Decreased FOXO1 Expression Is Correlated with Poor Prognosis in Myelodysplastic Syndromes
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Real-Time Quantitative PCR
2.3. Cell Culture and Sorting
2.4. Subset and Polarization of T Lymphocytes
2.5. Immunohistochemistry (IHC)
2.6. Statistical Analysis
3. Results
3.1. Patient General Features
3.2. FOXO1 Was Differentially Expressed in MDS with Different Clinical Parameters
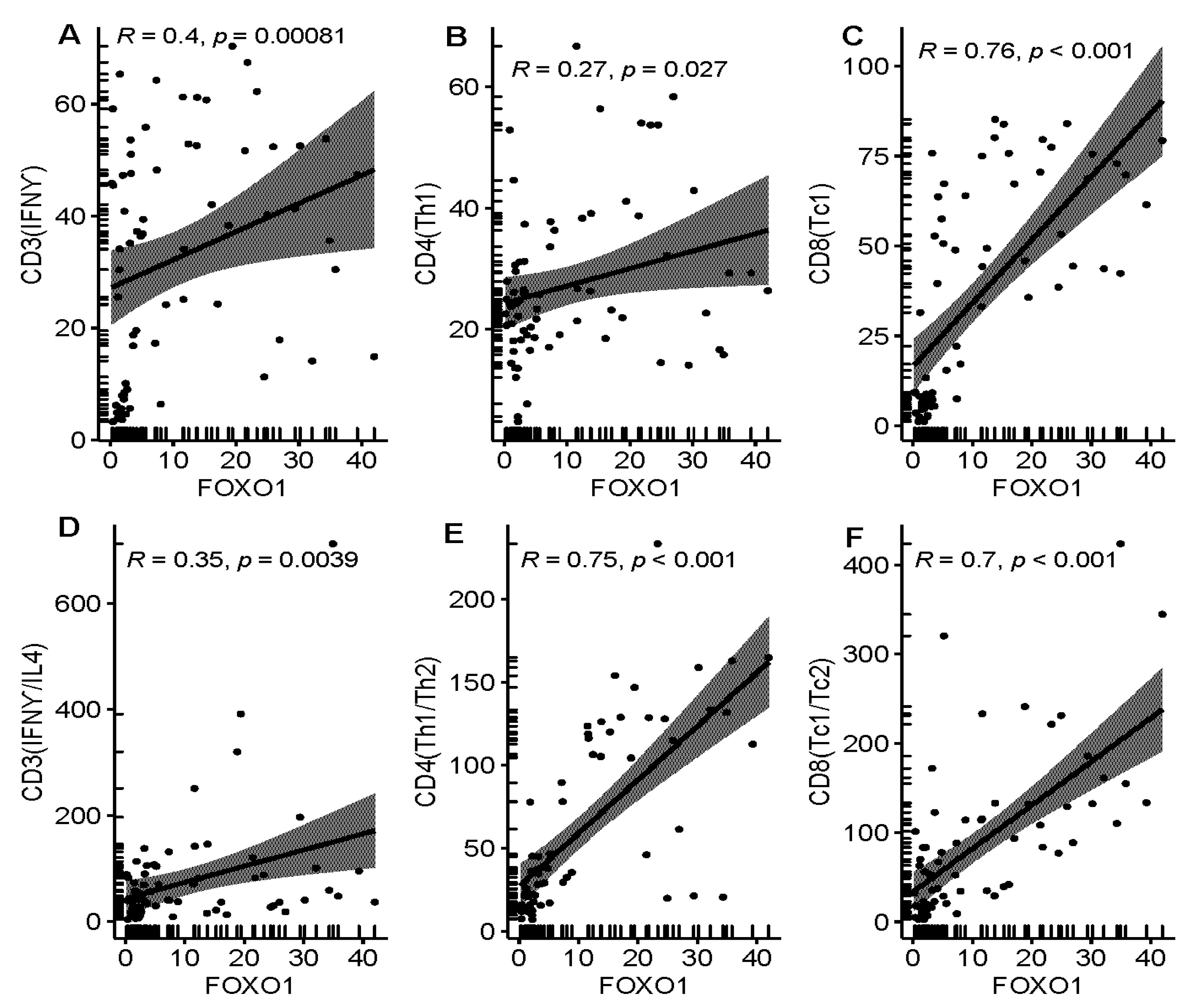
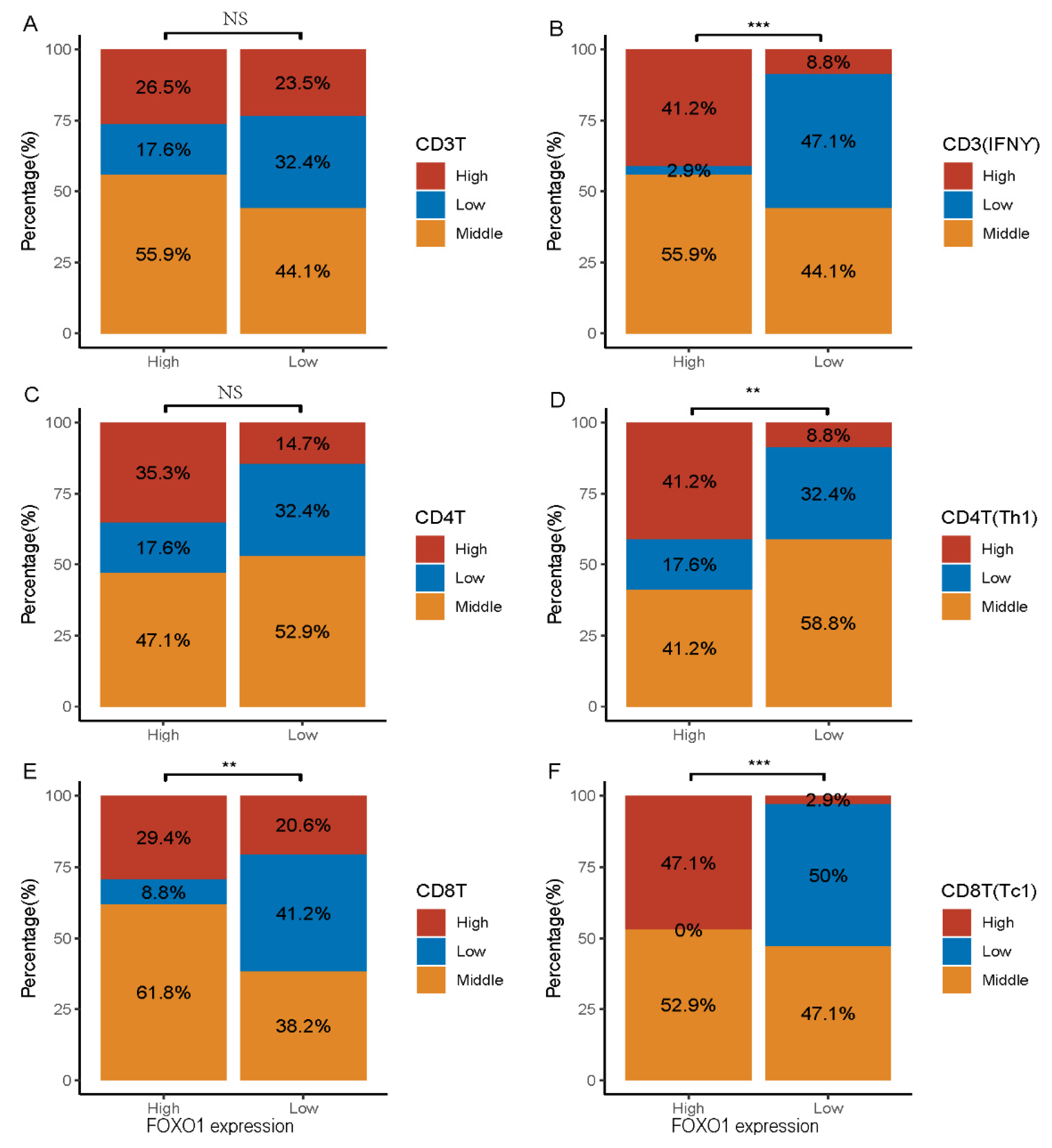
3.3. Relationship between FOXO1 Expression and Immunity Features in MDS Patients
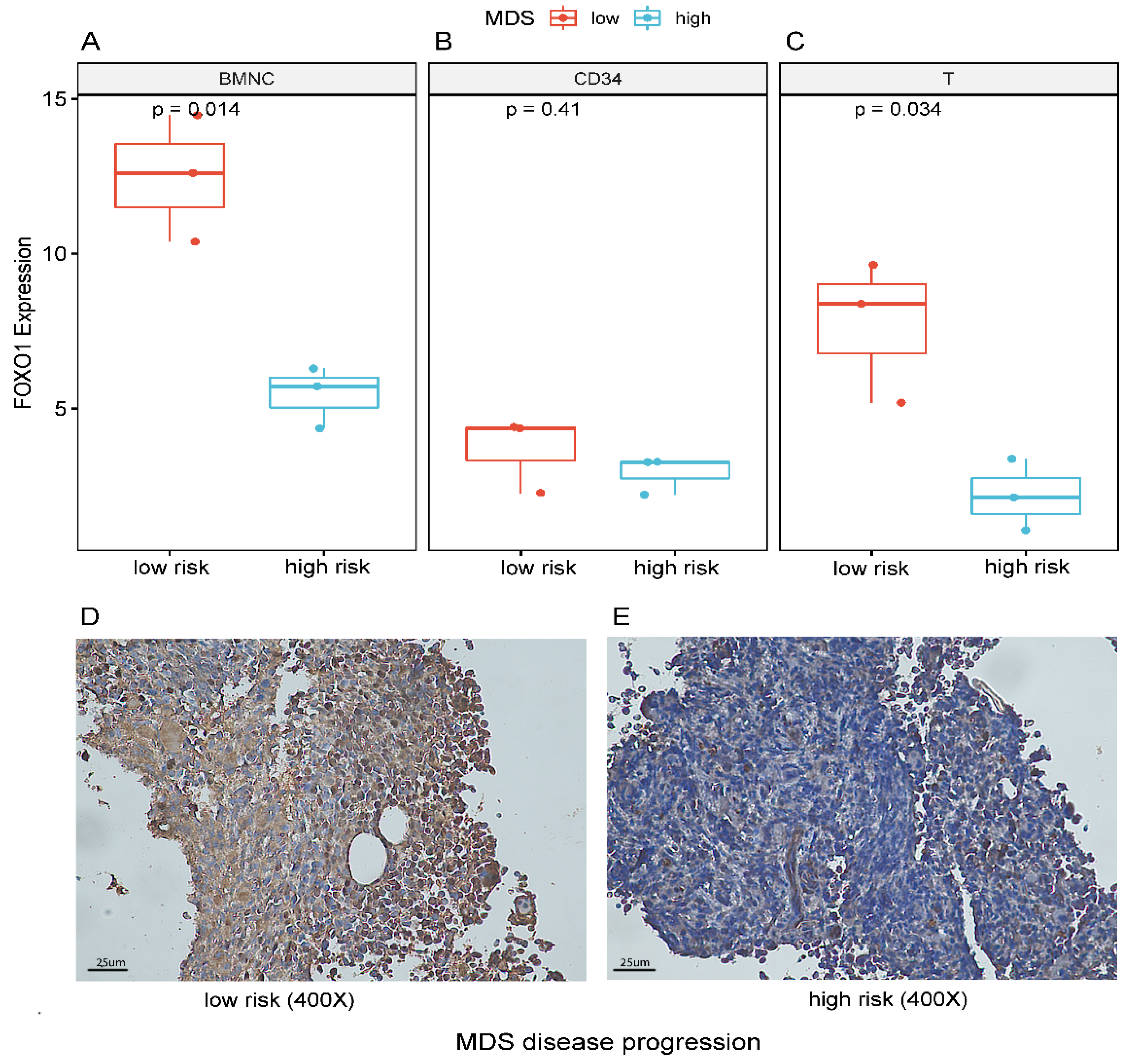
3.4. Decreased Expression of FOXO1 Correlates with Disease Progression
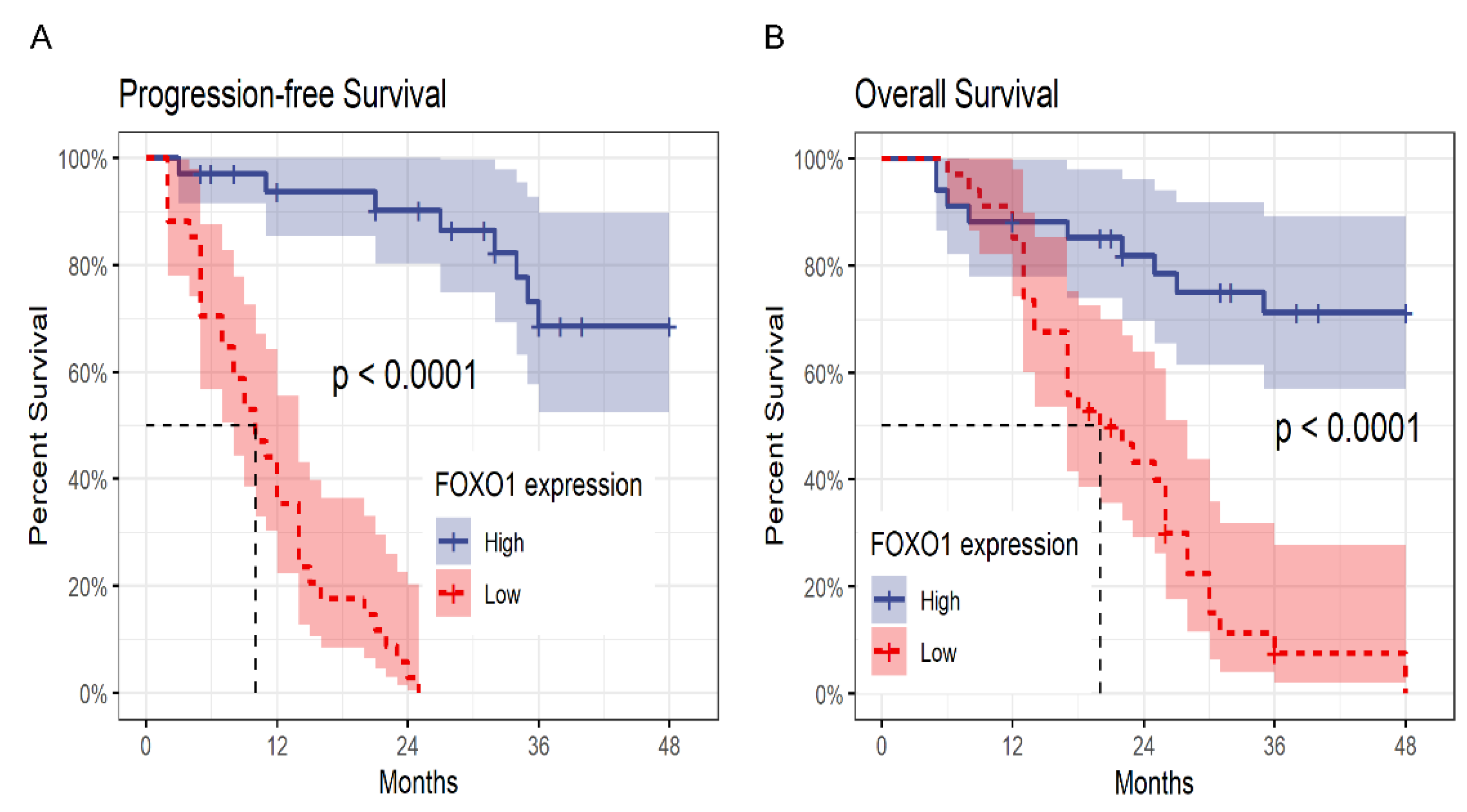
3.5. Low Expression of FOXO1 Correlates with Unfavorable Prognosis in MDS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cazzola, M. Myelodysplastic Syndromes. N. Engl. J. Med. 2020, 383, 1358–1374. [Google Scholar] [CrossRef] [PubMed]
- Saygin, C.; Carraway, H.E. Current and emerging strategies for management of myelodysplastic syndromes. Blood Rev. 2020, 48, 100791. [Google Scholar] [CrossRef] [PubMed]
- Platzbecker, U.; Kubasch, A.S.; Homer-Bouthiette, C.; Prebet, T. Current challenges and unmet medical needs in myelodysplastic syndromes. Leukemia 2021, 35, 2182–2198. [Google Scholar] [CrossRef] [PubMed]
- Chiereghin, C.; Travaglino, E.; Zampini, M.; Saba, E.; Saitta, C.; Riva, E.; Bersanelli, M.; Della Porta, M. The Genetics of Myelodysplastic Syndromes: Clinical Relevance. Genes 2021, 12, 1144. [Google Scholar] [CrossRef]
