Primary Vitreoretinal Lymphoma: Current Diagnostic Laboratory Tests and New Emerging Molecular Tools
Abstract
1. Introduction
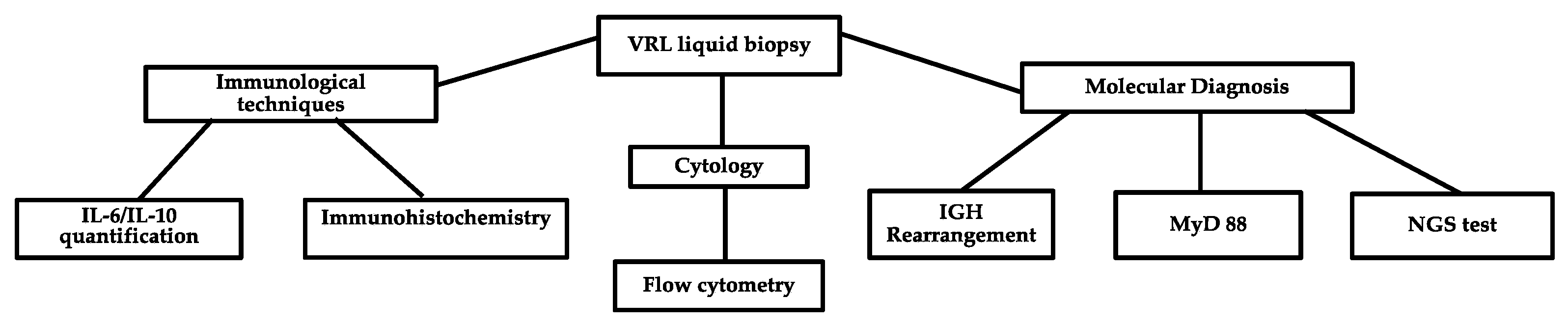
2. Current Laboratory Techniques Used in PVRL Diagnosis and the Main Problems with the Different Methods
2.1. Cytology plus Immunohistochemistry
2.2. IL-10 and IL-6
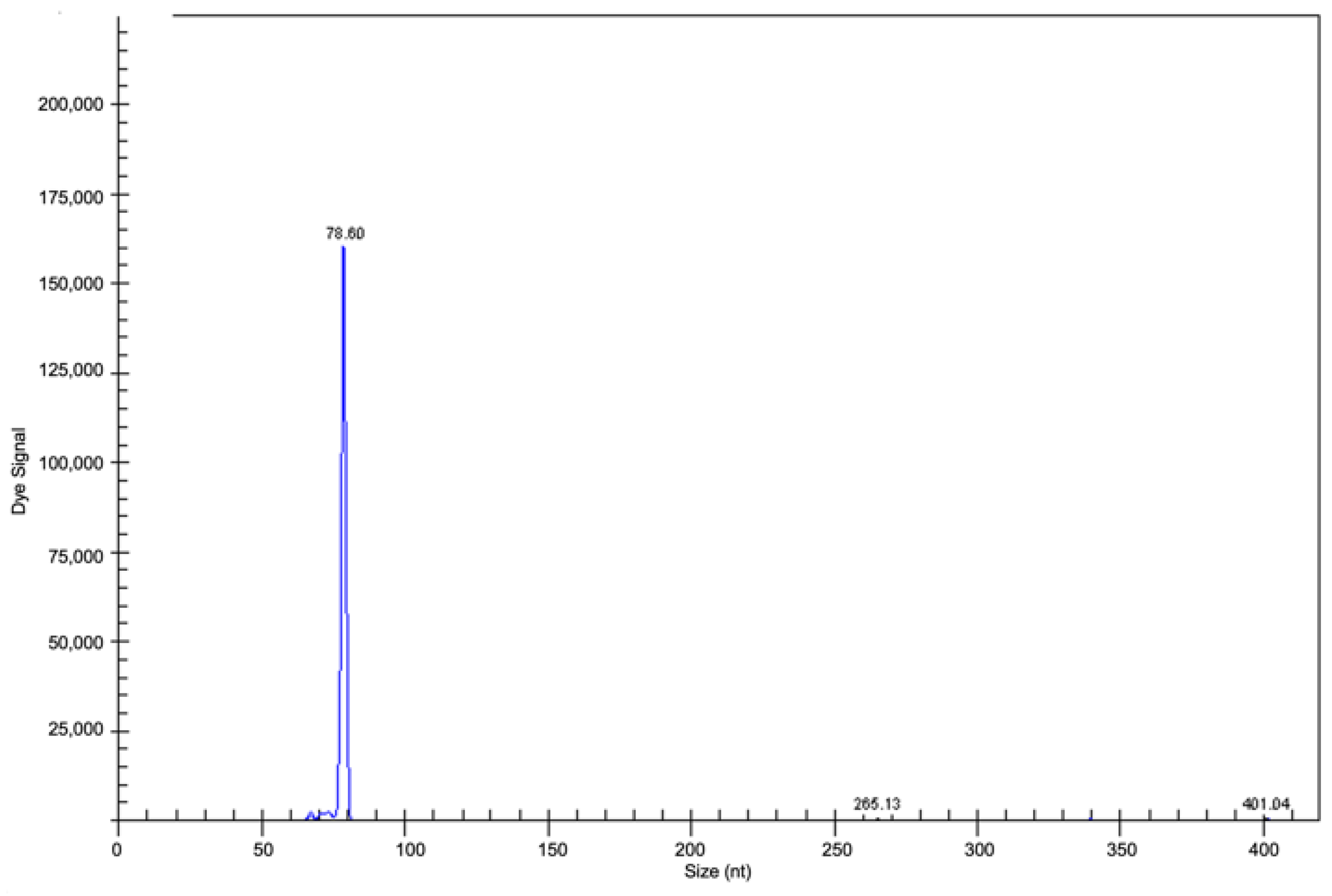
2.3. Clonality Analyses
2.4. MYD88 Mutation Analysis
2.5. Flow Cytometry
3. New Emerging Diagnostic Molecular Tools
3.1. VRL Mutational Profile Analysed with High-Throughput Techniques: What Is Known So Far
3.2. Quantification of microRNAs in Vitreous Specimens for Differential Diagnosis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sobolewska, B.; Chee, S.P.; Zaguia, F.; Goldstein, D.A.; Smith, J.R.; Fend, F.; Mochizuki, M.; Zierhut, M. Vitreoretinal Lymphoma. Cancers 2021, 13, 3921. [Google Scholar] [CrossRef] [PubMed]
- Carbonell, D.; Mahajan, S.; Chee, S.P.; Sobolewska, B.; Agrawal, R.; Bülow, T.; Gupta, V.; Jones, N.P.; Accorinti, M.; Agarwal, M.; et al. Consensus Recommendations for the Diagnosis of Vitreoretinal Lymphoma. Ocul. Immunol. Inflamm. 2021, 29, 507–520. [Google Scholar] [CrossRef]
- Alaggio, R.; Amador, C.; Anagnostopoulos, I.; Attygalle, A.D.; de Oliveira Araujo, I.B.; Borgs, A.M.; Boyer, D.; Calaminici, M.; Chadburn, A. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia 2022, 36, 1720–1748. [Google Scholar] [CrossRef] [PubMed]
- Gozzi, F.; Aldigeri, R.; Mastrofilippo, V.; de Simone, L.; Bolletta, E.; Marzano, J.; Iannetta, D.; Coassin, M.; Ilariucci, F.; Ferrari, A.; et al. Survival and Recurrence in Vitreoretinal Lymphoma Simulating Uveitis at Presentation: The Possible Role of Combined Chemotherapy. Ocul. Immunol. Inflamm. 2021, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Riemens, A.; Bromberg, J.; Touitou, V.; Sobolewska, B.; Missotten, T.; Baarsma, S.; Hoyng, C.; Cordero-Coma, M.; Tomkins-Netzer, O.; Rozalski, A.; et al. Treatment strategies in primary vitreoretinal lymphoma: A 17-center European collaborative study. JAMA Ophthalmol. 2015, 133, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Cimino, L.; Coassin, M.; Chan, C.C.; Marchi, S.; Belpoliti, M.; Fanti, A.; Iovieno, A.; Fontana, L. Vitreoretinal lymphomas misdiagnosed as uveitis: Lessons learned from a case series. Indian J. Ophthalmol. 2016, 64, 369–375. [Google Scholar] [CrossRef]
- Soussain, C.; Malaise, D.; Cassoux, N. Primary vitreoretinal lymphoma: A diagnostic and management challenge. Blood 2021, 138, 1519–1534. [Google Scholar] [CrossRef]
