Enhancing the Biological Activities of Food Protein-Derived Peptides Using Non-Thermal Technologies: A Review
Abstract
:1. Introduction
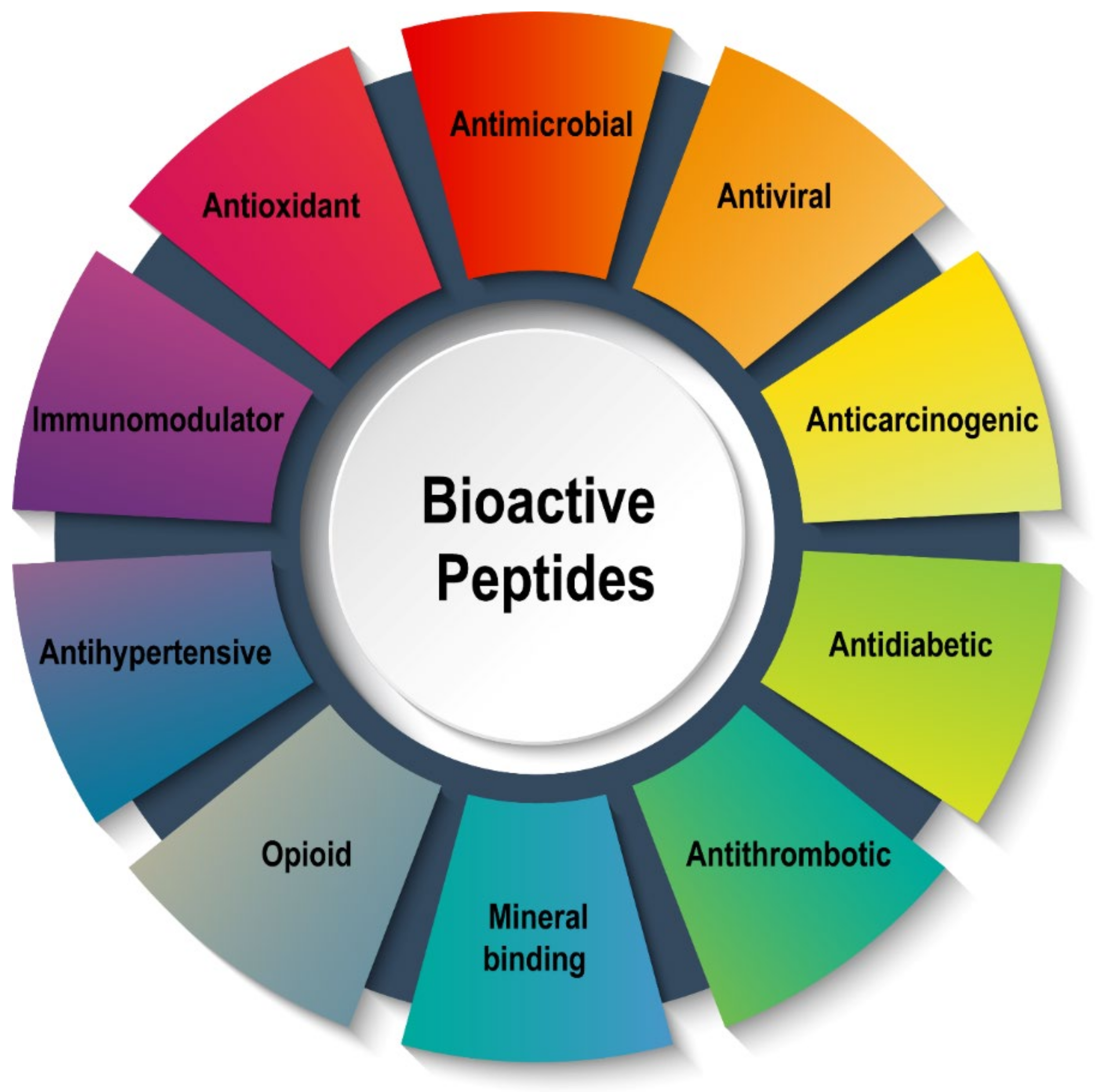
2. Production and Bioactivities of BPs by Enzymatic Hydrolysis
3. Bioactivities of BPs Derived from Food Proteins Using Enzymolysis
3.1. Antioxidant Properties of BPs
3.2. ACE-Inhibitory Properties of BPs
3.3. Antidiabetic Properties of BPs
3.4. Antiproliferative Properties of BPs
3.5. Antimicrobial Properties of BPs
4. Non-Thermal Pre-Treatments and Their Impacts on Yield and Bioactivities of BPs
4.1. Ultrasonication
4.1.1. Effect of Ultrasound on the Structure of Food Proteins
4.1.2. Effect of Ultrasound Pre-Treatment on the Bioactivities of BPs Produced Using Enzymolysis
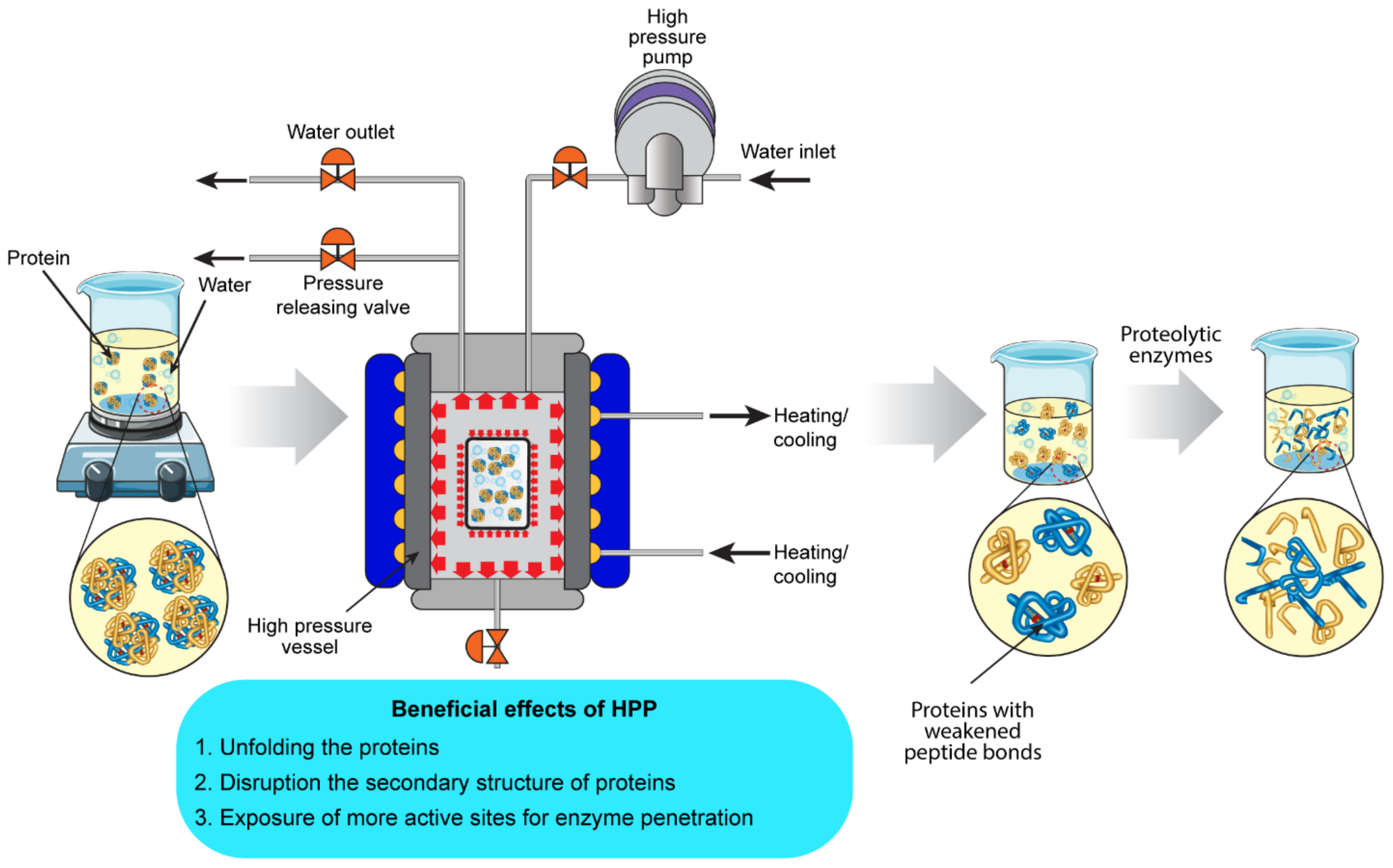
4.2. High-Pressure Processing (HPP)
4.2.1. Effect of HPP on the Structure of Food Proteins
4.2.2. Effect of HPP Pre-Treatment on the Bioactivity of BPs Produced Using Enzymolysis
4.3. Pulsed Electric Field (PEF)
4.3.1. Effect of PEF on the Structure of Food Protein
4.3.2. Effect of PEF on the Bioactivities of BPs Produced from Food Proteins Using Enzymolysis
5. Conclusions and Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Korhonen, H.; Pihlanto, A. Bioactive peptides: Production and functionality. Int. Dairy J. 2006, 16, 945–960. [Google Scholar] [CrossRef]
- Singh, B.P.; Vij, S.; Hati, S. Functional significance of bioactive peptides derived from soybean. Peptides 2014, 54, 171–179. [Google Scholar] [CrossRef]
- Kadam, S.U.; Tiwari, B.K.; Álvarez, C.; O’Donnell, C.P. Ultrasound applications for the extraction, identification and delivery of food proteins and bioactive peptides. Trends Food Sci. Technol. 2015, 46, 60–67. [Google Scholar] [CrossRef]
- Möller, N.P.; Scholz-Ahrens, K.E.; Roos, N.; Schrezenmeir, J. Bioactive peptides and proteins from foods: Indication for health effects. Eur. J. Nutr. 2008, 47, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, H. Milk-derived bioactive peptides: From science to applications. J. Funct. Foods 2009, 1, 177–187. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; Paolella, S.; Mudgil, P.; Maqsood, S.; FitzGerald, R.J. Dipeptidyl peptidase IV (DPP-IV) inhibitory properties of camel milk protein hydrolysates generated with trypsin. J. Funct. Foods 2017, 34, 49–58. [Google Scholar] [CrossRef] [Green Version]
- García-Tejedor, A.; Castelló-Ruiz, M.; Gimeno-Alcañíz, J.V.; Manzanares, P.; Salom, J.B. In vivo antihypertensive mechanism of lactoferrin-derived peptides: Reversion of angiotensin I-and angiotensin II-induced hypertension in Wistar rats. J. Funct. Foods 2015, 15, 294–300. [Google Scholar] [CrossRef] [Green Version]
- Idowu, A.T.; Benjakul, S.; Sinthusamran, S.; Sookchoo, P.; Kishimura, H. Protein hydrolysate from salmon frames: Production, characteristics and antioxidative activity. J. Food Biochem. 2019, 43, e12734. [Google Scholar] [CrossRef]
- Quan, T.H.; Benjakul, S. Production and characterisation of duck albumen hydrolysate using enzymatic process. Int. J. Food Sci. Technol. 2019, 54, 3015–3023. [Google Scholar] [CrossRef]
- Jang, A.; Jo, C.; Kang, K.-S.; Lee, M. Antimicrobial and human cancer cell cytotoxic effect of synthetic angiotensin-converting enzyme (ACE) inhibitory peptides. Food Chem. 2008, 107, 327–336. [Google Scholar] [CrossRef]
- Sangsawad, P.; Roytrakul, S.; Yongsawatdigul, J. Angiotensin converting enzyme (ACE) inhibitory peptides derived from the simulated in vitro gastrointestinal digestion of cooked chicken breast. J. Funct. Foods 2017, 29, 77–83. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; Paolella, S.; Mudgil, P.; Maqsood, S.; FitzGerald, R.J. Identification of novel dipeptidyl peptidase IV (DPP-IV) inhibitory peptides in camel milk protein hydrolysates. Food Chem. 2018, 244, 340–348. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wu, T.; Fang, L.; Liu, C.; Liu, X.; Li, H.; Shi, J.; Li, M.; Min, W. Anti-diabetic effect by walnut (Juglans mandshurica Maxim.)-derived peptide LPLLR through inhibiting α-glucosidase and α-amylase, and alleviating insulin resistance of hepatic HepG2 cells. J. Funct. Foods 2020, 69, 103944. [Google Scholar] [CrossRef]
- Fadimu, G.J.; Ghafoor, K.; Babiker, E.E.; Al-Juhaimi, F.; Abdulraheem, R.A.; Adenekan, M.K. Ultrasound-assisted process for optimal recovery of phenolic compounds from watermelon (Citrullus lanatus) seed and peel. J. Food Meas. Charact. 2020, 14, 1784–1793. [Google Scholar] [CrossRef]
- Abdel-Hamid, M.; Otte, J.; De Gobba, C.; Osman, A.; Hamad, E. Angiotensin I-converting enzyme inhibitory activity and antioxidant capacity of bioactive peptides derived from enzymatic hydrolysis of buffalo milk proteins. Int. Dairy J. 2017, 66, 91–98. [Google Scholar] [CrossRef]
- Wu, J.; Aluko, R.E.; Muir, A.D. Production of angiotensin I-converting enzyme inhibitory peptides from defatted canola meal. Bioresour. Technol. 2009, 100, 5283–5287. [Google Scholar] [CrossRef]
- Shu, G.; Huang, J.; Bao, C.; Meng, J.; Chen, H.; Cao, J. Effect of Different Proteases on the Degree of Hydrolysis and Angiotensin I-Converting Enzyme-Inhibitory Activity in Goat and Cow Milk. Biomolecules 2018, 8, 101. [Google Scholar] [CrossRef] [Green Version]
- De Noni, I.; Cattaneo, S. Occurrence of β-casomorphins 5 and 7 in commercial dairy products and in their digests following in vitro simulated gastro-intestinal digestion. Food Chem. 2010, 119, 560–566. [Google Scholar] [CrossRef]
- Losacco, M.; Gallerani, R.; Gobbetti, M.; Minervini, F.; De Leo, F. Production of active angiotensin-I converting enzyme inhibitory peptides derived from bovine β-casein by recombinant DNA technologies. Biotechnol. J. 2007, 2, 1425–1434. [Google Scholar] [CrossRef]
- Daliri, E.B.-M.; Oh, D.H.; Lee, B.H. Bioactive peptides. Foods 2017, 6, 32. [Google Scholar] [CrossRef]
- Lee, S.Y.; Hur, S.J. Antihypertensive peptides from animal products, marine organisms, and plants. Food Chem. 2017, 228, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Kou, X.; Gao, J.; Xue, Z.; Zhang, Z.; Wang, H.; Wang, X. Purification and identification of antioxidant peptides from chickpea (Cicer arietinum L.) albumin hydrolysates. LWT—Food Sci. Technol. 2013, 50, 591–598. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, Y.; Li, G. ACE-inhibitory and antioxidant peptides from coconut cake albumin hydrolysates: Purification, identification and synthesis. RSC Adv. 2019, 9, 5925–5936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pravda, L.; Berka, K.; Svobodová Vařeková, R.; Sehnal, D.; Banáš, P.; Laskowski, R.A.; Koča, J.; Otyepka, M. Anatomy of enzyme channels. BMC Bioinform. 2014, 15, 379. [Google Scholar] [CrossRef] [Green Version]
- Adjonu, R.; Doran, G.; Torley, P.; Agboola, S. Screening of whey protein isolate hydrolysates for their dual functionality: Influence of heat pre-treatment and enzyme specificity. Food Chem. 2013, 136, 1435–1443. [Google Scholar] [CrossRef]
- Aluko, R.E. Food protein-derived peptides: Production, isolation, and purification. In Proteins in Food Processing; Elsevier: Amsterdam, The Netherlands, 2018; pp. 389–412. [Google Scholar]
- Gauthier, S.; Pouliot, Y. Functional and biological properties of peptides obtained by enzymatic hydrolysis of whey proteins. J. Dairy Sci. 2003, 86, E78–E87. [Google Scholar] [CrossRef]
