Oppositely Charged Pickering Emulsion Co-Stabilized by Chitin Nanoparticles and Fucoidan: Influence of Environmental Stresses on Stability and Antioxidant Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Pickering Emulsion
2.2. Evaluation of the Pickering Emulsion Stability under Different Environmental Conditions
2.3. Particle Size and ζ-Potential
2.4. Confocal Laser Scanning Microscopy (CLSM)
2.5. Cryo-Scanning Electron Microscopy (Cryo-SEM)
2.6. Physical Stability of the Pickering Emulsion
2.7. Creaming Index (CI) of the Pickering Emulsion
2.8. Chemical Stability of the Pickering Emulsion
2.9. Storage Stability of the Pickering Emulsion
2.10. Antioxidant Activities of the Pickering Emulsion
2.11. Statistical Analysis
3. Results and Discussions
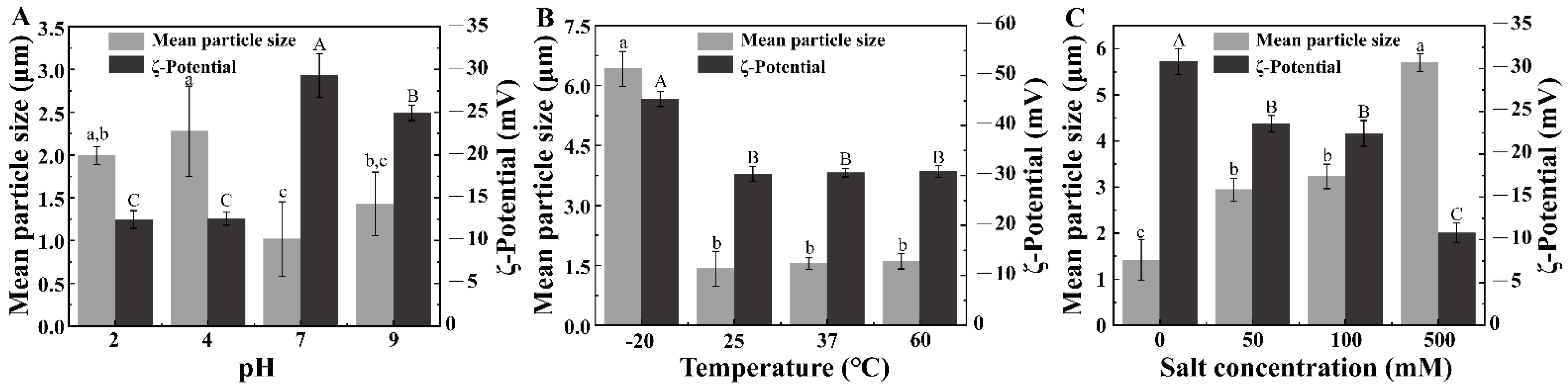
3.1. Mean Particle Size and ζ-Potential of the Pickering Emulsion
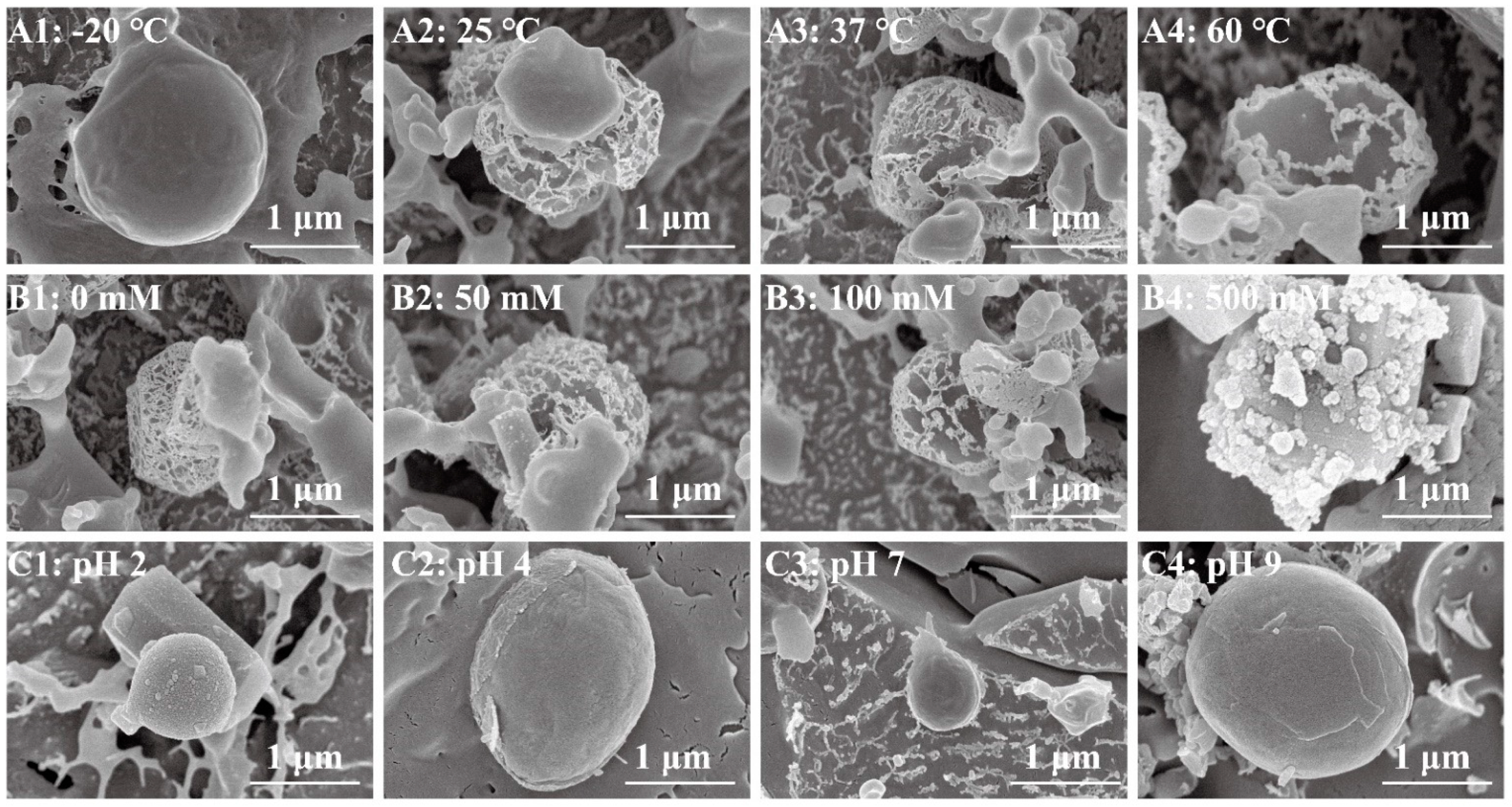
3.2. Microstructure of the Pickering Emulsion
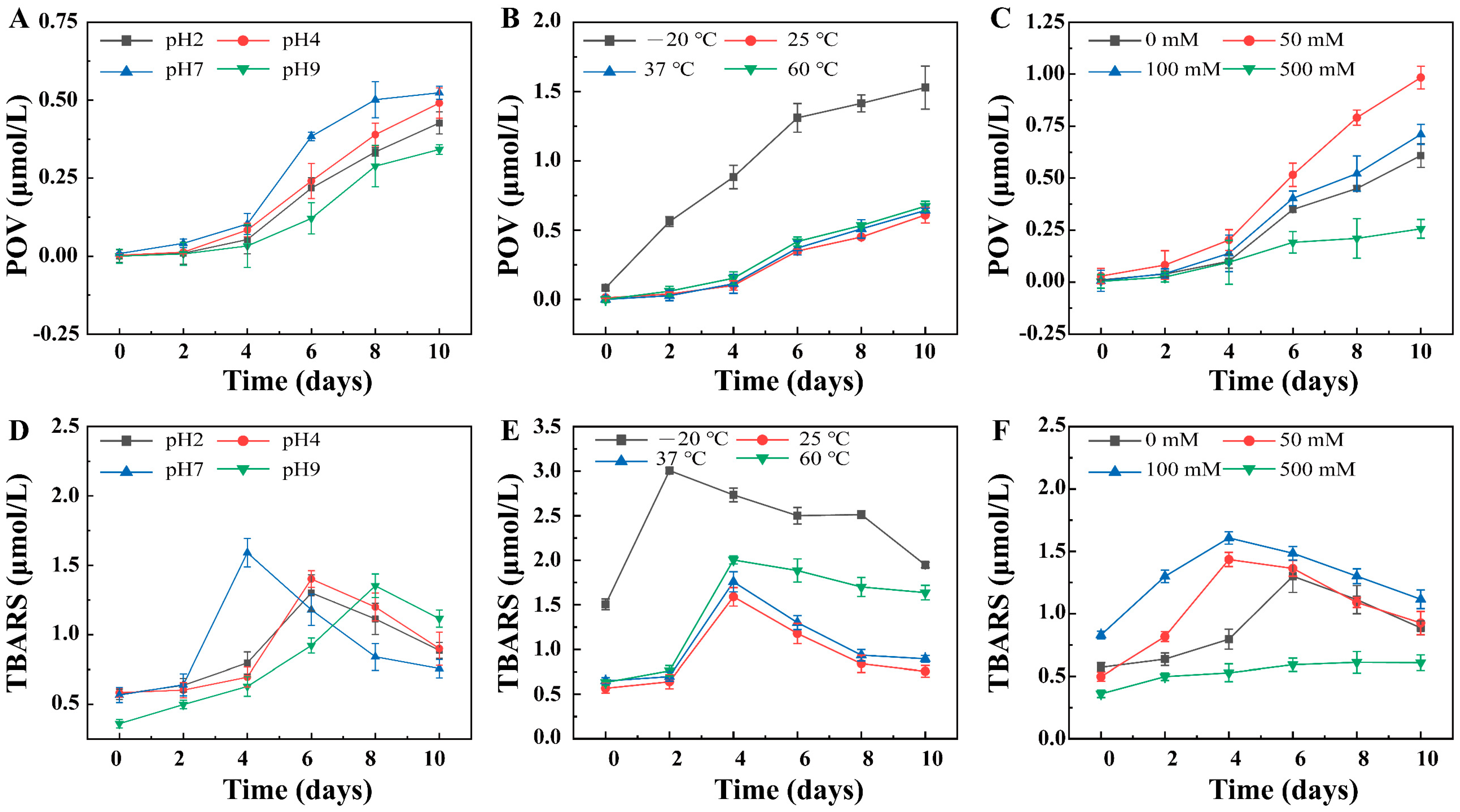
3.3. Chemical Stability of the Pickering Emulsion
3.4. Physical Stability of the Pickering Emulsion
3.5. Antioxidant Activities of the Pickering Emulsion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Binks, B.P. Particles as surfactants—similarities and differences. Curr. Opin. Colloid Interface Sci. 2002, 7, 21–41. [Google Scholar] [CrossRef]
- Wei, Y.; Sun, C.; Dai, L.; Mao, L.; Yuan, F.; Gao, Y. Novel bilayer emulsions costabilized by zein colloidal particles and propylene glycol alginate. 2. Influence of environmental stresses on stability and rheological properties. J. Agric. Food Chem. 2018, 67, 1209–1221. [Google Scholar] [CrossRef] [PubMed]
- Ramsden, W. Separation of solids in the surface-layers of solutions and ‘suspensions’(observations on surface-membranes, bubbles, emulsions, and mechanical coagulation)—Preliminary account. Proc. R. Soc. Lond. 1904, 72, 156–164. [Google Scholar]
