Extraction, Isolation and Identification of Low Molecular Weight Peptides in Human Milk
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Equipment
2.2. Sample Collection and Preparation
2.3. Solid Phase Extraction
2.4. Chip-QTOF Analysis
2.5. Data Analysis
2.6. Ethical Statement
3. Results
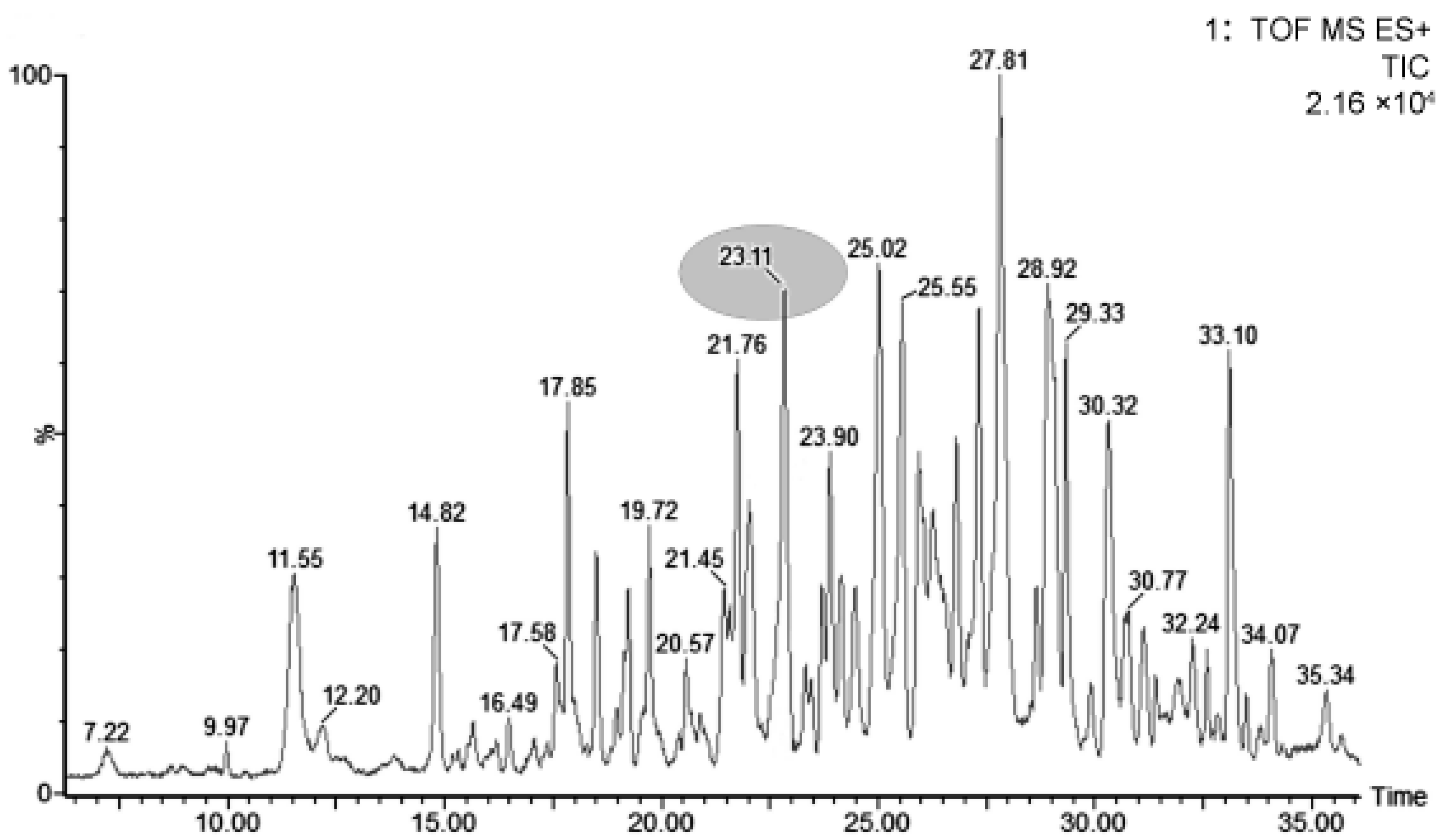
3.1. Extraction and Separation of LMWPs
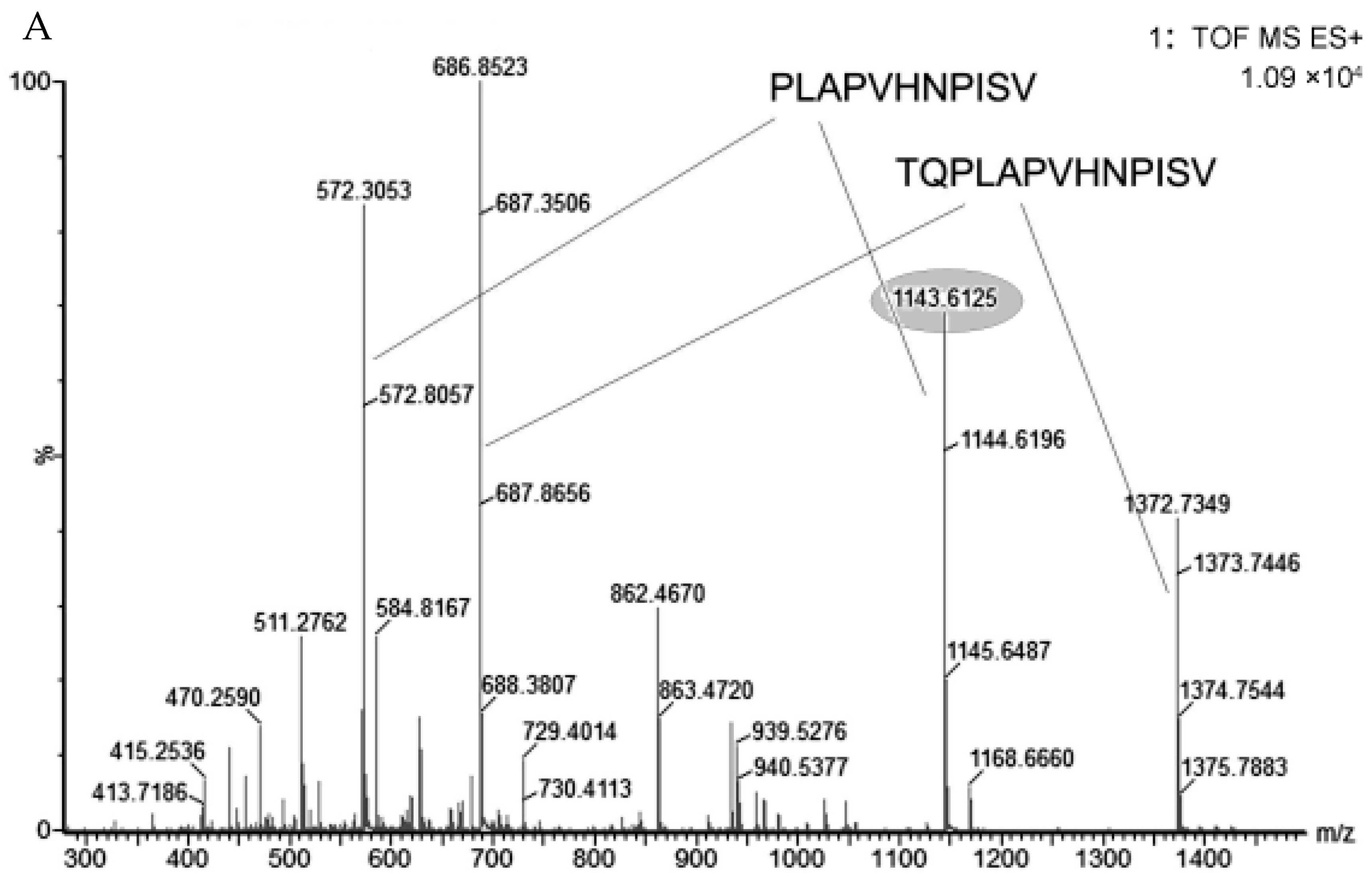
3.2. Sequencing Analysis and Quantification of LMWPs
3.3. Variations of Main LMWPs
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Korhonen, H. Milk-derived bioactive peptides: From science to applications. J. Funct. Foods 2009, 1, 177–187. [Google Scholar] [CrossRef]
- Muro-Urista, C.; Álvarez-Fernández, R.; Riera-Rodriguez, F.; Arana-Cuenca, A.; Téllez-Jurado, A. Review: Production and functionality of active peptides from milk. Food Sci. Technol. Int. 2011, 17, 293–317. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Li, X.; Jiang, X.; Zhou, H.; Jiang, X.; Kong, L.; Ye, M.; Zou, H. Comprehensive peptidome analysis of mouse livers by size exclusion chromatography prefractionation and nano LC-MS/MS identification. J. Proteome Res. 2007, 6, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Hölttä, M.; Zetterberg, H.; Mirgorodskaya, E.; Mattsson, N.; Blennow, K.; Gobom, J. Peptidome analysis of cerebrospinal fluid by LC-MALDI MS. PLoS ONE 2012, 7, e42555. [Google Scholar] [CrossRef] [Green Version]
- Dallas, D.C.; Guerrero, A.; Khaldi, N.; Castillo, P.A.; Martin, W.F.; Smilowitz, J.T.; Bevins, C.L.; Barile, D.; German, J.B.; Lebrilla, C.B. Extensive in vivo human milk peptidomics reveals specific proteolysis yielding protective antimicrobial peptides. J. Proteome Res. 2013, 12, 