Structure Characterization, Antioxidant and Immunomodulatory Activities of Polysaccharide from Pteridium aquilinum (L.) Kuhn
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
2.2. Polysaccharide Preparation from P. aquilinum
2.3. General Analysis of Purified Polysaccharide PAP-3
2.4. Determination of Monosaccharide Composition
2.5. Partial Acid Hydrolysis
2.6. Periodate Oxidation and Smith Degradation
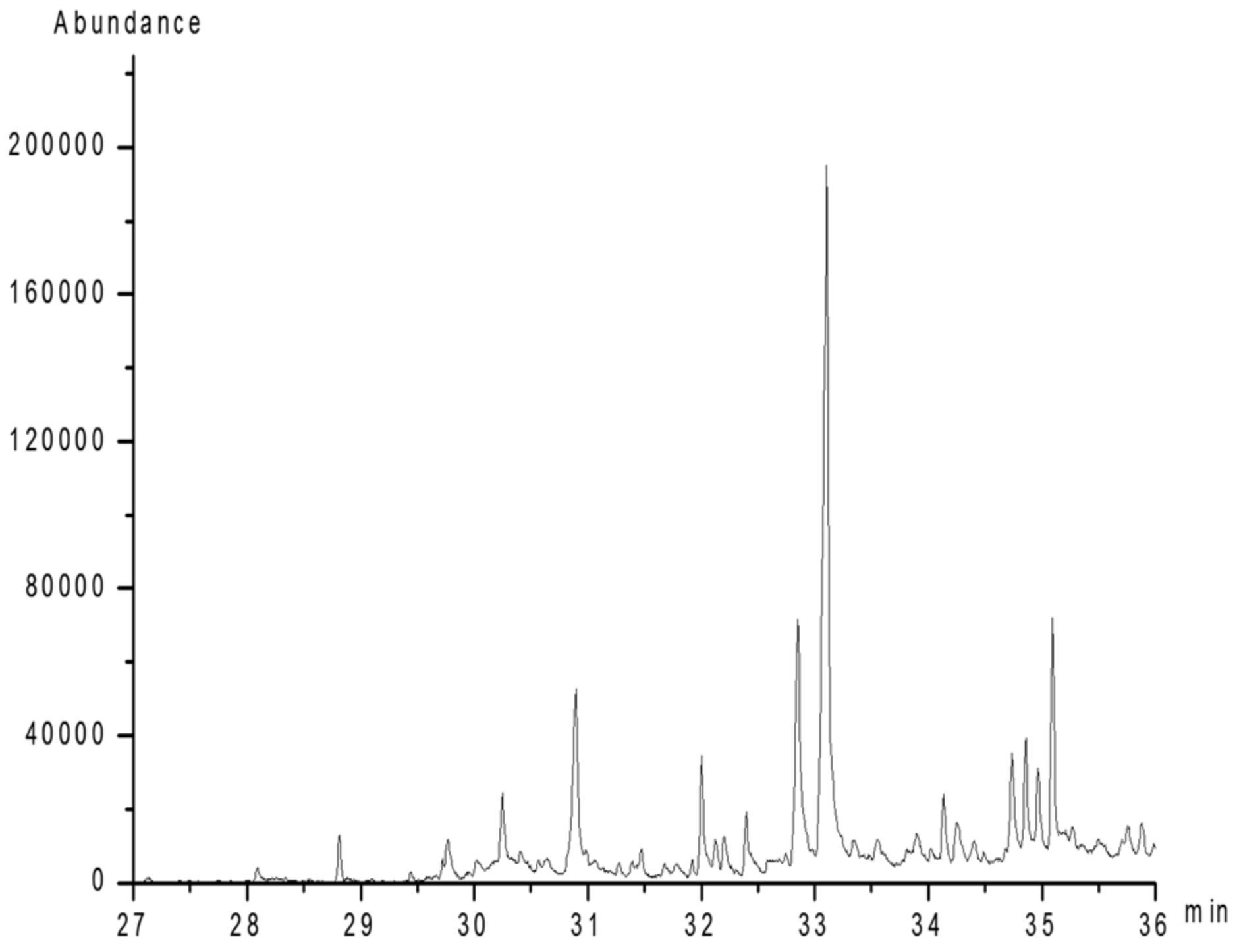
2.7. Methylation Analysis
2.8. In vitro Antioxidant Activities Assay
2.8.1. DPPH Free Radical Scavenging Activity
- Scavenging activity (%) = [1 − (A1 − A2)/A0] × 100%
- A0: Absorption (Abs) of the DPPH solution without PAP-3;
- A1: Abs of the reaction mixture;
- A2: Abs of PAP-3 without the DPPH solution.
2.8.2. ABTS Free Radical Scavenging Activity
- Scavenging activity (%) = [1 − (A1 − A2)/A0] × 100%
- A0: Abs of the ABTS solution without PAP-3;
- A1: Abs of the reaction mixture;
- A2: Abs of PAP-3 without the ABTS solution.