- Gerstner, E.R.; Batchelor, T.T. Primary central nervous system lymphoma. Arch. Neurol. 2010, 67, 291–297. [Google Scholar] [CrossRef]
- Chan, C.C.; Rubenstein, J.L.; Coupland, S.E.; Davis, J.L.; Harbour, J.W.; Johnston, P.B.; Cassoux, N.; Touitou, V.; Smith, J.R.; Batchelor, T.T.; et al. Primary vitreoretinal lymphoma: A report from an international primary central nervous system lymphoma collaborative group symposium. Oncologist 2011, 16, 1589–1599. [Google Scholar] [CrossRef]
- Péer, J.; Hochberg, F.H.; Foster, C.S. Clinical review: Treatment of vitreoretinal lymphoma. Ocul. Immunol. Inflamm. 2009, 17, 299–306. [Google Scholar] [CrossRef]
- Araujo, I.; Coupland, S.E. Primary Vitreoretinal Lymphoma—A Review. Asia Pac. J. Ophthalmol. (Phila) 2017, 6, 283–289. [Google Scholar]
- Balikov, D.A.; Hu, K.; Liu, C.J.; Betz, B.L.; Chinnaiyan, A.M.; Devisetty, L.V.; Venneti, S.; Tomlins, S.A.; Cani, A.K.; Rao, R.C. Comparative Molecular Analysis of Primary Central Nervous System Lymphomas and Matched Vitreoretinal Lymphomas by Vitreous Liquid Biopsy. Int. J. Mol. Sci. 2021, 22, 9992. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.L. Intraocular lymphoma: A clinical perspective. Eye 2013, 27, 153–162. [Google Scholar] [CrossRef]
- Costopoulos, M.; Touitou, V.; Golmard, J.L.; Darugar, A.; Fisson, S.; Bonnemye, P.; Le Lez, M.L.; Soussain, C.; Cassoux, N.; Lamy, T.; et al. ISOLD: A new highly sensitive interleukin score for intraocular lymphoma diagnosis. Ophthalmology 2016, 123, 1626–1628. [Google Scholar] [CrossRef] [PubMed]
- Frenkel, S.; Pe’Er, J.; Kaufman, R.; Maly, B.; Habot-Wilner, Z. The importance of cytokines analysis in the diagnosis of vitreoretinal lymphoma. Acta Ophthalmol. 2020, 98, e668–e673. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shen, D.; Wang, V.M.; Sen, H.N.; Chan, C.C. Molecular biomarkers for the diagnosis of primary vitreoretinal lymphoma. Int. J. Mol. Sci. 2011, 12, 5684–5697. [Google Scholar] [CrossRef] [PubMed]
- Bonzheim, I.; Giese, S.; Deuter, C.; Süsskind, D.; Zierhut, M.; Waizel, M.; Szurman, P.; Federmann, B.; Schmidt, J.; Quintanilla-Martinez, L.; et al. High frequency of MYD88 mutations in vitreoretinal B-cell lymphoma: A valuable tool to improve diagnostic yield of vitreous aspirates. Blood 2015, 126, 76–79. [Google Scholar] [CrossRef]
- Tan, W.J.; Wang, M.M.; Ricciardi-Castagnoli, P.; Tang, T.; Chee, S.P. Lim, T.S.; Chan, A.S.Y. Single cell MYD88 sequencing of isolated B cells from vitreous biopsies aids vitreoretinal lymphoma diagnosis. Blood 2019, 134, 709–712. [Google Scholar] [CrossRef]