- Garcia-Mora, P.; Peñas, E.; Frias, J.; Gomez, R.; Martinez-Villaluenga, C. High-pressure improves enzymatic proteolysis and the release of peptides with angiotensin I converting enzyme inhibitory and antioxidant activities from lentil proteins. Food Chem. 2015, 171, 224–232. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Sun, J.; Liu, S.; Bi, J.; Zhang, C.; Yang, Q. Ultrasonic-assisted enzymolysis to improve the antioxidant activities of peanut (Arachin conarachin L.) antioxidant hydrolysate. Int. J. Mol. Sci. 2012, 13, 9051–9068. [Google Scholar] [CrossRef] [Green Version]
- Fadimu, G.J.; Farahnaky, A.; Gill, H.; Truong, T. Influence of ultrasonic pretreatment on structural properties and biological activities of lupin protein hydrolysate. Int. J. Food Sci. Technol. 2022, 57, 1729–1738. [Google Scholar] [CrossRef]
- Fadimu, G.J.; Gill, H.; Farahnaky, A.; Truong, T. Investigating the Impact of Ultrasound Pretreatment on the Physicochemical, Structural, and Antioxidant Properties of Lupin Protein Hydrolysates. Food Bioprocess Technol. 2021, 14, 2004–2019. [Google Scholar] [CrossRef]
- Fadimu, G.J.; Gill, H.; Farahnaky, A.; Truong, T. Improving the enzymolysis efficiency of lupin protein by ultrasound pretreatment: Effect on antihypertensive, antidiabetic and antioxidant activities of the hydrolysates. Food Chem. 2022, 383, 132457. [Google Scholar] [CrossRef]
- Abadía-García, L.; Castaño-Tostado, E.; Ozimek, L.; Romero-Gómez, S.; Ozuna, C.; Amaya-Llano, S.L. Impact of ultrasound pretreatment on whey protein hydrolysis by vegetable proteases. Innov. Food Sci. Emerg. Technol. 2016, 37, 84–90. [Google Scholar] [CrossRef]
- Zhu, K.-X.; Su, C.-Y.; Guo, X.-N.; Peng, W.; Zhou, H.-M. Influence of ultrasound during wheat gluten hydrolysis on the antioxidant activities of the resulting hydrolysate. Int. J Food Sci. Technol. 2011, 46, 1053. [Google Scholar] [CrossRef]
- Chandrapala, J.; Zisu, B.; Kentish, S.; Ashokkumar, M. The effects of high-intensity ultrasound on the structural and functional properties of α-Lactalbumin, β-Lactoglobulin and their mixtures. Food Res. Int. 2012, 48, 940–943. [Google Scholar] [CrossRef]
- Moo-Young, M. Comprehensive Biotechnology; Elsevier: Amsterdam, The Netherlands, 2019; Volume 3, pp. 1–13. [Google Scholar]
- FitzGerald, R.; O’Cuinn, G. Enzymatic debittering of food protein hydrolysates. Biotechnol. Adv. 2006, 24, 234–237. [Google Scholar] [CrossRef]
- Zhang, T.; Jiang, B.; Miao, M.; Mu, W.; Li, Y. Combined effects of high-pressure and enzymatic treatments on the hydrolysis of chickpea protein isolates and antioxidant activity of the hydrolysates. Food Chem. 2012, 135, 904–912. [Google Scholar] [CrossRef]
- Ruiz-Ruiz, J.; Davila-Ortiz, G.; Chel-Guerrero, L.; Betancur-Aacona, D. Angiotensin I-converting enzyme inhibitory and antioxidant peptide fractions from hard-to-cook bean enzymatic hydrolysates. J. Food Biochem. 2013, 37, 26–35. [Google Scholar] [CrossRef]
- García-Tejedor, A.; Saánchez-Rivera, L.; Castelló-Ruiz, M.; Recio, I.; Salom, J.B.; Manzanares, P. Novel antihypertensive lactoferrin-derived peptides produced by Kluyveromyces marxianus: Gastrointestinal stability profile and in vivo angiotensin I-converting enzyme (ACE) inhibition. J. Agric. Food Chem. 2014, 62, 1609–1616. [Google Scholar] [CrossRef]
- He, R.; Alashi, A.; Malomo, S.A.; Girgih, A.T.; Chao, D.; Ju, X.; Aluko, R.E. Antihypertensive and free radical scavenging properties of enzymatic rapeseed protein hydrolysates. Food Chem. 2013, 141, 153–159. [Google Scholar] [CrossRef]
- Xue, Z.; Wen, H.; Zhai, L.; Yu, Y.; Li, Y.; Yu, W.; Cheng, A.; Wang, C.; Kou, X. Antioxidant activity and anti-proliferative effect of a bioactive peptide from chickpea (Cicer arietinum L.). Food Res. Int. 2015, 77, 75–81. [Google Scholar] [CrossRef]
- Shazly, A.B.; He, Z.; El-Aziz, M.A.; Zeng, M.; Zhang, S.; Qin, F.; Chen, J. Fractionation and identification of novel antioxidant peptides from buffalo and bovine casein hydrolysates. Food Chem. 2017, 232, 753–762. [Google Scholar] [CrossRef]
- Girgih, A.T.; He, R.; Malomo, S.; Offengenden, M.; Wu, J.; Aluko, R.E. Structural and functional characterization of hemp seed (Cannabis sativa L.) protein-derived antioxidant and antihypertensive peptides. J. Funct. Foods 2014, 6, 384–394. [Google Scholar] [CrossRef]
- Zhang, H.; Yokoyama, W.H.; Zhang, H. Concentration-dependent displacement of cholesterol in micelles by hydrophobic rice bran protein hydrolysates. J. Sci. Food Agric. 2012, 92, 1395–1401. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, R.; Ma, H.; Chen, S. Isolation and identification of dipeptidyl peptidase IV-inhibitory peptides from trypsin/chymotrypsin-treated goat milk casein hydrolysates by 2D-TLC and LC–MS/MS. J. Agric. Food Chem. 2015, 63, 8819–8828. [Google Scholar] [CrossRef]
- Ahmed, A.S.; El-Bassiony, T.; Elmalt, L.M.; Ibrahim, H.R. Identification of potent antioxidant bioactive peptides from goat milk proteins. Food Res. Int. 2015, 74, 80–88. [Google Scholar] [CrossRef]
- Wali, A.; Yanhua, G.; Ishimov, U.; Yili, A.; Aisa, H.A.; Salikhov, S. Isolation and identification of three novel antioxidant peptides from the Bactrian camel milk Hydrolysates. Int J. Pept. Res. Ther. 2019, 26, 641–650. [Google Scholar] [CrossRef]
- Sonklin, C.; Alashi, M.A.; Laohakunjit, N.; Kerdchoechuen, O.; Aluko, R.E. Identification of antihypertensive peptides from mung bean protein hydrolysate and their effects in spontaneously hypertensive rats. J. Funct Foods 2020, 64, 103635. [Google Scholar] [CrossRef]
- Li-Chan, E.C.; Hunag, S.-L.; Jao, C.-L.; Ho, K.-P.; Hsu, K.-C. Peptides derived from Atlantic salmon skin gelatin as dipeptidyl-peptidase IV inhibitors. J. Agric. Food Chem. 2012, 60, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Darewicz, M.; Borawska, J.; Vegarud, G.E.; Minkiewicz, P.; Iwaniak, A. Angiotensin I-converting enzyme (ACE) inhibitory activity and ACE inhibitory peptides of salmon (Salmo salar) protein hydrolysates obtained by human and porcine gastrointestinal enzymes. Int. J. Mol. Sci. 2014, 15, 14077–14101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikoo, M.; Benjakul, S.; Xu, X. Antioxidant and cryoprotective effects of Amur sturgeon skin gelatin hydrolysate in unwashed fish mince. Food Chem. 2015, 181, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Nakano, D.; Ogura, K.; Miyakoshi, M.; ISHII, F.; Kawanishi, H.; Kurumazuka, D.; Kwak, C.-j.; Ikemura, K.; Takaoka, M.; Moriguchi, S. Antihypertensive effect of angiotensin I-converting enzyme inhibitory peptides from a sesame protein hydrolysate in spontaneously hypertensive rats. Biosci. Biotechnol. Biochem. 2006, 70, 1118–1126. [Google Scholar] [CrossRef] [Green Version]
- Nimalaratne, C.; Bandara, N.; Wu, J. Purification and characterization of antioxidant peptides from enzymatically hydrolyzed chicken egg white. Food Chem. 2015, 188, 467–472. [Google Scholar] [CrossRef]
- Zamani, A.; Madani, R.; Rezaei, M.; Benjakul, S. Antioxidative activitiy of protein hydrolysate from the muscle of common kilka (Clupeonella cultriventris caspia) prepared using the purified trypsin from common kilka intestine. J. Aquat. Food Prod. Technol. 2017, 26, 2–16. [Google Scholar] [CrossRef]
- Pihlanto-Leppälä, A. Bioactive peptides derived from bovine whey proteins: Opioid and ace-inhibitory peptides. Trends Food Sci. Technol. 2000, 11, 347–356. [Google Scholar] [CrossRef]
- Sarmadi, B.H.; Ismail, A. Antioxidative peptides from food proteins: A review. Peptides 2010, 31, 1949–1956. [Google Scholar] [CrossRef]