2.9. In Vitro Immunomodulatory Activity Assay of PAP-3
3. Results and Discussion
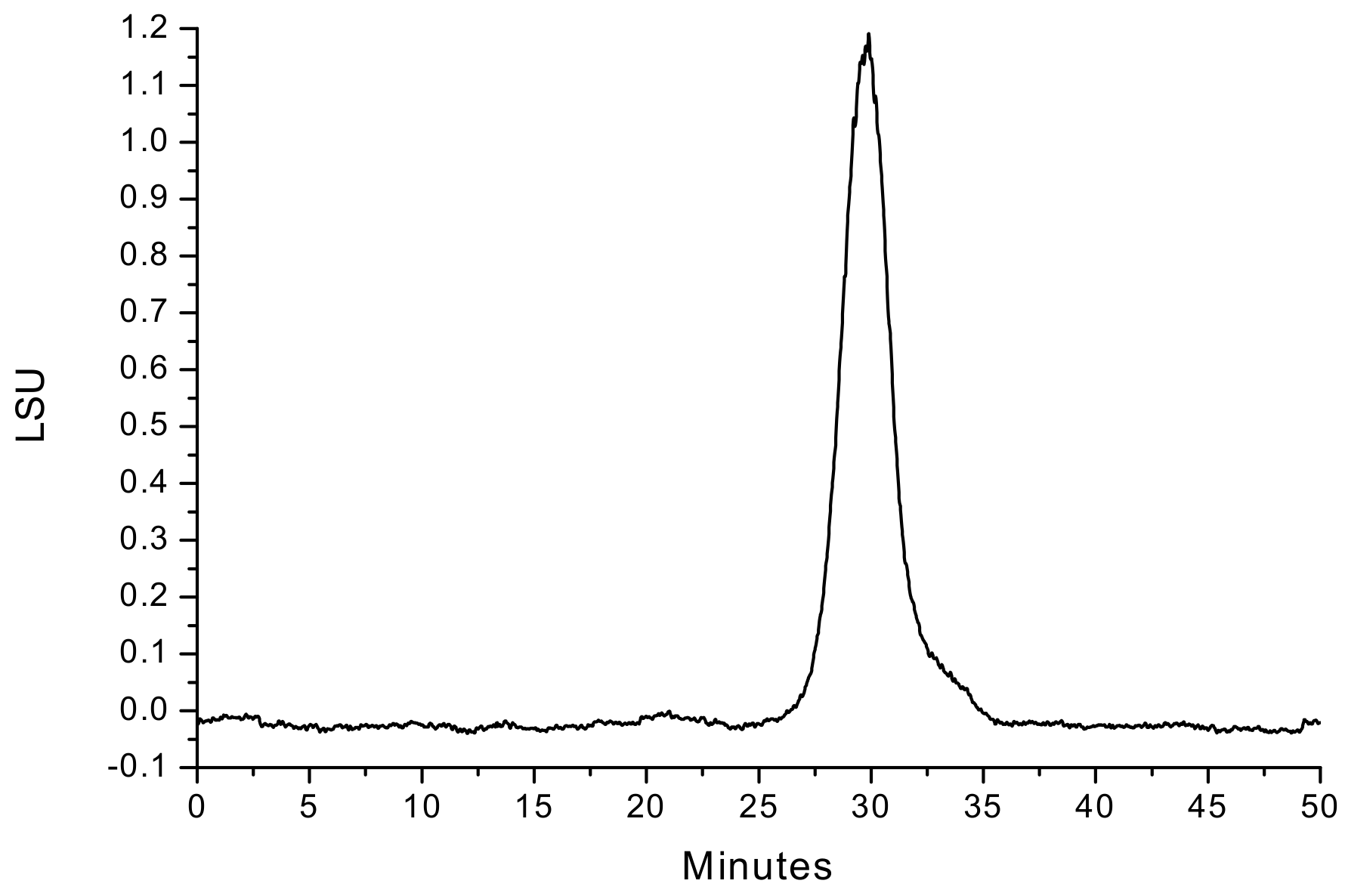
3.1. Extraction, Purification, and Molecular Weight of PAP-3
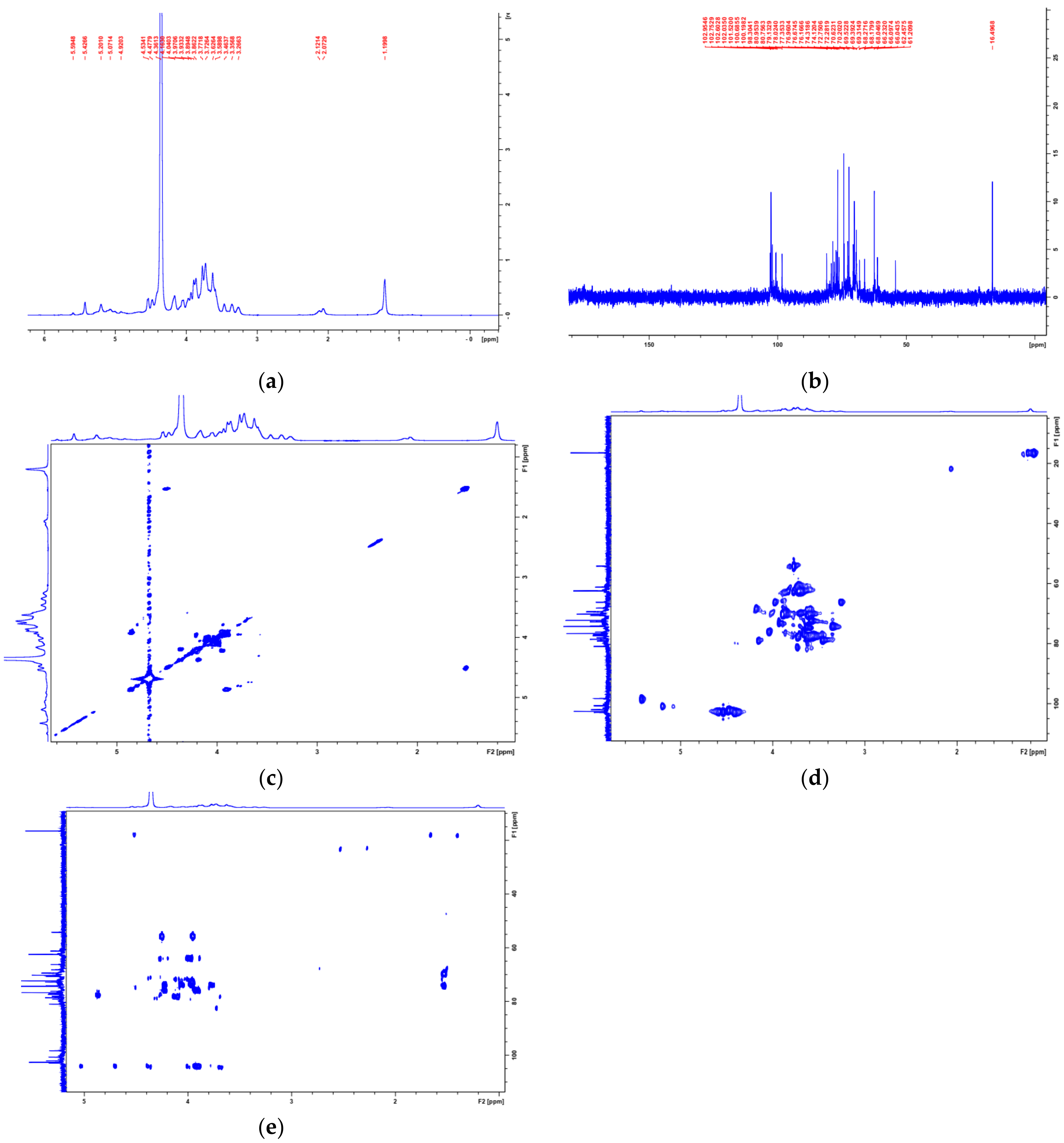
3.2. Structural Characterization of PAP-3
3.3. Antioxidant Activities Analysis
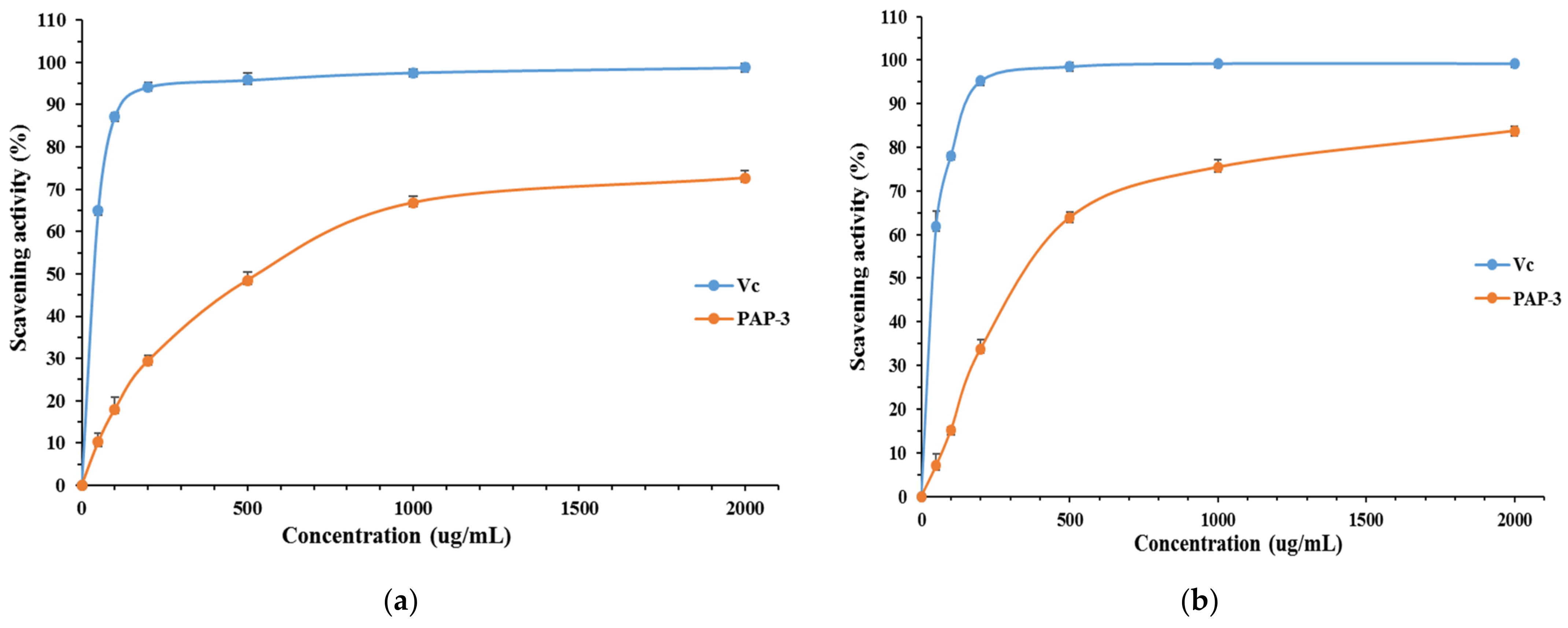
3.3.1. DPPH Free Radical Scavenging Activity of PAP-3
3.3.2. ABTS Free Radical Scavenging Activity of PAP-3
3.4. In Vitro Immunomodulatory Activity of PAP-3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kardong, D.; Upadhyaya, S.; Saikia, L.R. Screening of phytochemicals, antioxidant and antibacterial activity of crude extract of Pteridium aquilinum Kuhn. J. Pharm. Res. 2013, 6, 179–182. [Google Scholar] [CrossRef]
- Wang, H.; Wu, H. Preparation and antioxidant activity of Pteridium aquilinum-derived oligosaccharide. Int. J. Biol. Macromol. 2013, 61, 33–35. [Google Scholar] [CrossRef] [PubMed]
- Castillo, U.F.; Ojika, M.; Alonso-Amelot, M.; Sakagami, Y. Ptaquiloside Z, a New Toxic Unstable Sesquiterpene Glucoside from the Neotropical Bracken Fern Pteridium aquilinum Var. Caudatum. Bioorg. Med. Chem. 1998, 6, 2229–2233. [Google Scholar] [CrossRef]
- Fletcher, M.T.; Brock, I.J.; Reichmann, K.G.; McKenzie, R.A.; Blaney, B.J. Norsesquiterpene Glycosides in Bracken Ferns (Pteridium esculentum and Pteridium aquilinum subsp. wightianum) from Eastern Australia: Reassessed Poisoning Risk to Animals. J. Agric. Food Chem. 2011, 59, 5133–5138. [Google Scholar] [CrossRef] [PubMed]
- Castillo, U.F.; Wilkins, A.L.; Lauren, D.R.; Smith, B.L.; Alonso-Amelot, M. Pteroside A2-a New Illudane-Type Sesquiterpene Glucoside from Pteridium caudatum L. Maxon, and the Spectrometric Characterization of Caudatodienone. J. Agric. Food Chem. 2003, 51, 2559–2564. [Google Scholar] [CrossRef]