2295–2304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nonaka, A.; Nakamura, T.; Hirota, T.; Matsushita, A.; Asakura, M.; Ohki, K.; Kitakaze, M. The milk-derived peptides Val-Pro-Pro and Ile-Pro-Pro attenuate arterial dysfunction in L-NAME-treated rats. Hypertens. Res. 2014, 37, 703–707. [Google Scholar] [CrossRef]
- Baum, F.; Fedorova, M.; Ebner, J.; Hoffmann, R.; Pischetsrieder, M. Analysis of the endogenous peptide profile of milk: Identification of 248 mainly casein-derived peptides. J. Proteome Res. 2013, 12, 5447–5462. [Google Scholar] [CrossRef]
- Dallas, D.C.; Guerrero, A.; Khaldi, N.; Borghese, R.; Bhandari, A.; Underwood, M.A.; Lebrilla, C.B.; German, J.B.; Barile, D. A peptidomic analysis of human milk digestion in the infant stomach reveals protein-specific degradation patterns. J. Nutr. 2014, 144, 815–820. [Google Scholar] [CrossRef] [Green Version]
- Boersema, P.J.; Raijmakers, R.; Lemeer, S.; Mohammed, S.; Heck, A.J. Multiplex peptide stable isotope dimethyl labeling for quantitative proteomics. Nat. Protoc. 2009, 4, 484–494. [Google Scholar] [CrossRef]
- Li, M.; Kang, S.; Zheng, Y.; Shao, J.; Zhao, H.; An, Y.; Cao, G.; Li, Q.; Yue, X.; Yang, M. Comparative metabolomics analysis of donkey colostrum and mature milk using ultra-highperformance liquid tandem chromatography quadrupole time-of-flight mass spectrometry. J. Dairy Sci. 2019, 103, 992–1001. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Liu, Y.; Li, Q.; Yang, M.; Pi, Y.; Yang, N.; Zheng, Y.; Yue, X. Comparative exploration of free fatty acids in donkey colostrum and mature milk based on a metabolomics approach. J. Dairy Sci. 2020, 103, 6022–6031. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Logan, A.; Cocks, B.G.; Rochfort, S. Seasonal variation of polar lipid content in bovine milk. Food Chem. 2017, 237, 865–869. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, D.; FitzGerald, R.J. Production of caseinophosphopeptides (CPPs) from sodium caseinate using a range of commercial protease preparations. Int. Dairy J. 1998, 8, 39–45. [Google Scholar] [CrossRef]
- Vermeirssen, V.; Camp, J.V.; Verstraete, W. Bioavailability of angiotensin I converting enzyme inhibitory peptides. Br. J. Nutr. 2004, 92, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, S.F.; Pouliot, Y.; Saint-Sauveur, D. Immunomodulatory peptides obtained by the enzymatic hydrolysis of whey proteins. Int. Dairy J. 2006, 16, 1315–1323. [Google Scholar] [CrossRef]
- Lόpez-Expόsito, I.; Recio, I. Antibacterial activity of peptides and folding variants from milk proteins. Int. Dairy J. 2006, 16, 1294–1305. [Google Scholar] [CrossRef]
- López-Expósito, I.; Quirós, A.; Amigo, L.; Recio, I. Casein hydrolysates as a source of antimicrobial, antioxidant and antihypertensive peptides. Lait 2007, 87, 241–249. [Google Scholar] [CrossRef]
- Pihlanto, A. Antioxidative peptides derived from milk proteins. Int. Dairy J. 2006, 16, 1306–1314. [Google Scholar] [CrossRef]
- Rival, S.G.; Boeriu, C.G.; Wichers, H.J. Caseins and casein hydrolysates. 2. Antioxidative properties and relevance to lipoxygenase inhibition. J. Agric. Food Chem. 2001, 49, 295–302. [Google Scholar] [CrossRef]
- Migliore-Samour, D.; Floc’h, F.; Jollés, P. Biologically active casein peptides implicated in immunomodulation. J. Dairy Res. 1989, 56, 357–362. [Google Scholar] [CrossRef]