- Liu, S.; Gu, J.; Zhang, T.; Ping, B.; Zhou, M.; Huang, X.; Jiang, R.; Xu, G.; Chang, Q. Clinical features, diagnostic significance and prognosis of vitreoretinal lymphoma in young patients. Retina 2021, 41, 2596–2604. [Google Scholar] [CrossRef]
- Cani, A.K.; Hovelson, D.H.; Demirci, H.; Johnson, M.W.; Tomlins, S.A.; Rao, R.C. Next generation sequencing of vitreoretinal lymphomas from small-volume intraocular liquid biopsies: New routes to targeted therapies. Oncotarget 2017, 8, 989–998. [Google Scholar] [CrossRef]
- Bonzheim, I.; Sander, P.; Salmeron-Villalobos, J.; Süsskind, D.; Szurman, P.; Gekeler, F.; Spitzer, M.S.; Steinhilber, J.; Kohler, E.; Bussgen, M.; et al. The molecular hallmarks of primary and secondary vitreoretinal lymphoma. Blood Adv. 2022, 6, 1598–1607. [Google Scholar] [CrossRef] [PubMed]
- Yonese, I.; Takase, H.; Yoshimori, M.; Onozawa, E.; Tzuzura, A.; Miki, T.; Mochizuki, M.; Miura, O.; Arai, A. CD79B mutations in primary vitreoretinal lymphoma: Diagnostic and prognostic potential. Eur. J. Haematol. 2019, 102, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, B.; Lee, H.; Park, H.; Byeon, S.H.; Choi, J.R.; Lee, S.C.; Lee, S.T.; Lee, C.S. Whole exome sequencing identifies mutational signatures of vitreoretinal lymphoma. Haematologica 2020, 105, E458–E460. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Zhao, Z.; Chang, Q. Evaluation of cytologic specimens obtained during experimental vitreous biopsy using B-cell lymphoma line. Eur. J. Ophthalmol. 2014, 24, 911–917. [Google Scholar] [CrossRef]
- Pulido, J.S.; Johnston, P.B.; Nowakowski, G.S.; Castellino, A.; Raja, H. The diagnosis and treatment of primary vitreoretinal lymphoma: A review. Int. J. Retin. Vitr. 2018, 4, 4–22. [Google Scholar]
- Rousset, F.; Garcia, E.; Defrance, T.; Perrone, C.; Vezzio, N.; Hsu, D.H.; Kastelein, R.; Moore, K.W.; Banchereau, J. Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc. Natl. Acad. Sci. USA 1992, 89, 1890–1893. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.I.; Hoekzema, R.; van Haren, M.A.; de Hon, F.D.; Kijlstra, A. Aqueus humor interleukin-6 levels in uveitis. Inv. Ophthalmol. Vis. Sci. 1990, 31, 917–920. [Google Scholar]
- Fend, F.; Ferreri, A.J.M.; Coupland, S. How we diagnose and treat vitreoretinal lymphoma. Br. J. Haematol. 2016, 173, 680–692. [Google Scholar] [CrossRef]