- Liu, Q.; Ma, R.; Li, S.; Fei, Y.; Lei, J.; Li, R.; Pan, Y.; Liu, S.; Wang, L. Dietary Supplementation of Auricularia auricula-judae Polysaccharides Alleviate Nutritional Obesity in Mice via Regulating Inflammatory Response and Lipid Metabolism. Foods 2022, 11, 942. [Google Scholar] [CrossRef]
- Yu, Y.; Shen, M.; Song, Q.; Xie, J. Biological activities and pharmaceutical applications of polysaccharide from natural resources: A review. Carbohydr. Polym. 2018, 183, 91–101. [Google Scholar] [CrossRef]
- Qi, Y.; Wang, L.; You, Y.; Sun, X.; Wen, C.; Fu, Y.; Song, S. Preparation of Low-Molecular-Weight Fucoidan with Anticoagulant Activity by Photocatalytic Degradation Method. Foods 2022, 11, 822. [Google Scholar] [CrossRef]
- Pan, L.C.; Zhu, Y.M.; Zhu, Z.Y.; Xue, W.; Liu, C.Y.; Sun, H.Q.; Yin, Y. Chemical structure and effects of antioxidation and against α-glucosidase of natural polysaccharide from Glycyrrhiza inflata Batalin. Int. J. Biol. Macromol. 2020, 155, 560–571. [Google Scholar] [CrossRef]
- Li, S.; Huo, X.; Qi, Y.; Ren, D.; Li, Z.; Qu, D.; Sun, Y. The Protective Effects of Ginseng Polysaccharides and Their Effective Subfraction against Dextran Sodium Sulfate-Induced Colitis. Foods 2022, 11, 890. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, T.; Wang, H.; Cui, Z.; Cheng, F.; Wang, K. Structural characterization and in vitro antitumor activity of an acidic polysaccharide from Angelica sinensis (Oliv.) Diels. Carbohydr. Polym. 2016, 147, 401–408. [Google Scholar] [CrossRef]
- Samtiya, M.; Aluko, R.E.; Dhewa, T.; Moreno-Rojas, J.M. Potential Health Benefits of Plant Food-Derived Bioactive Components: An Overview. Foods 2021, 10, 839. [Google Scholar] [CrossRef]
- Jiang, L.L.; Gong, X.; Ji, M.Y.; Wang, C.C.; Wang, J.H.; Li, M.H. Bioactive Compounds from Plant-Based Functional Foods: A Promising Choice for the Prevention and Management of Hyperuricemia. Foods 2020, 9, 973. [Google Scholar] [CrossRef]