- Teschemacher, H. Opioid receptor ligands derived from food proteins. Curr. Pharm. Des. 2003, 9, 1331–1344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phelan, M.; Aherne, A.; FitzGerald, R.J.; O’Brien, N.M. Casein-derived bioactive peptides: Biological effects, industrial uses, safety aspects and regulatory status. Int. Dairy J. 2009, 19, 643–654. [Google Scholar] [CrossRef]
- Wada, Y.; Lönnerdal, B. Bioactive peptides derived from human milk proteins—Mechanisms of action. J. Nutr. Biochem. 2014, 22, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Andrea, M.; Daniela, B.; Jaime, S.; Javier, M.; Alfonso, C.; Isabel, R.M.; Maria, L.S. Changes in caprine milk oligosaccharides at different lactation stages analyzed by high performance liquid chromatography coupled to mass spectrometry. J. Agric. Food Chem. 2017, 65, 3523–3531. [Google Scholar]
- Wu, S.; Grimm, R.; German, J.B.; Lebrilla, C.B. Annotation and Structural Analysis of Sialylated Human Milk Oligosaccharides. J. Proteome Res. 2011, 10, 856–868. [Google Scholar] [CrossRef] [Green Version]
- Ferranti, P.; Traisci, M.V.; Picariello, G.; Nasi, A.; Boschi, V.; Sievo, M.; Falconi, C.; Chianese, L.; Addeo, F. Casein proteolysis in human milk: Tracing the pattern of casein breakdown and the formation of potential bioactive peptides. J. Dairy Res. 2004, 71, 74–87. [Google Scholar] [CrossRef]
- Minervini, F.; Algaron, F.; Rizzello, C.G.; Fox, P.F.; Monnet, V.; Gobbetti, M. Angiotensin I-converting-enzyme-inhibitory and antibacterial peptides from Lactobacillus helveticus PR4 proteinase-hydrolyzed caseins of milk from six species. Appl. Environ. Microbiol. 2003, 69, 5297–5305. [Google Scholar] [CrossRef] [Green Version]
- Asano, M.; Nio, N.; Ariyoshi, Y. Inhibition of prolyl endopeptidase by synthetic peptide fragments of human beta-casein. Agric. Biol. Chem. 1991, 55, 825–828. [Google Scholar] [CrossRef] [Green Version]