- Rizzello, C.; Cassone, A.; Di Cagno, R.; Gobbetti, M. Synthesis of angiotensin I-converting enzyme (ACE)-inhibitory peptides and γ-aminobutyric acid (GABA) during sourdough fermentation by selected lactic acid bacteria. J. Agric. Food Chem. 2008, 56, 6936–6943. [Google Scholar] [CrossRef]
- Gan, R.Y.; Li, H.B.; Gunaratne, A.; Sui, Z.Q.; Corke, H. Effects of fermented edible seeds and their products on human health: Bioactive components and bioactivities. Compr. Rev. Food Sci. 2017, 16, 489–531. [Google Scholar] [CrossRef]
- Fernández-Musoles, R.; Manzanares, P.; Burguete, M.C.; Alborch, E.; Salom, J.B. In vivo angiotensin I-converting enzyme inhibition by long-term intake of antihypertensive lactoferrin hydrolysate in spontaneously hypertensive rats. Food Res. Int. 2013, 54, 627–632. [Google Scholar] [CrossRef]
- Wattanasiritham, L.; Theerakulkait, C.; Wickramasekara, S.; Maier, C.S.; Stevens, J.F. Isolation and identification of antioxidant peptides from enzymatically hydrolyzed rice bran protein. Food Chem. 2016, 192, 156–162. [Google Scholar] [CrossRef]
- Pihlanto, A.; Korhonen, H. Bioactive peptides from fermented foods and health promotion. In Advances in Fermented Foods and Beverages; Elsevier: Amsterdam, The Netherlands, 2015; pp. 39–74. [Google Scholar]
- Rahman, T.; Hosen, I.; Islam, M.T.; Shekhar, H.U. Oxidative stress and human health. Adv. Biosci. Biotechnol. 2012, 3, 997. [Google Scholar] [CrossRef] [Green Version]
- Ito, N.; Fukushima, S.; Tsuda, H. Carcinogenicity and modification of the carcinogenic response by BHA, BHT, and other antioxidants. CRC Crit. Rev. Toxicol. 1985, 15, 109–150. [Google Scholar] [CrossRef]
- Zou, T.-B.; He, T.-P.; Li, H.-B.; Tang, H.-W.; Xia, E.-Q. The structure-activity relationship of the antioxidant peptides from natural proteins. Molecules 2016, 21, 72. [Google Scholar] [CrossRef]
- Karnjanapratum, S.; O’Callaghan, Y.C.; Benjakul, S.; O’Brien, N. Antioxidant, immunomodulatory and antiproliferative effects of gelatin hydrolysate from unicorn leatherjacket skin. J. Sci. Food Agric. 2016, 96, 3220–3226. [Google Scholar] [CrossRef]
- Sae-leaw, T.; O’Callaghan, Y.C.; Benjakul, S.; O’Brien, N.M. Antioxidant, immunomodulatory and antiproliferative effects of gelatin hydrolysates from seabass (Lates calcarifer) skins. Int. J. Food Sci. Technol. 2016, 51, 1545–1551. [Google Scholar] [CrossRef]
- Ghafoor, K.; Özcan, M.M.; Al-Juhaimi, F.; Babiker, E.E.; Fadimu, G.J. Changes in quality, bioactive compounds, fatty acids, tocopherols, and phenolic composition in oven- and microwave-roasted poppy seeds and oil. LWT 2019, 99, 490–496. [Google Scholar] [CrossRef]
- Juhaimi, F.A.; Ghafoor, K.; Uslu, N.; Mohamed Ahmed, I.A.; Babiker, E.E.; Özcan, M.M.; Fadimu, G.J. The effect of harvest times on bioactive properties and fatty acid compositions of prickly pear (Opuntia ficus-barbarica A. Berger) fruits. Food Chem. 2020, 303, 125387. [Google Scholar] [CrossRef]
- Al-Shamsi, K.A.; Mudgil, P.; Hassan, H.M.; Maqsood, S. Camel milk protein hydrolysates with improved technofunctional properties and enhanced antioxidant potential in in vitro and in food model systems. J. Dairy Sci. 2018, 101, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Shen, Y.; Li, Y. Antioxidant activities of sorghum kafirin alcalase hydrolysates and membrane/gel filtrated fractions. Antioxidants 2019, 8, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, Q.; Ren, X.; Ma, H.; Li, S.; Xu, K.; Oladejo, A.O. Effect of low-frequency ultrasonic-assisted enzymolysis on the physicochemical and antioxidant properties of corn protein hydrolysates. J. Food Qual. 2017, 2017, 2784146. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Jin, Y.; Lin, S.; Jones, G.S.; Chen, F. Purification and identification of novel antioxidant peptides from egg white protein and their antioxidant activities. Food Chem. 2015, 175, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Wu, X.; Zhang, Y.; Li, X.; Ma, S.; Li, J. Purification and characterization of three antioxidant peptides from protein hydrolysate of grass carp (Ctenopharyngodon idella) skin. J. Funct. Foods 2015, 16, 234–242. [Google Scholar] [CrossRef]
- Chi, C.-F.; Hu, F.-Y.; Wang, B.; Ren, X.-J.; Deng, S.-G.; Wu, C.-W. Purification and characterization of three antioxidant peptides from protein hydrolyzate of croceine croaker (Pseudosciaena crocea) muscle. Food Chem. 2015, 168, 662–667. [Google Scholar] [CrossRef]
- Tkaczewska, J.; Bukowski, M.; Mak, P. Identification of antioxidant peptides in enzymatic hydrolysates of carp (Cyprinus carpio) skin gelatin. Molecules 2019, 24, 97. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; Li, B.; Hou, H.; Zhang, H.; Zhao, X. Identification of iron-chelating peptides from Pacific cod skin gelatin and the possible binding mode. J. Funct. Foods 2017, 35, 418–427. [Google Scholar] [CrossRef]
- Chi, C.F.; Wang, B.; Hu, F.Y.; Wang, Y.M.; Zhang, B.; Deng, S.G.; Wu, C.W. Purification and identification of three novel antioxidant peptides from protein hydrolysate of bluefin leatherjacket (Navodon septentrionalis) skin. Food Res. Int. 2015, 73, 124–129. [Google Scholar] [CrossRef]
- Guang, C.; Phillips, R.D.; Jiang, B.; Milani, F. Three key proteases–angiotensin-I-converting enzyme (ACE), ACE2 and renin–within and beyond the renin-angiotensin system. Arch. Cardiovasc. Dis. 2012, 105, 373–385. [Google Scholar] [CrossRef] [Green Version]
- Hong, F.; Ming, L.; Yi, S.; Zhanxia, L.; Yongquan, W.; Chi, L. The antihypertensive effect of peptides: A novel alternative to drugs? Peptides 2008, 29, 1062–1071. [Google Scholar] [CrossRef]
- Daliri, E.B.-M.; Lee, B.H.; Oh, D.H. Current perspectives on antihypertensive probiotics. Probiotics Antimicrob. 2017, 9, 91–101. [Google Scholar] [CrossRef]
- Ichimura, T.; Hu, J.; Aita, D.Q.; Maruyama, S. Angiotensin I-converting enzyme inhibitory activity and insulin secretion stimulative activity of fermented fish sauce. J. Biosci. Bioeng. 2003, 96, 496–499. [Google Scholar] [CrossRef]
- Martinez-Villaluenga, C.; Torino, M.I.; Martin, V.; Arroyo, R.; Garcia-Mora, P.; Estrella Pedrola, I.; Vidal-Valverde, C.; Rodriguez, J.M.; Frias, J. Multifunctional properties of soy milk fermented by Enterococcus faecium strains isolated from raw soy milk. J. Agric. Food Chem. 2012, 60, 10235–10244. [Google Scholar] [CrossRef]
- Phelan, M.; Kerins, D. The potential role of milk-derived peptides in cardiovascular disease. Food Funct. 2011, 2, 153–167. [Google Scholar] [CrossRef]
- Ono, S.; Hosokawa, M.; Miyashita, K.; Takahashi, K. Isolation of peptides with angiotensin I-converting enzyme inhibitory effect derived from hydrolysate of upstream chum salmon muscle. J. Food Sci. 2003, 68, 1611–1614. [Google Scholar] [CrossRef]
- Miner-Williams, W.M.; Stevens, B.R.; Moughan, P.J. Are intact peptides absorbed from the healthy gut in the adult human? Nutr. Res. Rev. 2014, 27, 308–329. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Feng, X.; Lan, X.; Xu, Y.; Liao, D. Purification and identification of Angiotensin-I Converting Enzyme (ACE) inhibitory peptide from lizard fish (Saurida elongata) hydrolysate. J. Funct. Foods 2015, 13, 295–299. [Google Scholar] [CrossRef]