- Silva, G.B.; Ionashiro, M.; Carrara, T.B.; Crivellari, A.C.; Tiné, M.A.S.; Prado, J.; Carpita, N.C.; Buckeridge, M.S. Cell wall polysaccharides from fern leaves: Evidence for a mannan-rich Type III cell wall in Adiantum raddianum. Phytochemistry 2011, 72, 2352–2360. [Google Scholar] [CrossRef]
- Wu, M.J.; Weng, C.Y.; Wang, L.; Lianm, T.W. Immunomodulatory mechanism of the aqueous extract of sword brake fern (Pteris ensiformis Burm.). J. Ethnopharmacol. 2005, 98, 73–81. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, F.; Luo, Y.; Ma, L.; Kou, X.; Huang, K. Antioxidant activity of a water-soluble polysaccharide purified from Pteridium aquilinum. Carbohydr. Res. 2009, 344, 217–222. [Google Scholar] [CrossRef]
- Song, G.L.; Wang, K.W.; Zhang, H.; Sun, H.X.; Wu, B.; Ju, X.Y. Structural characterization and immunomodulatory activity of a novel polysaccharide from Pteridium aquilinum. Int. J. Biol. Macromol. 2017, 102, 599–604. [Google Scholar] [CrossRef]
- Yu, X.H.; Liu, Z.Y.; Yang, J.S.; Yang, X.Y.; Wan, R.L. Controlling Quality of Astragalus polysaccharide Meal by Combined TLC and Phenol-Sulfuric Acid Method. Med. Plant 2010, 1, 54–57. [Google Scholar]
- Zhang, H.; Ye, L.; Wang, K.W. Structural characterization and anti-inflammatory activity of two water-soluble polysaccharides from Bellamya purificata. Carbohydr. Polym. 2010, 81, 953–960. [Google Scholar] [CrossRef]
- Hu, J.; Gao, J.; Zhao, Z.; Yang, X. Response surface optimization of polysaccharide extraction from Galla Chinensis and determination of its antioxidant activity in vitro. Food Sci. Technol. 2021, 41, 188–194. [Google Scholar] [CrossRef]
- Chen, L.; Huang, G. Antioxidant activities of phosphorylated pumpkin polysaccharide. Int. J. Biol. Macromol. 2019, 125, 256–261. [Google Scholar] [CrossRef]
- Sun, H.X.; Zhang, J.; Chen, F.Y.; Chen, X.F.; Zhou, Z.H.; Wang, H. Activation of RAW264.7 macrophages by the polysaccharide from the roots of Actinidia eriantha and its molecular mechanisms. Carbohydr. Polym. 2015, 121, 388–402. [Google Scholar] [CrossRef]