- Miguel, M.; Recio, I.; Ramos, M.; Delgado, M.A.; Aleixandre, M.A. Antihypertensive effect of peptides obtained from Enterococcus faecalis-fermented milk in rats. J. Dairy Sci. 2006, 89, 3352–3359. [Google Scholar] [CrossRef] [Green Version]
- Koch, G.; Wiedemann, K.; Teschemacher, H. Opioid activities of human betacasomorphins. Naunyn-Schmiedebergs Arch. Pharmacol. 1985, 331, 351–354. [Google Scholar] [CrossRef]
- Brantl, V. Novel opioid peptides derived from human β-casein: Human β-casomorphins. Eur. J. Pharmacol. 1984, 106, 213–214. [Google Scholar] [CrossRef]
- Kampa, M.; Loukas, S.; Hatzoglou, A.; Martin, P.; Martin, P.M.; Castanas, E. Identification of a novel opioid peptide (Tyr-Val-Pro-Phe-Pro) derived from human αS1 casein (αS1-casomorphin, and αS1-casomorphin amide). Biochem. J. 1996, 319, 903–908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teschemacher, H.; Koch, G.; Brantl, V. Milk protein-derived opioid receptor ligands. Biopolymers 1997, 43, 99–117. [Google Scholar] [CrossRef]
- Parker, F.; Migliore-Samour, D.; Floc’h, F.; Zerial, A.; Werner, G.H.; Jollès, J.; Casaretto, M.; Zahn, H.; Jollès, P. Immunostimulating hexapeptide from human casein: Amino acid sequence, synthesis and biological properties. Eur. J. Biochem. 1984, 145, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Berthou, J.; Migliore-Samour, D.; Lifchitz, A.; Delettré, J.; Floc’h, F.; Jollès, P. Immunostimulating properties and three-dimensional structure of two tripeptides from human and cow caseins. FEBS Lett. 1987, 218, 55–58. [Google Scholar] [CrossRef]

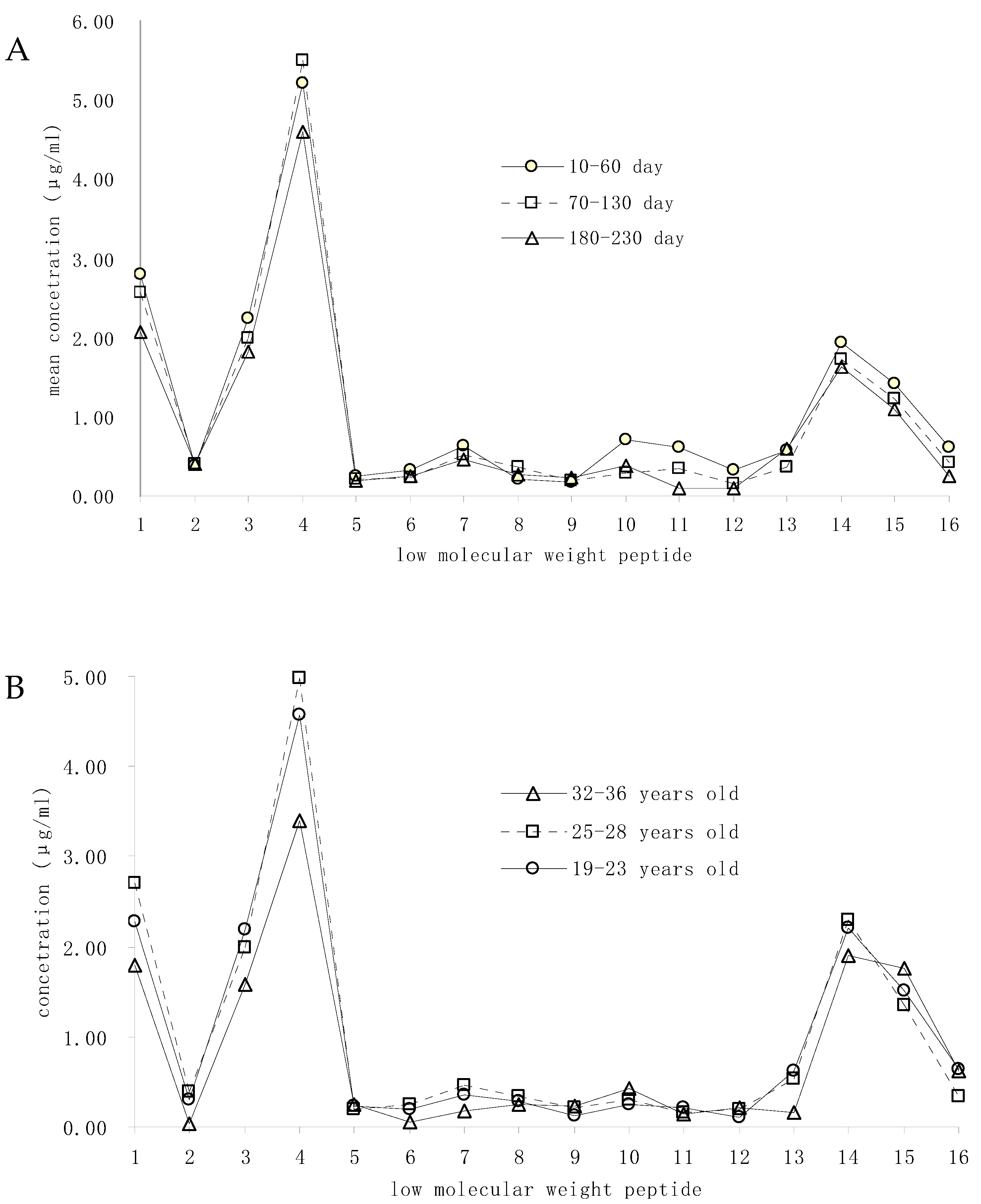
| Protein Precursor | Residue | Sequence | MH+ (Da) | Charge State | RT (min) | Concentration μg/mL |
|---|---|---|---|---|---|---|