- Hosono, N. Genetic abnormalities and pathophysiology of MDS. Int. J. Clin. Oncol. 2019, 24, 885–892. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.; Lv, N.; Li, Y.; Wang, L.; Yu, L. Predictors of clinical responses to hypomethylating agents in acute myeloid leukemia or myelodys-plastic syndromes. Ann. Hematol. 2018, 97, 2025–2038. [Google Scholar] [CrossRef]
- Xing, Y.-Q.; Li, A.; Yang, Y.; Li, X.-X.; Zhang, L.-N.; Guo, H.-C. The regulation of FOXO1 and its role in disease progression. Life Sci. 2018, 193, 124–131. [Google Scholar] [CrossRef]
- Hou, T.; Li, Z.; Zhao, Y.; Zhu, W.-G. Mechanisms controlling the anti-neoplastic functions of FoxO proteins. Semin. Cancer Biol. 2017, 50, 101–114. [Google Scholar] [CrossRef]
- Tan, Y.-F.; Chen, Z.-Y.; Wang, L.; Wang, M.; Liu, X.-H. MiR-142-3p functions as an oncogene in prostate cancer by targeting FOXO1. J. Cancer 2020, 11, 1614–1624. [Google Scholar] [CrossRef]
- Luo, C.T.; Li, M.O. Foxo transcription factors in T cell biology and tumor immunity. Semin. Cancer Biol. 2018, 50, 13–20. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, F.; Hughes, T.; Yu, J. FOXOs in cancer immunity: Knowns and unknowns. Semin. Cancer Biol. 2018, 50, 53–64. [Google Scholar] [CrossRef]
- Hedrick, S.M.; Michelini, R.H.; Doedens, A.L.; Goldrath, A.W.; Stone, E.L. FOXO transcription factors throughout T cell biology. Nat. Rev. Immunol. 2012, 12, 649–661. [Google Scholar] [CrossRef] [PubMed]
- Ushmorov, A.; Wirth, T. FOXO in B-cell lymphopoiesis and B cell neoplasia. Semin. Cancer Biol. 2018, 50, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Li, T.; Yang, Z.; Hu, W.; Yang, Y. Deciphering the roles of FOXO1 in human neoplasms. Int. J. Cancer 2018, 143, 1560–1568. [Google Scholar] [CrossRef] [PubMed]
- Jiramongkol, Y.; Lam, E.W.-F. FOXO transcription factor family in cancer and metastasis. Cancer Metastasis Rev. 2020, 39, 681–709. [Google Scholar] [CrossRef]