- Pickering, S.U. Cxcvi.—emulsions. J. Chem. Society. Trans. 1907, 91, 2001–2021. [Google Scholar] [CrossRef] [Green Version]
- Frelichowska, J.; Bolzinger, M.A.; Pelletier, J.; Valour, J.P.; Chevalier, Y. Topical delivery of lipophilic drugs from o/w Pickering emulsions. Int. J. Pharm. 2009, 371, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Low, L.E.; Sangeetaprivya, P.S.; Yong, K.H.; Eng, S.C.; Beng, T.T. Recent advances of characterization techniques for the formation, physical properties, and stability of Pickering emulsion. Adv. Colloid Interface Sci. 2020, 277, 102117. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Quinlan, P.J.; Tam, K.C. Stimuli-responsive Pickering emulsions: Recent advances and potential applications. Soft Matter 2015, 11, 3512–3529. [Google Scholar] [CrossRef]
- Tzoumaki, M.V.; Moschakis, T.; Kiosseoglou, V.; Biliaderis, C.G. Oil-in-water emulsions stabilized by chitin nanocrystal particles. Food Hydrocoll. 2011, 25, 1521–1529. [Google Scholar] [CrossRef]
- Cho, M.S.; Oh, S.G. Size effect of carboxymethyl chitin nanocrystals on the properties of foams in aqueous surfactant solutions. Colloid Surf. A 2020, 604, 1253062020. [Google Scholar] [CrossRef]
- Jiménez-Saelices, C.; Trongsatitkul, T.; Lourdin, D.; Capron, I. Chitin Pickering emulsion for oil inclusion in composite films. Carbohyd. Polym. 2020, 242, 116366. [Google Scholar] [CrossRef]
- Chang, Y.; McClements, D.J. Interfacial deposition of an anionic polysaccharide (fucoidan) on protein-coated lipid droplets: Impact on the stability of fish oil-in-water emulsions. Food Hydrocoll. 2015, 51, 252–260. [Google Scholar] [CrossRef]
- Kim, D.Y.; Shin, W.S.; Hong, W.S. The unique behaviors of biopolymers, BSA and fucoidan, in a model emulsion system under different pH circumstances. Macromol. Res. 2010, 18, 1103–1108. [Google Scholar] [CrossRef]
- Huang, Y.C.; Chen, J.K.; Lam, U.L.; Chen, S.Y. Preparing, characterizing, and evaluating chitosan/fucoidan nanoparticles as oral delivery carriers. J. Polym. Res. 2014, 21, 415. [Google Scholar] [CrossRef]