- Yin, Y.; Yu, R.; Yang, W.; Yuan, F.; Yan, C.; Song, L. Structural characterization and anti-tumor activity of a novel heteropolysaccharide isolated from Taxus yunnanensis. Carbohydr. Polym. 2010, 82, 543–548. [Google Scholar] [CrossRef]
- Tu, J.; Liu, H.; Wen, Y.; Chen, P.; Liu, Z. A novel polysaccharide from Hericium erinaceus: Preparation, structural characteristics, thermal stabilities, and antioxidant activities in vitro. J. Food Biochem. 2021, 45, e13871. [Google Scholar] [CrossRef] [PubMed]
- Heiss, C.; Burtnick, M.N.; Roberts, R.A.; Black, I.; Azadi, P.; Brett, P.J. Revised structures for the predominant O-polysaccharides expressed by Burkholderia pseudomallei and Burkholderia mallei. Carbohydr. Res. 2013, 381, 6–11. [Google Scholar] [CrossRef] [Green Version]
- Yao, H.Y.Y.; Wang, J.Q.; Yin, J.Y.; Nie, S.P.; Xie, M.Y. A review of NMR analysis in polysaccharide structure and conformation: Progress, challenge and perspective. Food Res. Int. 2021, 143, 110290. [Google Scholar] [CrossRef] [PubMed]
- Carlotto, J.; De Almeida Veiga, A.; De Souza, L.M.; Cipriani, T.R. Polysaccharide fractions from Handroanthus heptaphyllus and Handroanthus albus barks: Structural characterization and cytotoxic activity. Int. J. Biol. Macromol. 2020, 165, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Chen, W.; He, P.; Zhan, Q.; Wang, Q.; Wu, H.; Zhang, M. Structural characterization of polysaccharides with potential antioxidant and immunomodulatory activities from Chinese water chestnut peels. Carbohydr. Polym. 2020, 246, 116551. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, J.; Zhang, Y.; Dai, B.; An, Y.; Yu, L. Structural, Thermal, and Anti-inflammatory Properties of a Novel Pectic Polysaccharide from Alfalfa (Medicago sativa L.) Stem. J. Agric. Food Chem. 2015, 63, 3219–3228. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, L.; Zhang, X.; Wan, L.; Zheng, Q.; Xu, D.; Li, Y.; Liang, Y.; Chen, M.; Li, B.; et al. Structural characterization of polysaccharide from Centipeda minima and its hypoglycemic activity through alleviating insulin resistance of hepatic HepG2 cells. J. Funct. Foods 2021, 82, 104478. [Google Scholar] [CrossRef]
- Makarova, E.N.; Shakhmatov, E.G. Characterization of pectin-xylan-glucan-arabinogalactan proteins complex from Siberian fir Abies sibirica Ledeb. Carbohydr. Polym. 2021, 260, 117825. [Google Scholar] [CrossRef] [PubMed]
- San-Blas, G.; Prieto, A.; Bernabe, M.; Ahrazem, O.; Moreno, B.; Leal, J.A. α-galf 1→6-α-mannopyranoside side chains in Paracoccidioides brasiliensis cell wall are shared by members of the Onygenales, but not by galactomannans of other fungal genera. Med. Mycol. 2005, 43, 153–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smiderle, F.R.; Sassaki, G.L.; Van Griensven, L.J.L.D.; Iacomini, M. Isolation and chemical characterization of a glucogalactomannan of the medicinal mushroom Cordyceps militaris. Carbohydr. Polym. 2013, 97, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.; Yin, J.Y.; Zhang, T.; Xin, Y.; Huang, X.J.; Nie, S.P. Utilizing relative ordered structure theory to guide polysaccharide purification for structural characterization. Food Hydrocoll. 2021, 115, 106603. [Google Scholar] [CrossRef]
- Sigida, E.N.; Fedonenko, Y.P.; Shashkov, A.S.; Zdorovenko, E.L.; Konnova, S.A.; Ignatov, V.V.; Knirel, Y.A. Structural studies of the O-specific polysaccharide(s) from the lipopolysaccharide of Azospirillum brasilense type strain Sp7. Carbohydr. Res. 2013, 380, 76–80. [Google Scholar] [CrossRef]