- Shi, F.; Li, T.; Liu, Z.; Qu, K.; Shi, C.; Li, Y.; Qin, Q.; Cheng, L.; Jin, X.; Yu, T.; et al. FOXO1: Another avenue for treating digestive malignancy? Semin. Cancer Biol. 2018, 50, 124–131. [Google Scholar] [CrossRef]
- Pyrzynska, B.; Dwojak, M.; Zerrouqi, A.; Morlino, G.; Zapala, P.; Miazek, N.; Zagozdzon, A.; Bojarczuk, K.; Bobrowicz, M.; Siernicka, M.; et al. FOXO1 promotes resistance of non-Hodgkin lymphomas to anti-CD20-based therapy. OncoImmunology 2018, 7, e1423183. [Google Scholar] [CrossRef]
- Long, J.; Jia, M.-Y.; Fang, W.-Y.; Chen, X.-J.; Mu, L.-L.; Wang, Z.-Y.; Shen, Y.; Xiang, R.-F.; Wang, L.-N.; Wang, L.; et al. FLT3 inhibition upregulates HDAC8 via FOXO to inactivate p53 and promote maintenance of FLT3-ITD+ acute myeloid leukemia. Blood 2020, 135, 1472–1483. [Google Scholar] [CrossRef]
- Zhang, Z.; Jia, Y.; Xv, F.; Song, L.-X.; Shi, L.; Guo, J.; Chang, C.-K. Decitabine Induces Change of Biological Traits in Myelodysplastic Syndromes via FOXO1 Activation. Front. Genet. 2021, 11, 1712. [Google Scholar] [CrossRef]
- Vardiman, J.W.; Thiele, J.; Arber, D.A.; Brunning, R.D.; Borowitz, M.J.; Porwit, A.; Harris, N.L.; Le Beau, M.M.; Hellström-Lindberg, E.; Tefferi, A.; et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: Rationale and important changes. Blood 2009, 114, 937–951. [Google Scholar] [CrossRef]
- Greenberg, P.L.; Tuechler, H.; Schanz, J.; Sanz, G.; Garcia-Manero, G.; Solé, F.; Bennett, J.M.; Bowen, D.; Fenaux, P.; Dreyfus, F.; et al. Revised International Prognostic Scoring System for Myelodysplastic Syndromes. Blood 2012, 120, 2454–2465. [Google Scholar] [CrossRef] [PubMed]