- Huang, Y.C.; Li, R.Y. Preparation, and characterization of antioxidant nanoparticles composed of chitosan and fucoidan for antibiotics delivery. Mar. Drugs 2014, 12, 4379–4398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tambe, D.E.; Sharma, M.M. The effect of colloidal particles on fluid-fluid interfacial properties and emulsion stability. Adv. Colloid Interface Sci. 1994, 52, 1–63. [Google Scholar] [CrossRef]
- Bouyer, E.; Mekhloufi, G.; Rosilio, V.; Grossiord, J.L.; Agnely, F. Proteins, polysaccharides, and their complexes used as stabilizers for emulsions: Alternatives to synthetic surfactants in the pharmaceutical field? Int. J. Pharm. 2012, 436, 359–378. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Heuzey, M.C. Pickering emulsion gels based on insoluble chitosan/gelatin electrostatic complexes. RSC Adv. 2016, 6, 89776–89784. [Google Scholar] [CrossRef]
- Doost, A.S.; Nasrabadi, M.N.; Kassozi, V.; Dewettinck, K.; Stevens, C.V.; Van der Meeren, P. Pickering stabilization of thymol through green emulsification using soluble fraction of almond gum–Whey protein isolate nano-complexes. Food Hydrocoll. 2018, 88, 218–227. [Google Scholar] [CrossRef] [Green Version]
- Akhtar, G.; Masoodi, F. Structuring functional mayonnaise incorporated with Himalayan walnut oil Pickering emulsions by ultrasound assisted emulsification. Ultrason. Sonochem. 2022, 86, 106022. [Google Scholar] [CrossRef]
- Li, K.-Y.; Zhang, X.-R.; Huang, G.-Q.; Teng, J.; Guo, L.-P.; Li, X.-D.; Xiao, J.-X. Complexation between ovalbumin and gum Arabic in high total biopolymer concentrations and the emulsifying ability of the complexes. Colloids Surf. A Physicochem. Eng. Asp. 2022, 642, 128624. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, M.; Zhang, S.; Jiang, L.; Xie, F.; Li, Y. Oil-in-water Pickering emulsion stabilization with oppositely charged polysaccharide particles: Chitin nanocrystals/fucoidan complexes. J. Sci. Food Agric. 