieving complete remission. Clin. Cancer Res. 2013, 19, 4770–4779. [Google Scholar] [CrossRef] [Green Version]
- Saltarella, I.; Lamanuzzi, A.; Apollonio, B.; Desantis, V.; Bartoli, G.; Vacca, A.; Frassanito, M.A. Role of extracellular vesicle-based cell-to-cell communication in multiple myeloma progression. Cells 2021, 10, 3185. [Google Scholar] [CrossRef]
- Godin, G.; Shephard, R.J. A simple method to assess exercise behavior in the community. Can. J. Appl. Sport Sci. 1985, 10, 141–146. [Google Scholar]
- Amireault, S.; Godin, G.; Lacombe, J.; Sabiston, C.M. Validation of the Godin-Shephard Leisure-time Physical Activity Questionnaire classification coding system using accelerometer assessment among breast cancer survivors. J. Cancer Surviv. 2015, 9, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Rauh, M.J.; Hovell, M.F.; Hofstetter, C.R.; Sallis, J.F.; Gleghorn, A. Reliability and validity of self-reported physical activity in Latinos. Int. J. Epidemiol. 1992, 21, 966–971. [Google Scholar] [CrossRef] [PubMed]
- Kozey-Keadle, S.; Libertine, A.; Lyden, K.; Staudenmayer, J.; Freedson, P.S. Validation of wearable monitors for assessing sedentary behavior. Med. Sci. Sports Exerc. 2011, 43, 1561–1567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryde, G.C.; Gilson, N.D.; Suppini, A.; Brown, W.J. Validation of a novel, objective measure of occupational sitting. J. Occup. Health 2012, 54, 383–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grant, P.M.; Ryan, C.G.; Tigbe, W.W.; Granat, M.H. The validation of a novel activity monitor in the measurement of posture and motion during everyday activities. Br. J. Sports Med. 2006, 40, 992–997. [Google Scholar] [CrossRef] [Green Version]
- Troiano, R.P.; Berrigan, D.; Dodd, K.W.; Masse, L.C.; Tilert, T.; McDowell, M. Physical activity in the United States measured by accelerometer. Med. Sci. Sports Exerc. 2008, 40, 181–188. [Google Scholar] [CrossRef]
- Migueles, J.H.; Rowlands, V.; Huber, F.; Sabia, S.V.; van Hees, V.T. GGIR: A research community driven open source R package for generating physical activity and sleep outcomes from multi-day raw accelerometer data. J. Meas. Phys. Behav. 2019, 2, 188. [Google Scholar] [CrossRef] [Green Version]
- Hildebrand, M.; VT, V.A.N.H.; Hansen, B.H.; Ekelund, U. Age group comparability of raw accelerometer output from wrist- and hip-worn monitors. Med. Sci. Sports Exerc. 2014, 46, 1816–1824. [Google Scholar] [CrossRef]
- Kendzierski, D.; DeCarlo, K. Physical Activity Enjoyment Scale: Two validation studies. J. Sport Exerc. Psychol. 1991, 13, 50–64. [Google Scholar] [CrossRef]
- Mullen, S.P.; Olson, E.A.; Phillips, S.M.; Szabo, A.N.; Wojcicki, T.R.; Mailey, E.L.; Gothe, N.P.; Fanning, J.T.; Kramer, A.F.; McAuley, E. Measuring enjoyment of physical activity in older adults: Invariance of the Physical Activity Enjoyment Scale (PACES) across groups and time. Int. J. Behav. Nutr. Phys. Act. 2011, 8, 103. [Google Scholar] [CrossRef] [Green Version]
- Miles, M.B.; Huberman, A.M. Qualitative Data Analysis: An Expanded Sourcebook, 2nd ed.; SAGE: Thousand Oaks, CA, USA, 1994. [Google Scholar]
- De Jesus, S.; Fitzgeorge, L.; Unsworth, K.; Massel, D.; Suskin, N.; Prapavessis, H.; Sanatani, M. Feasibility of an exercise intervention for fatigued breast cancer patients at a community-based cardiac rehabilitation program. Cancer Manag. Res. 2017, 9, 29–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walters, S.J.; Bonacho dos Anjos Henriques-Cadby, I.; Bortolami, O.; Flight, L.; Hind, D.; Jacques, R.M.; Knox, C.; Nadin, B.; Rothwell, J.; Surtees, M.; et al. Recruitment and retention of participants in randomised controlled trials: A review of trials funded and published by the United Kingdom Health Technology Assessment Programme. BMJ Open 2017, 7, e015276. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.A.; Sinden, A.R. Who will stay and who will go? A review of older adults’ adherence to randomized controlled trials of exercise. J. Aging Phys. Act. 2001, 9, 91–114. [Google Scholar] [CrossRef] [Green Version]