- Sila, A.; Haddar, A.; Martinez-Alvarez, O.; Bougatef, A. Angiotensin-I-converting enzyme inhibitory and antioxidant activities of protein hydrolysate from muscle of barbel (Barbus callensis). J. Chem. 2013, 2013, 545303. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, H.R.; Ahmed, A.S.; Miyata, T. Novel angiotensin-converting enzyme inhibitory peptides from caseins and whey proteins of goat milk. J. Adv. Res. 2017, 8, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Alhaj, O.A. Identification of potential ACE-inhibitory peptides from dromedary fermented camel milk. CyTA J. Food 2017, 15, 191–195. [Google Scholar] [CrossRef] [Green Version]
- Suwannapan, O.; Wachirattanapongmetee, K.; Thawornchinsombut, S.; Katekaew, S. Angiotensin I converting enzyme (ACE)-inhibitory peptides from Thai jasmine rice bran protein hydrolysates. Int. J. Food Sci. Technol. 2019, 55, 2441–2450. [Google Scholar] [CrossRef]
- Geerlings, A.; Villar, I.; Zarco, F.H.; Sánchez, M.; Vera, R.; Gomez, A.Z.; Boza, J.; Duarte, J. Identification and characterization of novel angiotensin-converting enzyme inhibitors obtained from goat milk. J. Dairy Sci. 2006, 89, 3326–3335. [Google Scholar] [CrossRef]
- Mojica, L.; de Mejia, E.G.; Granados-Silvestre, M.Á.; Menjivar, M. Evaluation of the hypoglycemic potential of a black bean hydrolyzed protein isolate and its pure peptides using in silico, in vitro and in vivo approaches. J. Funct. Foods 2017, 31, 274–286. [Google Scholar] [CrossRef]
- Ennaas, N.; Hammami, R.; Beaulieu, L.; Fliss, I. Purification and characterization of four antibacterial peptides from protamex hydrolysate of Atlantic mackerel (Scomber scombrus) by-products. Biochem. Biophys. Res. Commun. 2015, 462, 195–200. [Google Scholar] [CrossRef]
- Tang, W.; Zhang, H.; Wang, L.; Qian, H.; Qi, X. Targeted separation of antibacterial peptide from protein hydrolysate of anchovy cooking wastewater by equilibrium dialysis. Food Chem. 2015, 168, 115–123. [Google Scholar] [CrossRef]
- Théolier, J.; Hammami, R.; Labelle, P.; Fliss, I.; Jean, J. Isolation and identification of antimicrobial peptides derived by peptic cleavage of whey protein isolate. J. Funct. Foods 2013, 5, 706–714. [Google Scholar] [CrossRef]
- Li, M.; Zhang, Y.; Xia, S.; Ding, X. Finding and isolation of novel peptides with anti-proliferation ability of hepatocellular carcinoma cells from mung bean protein hydrolysates. J. Funct. Foods 2019, 62, 103557. [Google Scholar] [CrossRef]
- Marcela, G.-M.; Eva, R.-G.; Del Carmen, R.-R.M.; Rosalva, M.-E. Evaluation of the antioxidant and antiproliferative effects of three peptide fractions of germinated soybeans on breast and cervical cancer cell lines. Plant Foods Hum. Nutr. 2016, 71, 368–374. [Google Scholar] [CrossRef]
- Lee, S.H.; Qian, Z.J.; Kim, S.K. A novel angiotensin I converting enzyme inhibitory peptide from tuna frame protein hydrolysate and its antihypertensive effect in spontaneously hypertensive rats. Food Chem. 2010, 118, 96–102. [Google Scholar] [CrossRef]
- Chalamaiah, M.; Jyothirmayi, T.; Diwan, P.V.; Kumar, B.D. Antiproliferative, ACE-inhibitory and functional properties of protein hydrolysates from rohu (Labeo rohita) roe (egg) prepared by gastrointestinal proteases. J. Food Sci. Technol. 2015, 52, 8300–8307. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Zhao, J.; Wang, X.; Qayum, A.; Hussain, M.A.; Liang, G.; Hou, J.; Jiang, Z. Novel angiotensin converting enzyme inhibitory peptides from fermented bovine milk started by Lactobacillus helveticus KLDS. 31 and Lactobacillus casei KLDS. 105: Purification, identification and interaction mechanism. Front. Microbiol. 2019, 10, 2643. [Google Scholar] [CrossRef]
- Stadnik, J.; Kęska, P. Meat and fermented meat products as a source of bioactive peptides. Acta Sci. Pol. Technol. Aliment. 2015, 14, 181–190. [Google Scholar] [CrossRef]
- Priyanto, A.D.; Doerksen, R.J.; Chang, C.-I.; Sung, W.-C.; Widjanarko, S.B.; Kusnadi, J.; Lin, Y.-C.; Wang, T.-C.; Hsu, J.-L. Screening, discovery, and characterization of angiotensin-I converting enzyme inhibitory peptides derived from proteolytic hydrolysate of bitter melon seed proteins. J. Proteom. 2015, 128, 424–435. [Google Scholar] [CrossRef]
- Tan, S.Y.; Wong, J.L.M.; Sim, Y.J.; Wong, S.S.; Elhassan, S.A.M.; Tan, S.H.; Lim, G.P.L.; Tay, N.W.R.; Annan, N.C.; Bhattamisra, S.K. Type 1 and 2 diabetes mellitus: A review on current treatment approach and gene therapy as potential intervention. Diabetes Metab Syndr. Clin. Res. Rev. 2019, 13, 364–372. [Google Scholar] [CrossRef]
- Gharravi, A.M.; Jafar, A.; Ebrahimi, M.; Mahmodi, A.; Pourhashemi, E.; Haseli, N.; Talaie, N.; Hajiasgarli, P. Current status of stem cell therapy, scaffolds for the treatment of diabetes mellitus. Diabetes Metab. Syndr. Clin. Res. Rev. 2018, 12, 1133–1139. [Google Scholar] [CrossRef]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2009, 33, S62–S67. [Google Scholar] [CrossRef] [Green Version]
- Dujic, T.; Causevic, A.; Bego, T.; Malenica, M.; Velija-Asimi, Z.; Pearson, E.; Semiz, S. Organic cation transporter 1 variants and gastrointestinal side effects of metformin in patients with Type 2 diabetes. Diabet. Med. 2016, 33, 511–514. [Google Scholar] [CrossRef] [Green Version]
- Thulé, P.M.; Umpierrez, G. Sulfonylureas: A new look at old therapy. Curr. Diabetes Rep. 2014, 14, 473. [Google Scholar] [CrossRef]
- Thong, K.; Gupta, P.S.; Blann, A.; Ryder, R. The influence of age and metformin treatment status on reported gastrointestinal side effects with liraglutide treatment in type 2 diabetes. Diabetes Res. Clin. Pract. 2015, 109, 124–129. [Google Scholar] [CrossRef]
- Meier, J.J.; Nauck, M.A. Risk of pancreatitis in patients treated with incretin-based therapies. Diabetologia 2014, 57, 1320–1324. [Google Scholar] [CrossRef] [Green Version]
- Ahrén, B. Dipeptidyl peptidase-4 inhibitors: Clinical data and clinical implications. Diabetes Care 2007, 30, 1344–1350. [Google Scholar] [CrossRef] [Green Version]
- Lacroix, I.M.; Li-Chan, E.C. Food-derived dipeptidyl-peptidase IV inhibitors as a potential approach for glycemic regulation—Current knowledge and future research considerations. Trends Food Sci. Technol. 2016, 54, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.-Y.; Wang, H.-P.; Wang, H.-J.; Yao, J.-M.; Wu, X.-Y.; Dong, J.-J.; Liao, L. Efficacy and safety of sitagliptin in patients with type 2 diabetes mellitus: A meta-analysis. Int. J. Clin. Exp. Med. 2016, 9, 11202–11210. [Google Scholar]
- Jao, C.-L.; Hung, C.-C.; Tung, Y.-S.; Lin, P.-Y.; Chen, M.-C.; Hsu, K.-C. The development of bioactive peptides from dietary proteins as a dipeptidyl peptidase IV inhibitor for the management of type 2 diabetes. Biomedicine 2015, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Mudgil, P.; Kamal, H.; Yuen, G.C.; Maqsood, S. Characterization and identification of novel antidiabetic and anti-obesity peptides from camel milk protein hydrolysates. Food Chem. 2018, 259, 46–54. [Google Scholar] [CrossRef] [PubMed]
- You, L.; Zhao, M.; Liu, R.H.; Regenstein, J.M. Antioxidant and antiproliferative activities of loach (Misgurnus anguillicaudatus) peptides prepared by papain digestion. J. Agric. Food Chem. 2011, 59, 7948–7953. [Google Scholar] [CrossRef] [PubMed]
- Hsu, K.-C.; Li-Chan, E.C.; Jao, C.-L. Antiproliferative activity of peptides prepared from enzymatic hydrolysates of tuna dark muscle on human breast cancer cell line MCF-7. Food Chem. 2011, 126, 617–622. [Google Scholar] [CrossRef]
- Naqash, S.Y.; Nazeer, R. In vitro antioxidant and antiproliferative activities of bioactive peptide isolated from Nemipterus Japonicus Backbone. Int. J. Food Prop. 2012, 15, 1200–1211. [Google Scholar] [CrossRef] [Green Version]