2021, 101, 3003–3012. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Gonzalez, A.J.P.; Huang, Q. Kafirin nanoparticles-stabilized Pickering emulsions: Microstructure and rheological behavior. Food Hydrocoll. 2016, 54, 30–39. [Google Scholar] [CrossRef]
- Hu, M.; Xie, F.; Zhang, S.; Li, Y.; Qi, B. Homogenization pressure and soybean protein concentration impact the stability of perilla oil nanoemulsions. Food Hydrocoll. 2020, 101, 105575. [Google Scholar] [CrossRef]
- Villiere, A.; Viau, M.; Bronnec, I.; Moreau, N.; Genot, C. Oxidative stability of bovine serum albumin-and sodium caseinate-stabilized emulsions depends on metal availability. J. Agric. Food Chem. 2005, 53, 1514–1520. [Google Scholar] [CrossRef]
- Mcdonald, R.E.; Hultin, H.O. Some characteristics of the enzymic lipid peroxidation system in the microsomal fraction of flounder skeletal muscle. J. Food Sci. 1987, 52, 15–21. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, X.; Ye, Y.; Wang, M.; Wang, J. Emulsifying properties of wheat germ: Influence of pH and NaCl. Food Hydrocoll. 2020, 100, 105431. [Google Scholar] [CrossRef]
- Mwangi, W.W.; Ho, K.W.; Tey, B.T.; Chan, E.S. Effects of environmental factors on the physical stability of pickering-emulsions stabilized by chitosan particles. Food Hydrocoll. 2016, 60, 543–550. [Google Scholar] [CrossRef]
- Aoki, T.; Decker, E.A.; Mc Clements, D.J. Influence of environmental stresses on stability of O/W emulsions containing droplets stabilized by multilayered membranes produced by a layer-by-layer electrostatic deposition technique. Food Hydrocoll. 2005, 19, 209–220. [Google Scholar] [CrossRef]
- Li, Y.; Zhong, M.; Xie, F.; Sun, Y.; Zhang, S.; Qi, B. The effect of pH on the stabilization and digestive characteristics of soybean lipophilic protein oil-in-water emulsions with Hypromellose. Food Chem. 2020, 309, 1255792020. [Google Scholar] [CrossRef]