- Ponzoni, M. Look into my eyes, please. Blood 2015, 126, 4. [Google Scholar] [CrossRef][Green Version]
- Ngo, V.N.; Young, R.M.; Schmitz, R.; Jhavar, S.; Xiao, W.; Lim, K.H.; Kohlhammer, H.; Xu, W.; Yang, Y.; Zhao, H.; et al. Oncogenically active MYD88 mutations in human lymphoma. Nature 2011, 470, 115–121. [Google Scholar] [CrossRef]
- Deguine, J.; Barton, G.M. MYD88: A central player in innate immune signaling. F1000 Prim. Rep. 2014, 6, 97. [Google Scholar] [CrossRef] [PubMed]
- Raja, H.; Salomão, D.R.; Viswanatha, D.S.; Pulido, J.S. Prevalence of MYD88 L265P mutation in histologically proven, diffuse large B-cell vitreoretinal lymphoma. Retina 2016, 36, 624–628. [Google Scholar] [CrossRef]
- Narasimhan, S.; Joshi, M.; Parameswaran, S.; Rishi, P.; Khetan, V.; Ganesan, S.; Biswas, J.; Sundaram, N.; Sreenivasan, J.; Verma, S.; et al. MYD88 L265P mutation in intraocular lymphoma: A potential diagnostic marker. Indian J. Ophthalmol. 2020, 68, 2160–2165. [Google Scholar]
- de Groen, R.A.L.; Schrader, A.M.R.; Kersten, M.J.; Pals, S.T.; Vermaat, J.S.P. MYD88 in the driver’s seat of B-cell lymphomagenesis: From molecular mechanisms to clinical implications. Haematologica 2019, 104, 2337–2348. [Google Scholar] [CrossRef] [PubMed]
- Tsang, M.; Cleveland, J.; Rubenstein, J.L. On point in primary CNS lymphoma. Hematol. Oncol. 2020, 38, 640. [Google Scholar] [CrossRef]
- Montesinos-Rongen, M.; Godlewka, E.; Brunn, A.; Wiestler, O.D.; Siebert, R.; Deckert, M. Activating L265P mutations of the MYD88 gene are common in primary central nervous system lymphoma. Acta Neuropathol. 2011, 122, 791–792. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, A.; Pulido, J.S.; Mashayekhi, A.; Milman, T.; Deák, G.G. Diagnosing Vitreoretinal Lymphomas: An Analysis of the Sensitivity of Existing Tools. Cancers 2022, 14, 598. [Google Scholar] [CrossRef]
- Takhar, J.; Doan, T.; Gonzales, J.A. Vitreoretinal Lymphoma: A Literature Review and Introduction of a New Diagnostic. Method. Asia Pac. J. Ophthalmol. 2021, 10, 93–98. [Google Scholar] [CrossRef]
- Takase, H.; Arai, A.; Iwasaki, Y.; Imai, A.; Nagao, T.; Kawagishi, M.; Ishida, T.; Mochizuki, M. Challenges in the diagnosis and management of vitreoretinal lymphoma—Clinical and basic approaches. Prog. Retin. Eye Res. 2022, 90, 101053. [Google Scholar] [CrossRef]
- Davis, J.L.; Viciana, A.L.; Ruiz, P. Diagnosis of intraocular lymphoma by flow cytometry. Am. J. Ophthalmol. 1997, 124, 362–372. [Google Scholar] [CrossRef]