- Gashti, A.B.; Prakash, H. Characterization of antioxidant and antiproliferative activities of indian salmon (eleutheronema tetradactylum) protein hydrolysates. Int. J. Pharm. Pharm. Sci. 2016, 8, 102–108. [Google Scholar]
- Kandyliari, A.; Golla, J.P.; Chen, Y.; Papandroulakis, N.; Kapsokefalou, M.; Vasiliou, V. Antiproliferative activity of protein hydrolysates derived from fish by-products on human colon and breast cancer cells. In Proceedings of the Nutrition Society; Cambridge Press: Cambridge, UK, 2020. [Google Scholar]
- Rai, M.; Pandit, R.; Gaikwad, S.; Kövics, G. Antimicrobial peptides as natural bio-preservative to enhance the shelf-life of food. J. Food Sci. Technol. 2016, 53, 3381–3394. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Cui, X.; Fu, Y.; Zhang, J.; Zhou, Y.; Sun, Y.; Wang, X.; Li, Y.; Liu, Q.; Chen, T. Antimicrobial activity and mechanism of the human milk-sourced peptide casein201. Biochem. Biophys. Res. Commun. 2017, 485, 698–704. [Google Scholar] [CrossRef]
- Olatunde, O.O.; Benjakul, S. Natural preservatives for extending the shelf-life of seafood: A revisit. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1595–1612. [Google Scholar] [CrossRef] [Green Version]
- Floris, R.; Recio, I.; Berkhout, B.; Visser, S. Antibacterial and antiviral effects of milk proteins and derivatives thereof. Curr. Pharm. Des. 2003, 9, 1257–1275. [Google Scholar] [CrossRef]
- Piotto, S.P.; Sessa, L.; Concilio, S.; Iannelli, P. YADAMP: Yet another database of antimicrobial peptides. Int. J. Antimicrob. Agents 2012, 39, 346–351. [Google Scholar] [CrossRef]
- Guinane, C.M.; Kent, R.M.; Norberg, S.; O’Connor, P.M.; Cotter, P.D.; Hill, C.; Fitzgerald, G.F.; Stanton, C.; Ross, R.P. Generation of the antimicrobial peptide caseicin A from casein by hydrolysis with thermolysin enzymes. Int. Dairy J. 2015, 49, 1–7. [Google Scholar] [CrossRef]
- Seo, J.-K.; Lee, M.J.; Go, H.-J.; Kim, Y.J.; Park, N.G. Antimicrobial function of the GAPDH-related antimicrobial peptide in the skin of skipjack tuna, Katsuwonus pelamis. Fish Shellfish Immunol. 2014, 36, 571–581. [Google Scholar] [CrossRef]
- Abuine, R.; Rathnayake, A.U.; Byun, H.-G. Biological activity of peptides purified from fish skin hydrolysates. Fish Aquat. Sci. 2019, 22, 10. [Google Scholar] [CrossRef] [Green Version]
- Dissanayake, M.; Vasiljevic, T. Functional properties of whey proteins affected by heat treatment and hydrodynamic high-pressure shearing. J. Dairy Sci. 2009, 92, 1387–1397. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Yang, J.; Abliz, A.; Yuan, F.; Gao, Y. Influence of thermal treatment on physical, structural characteristics and stability of lactoferrin, EGCG and high methoxylated pectin aggregates. LWT 2020, 125, 109221. [Google Scholar] [CrossRef]
- Korczek, K.R.; Tkaczewska, J.; Duda, I.; Migdał, W. Effect of Heat Treatment on the Antioxidant and Antihypertensive Activity as Well as in vitro Digestion Stability of Mackerel (Scomber scombrus) Protein Hydrolysates. J. Aquat. Food Prod. Technol. 2020, 29, 73–89. [Google Scholar] [CrossRef]
- Islam, M.N.; Zhang, M.; Adhikari, B. The inactivation of enzymes by ultrasound—A review of potential mechanisms. Food Rev. Int. 2014, 30, 1–21. [Google Scholar] [CrossRef]
- Han, Z.; Cai, M.-J.; Cheng, J.-H.; Sun, D.-W. Effects of electric fields and electromagnetic wave on food protein structure and functionality: A review. Trends Food Sci. Technol. 2018, 75, 1–9. [Google Scholar] [CrossRef]
- Uluko, H.; Zhang, S.; Liu, L.; Chen, J.; Sun, Y.; Su, Y.; Li, H.; Cui, W.; Lv, J. Effects of microwave and ultrasound pretreatments on enzymolysis of milk protein concentrate with different enzymes. Int. J. Food Sci. Technol. 2013, 48, 2250–2257. [Google Scholar] [CrossRef]
- Guerra-Almonacid, C.M.; Torruco-Uco, J.G.; Murillo-Arango, W.; Méndez-Arteaga, J.J.; Rodríguez-Miranda, J. Effect of ultrasound pretreatment on the antioxidant capacity and antihypertensive activity of bioactive peptides obtained from the protein hydrolysates of Erythrina edulis. Emir. J. Food Agric. 2019, 31, 288–296. [Google Scholar] [CrossRef]
- Wali, A.; Ma, H.; Shahnawaz, M.; Hayat, K.; Xiaong, J.; Jing, L. Impact of Power Ultrasound on Antihypertensive Activity, Functional Properties, and Thermal Stability of Rapeseed Protein Hydrolysates. J. Chem. 2017, 2017, 4373859. [Google Scholar] [CrossRef]
- Lin, S.; Liang, R.; Li, X.; Xing, J.; Yuan, Y. Effect of pulsed electric field (PEF) on structures and antioxidant activity of soybean source peptides-SHCMN. Food Chem. 2016, 213, 588–594. [Google Scholar] [CrossRef]
- Lin, S.; Liang, R.; Xue, P.; Zhang, S.; Liu, Z.; Dong, X. Antioxidant activity improvement of identified pine nut peptides by pulsed electric field (PEF) and the mechanism exploration. LWT 2017, 75, 366–372. [Google Scholar] [CrossRef]
- Koirala, S.; Prathumpai, W.; Anal, A.K. Effect of ultrasonication pretreatment followed by enzymatic hydrolysis of caprine milk proteins on peptide antioxidant and angiotensin converting enzyme (ACE) inhibitory activity. Int. Dairy J. 2021, 118, 105026. [Google Scholar] [CrossRef]
- Mason, T.J.; Riera, E.; Vercet, A.; Lopez-Buesa, P. Application of ultrasound. In Emerging Technologies for Food Processing; Elsevier: Amsterdam, The Netherlands, 2005; pp. 323–351. [Google Scholar]
- Gulzar, S.; Raju, N.; Nagarajarao, R.C.; Benjakul, S. Oil and pigments from shrimp processing by-products: Extraction, composition, bioactivities and its application-A review. Trends Food Sci. Technol. 2020, 100, 307–319. [Google Scholar] [CrossRef]
- O’Donnell, C.; Tiwari, B.; Bourke, P.; Cullen, P. Effect of ultrasonic processing on food enzymes of industrial importance. Trends Food Sci. Technol. 2010, 21, 358–367. [Google Scholar] [CrossRef] [Green Version]
- Mitragotri, S.; Blankschtein, D.; Langer, R. Ultrasound-mediated transdermal protein delivery. Science 1995, 269, 850–853. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Park, H.; Seo, J.; Lee, S. Sonophoresis in transdermal drug deliverys. Ultrasonics 2014, 54, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Gallo, M.; Ferrara, L.; Naviglio, D. Application of ultrasound in food science and technology: A perspective. Foods 2018, 7, 164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torley, P.J.; Truong, T.T.; Bhandari, B. Ultrasound in Food Processing and Preservation. In Handbook of Food Preservation, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2020; p. 1072. [Google Scholar]
- Branden, C.; Tooze, J. Introduction to Protein Structure; Garland Science: New York, NY, USA, 1999; Volume 54, pp. 905–921. [Google Scholar]
- Jiang, L.; Wang, J.; Li, Y.; Wang, Z.; Liang, J.; Wang, R.; Chen, Y.; Ma, W.; Qi, B.; Zhang, M. Effects of ultrasound on the structure and physical properties of black bean protein isolates. Food Res. Int. 2014, 62, 595–601. [Google Scholar] [CrossRef]
- Saleem, R.; Ahmad, R. Effect of ultrasonication on secondary structure and heat induced gelation of chicken myofibrils. J. Food Sci. Technol. 2016, 53, 3340–3348. [Google Scholar] [CrossRef] [Green Version]
- Vidal, A.R.; da Fonseca Cechin, C.; Cansian, R.L.; de Oliveira Mello, R.; Schmidt, M.M.; Demiate, I.M.; Kempka, A.P.; Dornelles, R.C.P. Enzymatic hydrolysis (pepsin) assisted by ultrasound in the functional Properties of hydrolyzates from different collagens. Ciênc. Rural 2018, 48. [Google Scholar] [CrossRef] [Green Version]