- Bell, M.L.; Fiero, M.; Horton, N.J.; Hsu, C.H. Handling missing data in RCTs; a review of the top medical journals. BMC Med. Res. Methodol. 2014, 14, 118. [Google Scholar] [CrossRef] [Green Version]
- King, M.T.; Viney, R.; Pickard, A.S.; Rowen, D.; Aaronson, N.K.; Brazier, J.E.; Cella, D.; Costa, D.S.J.; Fayers, P.M.; Kemmler, G.; et al. Australian utility weights for the EORTC QLU-C10D, a multi-attribute utility instrument derived from the cancer-specific quality of life questionnaire, EORTC QLQ-C30. Pharmacoeconomics 2018, 36, 225–238. [Google Scholar] [CrossRef] [Green Version]
- Terpos, E.; Mikhael, J.; Hajek, R.; Chari, A.; Zweegman, S.; Lee, H.C.; Mateos, M.V.; Larocca, A.; Ramasamy, K.; Kaiser, M.; et al. Management of patients with multiple myeloma beyond the clinical-trial setting: Understanding the balance between efficacy, safety and tolerability, and quality of life. Blood Cancer J. 2021, 11, 40. [Google Scholar] [CrossRef]
| Timepoint | ||||
|---|---|---|---|---|
| Assessment or Outcome | Baseline a | 3-Months | 6-Months | 12-Months |
| Screening | ||||
| Participant eligibility | ✓ | |||
| Informed consent | ✓ | |||
| Doctor’s consent | ✓ | |||
| Health history and disease status | ✓ | ✓ | ✓ | ✓ |
| Demographics | ✓ | |||
| Concomitant research study participation | ✓ | ✓ | ✓ | ✓ |
| Primary Outcomes | ||||
| Quality of life (EORTC QLQ-C30 and QLQ-MY20) | ✓ | ✓ | ✓ | ✓ |
| Secondary Outcomes | ||||
| Cardiorespiratory fitness (V̇O2peak) | ✓ | ✓ | ✓ | ✓ |
| Body composition and bone mineral density (DXA) | ✓ | ✓ | ✓ | ✓ |
| Bone architecture (pQCT) | ✓ | ✓ | ✓ | ✓ |
| Anthropometry (BMI, waist, and hip circumferences) | ✓ | ✓ | ✓ | ✓ |
| Strength (30STS, grip strength, mid-thigh pull) | ✓ | ✓ | ✓ | ✓ |
| Balance (Single leg stance, YBT) | ✓ | ✓ | ✓ | ✓ |
| Pain and bone pain (BPI, FACT-BP) | ✓ | ✓ | ✓ | ✓ |
| Fatigue (FACIT-F) | ✓ | ✓ | ✓ | ✓ |
| Functional disability (OLBPDQ) | ✓ | ✓ | ✓ | ✓ |
| Myeloma-specific PROs (FACT-MM, MyPOS) | ✓ | ✓ | ✓ | ✓ |
| Falls self-efficacy (FES-I) | ✓ | ✓ | ✓ | ✓ |
| Blood collection for blood biomarkers, metabolomics, and lipidomics | ✓ | ✓ | ✓ | ✓ |
| Self-reported physical activity (Godin) | ✓ | ✓ | ✓ | ✓ |
| Sedentary behavior and physical activity (ActiGraph™ accelerometry) | ✓ | ✓ | ✓ | ✓ |
| Exercise enjoyment (PACES-8) b | ✓ | |||
| Tertiary Outcomes | ||||
| Qualitative analysis (Semi-structured interviews) | ✓ | |||
| Adherence c | ✓ | ✓ | ||
| Safety (Adverse and serious adverse events) c | ✓ | ✓ | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicol, J.L.; Woodrow, C.; Cunningham, B.J.; Mollee, P.; Weber, N.; Smith, M.D.; Nicol, A.J.; Gordon, L.G.; Hill, M.M.; Skinner, T.L. An Individualized Exercise Intervention for People with Multiple Myeloma—Study Protocol of a Randomized Waitlist-Controlled Trial. Curr. Oncol. 2022, 29, 901-923. https://doi.org/10.3390/curroncol29020077
Nicol JL, Woodrow C, Cunningham BJ, Mollee P, Weber N, Smith MD, Nicol AJ, Gordon LG, Hill MM, Skinner TL. An Individualized Exercise Intervention for People with Multiple Myeloma—Study Protocol of a Randomized Waitlist-Controlled Trial. Current Oncology. 2022; 29(2):901-923. https://doi.org/10.3390/curroncol29020077
Chicago/Turabian StyleNicol, Jennifer L., Carmel Woodrow, Brent J. Cunningham, Peter Mollee, Nicholas Weber, Michelle D. Smith, Andrew J. Nicol, Louisa G. Gordon, Michelle M. Hill, and Tina L. Skinner. 2022. "An Individualized Exercise Intervention for People with Multiple Myeloma—Study Protocol of a Randomized Waitlist-Controlled Trial" Current Oncology 29, no. 2: 901-923. https://doi.org/10.3390/curroncol29020077
APA StyleNicol, J. L., Woodrow, C., Cunningham, B. J., Mollee, P., Weber, N., Smith, M. D., Nicol, A. J., Gordon, L. G., Hill, M. M., & Skinner, T. L. (2022). An Individualized Exercise Intervention for People with Multiple Myeloma—Study Protocol of a Randomized Waitlist-Controlled Trial. Current Oncology, 29(2), 901-923. https://doi.org/10.3390/curroncol29020077