- Tefferi, A.; Cervantes, F.; Mesa, R.; Passamonti, F.; Verstovsek, S.; Vannucchi, A.M.; Gotlib, J.; Dupriez, B.; Pardanani, A.; Harrison, C.; et al. Revised response criteria for myelofibrosis: International Working Group-Myeloproliferative Neoplasms Research and Treatment (IWG-MRT) and European LeukemiaNet (ELN) consensus report. Blood 2013, 122, 1395–1398. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chang, C.K.; He, Q.; Guo, J.; Tao, Y.; Wu, L.Y.; Xu, F.; Wu, D.; Zhou, L.Y.; Su, J.Y.; et al. Increased PD-1/STAT1 ratio may account for the survival benefit in decitabine therapy for lower risk myelodysplastic syndrome. Leuk. Lymphoma 2017, 58, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Shi, A.; Zou, Y.; Sun, M.; Zhan, Y.; Dong, Y.; Fan, Z. EZH2-Mediated microRNA-375 Upregulation Promotes Progression of Breast Cancer via the Inhibition of FOXO1 and the p53 Signaling Pathway. Front. Genet. 2021, 12, 633756. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Pan, Y.; Zheng, L.; Choe, C.; Lindgren, B.; Jensen, E.D.; Westendorf, J.J.; Cheng, L.; Huang, H. FOXO1 Inhibits Runx2 Transcriptional Activity and Prostate Cancer Cell Migration and Invasion. Cancer Res. 2011, 71, 3257–3267. [Google Scholar] [CrossRef]
- Chen, Y.H.; Li, C.L.; Chen, W.J.; Liu, J.; Wu, H.T. Diverse roles of FOXO family members in gastric cancer. World J. Gastrointest. Oncol. 2021, 13, 1367–1382. [Google Scholar] [CrossRef]
- Köhrer, S.; Havranek, O.; Seyfried, F.; Hurtz, C.; Coffey, G.P.; Kim, E.; Ten Hacken, E.; Jäger, U.; Vanura, K.; O’Brien, S.; et al. Pre-BCR signaling in precursor B-cell acute lympho-blastic leukemia regulates PI3K/AKT, FOXO1 and MYC, and can be targeted by SYK inhibition. Leukemia 2016, 30, 1246–1254. [Google Scholar] [CrossRef]