- Yuanqing, H.; Min, C.; Lingling, S.; Quancai, S.; Pengyao, Y.; Rui, G.; Sijia, W.; Yuqing, D.; Haihui, Z.; Haile, M. Ultrasound Pretreatment Increases the Bioavailability of Dietary Proteins by Dissociating Protein Structure and Composition. Food Biophys. 2020, 15, 409–415. [Google Scholar] [CrossRef]
- Jin, J.; Ma, H.; Wang, K.; Yagoub, A.E.-G.A.; Owusu, J.; Qu, W.; He, R.; Zhou, C.; Ye, X. Effects of multi-frequency power ultrasound on the enzymolysis and structural characteristics of corn gluten meal. Ultrason. Sonochem. 2015, 24, 55–64. [Google Scholar] [CrossRef]
- Tao, Y.; Sun, D.-W. Enhancement of food processes by ultrasound: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 570–594. [Google Scholar] [CrossRef]
- Ozuna, C.; Paniagua-Martínez, I.; Castaño-Tostado, E.; Ozimek, L.; Amaya-Llano, S.L. Innovative applications of high-intensity ultrasound in the development of functional food ingredients: Production of protein hydrolysates and bioactive peptides. Food Res. Int. 2015, 77, 685–696. [Google Scholar] [CrossRef]
- Ashokkumar, M. Applications of ultrasound in food and bioprocessing. Ultrason. Sonochem. 2015, 25, 17–23. [Google Scholar] [CrossRef]
- Flores-Jiménez, N.T.; Ulloa, J.A.; Silvas, J.E.U.; Ramírez, J.C.R.; Ulloa, P.R.; Rosales, P.U.B.; Carrillo, Y.S.; Leyva, R.G. Effect of high-intensity ultrasound on the compositional, physicochemical, biochemical, functional and structural properties of canola (Brassica napus L.) protein isolate. Food Res. Int. 2019, 121, 947–956. [Google Scholar] [CrossRef]
- O’Sullivan, J.; Murray, B.; Flynn, C.; Norton, I. The effect of ultrasound treatment on the structural, physical and emulsifying properties of animal and vegetable proteins. Food Hydrocoll. 2016, 53, 141–154. [Google Scholar] [CrossRef] [Green Version]
- Zhao, F.; Liu, X.; Ding, X.; Dong, H.; Wang, W. Effects of High-Intensity Ultrasound Pretreatment on Structure, Properties, and Enzymolysis of Soy Protein Isolate. Molecules 2019, 24, 3637. [Google Scholar] [CrossRef] [Green Version]
- Xue, F.; Wu, Z.; Tong, J.; Zheng, J.; Li, C. Effect of combination of high-intensity ultrasound treatment and dextran glycosylation on structural and interfacial properties of buckwheat protein isolates. Biosci. Biotechnol. Biochem. 2017, 81, 1891–1898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanga, S.K.; Wang, J.; Orsat, V.; Raghavan, V. Effect of pulsed ultrasound, a green food processing technique, on the secondary structure and in-vitro digestibility of almond milk protein. Food Res. Int. 2020, 137, 109523. [Google Scholar] [CrossRef]
- Munir, M.; Nadeem, M.; Qureshi, T.M.; Gamlath, C.J.; Martin, G.J.; Hemar, Y.; Ashokkumar, M. Effect of sonication, microwaves and high-pressure processing on ACE-inhibitory activity and antioxidant potential of Cheddar cheese during ripening. Ultrason. Sonochem. 2020, 47, 105140. [Google Scholar] [CrossRef]
- Jia, J.; Ma, H.; Zhao, W.; Wang, Z.; Tian, W.; Luo, L.; He, R. The use of ultrasound for enzymatic preparation of ACE-inhibitory peptides from wheat germ protein. Food Chem. 2010, 119, 336–342. [Google Scholar] [CrossRef]
- Dabbour, M.; He, R.; Mintah, B.; Golly, M.K.; Ma, H. Ultrasound pretreatment of sunflower protein: Impact on enzymolysis, ACE-inhibition activity, and structure characterization. J. Food Process. Preserv. 2020, 44, e14398. [Google Scholar] [CrossRef]
- Jovanović, J.; Stefanović, A.; Žuža, M.; Jakovetić, S.; Šekuljica, N.; Bugarski, B.; Knežević-Jugović, Z. Improvement of antioxidant properties of egg white protein enzymatic hydrolysates by membrane ultrafiltration. Hem. Ind. 2016, 70, 419–428. [Google Scholar] [CrossRef]
- Norton, T.; Sun, D.-W. Recent advances in the use of high pressure as an effective processing technique in the food industry. Food Bioprocess Technol. 2008, 1, 2–34. [Google Scholar] [CrossRef]
- Lou, F.; Neetoo, H.; Chen, H.; Li, J. High hydrostatic pressure processing: A promising nonthermal technology to inactivate viruses in high-risk foods. Annu. Rev. Food Sci. Technol. 2015, 6, 389–409. [Google Scholar] [CrossRef]
- Abera, G. Review on high-pressure processing of foods. Cogent Food Agric. 2019, 5, 1568725. [Google Scholar] [CrossRef]
- Goyal, A.; Sharma, V.; Upadhyay, N.; Sihag, M.; Kaushik, R. High pressure processing and its impact on milk proteins: A review. J. Dairy Sci. Technol. 2018, 2, 12–20. [Google Scholar]
- Balny, C.; Masson, P. Effects of high pressure on proteins. Food Rev. Int. 1993, 9, 611–628. [Google Scholar] [CrossRef]
- Messens, W.; Van Camp, J.; Huyghebaert, A. The use of high pressure to modify the functionality of food proteins. Trends Food Sci. Technol. 1997, 8, 107–112. [Google Scholar] [CrossRef]
- Chakraborty, S.; Kaushik, N.; Rao, P.S.; Mishra, H. High-pressure inactivation of enzymes: A review on its recent applications on fruit purees and juices. Compr. Rev. Food Sci. Food Saf. 2014, 13, 578–596. [Google Scholar] [CrossRef]
- Hendrickx, M.E.; Knorr, D. Ultra High Pressure Treatment of Foods; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Chicón, R.; Belloque, J.; Recio, I.; López-Fandiño, R. Influence of high hydrostatic pressure on the proteolysis of [Beta]-lactoglobulin A by trypsin. J. Dairy Res. 2006, 73, 121. [Google Scholar] [CrossRef]
- Quirós, A.; Chichón, R.; Recio, I.; López-Fandiño, R. The use of high hydrostatic pressure to promote the proteolysis and release of bioactive peptides from ovalbumin. Food Chem. 2007, 104, 1734–1739. [Google Scholar] [CrossRef]
- Olatunde, O.O.; Benjakul, S. Nonthermal Processes for Shelf-Life Extension of Seafoods: A Revisit. Compr. Rev. Food Sci. Food Saf. 2018, 17, 892–904. [Google Scholar] [CrossRef] [Green Version]
- Shiekh, K.A.; Benjakul, S. Effect of pulsed electric field treatments on melanosis and quality changes of Pacific white shrimp during refrigerated storage. J. Food Process. Preserv. 2020, 44, e14292. [Google Scholar] [CrossRef]
- Kumar, D.; Chatli, M.K.; Singh, R.; Mehta, N.; Kumar, P. Antioxidant and antimicrobial activity of camel milk casein hydrolysates and its fractions. Small Rumin. Res. 2016, 139, 20–25. [Google Scholar] [CrossRef]
- Eissa, A.A. Structure and Function of Food Engineering; BoD–Books on Demand: Norderstedt, Germany, 2012. [Google Scholar]
- Zhong, K.; Hu, X.; Zhao, G.; Chen, F.; Liao, X. Inactivation and conformational change of horseradish peroxidase induced by pulsed electric field. Food Chem. 2005, 92, 473–479. [Google Scholar] [CrossRef]
- Riener, J.; Noci, F.; Cronin, D.A.; Morgan, D.J.; Lyng, J.G. Effect of high intensity pulsed electric fields on enzymes and vitamins in bovine raw milk. Int. J. Dairy Technol. 2009, 62, 1–6. [Google Scholar] [CrossRef]
- Gomaa, A.; Sedman, J.; Ismail, A. An investigation of the effect of microwave treatment on the structure and unfolding pathways of β-lactoglobulin using FTIR spectroscopy with the application of two-dimensional correlation spectroscopy (2D-COS). Vib. Spectrosc. 2013, 65, 101–109. [Google Scholar] [CrossRef]
- Li, Y.-Q. Structure changes of soybean protein isolates by pulsed electric fields. Phys. Procedia 2012, 33, 132–137. [Google Scholar] [CrossRef] [Green Version]
- Olatunde, O.O.; Singh, A.; Shiekh, K.A.; Nuthong, P.; Benjakul, S. Effect of High Voltage Cold Plasma on Oxidation, Physiochemical, and Gelling Properties of Myofibrillar Protein Isolate from Asian Sea Bass (Lates calcarifer). Foods 2021, 10, 326. [Google Scholar] [CrossRef]