- Tsumbu, C.N.; Deby-Dupont, G.; TitsZ, M.; Angenot, L.; Frederich, M.; Kohnen, S.; Mouithys-Mickalad, A.; Serteyn, D.; Franck, T. Polyphenol Content and Modulatory Activities of Some Tropical Dietary Plant Extracts on the Oxidant Activities of Neutrophils and Myeloperoxidase. Int. J. Mol. Sci. 2012, 13, 628–650. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.; He, X.; Zhou, S.; Luo, A.; He, T.; Chun, Z. Composition analysis and antioxidant activity of polysaccharide from Den drobium denneanum. Int. J. Biol. Macromol. 2009, 45, 169–173. [Google Scholar] [CrossRef]
- Luo, A.; Ge, Z.; Fan, Y.; Luo, A.; Chun, Z.; He, X. In Vitro and In Vivo Antioxidant Activity of a Water-Soluble Polysaccharide from Dendrobium denneanum. Molecules 2011, 16, 1579–1592. [Google Scholar] [CrossRef] [Green Version]
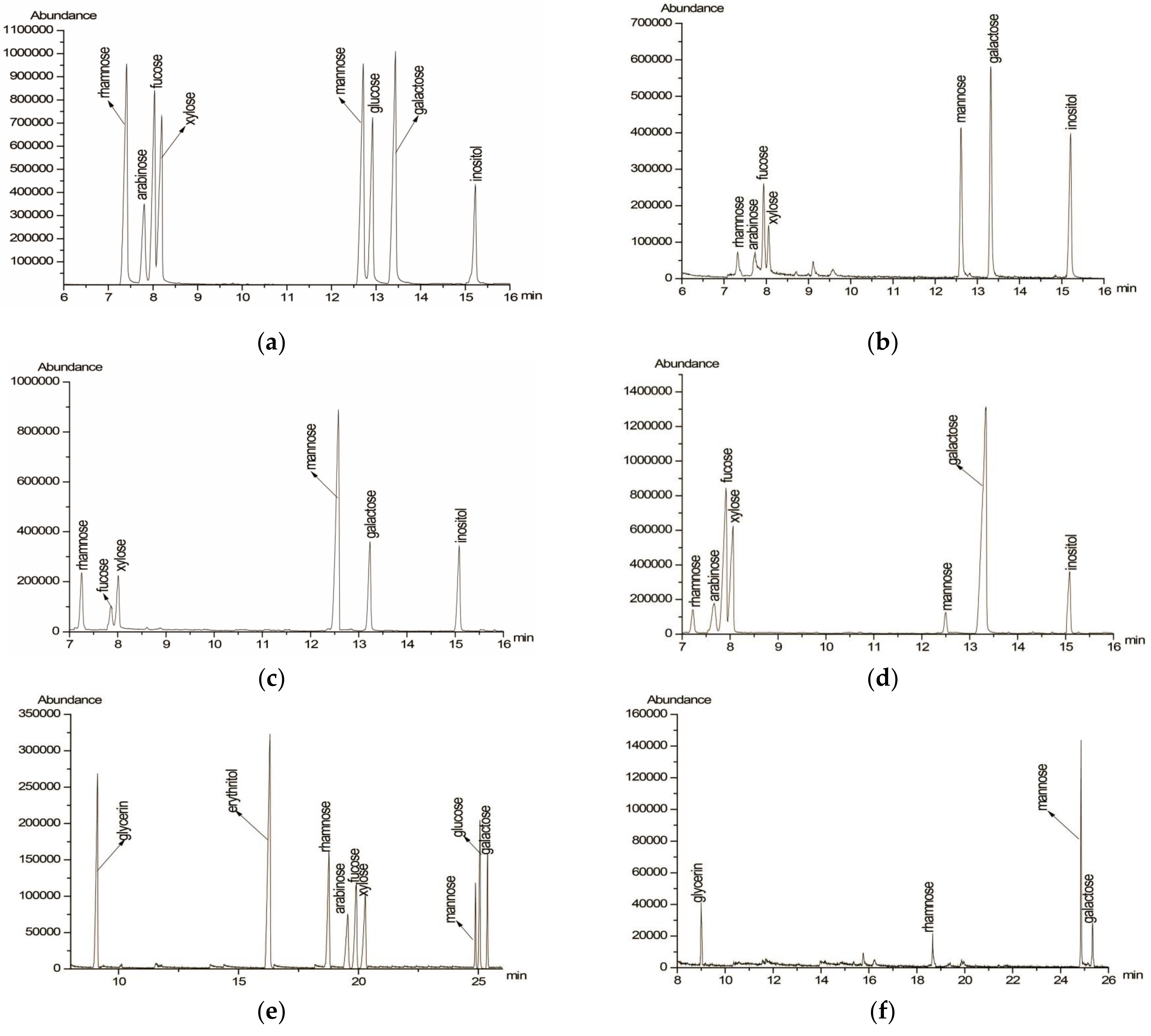

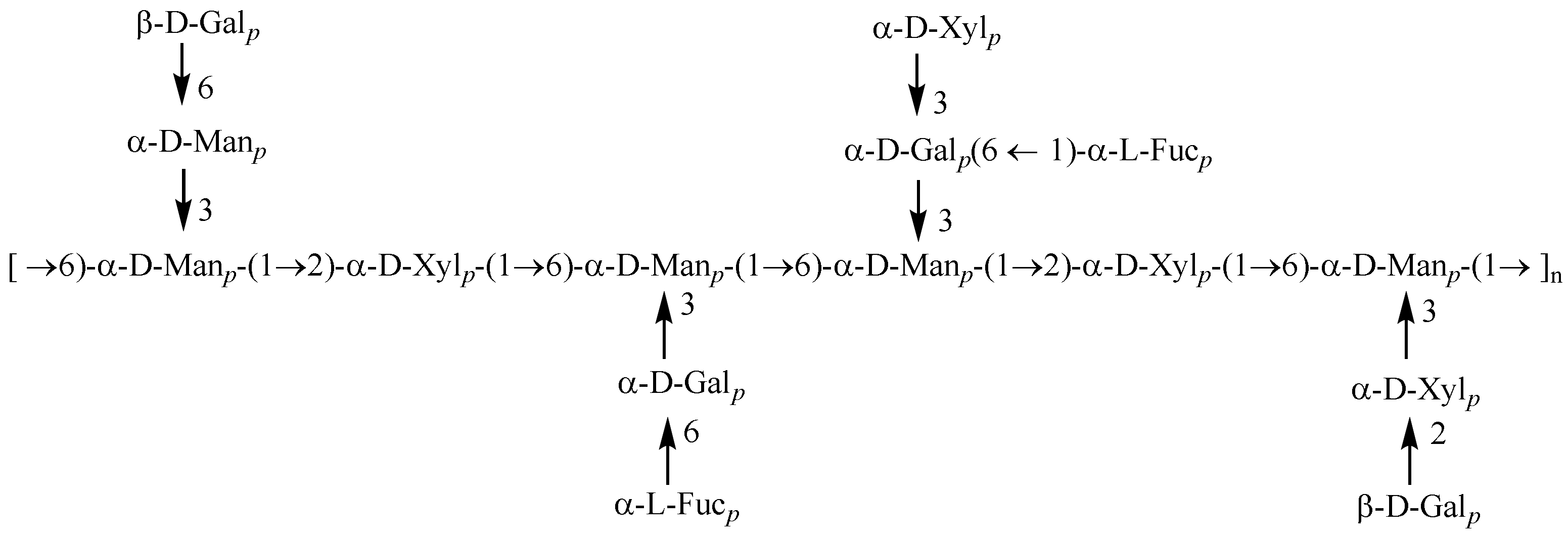

| Gly | Ery | L-Ara | L-Rha | D-Fuc | D-Gal | D-Man | D-Xyl | |
|---|---|---|---|---|---|---|---|---|
| PAP-3 | / | / | 1.58 | 1.00 | 3.26 | 4.57 | 4.81 | 3.33 |
| PAP-3-I | / | / | - | 2.25 | 1.00 | 1.87 | 8.92 | 4.02 |
| PAP-3-O | / | / | 7.53 | 1.66 | 12.62 | 13.48 | 1.00 | 15.66 |
| Smith degradation | + | - | - | + | - | + | + | - |
| Methylated Sugar | Deduced Linkage Patten | Major Mass Ion Fragmentation | Relative Molar Ratio | |
|---|---|---|---|---|
| A | 2,3,4,6-Me4-Galp | 1-Linked Galp | 87, 101, 117, 129, 145, 161, 205 | 1.0 |
| B | 2,3,4-Me3-Galp | 1,6-Linked Galp | 87, 101, 117, 129, 145, 161, 187, 205 | 1.3 |
| C | 2,4-Me2-Galp | 1,3,6-Linked Galp | 87, 99, 101, 129, 149, 161, 233 | 2.1 |
| D | 2,3,4-Me3-Fucp | 1-Linked Fucp | 81, 101, 117, 126, 155, 207, 233 | 2.2 |
| E | 2,3,4-Me3-Manp | 1,6-Linked Manp | 89, 101, 117, 129, 145, 161, 173, 205 | 1.5 |
| F | 2,4-Me2-Manp | 1,3,6-Linked Manp | 87, 99, 117, 129, 173, 189, 207, 233 | 2.5 |
| G | 2,3,4-Me3-Xylp | 1-Linked Xylp | 71, 87, 101, 117, 129, 161 | 1.2 |
| H | 3,4-Me2-Xylp | 1,2-Linked Xylp | 87, 101, 129, 145, 161, 189 | 3.2 |
| Deduced Linkage | NMR Data (δ in ppm) | ||||||
|---|---|---|---|---|---|---|---|
| H1/C1 | H2/C2 | H3/C3 | H4/C4 | H5/C5 | H6/C6 | ||
| A | T-β-d-Galp-(1→ | 4.48/102.04 | 3.36/74.12 | 3.59/74.32 | 3.70/69.39 | 3.58/77.35 | 3.72/61.21 |