- Makeri, M.; Muhammad, K.; Ghazali, H.; Mohammed, A. Influence of temperature and ionic conditions on the rheology and droplets characteristics of winged bean protein stabilized oil-in-water emulsion. J. Food Meas. Charact. 2019, 13, 97–106. [Google Scholar] [CrossRef]
- Liu, G.; Li, W.; Qin, X.; Zhong, Q. Pickering emulsions stabilized by amphiphilic anisotropic nanofibrils of glycated whey proteins. Food Hydrocoll. 2020, 101, 105503. [Google Scholar] [CrossRef]
- Cao, X.; Xiong, C.; Zhao, X.; Yang, S.; Wen, Q.; Tang, H.; Zeng, Q.; Feng, Y.; Li, J. Tuning self-assembly of amphiphilic sodium alginate-decorated selenium nanoparticle surfactants for antioxidant Pickering emulsion. Int. J. Biol. Macromol. 2022, 210, 600–613. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, M.; Du, X.; Liu, G.; Huang, Y.; Liu, Z.; Sun, S.; Li, Y. Oppositely Charged Pickering Emulsion Co-Stabilized by Chitin Nanoparticles and Fucoidan: Influence of Environmental Stresses on Stability and Antioxidant Activity. Foods 2022, 11, 1835. https://doi.org/10.3390/foods11131835
Hu M, Du X, Liu G, Huang Y, Liu Z, Sun S, Li Y. Oppositely Charged Pickering Emulsion Co-Stabilized by Chitin Nanoparticles and Fucoidan: Influence of Environmental Stresses on Stability and Antioxidant Activity. Foods. 2022; 11(13):1835. https://doi.org/10.3390/foods11131835
Chicago/Turabian StyleHu, Miao, Xiaoqian Du, Guannan Liu, Yuyang Huang, Zhao Liu, Shukun Sun, and Yang Li. 2022. "Oppositely Charged Pickering Emulsion Co-Stabilized by Chitin Nanoparticles and Fucoidan: Influence of Environmental Stresses on Stability and Antioxidant Activity" Foods 11, no. 13: 1835. https://doi.org/10.3390/foods11131835
APA StyleHu, M., Du, X., Liu, G., Huang, Y., Liu, Z., Sun, S., & Li, Y. (2022). Oppositely Charged Pickering Emulsion Co-Stabilized by Chitin Nanoparticles and Fucoidan: Influence of Environmental Stresses on Stability and Antioxidant Activity. Foods, 11(13), 1835. https://doi.org/10.3390/foods11131835