- Palacios, F.; Prieto, D.; Abreu, C.; Ruiz, S.; Morande, P.; Fernández-Calero, T.; Libisch, G.; Landoni, A.I.; Oppezzo, P. Dissecting chronic lymphocytic leukemia microenvironment signals in patients with unmutated disease: microRNA-22 regulates phosphatase and tensin homolog/AKT/FOXO1 pathway in proliferative leukemic cells. Leuk. Lymphoma 2015, 56, 1560–1565. [Google Scholar] [CrossRef]
- Pellicano, F.; Scott, M.T.; Helgason, G.V.; Hopcroft, L.E.M.; Allan, E.K.; Aspinall-O’Dea, M.; Copland, M.; Pierce, A.; Huntly, B.J.P.; Whetton, A.D.; et al. The Antiproliferative Activity of Kinase Inhibitors in Chronic Myeloid Leukemia Cells Is Mediated by FOXO Transcription Factors. Stem Cells 2014, 32, 2324–2337. [Google Scholar] [CrossRef]
- Trowbridge, J.J.; Starczynowski, D.T. Innate immune pathways and inflammation in hematopoietic aging, clonal hematopoiesis, and MDS. J. Exp. Med. 2021, 218, e20201544. [Google Scholar] [CrossRef]
- Matos, A.; Magalhães, S.M.M.; Rauh, M.J. Immune Dysregulation and Recurring Mutations in Myelodysplastic Syndromes Pathogenesis. Adv. Exp. Med. Biol. 2021, 1326, 1–10. [Google Scholar] [PubMed]
- Barreyro, L.; Chlon, T.; Starczynowski, D.T. Chronic immune response dysregulation in MDS pathogenesis. Blood 2018, 132, 1553–1560. [Google Scholar] [CrossRef] [PubMed]
- Newton, R.H.; Shrestha, S.; Sullivan, J.M.; Yates, K.B.; Compeer, E.B.; Ron-Harel, N.; Blazar, B.R.; Bensinger, S.J.; Haining, W.N.; Dustin, M.L.; et al. Maintenance of CD4 T cell fitness through regulation of Foxo1. Nat. Immunol. 2018, 19, 838–848. [Google Scholar] [CrossRef] [PubMed]