- Missotten, T.; Tielemans, D.; Bromberg, J.E.; van Hagen, P.M.; van Lochem, E.G.; van Dongen, J.J.M.; Baarsma, G.S.; Langerak, A.W. Multicolor flowcytometric immunophenotyping is a valuable tool for detection of intraocular lymphoma. Ophthalmology. 2013, 120, 991–996. [Google Scholar] [CrossRef]
- Tan, W.J.; Wang, M.M.; Ricciardi-Castagnoli, P.; Chan, A.S.Y.; Lim, T.S. Cytologic and molecular diagnostics for vitreoretinal lymphoma: Current approaches and emerging single-cell analyses. Front. Mol. Biosci. 2021, 7, 611017. [Google Scholar] [CrossRef] [PubMed]
- WHO Classification of Tumours Editorial Board. WHO Classification of Tumours Haematopoietic and Lymphoid Tissues, Revised, 4th ed.; WHO Classification of Tumours Editorial Board, Ed.; IARC: Lyon, France, 2017. [Google Scholar]
- Camilleri -Broet, S.; Criniere, E.; Broet, P.; Delwail, V.; Mokhtari, K.; Moreau, A.; Kujas, M.; Raphael, M.; Iraqi, W.; Sautes-Fridman, C.; et al. A uniform activated B-cell-like immunophenotype might explain the poor prognosis of primary central nervous system lymphomas: Analysis of 83 cases. Blood 2006, 107, 90–196. [Google Scholar] [CrossRef] [PubMed]
- Montesinos-Rongen, M.; Brunn, A.; Bentik, S.; Basso, K.; Lim, W.K.; Klapper, W.; Schaller, C.; Reifenberger, G.; Rubenstein, J.; Wiestler, O.D.; et al. Gene expression profiling suggests primary central nervous system lymphomas to be derived from a late germinal center B cell. Leukemia 2008, 22, 400–405. [Google Scholar] [CrossRef]
- Ho, K.G.; Grommes, G. Molecular profiling of primary central nervous system lymphomas: Predictive and prognostic value? Curr. Opin. Neurol. 2019, 32, 886–894. [Google Scholar] [CrossRef] [PubMed]
- Sung, C.O.; Kim, S.C.; Karnan, S.; Karube, K.; Shin, H.J.; Nam, D.H.; Suh, Y.L.; Kim, S.H.; Kim, J.Y.; Kim, S.J.; et al. Genomic profiling combined with gene expression profiling in primary central nervous system lymphoma. Blood 2011, 117, 1291–1300. [Google Scholar] [CrossRef]
- Chapuy, B.; Stewart, C.; Dunford, A.J.; Kim, J.; Kamburov, A.; Redd, R.A.; Lawrence, M.S.; Roemer, M.G.M.; Li, A.J.; Ziepert, M.; et al. Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nat. Med. 2018, 24, 1290–1292. [Google Scholar] [CrossRef]
- Schmitz, R.; Wright, G.W.; Huang, D.W.; Johnson, C.A.; Phelan, J.D.; Wang, J.Q.; Roulland, S.; Kasbekar, M.; Young, R.M.; Shaffer, A.L.; et al. Genetics and pathogenesis of diffuse large B-cell lymphoma. N. Engl. J. Med. 2018, 378, 1396–1407. [Google Scholar] [CrossRef]