- Battisti, A.; Ciasca, G.; Grottesi, A.; Tenenbaum, A. Thermal compaction of the intrinsically disordered protein tau: Entropic, structural, and hydrophobic factors. Phys. Chem. Chem. Phys. 2017, 19, 8435–8446. [Google Scholar] [CrossRef]
- Karoui, R.; Blecker, C. Fluorescence spectroscopy measurement for quality assessment of food systems—A review. Food Bioprocess Technol. 2011, 4, 364–386. [Google Scholar] [CrossRef]
- Qian, J.-Y.; Ma, L.-J.; Wang, L.-J.; Jiang, W. Effect of pulsed electric field on structural properties of protein in solid state. LWT 2016, 74, 331–337. [Google Scholar] [CrossRef]
- Xiang, B.Y.; Ngadi, M.O.; Ochoa-Martinez, L.A.; Simpson, M.V. Pulsed electric field-induced structural modification of whey protein isolate. Food Bioprocess Technol. 2011, 4, 1341–1348. [Google Scholar] [CrossRef]
- Lin, S.; Guo, Y.; You, Q.; Yin, Y.; Liu, J. Preparation of antioxidant peptide from egg white protein and improvement of its activities assisted by high-intensity pulsed electric field. J. Sci. Food Agric. 2012, 92, 1554–1561. [Google Scholar] [CrossRef]
- Perez, O.E.; Pilosof, A.M. Pulsed electric fields effects on the molecular structure and gelation of β-lactoglobulin concentrate and egg white. Food Res. Int. 2004, 37, 102–110. [Google Scholar] [CrossRef]


| Proteases | References |
|---|---|
| Flavourzyme | [22,23] |
| Trypsin | [23,42] |
| Chymotrypsin | [43] |
| Alcalase | [13,22,43,44,45] |
| Flavorase | [45] |
| Papain | [15,45] |
| Protamex | [30,46] |
| Pepsin | [15,23,47,48] |
| Neutrase | [43] |
| Bromelain | [33,49,50] |
| Corolase | [28,51] |
| Neutral protease | [52] |
| Savinase | [28] |
| Thermolysin | [53] |
| Protease P | [54] |
| Group | Source | Method of Preparation | Amino Acid Sequence | Major Findings | References |
|---|---|---|---|---|---|
| Plant protein | Corn protein | Hydrolysis using alcalase | SGV, LLPH, NGGGA | Peptides identified in the study showed strong antioxidant activity | Liang, Ren, Ma, Li, Xu, and Oladejo [72] |
| Chickpea albumin | Hydrolysis using flavourzyme and alcalase | RQSHFANAQP | Strongest antioxidative peptides were identified in the fractionated chickpea hydrolysate | Kou, Gao, Xue, Zhang, Wang, and Wang [22] | |
| Coconut cake albumin hydrolysate | Hydrolysis using alcalase, trypsin, pepsin, and flavourzyme | KAQYPYV, KIIIYN, KILIYG | Identified peptides showed strong ion chelating ability and substantial superoxide radical scavenging activity | Zheng, Li, and Li [23] | |
| Rice endosperm protein | Hydrolysis using alcalase, neutrase, papain, flavorase, and chymotrypsin | FRDEHKK, KHDRGDEF | Most potent antioxidant peptides were released by neutrase | Zhang et al. [45] | |
| Animal protein | Goat milk casein | Hydrolysis using pepsin | TVNREQL, VNQELAYFYPQLFRQ, DMESTEVF, QSLVYPFTGPI | Protein hydrolysates showed strong antioxidant activity. Four potent antioxidant peptides were identified | Ahmed et al. [47] |
| Buffalo milk | Hydrolysis using papain, pepsin, and trypsin | KFQ, YPSG, HPFA | Antioxidant peptides were identified in papain hydrolysates | Abdel-Hamid, Otte, De Gobba, Osman, and Hamad [15] | |
| Buffalo casein | Hydrolysis using alcalase and trypsin | RELEE, TVA, MEDNKQ, EQL | Peptides with molecular weight below 1 KDa exhibited strongest antioxidant activity | Shazly et al. [43] | |
| Egg white protein | Hydrolysis using alcalase | FFGFN, DHTKE, MPDAHL | Out of the three identified peptides, DHTKE showed highest ORAC | Liu et al. [73] | |
| Chicken egg white | Hydrolysis using protease P | DEDTQAMP, AEERYP | Only two of the sixteen identified peptides showed very strong oxygen radical absorbance capacity (ORAC) | Nimalaratne et al. [54] | |
| Camel milk | Hydrolysis using papain, trypsin, alcalase, and pepsin | RLDGQGRPRVWLGR, TPDNIDIWLGGIAEPQVKR, VAYSDDGENWTEYRDQGAVEGK | Peptides showed strong DPPH and ABTS activities | Wali et al. [48] | |
| Fish and fish products | Seabass skin | Hydrolysis using alcalase | GLPGPA, GATGPGGPLGPA, VLGPP, GLGPLGPV | Hydrolysates showed strong DPPH radical scavenging activity | Sae-leaw et al. [67] |
| Grass carp | Hydrolysis using alcalase | PYSFK, GFGPEL, GGRP | Three isolated peptides exhibited high ABTS, DPPH, and hydroxyl radical activities in a dose-dependent manner | Cai et al. [74] | |
| Unicorn leatherjacket | - | GPGPVG, LPGPAG, LAGPVG, GGPLG | Isolated peptides showed cellular antioxidant activity by protecting H2O2-induced DNA damage | Karnjanapratum et al. [66] | |
| Croceine croaker muscle | Hydrolysis using alcalase | VLYEE, YLMSR, MILMR | YLMSR exhibited highest DPPH, superoxide, and ABTS activity | Chi et al. [75] | |
| Carp skin gelatin | Hydrolysis using protamex | AY | Peptide identified is responsible for high antioxidant activity recorded | Tkaczewska et al. [76] | |
| Pacific cod | Hydrolysis using trypsin | AGPAGPAGAR, GPAGPHGPPGKDGR, AGPHGPPGKDGR | Identified peptide exhibited high iron chelating activity | Wu et al. [77] | |
| Bluefin leatherjacket (Navodon septentrionalis) | Hydrolysis using alcalase, neutrase, and flavourzyme | FIGP, GPGGFI, GSGGL | All identified peptides contributed to the DPPH and hydroxyl radical scavenging activity of the hydrolysate | Chi et al. [78] | |
| Amur sturgeon | Hydrolysis using alcalase and flavourzyme | PAGT | The peptide exhibited DPPH, ABTS, and hydroxyl radical scavenging abilities | Nikoo et al. [52] |
| Biological Activity | Food Source | Method of Preparation | Peptide Sequence | Major Findings | References |
|---|---|---|---|---|---|
| ACE-inhibitory peptides | Fermented camel milk | Fermentation using L. helveticus and L. acidophilus | LSLSQF, KVLVPQ, FQEPVPDPVR, LENLHLPLPL, KVLPVPQQMVPYPQ, VMVPFLQPK | Seven ACE-inhibitory peptides identified in L. helveticus sample and 3 from L. acidophilus sample | Alhaj [90] |
| Sesame protein powder | Enzymatic hydrolysis using thermolysin | LVY, LSA, LKY, IVY, MLPAY | Identified peptides had strong antihypertensive effect on spontaneously hypertensive rats | Nakano, Ogura, Miyakoshi, ISHII, Kawanishi, Kurumazuka, Kwak, Ikemura, Takaoka, and Moriguchi [53] | |
| Mung bean protein | Enzymatic hydrolysis using bromelain | LRLESF, LPRL, HLNVVHEN, YADLVF, PGSGCAGTDL | Five ACE-inhibitory peptides identified from molecular weight fraction below 1 KDa and LRLESF was the most potent peptide | Sonklin, Alashi, Laohakunjit, Kerdchoechuen, and Aluko [49] | |
| Salmon protein | Enzymatic hydrolysis using corolase | IWHHT, ALPHA, IVY, VW, VPW, TVY, IW, IY | Eleven ACE-inhibitory peptides identified from in vitro, ex vivo, and in silico studies of salmon protein hydrolysate | Darewicz, Borawska, Vegarud, Minkiewicz, and Iwaniak [51] | |
| Rice bran protein | Enzymatic fermentation using protease G6 | GSGYF | Identified peptide showed strong ACE-inhibitory activity with IC50 value close to that of captopril | Suwannapan, Wachirattanapongmetee, Thawornchinsombut, and Katekaew [91] | |
| Buffalo milk | Enzymatic hydrolysis using pepsin, papain, and trypsin | IPPK, QPPQ, FPGPIPK, IVPN | ACE-inhibitory peptides were identified only in papain hydrolysates | Abdel-Hamid, Otte, De Gobba, Osman, and Hamad [15] | |
| Coconut cake albumin hydrolysate | Enzymatic hydrolysis using alcalase, trypsin, and pepsin | KAQYPYV, KIIIYN, KILIYG | Identified peptides showed strong ACE-inhibitory potential and KAQYPYV was stable against gastrointestinal digestion enzymes | Zheng, Li, and Li [23] | |