- Delpoux, A.; Michelini, R.H.; Verma, S.; Lai, C.-Y.; Omilusik, K.D.; Utzschneider, D.T.; Redwood, A.J.; Goldrath, A.W.; Benedict, C.A.; Hedrick, S.M. Continuous activity of Foxo1 is required to prevent anergy and maintain the memory state of CD8+ T cells. J. Exp. Med. 2017, 215, 575–594. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Ortega, A.A.; Feinberg, D.; Liang, Y.; Rossa, J.C.; Graves, D.T. The Role of Forkhead Box 1 (FOXO1) in the Immune System: Dendritic Cells, T Cells, B Cells, and Hematopoietic Stem Cells. Crit. Rev. Immunol. 2017, 37, 1–13. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | n = 68 1 |
|---|---|
| Age(years) | |
| old (≥60) | 48 (71%) |
| young (<60) | 20 (29%) |
| Gender | |
| female | 25 (37%) |
| male | 43 (63%) |
| Neu (×109/L) | |
| ≥0.8 | 37 (54%) |
| <0.8 | 31 (46%) |
| Hb(g/L) | |
| <80 | 45 (66%) |
| 80~<100 | 11 (16%) |
| ≥100 | 12 (18%) |
| Plt (×109/L) | |
| <50 | 37 (54%) |
| 50~<100 | 14 (21%) |
| ≥100 | 17 (25%) |
| Blast (%) | |
| ≤2 | 24 (35%) |
| 2~5 | 11 (16%) |
| 5~10 | 22 (32%) |
| >10 | 11 (16%) |
| Chromosome * | |
| good, very good | 50 (74%) |
| intermediate | 13 (19%) |
| poor, very poor | 5 (7.4%) |
| WHO (2016) | |
| SLD, MLD, RS-MLD, 5q- | 33 (49%) |
| EB-1 | 24 (35%) |
| EB-2 | 11 (16%) |
| WPSS | |
| <3 | 30 (44%) |
| ≥3 | 38 (56%) |
| IPSSR | |
| ≤1.5 | 1 (1.5%) |
| >1.5~3 | 15 (22%) |
| >3~4.5 | 19 (28%) |
| >4.5~6 | 22 (32%) |
| >6 | 11 (16%) |
| Gene mutation | |
| <3 | 46 (71%) |
| ≥3 | 19 (29%) |
| NA | 3 |
| Treatment | |
| cytokine | 22 (32%) |
| immunosuppressive therapy | 5 (8%) |
| lenalidomide | 7 (10%) |
| hypomethylating agent | 34 (50%) |
| Characteristic | High(N = 34) 1 | Low(N = 34) 1 | p-Value 2 |
|---|---|---|---|
| Age | 0.033 | ||
| Old (≥60) | 20 (59%) | 28 (82%) | |
| Young (<60) | 14 (41%) | 6 (18%) | |
| Gender | 0.8 | ||
| Female | 12 (35%) | 13 (38%) | |
| Male | 22 (65%) | 21 (62%) | |
| Blast (%) | <0.001 | ||