| *β-casein | 16–33 | RETIESLSSSEESITEYK | 2247.9573 | 2 | 19.16 | 5.315 ± 1.267 |
| β-casein | 16–38 | RETIESLSSSEESITEYKQKVEK | 2700.3093 | 3 | 18.97 | 0.323 ± 0.109 |
| β-casein | 16–40 | RETIESLSSSEESITEYKQKVEKVK | 2860.2437 | 4 | 17.77 | 2.044 ± 0.813 |
| β-casein | 17–33 | ETIESLSSSEESITEYK | 2091.8591 | 2 | 19.71 | 4.065 ± 1.215 |
| β-casein | 17–38 | ETIESLSSSEESITEYKQKVEK | 2544.0308 | 2 | 27.84 | 0.170 ± 0.083 |
| β-casein | 23–33 | SSSEESITEYK | 1259.585 | 2 | 22.86 | 0.003 ± 0.001 |
| β-casein | 27–33 | ESITEYK | 869.4235 | 1 | 22.43 | 0.093 ± 0.037 |
| β-casein | 28–33 | SITEYK | 740.378 | 1 | 17.91 | 0.210 ± 0.069 |
| β-casein | 30–32 | TEY | 412.2193 | 1 | 21.09 | 0.019 ± 0.005 |
| β-casein | 30–33 | TEYK | 540.2654 | 1 | 22.43 | 0.489 ± 0.071 |
| β-casein | 31–33 | EYK | 439.218 | 1 | 19.2 | 0.042 ± 0.009 |
| β-casein | 85–103 | AQPAVVLPVPQPEIMEVPK | 2042.168 | 2 | 38.86 | 0.028 ± 0.006 |
| β-casein | 86–103 | QPAVVLPVPQPEIMEVPK | 1971.1284 | 2 | 38.82 | 0.048 ± 0.015 |
| β-casein | 86–112 | QPAVVLPVPQPEIMEVPKAKDTVYTKG | 2934.5535 | 3 | 37.29 | 0.274 ± 0.067 |
| β-casein | 89–103 | VVLPVPQPEIMEVPK | 1674.97 | 3 | 38.08 | 0.177 ± 0.033 |
| β-casein | 89–110 | VVLPVPQPEIMEVPKAKDTVYT | 2453.3762 | 3 | 37.79 | 0.34 ± 0.086 |
| β-casein | 91–103 | LPVPQPEIMEVPK | 1476.8384 | 2 | 30.94 | 0.188 ± 0.054 |
| β-casein | 94–98 | PQPEI | 583.3124 | 1 | 36.25 | 0.003 ± 0.001 |
| β-casein | 94–103 | PQPEIMEVPK | 1167.6152 | 2 | 38.82 | 0.028 ± 0.009 |
| β-casein | 126–130 | FDPQI | 601.3356 | 1 | 36.91 | 0.074 ± 0.019 |
| β-casein | 127–140 | DPQIPKLTDLENLH | 1632.8809 | 2 | 33.68 | 0.004 ± 0.001 |
| β-casein | 150–154 | QQVPQ | 564.2857 | 1 | 19.99 | 0.055 ± 0.011 |
| β-casein | 154–158 | PQPIP | 551.3185 | 1 | 1.77 | 0.088 ± 0.023 |