| B | → 6)-β-d-Galp-(1→ | 4.42/102.95 | 3.46/72.75 | 3.36/74.12 | 3.89/70.63 | 4.05/76.17 | 3.27, 3.97/66.04 |
| C | →3,6)-β-d-Galp-(1→ | 4.53/102.60 | 3.63/74.32 | 3.73/80.95 | 4.02/69.52 | 3.73/76.67 | 3.27, 3.97/66.23 |
| D | T-α-l-Fucp-(1→ | 4.92/101.52 | 3.71/69.39 | 3.90/69.52 | 3.65/74.32 | 4.18/68.05 | 1.20/16.50 |
| E | →6)-α-d-Manp-(1→ | 5.07/100.20 | 3.67/74.32 | 3.83/78.02 | 3.73/69.32 | 3.59/72.28 | 3.27, 3.97/66.09 |
| F | →3,6)-α-d-Manp-(1→ | 5.42/98.30 | 3.69/72.28 | 3.75/80.75 | 3.67/74.32 | 3.58/72.28 | 3.27, 3.97/66.10 |
| G | T-α-d-Xylp-(1→ | 4.90/102.75 | 3.59/74.32 | 3.86/76.96 | 3.62/72.28 | 3.77, 3.86/62.46 | |
| H | →2)-α-d-Xylp-(1→ | 5.20/100.69 | 4.16/79.13 | 3.59/74.32 | 3.58/72.28 | 3.77, 3.86/62.46 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.-H.; Ju, X.-Y.; Wang, K.-W.; Chen, X.-J.; Sun, H.-X.; Cheng, K.-J. Structure Characterization, Antioxidant and Immunomodulatory Activities of Polysaccharide from Pteridium aquilinum (L.) Kuhn. Foods 2022, 11, 1834. https://doi.org/10.3390/foods11131834
Zhao Z-H, Ju X-Y, Wang K-W, Chen X-J, Sun H-X, Cheng K-J. Structure Characterization, Antioxidant and Immunomodulatory Activities of Polysaccharide from Pteridium aquilinum (L.) Kuhn. Foods. 2022; 11(13):1834. https://doi.org/10.3390/foods11131834
Chicago/Turabian StyleZhao, Zhe-Han, Xian-Yan Ju, Kui-Wu Wang, Xin-Juan Chen, Hong-Xiang Sun, and Ke-Jun Cheng. 2022. "Structure Characterization, Antioxidant and Immunomodulatory Activities of Polysaccharide from Pteridium aquilinum (L.) Kuhn" Foods 11, no. 13: 1834. https://doi.org/10.3390/foods11131834
APA StyleZhao, Z.-H., Ju, X.-Y., Wang, K.-W., Chen, X.-J., Sun, H.-X., & Cheng, K.-J. (2022). Structure Characterization, Antioxidant and Immunomodulatory Activities of Polysaccharide from Pteridium aquilinum (L.) Kuhn. Foods, 11(13), 1834. https://doi.org/10.3390/foods11131834