| ≤2 | 22 (65%) | 2 (5.9%) | |
| >2~<5 | 3 (8.8%) | 8 (24%) | |
| 5~10 | 7 (21%) | 15 (44%) | |
| >10 | 2 (5.9%) | 9 (26%) | |
| Chromosome | 0.022 | ||
| good | 30 (88%) | 20 (59%) | |
| inter | 3 (8.8%) | 10 (29%) | |
| poor | 1 (2.9%) | 4 (12%) | |
| WHO (2016) | <0.001 | ||
| SLD, MLD, RS-MLD, 5q- | 25 (74%) | 8 (24%) | |
| EB-1 | 7 (21%) | 17 (50%) | |
| EB-2 | 2 (5.9%) | 9 (26%) | |
| IPSSR | <0.001 | ||
| ≤1.5 | 1 (2.9%) | 0 (0%) | |
| >1.5~3 | 15 (44%) | 0 (0%) | |
| >3~4.5 | 11 (32%) | 8 (24%) | |
| >4.5~6 | 5 (15%) | 17 (50%) | |
| >6 | 2 (5.9%) | 9 (26%) | |
| WPSS | <0.001 | ||
| <3 | 26 (76%) | 4 (12%) | |
| ≥3 | 8 (24%) | 30 (88%) | |
| Gene mutation | 0.047 | ||
| <3 | 27 (82%) | 19 (59%) | |
| ≥3 | 6 (18%) | 13 (41%) | |
| NA | 1 | 2 |
| Variable | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| p | 95% Confidence Interval | p | 95% Confidence Interval | |
| FOXO1 | ||||
| Low expression | <0.001 | 9.66 (4.33–21.57) | 0.001 | 5.24 (2.00–13.78) |
| Blast | ||||
| >2~5 | 0.006 | 3.85 (1.47–10.07) | 0.519 | 1.79 (0.31–10.45) |
| >5~10 | <0.001 | 7.16 (3.21–15.98) | 0.180 | 3.88 (0.53–28.13) |
| >10 | <0.001 | 16.16 (5.95–43.87) | 0.047 | 1.55 (1.05–41.45] |
| Chromosome | ||||
| poor | <0.001 | 9.11 (3.08–26.99) | 0.002 | 6.61 (1.99–21.97) |
| WHO | ||||
| EB-1 | <0.001 | 4.30 (2.23–8.29) | 0.75 | 0.714 (0.16–3.45) |
| EB-2 | <0.001 | 9.91 (4.17–23.95) | NA | NA |
| IPSSR | ||||
| >3.5 | <0.001 | 5.77 (2.52–13.19) | 0.720 | 1.34 (0.27–6.65) |
| WPSS | ||||
| ≥3 | <0.001 | 5.07 (2.63–9.76) | 0.923 | 1.06 (0.34–3.26) |
| Variable | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| p | 95% Confidence Interval | p | 95% Confidence Interval | |
| FOXO1 | ||||
| Low expression | <0.001 | 8.37 (3.53–19.85) | 0.029 | 4.17 (1.16–15.06) |