| β-casein | 157–161 | PQTLA | 529.2968 | 1 | 19.98 | 0.164 ± 0.036 |
| β-casein | 171–189 | SVPQPKVLPIPQQVVPYPQR | 2270.33 | 2 | 33.42 | 0.005 ± 0.001 |
| β-casein | 176–194 | VLPIPQQVVPYPQRAVPVQ | 2128.2644 | 2 | 36.52 | 0.030 ± 0.008 |
| β-casein | 183–194 | VVPYPQRAVPVQ | 1352.7789 | 2 | 11.64 | 0.035 ± 0.014 |
| β-casein | 190–194 | VPVQA | 495.2942 | 1 | 25.06 | 0.018 ± 0.005 |
| β-casein | 191–196 | VPVQAL | 608.3761 | 1 | 25.06 | 0.003 ± 0.001 |
| β-casein | 196–200 | LLLNQ | 582.358 | 1 | 26.81 | 0.005 ± 0.001 |
| β-casein | 199–203 | NQELL | 598.3207 | 1 | 19.12 | 0.004 ± 0.001 |
| β-casein | 204–207 | NPTH | 468.2182 | 1 | 12.71 | 0.005 ± 0.001 |
| β-casein | 205–208 | PTHQ | 482.2364 | 1 | 28.86 | 0.002 ± 0.001 |
| β-casein | 205–226 | NPTHQIYPVTQPLAPVHNPISV | 2422.3235 | 3 | 33.88 | 0.319 ± 0.081 |
| β-casein | 209–226 | QIYPVTQPLAPVHNPISV | 1973.1118 | 2 | 36.92 | 0.017 ± 0.005 |
| β-casein | 211–226 | YPVTQPLAPVHNPISV | 1731.9642 | 2 | 31.9 | 0.030 ± 0.007 |
| β-casein | 212–226 | PVTQPLAPVHNPISV | 1568.8969 | 2 | 27.53 | 0.042 ± 0.015 |
| β-casein | 213–226 | VTQPLAPVHNPISV | 1471.8435 | 2 | 25.41 | 0.071 ± 0.023 |
| β-casein | 214–218 | TQPLA | 493.2703 | 1 | 7.26 | 0.006 ± 0.002 |
| β-casein | 214–226 | TQPLAPVHNPISV | 1372.7666 | 2 | 23.09 | 2.59 ± 0.951 |
| β-casein | 215–226 | QPLAPVHNPISV | 1271.7212 | 2 | 21.96 | 1.65 ± 0.432 |
| β-casein | 216–226 | PLAPVHNPISV | 1143.6647 | 1 | 36.91 | 0.460 ± 0.105 |
| β-casein | 218–224 | APVHNPI | 729.4062 | 1 | 9.75 | 0.033 ± 0.006 |
| β-casein | 218–226 | APVHNPISV | 933.5039 | 2 | 9.78 | 0.066 ± 0.015 |
| β-casein | 222–226 | NPISV | 529.2968 | 1 | 19.98 | 0.004 ± 0.001 |
| β-casein | 223–226 | PISV | 415.2347 | 1 | 28.6 | 0.024 ± 0.009 |