- Gu, J.; Jiang, T.; Ping, B.; Li, R.; Chen, W.; Wang, L.; Huang, X.; Xu, G.; Chang, Q. Cell-free DNA sequencing of intraocular fluid as liquid biopsy in the diagnosis of vitreoretinal lymphoma. Front. Oncol. 2022, 12, 932674. [Google Scholar] [CrossRef]
- Tuo, J.; Shen, D.; Yang, H.H.; Chan, C.C. Distinct microRNA-155 expression in the vitreous of patients with primary vitreoretinal lymphoma and uveitis. Am. J. Ophthalmol. 2014, 157, 728–734. [Google Scholar] [CrossRef]
- Kakkassery, V.; Schroers, R.; Coupland, S.E.; Wunderlich, M.I.; Schargus, M.; Heinz, C.; Wasmuth, S.; Heiligenhaus, A.; Ahle, G.; Lenoble, P.; et al. Vitreous microRNA levels as diagnostic biomarkers for vitreoretinal lymphoma. Blood 2017, 129, 3130–3133. [Google Scholar] [CrossRef] [PubMed]
- Minezaki, T.; Usui, Y.; Asakage, M.; Takanashi, M.; Shimizu, H.; Nezu, N.; Narimatsu, A.; Tsubota, K.; Umazume, K.; Yamakawa, N.; et al. High-Throughput MicroRNA Profiling of Vitreoretinal Lymphoma: Vitreous and Serum MicroRNA Profiles Distinct from Uveitis. J. Clin. Med. 2020, 9, 1844. [Google Scholar] [CrossRef] [PubMed]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melli, B.; Gentile, P.; Nicoli, D.; Farnetti, E.; Croci, S.; Gozzi, F.; Bolletta, E.; De Simone, L.; Sanguedolce, F.; Palicelli, A.; et al. Primary Vitreoretinal Lymphoma: Current Diagnostic Laboratory Tests and New Emerging Molecular Tools. Curr. Oncol. 2022, 29, 6908-6921. https://doi.org/10.3390/curroncol29100543
Melli B, Gentile P, Nicoli D, Farnetti E, Croci S, Gozzi F, Bolletta E, De Simone L, Sanguedolce F, Palicelli A, et al. Primary Vitreoretinal Lymphoma: Current Diagnostic Laboratory Tests and New Emerging Molecular Tools. Current Oncology. 2022; 29(10):6908-6921. https://doi.org/10.3390/curroncol29100543
Chicago/Turabian StyleMelli, Beatrice, Pietro Gentile, Davide Nicoli, Enrico Farnetti, Stefania Croci, Fabrizio Gozzi, Elena Bolletta, Luca De Simone, Francesca Sanguedolce, Andrea Palicelli, and et al. 2022. "Primary Vitreoretinal Lymphoma: Current Diagnostic Laboratory Tests and New Emerging Molecular Tools" Current Oncology 29, no. 10: 6908-6921. https://doi.org/10.3390/curroncol29100543
APA StyleMelli, B., Gentile, P., Nicoli, D., Farnetti, E., Croci, S., Gozzi, F., Bolletta, E., De Simone, L., Sanguedolce, F., Palicelli, A., Zizzo, M., Ricci, S., Ilariucci, F., Rossi, C., Cavazza, A., Ascani, S., Cimino, L., & Zanelli, M. (2022). Primary Vitreoretinal Lymphoma: Current Diagnostic Laboratory Tests and New Emerging Molecular Tools. Current Oncology, 29(10), 6908-6921. https://doi.org/10.3390/curroncol29100543