| Blast | ||||
| >2~5 | 0.015 | 4.15 (1.31–13.15) | 0.798 | 1.25 (0.22– 6.97) |
| >5~10 | 0.001 | 5.50 (1.97–15.37) | 0.219 | 4.14 (0.43–39.85) |
| >10 | <0.001 | 12.04 (4.13–35.12) | 0.294 | 2.38 (0.47–12.03) |
| Chromosome | ||||
| intermediate | 0.018 | 2.45 (1.17–5.15) | 0.356 | 1.59 (0.59–4.28) |
| poor | 0.004 | 4.50 (1.64–12.37) | 0.039 | 3.29 (1.67–7.88) |
| WHO | ||||
| EB-1 | 0.013 | 2.70 (1.24–5.91) | 0.296 | 0.36 (0.05–2.46) |
| EB-2 | <0.001 | 6.62 (2.83–15.44) | NA | NA |
| IPSSR | ||||
| >3.5 | <0.001 | 8.33 (2.53–27.44) | 0.938 | 1.08 (0.15–8.06) |
| WPSS | ||||
| ≥3 | <0.001 | 8.05 (3.12–20.79) | 0.021 | 4.26 (1.50–10.11) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Huang, N.; Xv, F.; Zhao, S.; Guo, J.; Zhao, Y.; Chang, C. Decreased FOXO1 Expression Is Correlated with Poor Prognosis in Myelodysplastic Syndromes. Curr. Oncol. 2022, 29, 6933-6946. https://doi.org/10.3390/curroncol29100545
Zhang Z, Huang N, Xv F, Zhao S, Guo J, Zhao Y, Chang C. Decreased FOXO1 Expression Is Correlated with Poor Prognosis in Myelodysplastic Syndromes. Current Oncology. 2022; 29(10):6933-6946. https://doi.org/10.3390/curroncol29100545
Chicago/Turabian StyleZhang, Zheng, Nanfang Huang, Feng Xv, Sida Zhao, Juan Guo, Youshan Zhao, and Chunkang Chang. 2022. "Decreased FOXO1 Expression Is Correlated with Poor Prognosis in Myelodysplastic Syndromes" Current Oncology 29, no. 10: 6933-6946. https://doi.org/10.3390/curroncol29100545
APA StyleZhang, Z., Huang, N., Xv, F., Zhao, S., Guo, J., Zhao, Y., & Chang, C. (2022). Decreased FOXO1 Expression Is Correlated with Poor Prognosis in Myelodysplastic Syndromes. Current Oncology, 29(10), 6933-6946. https://doi.org/10.3390/curroncol29100545