| *κ-casein | 73–79 | NPYVPRT | 846.4499 | 1 | 18.35 | 0.046 ± 0.018 |
| κ-casein | 82–89 | ANPAVVRP | 823.484 | 1 | 19.01 | 0.008 ± 0.003 |
| κ-casein | 82–91 | ANPAVVRPHA | 1031.5775 | 2 | 21.25 | 0.037 ± 0.019 |
| κ-casein | 83–89 | NPAVRP | 752.4417 | 1 | 17.81 | 0.026 ± 0.011 |
| κ-casein | 83–91 | NPAVRPHA | 960.5374 | 2 | 18.66 | 0.029 ± 0.015 |
| κ-casein | 84–89 | PAVVRP | 638.403 | 1 | 25.34 | 0.008 ± 0.005 |
| κ-casein | 99–106 | LPNSHPPT | 862.4427 | 2 | 26.83 | 0.018 ± 0.010 |
| κ-casein | 99–109 | LPNSHPPTVVR | 1216.6873 | 2 | 22.07 | 0.089 ± 0.026 |
| κ-casein | 100–111 | PNSHPPTVVRRP | 1356.7617 | 3 | 30.15 | 0.036 ± 0.021 |
| κ-casein | 104–116 | PPTVVRRNLHPS | 1469.847 | 3 | 33.02 | 0.041 ± 0.0013 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, H.; Jiang, H.; Tu, H.; Jia, Y.; Wang, H.; Lü, X.; Fang, R.; Xiao, G. Extraction, Isolation and Identification of Low Molecular Weight Peptides in Human Milk. Foods 2022, 11, 1836. https://doi.org/10.3390/foods11131836
Xiao H, Jiang H, Tu H, Jia Y, Wang H, Lü X, Fang R, Xiao G. Extraction, Isolation and Identification of Low Molecular Weight Peptides in Human Milk. Foods. 2022; 11(13):1836. https://doi.org/10.3390/foods11131836
Chicago/Turabian StyleXiao, Hailong, He Jiang, Haiyun Tu, Yanbo Jia, Hongqing Wang, Xin Lü, Ruosi Fang, and Gongnian Xiao. 2022. "Extraction, Isolation and Identification of Low Molecular Weight Peptides in Human Milk" Foods 11, no. 13: 1836. https://doi.org/10.3390/foods11131836
APA StyleXiao, H., Jiang, H., Tu, H., Jia, Y., Wang, H., Lü, X., Fang, R., & Xiao, G. (2022). Extraction, Isolation and Identification of Low Molecular Weight Peptides in Human Milk. Foods, 11(13), 1836. https://doi.org/10.3390/foods11131836