| Goat milk | Enzymatic hydrolysis using alcalase | SLPQ, TGPIPN, SQPK | ACE-inhibitory peptides identified had high IC50 values and TGPIPN passed monolayer of Caco-2 cells intact in small quantity | Geerlings, Villar, Zarco, Sánchez, Vera, Gomez, Boza, and Duarte [92] | |
| Antidiabetic peptides | Camel milk protein | Enzymatic hydrolysis using trypsin | LPVPQWK | Potent and unique peptide with DPP-IV-inhibitory activity was identified in camel milk protein hydrolysate for the first time | Nongonierma, Paolella, Mudgil, Maqsood, and FitzGerald [6] |
| Black bean | Enzymatic hydrolysis using alcalase | AKSPLF, ATNPLF, FEELN, LSVSVL | Protein hydrolysate from black bean caused a reduction in blood glucose of hyperglycemic rat | Mojica, de Mejia, Granados-Silvestre, and Menjivar [93] | |
| Atlantic salmon skin gelatin | Enzymatic hydrolysis using bromelain, flavourzyme, and alcalase | GPGA, GPAE | Flavourzyme hydrolysate at 6% enzyme substrate ratio showed higher dipeptidyl peptidase activity than alcalase and bromelain hydrolysate | Li-Chan, Hunag, Jao, Ho, and Hsu [50] | |
| Camel milk protein | Enzymatic hydrolysis using trypsin | LPVP, MPVQA | Nine novel DPP-IV peptides were identified in the hydrolysate and the most potent two had IC50 values comparable to that of pure peptides | Nongonierma, Paolella, Mudgil, Maqsood, and FitzGerald [12] | |
| Goat milk casein | Enzymatic hydrolysis using trypsin | AWPQYL, SPTVMFPPQSVL, MHQPPQPL, VMFPPQSVL, INNQFLPYPY | Five new peptides with DPP-IV-inhibitory activity were identified and isolated using 2D-TLC. One of the peptides (INNQFLPYPY) showed remarkable IC50 | Zhang et al. [46] | |
| Walnut protein | Enzymatic hydrolysis using alcalase | LPLLR | The identified peptide showed strong inhibitory action against α-glucosidase and α-amylase | Wang, Wu, Fang, Liu, Liu, Li, Shi, Li, and Min [13] | |
| Antimicrobial peptides | Mackerel hydrolysate | Enzymatic hydrolysis using protamex | SIFIQRFTT | All four identified peptides partially inhibited Gram positive (Listeria innocua) and Gram negative (Escherichia coli) bacterial strains while SIFIQRFTT totally inhibited both strains | Ennaas et al. [94] |
| Anchovy cooking waste | Enzymatic hydrolysis using protamex | GLSRLFTALK | Identified peptide showed no hemolytic activity and exhibited bactericidal effect in reconstituted milk | Tang et al. [95] | |
| Whey protein | Enzymatic hydrolysis using pepsin, chymotrypsin, and trypsin | VRT, KVGIN, PGDL, KVAGT, EKF, LPMH | Trypsin and chymotryptic hydrolysates did not exhibit antibacterial activity; only hydrolysate from pepsin showed significant activity | Théolier et al. [96] | |
| Antiproliferative peptides | Mung bean protein | Enzymatic hydrolysis using papain | PQG, LAF, EGA, VEG | Identified peptides exhibited in vitro and in vivo anticancer activities | Li, Zhang, Xia, and Ding [97] |
| Chickpea protein | Enzymatic hydrolysis using trypsin and pepsin | RQSHFANAQP | Identified peptide inhibited breast cancer cells | Xue et al. [42] | |
| Germinated soybean | Enzymatic hydrolysis using pepsin | - | Inhibited cervical and breast cancer cells | Marcela, Eva, Del Carmen, and Rosalva [98] |
| Source of Peptides | Non-Thermal Treatment | Enzyme Used for Preparation | Major Findings | Reference |
|---|---|---|---|---|
| Lentil protein hydrolysate | High-pressure processing (HPP); 100 to 300 MPa at 40 °C for 15 min | Alcalase, protamex, savinase, corolase | HPP increased ACE-inhibitory activity of hydrolysates from all the enzymes when compared with control, with exception of alcalase In comparison to control, HPP increased oxygen radical absorbance capacity of all hydrolysate samples | Garcia-Mora, Peñas, Frias, Gomez, and Martinez-Villaluenga [28] |
| Lupin protein hydrolysate | Ultrasound (ultrasonic power: 400 W, frequency: 20 kHz, time: 10 min) | Flavourzyme (pH: 6.0 at 60 °C), alcalase (pH: 8.0 at 50 °C), protamex (pH: 8.0 at 50 °C) for 4 h | Ultrasound increased antioxidant, antihypertensive, α-amylase, and α-glucosidase activities when compared with control | Fadimu, Gill, Farahnaky and Truong [31], Fadimu, Gill, Farahnaky, and Truong [32], Fadimu, Farahnaky, Gill, and Truong [30] |
| Peanut protein hydrolysate | Ultrasound (ultrasonic power: 150 W, time: 25 min) | Alcalase (pH: 8.5 at 60 °C) | DPPH radical scavenging activity increased up to 90% after ultrasonic treatment | Yu et al. [29] |
| Wheat gluten hydrolysate | Ultrasound (ultrasonic frequency: 25–69 kHz, ultrasound intensity: 0.707 W/cm2) | Alcalase (pH: 9.0; temperature: 50 °C for 30 min) | Hydrolysate obtained under ultrasound treatment exhibited highest iron chelating and reducing power in a dose-dependent manner | Zhu, Su, Guo, Peng, and Zhou [34] |
| Whey protein hydrolysate | Ultrasound (ultrasound density: 0.092 W/mL, time: 5 min) | Bromelain (pH 7.0 at 50 °C); papain (pH 7.0 at 60 °C for 180 min) | ACE-inhibitory activity increased from 13 to 95% in bromelain hydrolysate, but did not improve in papain hydrolysate Ultrasound treatment did not improve antioxidant activity | Abadía-García, Castaño-Tostado, Ozimek, Romero-Gómez, Ozuna, and Amaya-Llano [33] |
| Duck albumen hydrolysate | Ultrasound (amplitude: 60%, time: 10 min, power: 750 W) | Papain, alcalase (pH 8.0 at 50 °C for 4 h) | Ultrasound pre-treatment improved the antioxidant activity after 90 min of hydrolysis | Quan and Benjakul [9] |
| Corn protein hydrolysate | Ultrasound (frequency: 28 kHz, time: 25 min, power: 65 W/L) | Alcalase (pH 9.0 at 50 °C) | Sonication treatment increased antioxidant activity from 60 to 65% | Liang, Ren, Ma, Li, Xu, and Oladejo [72] |
| Erythrina edulis hydrolysate | Ultrasound (amplitude: 100%, time: 10 min, frequency: 80 kHz) | Flavourzyme, alcalase | DPPH and ABTS radical scavenging activity significantly increased following ultrasonic pre-treatment in comparison to untreated hydrolysates ACE-inhibitory activity increased in a dose-dependent manner after ultrasonic treatment | Guerra-Almonacid et al. [135] |
| Rapeseed protein hydrolysate | Ultrasound (power: 600 W, time: 12 min) | Alcalase (pH 9.0 at 50 °C for 120 min) | Ultrasonic treatment increased ACE inhibitory activity from 51.10 to 72.13% | Wali et al. [136] |
| Egg white protein | Pulse electric field (electric field: 10 kV/cm, frequency: 3000 Hz, pulse number: 300) | Alcalase, pepsin, trypsin | PEF treatment increased antioxidant activity from 3.0 to 3.5%, with alcalase hydrolysate having highest activity | Lin, Guo, You, Yin, and Liu [137] |
| Pine nut protein | Pulse electric field (frequency: 1800 Hz, electric field: 15 kV/cm) | - | PEF increased DPPH scavenging activity from 89.10 to 93.22%. | Lin et al. [138] |
| Caprine milk protein | Ultrasound (power: 200 W, amplitude: 80%, time: 20 min) | Neutral protease (50 °C for 6 h), pepsin (37 °C for 6 h) | Ultrasound pre-treatment caused significant increase in DPPH and ACE-inhibitory activity | Koirala et al. [139] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fadimu, G.J.; Le, T.T.; Gill, H.; Farahnaky, A.; Olatunde, O.O.; Truong, T. Enhancing the Biological Activities of Food Protein-Derived Peptides Using Non-Thermal Technologies: A Review. Foods 2022, 11, 1823. https://doi.org/10.3390/foods11131823
Fadimu GJ, Le TT, Gill H, Farahnaky A, Olatunde OO, Truong T. Enhancing the Biological Activities of Food Protein-Derived Peptides Using Non-Thermal Technologies: A Review. Foods. 2022; 11(13):1823. https://doi.org/10.3390/foods11131823
Chicago/Turabian StyleFadimu, Gbemisola J., Thao T. Le, Harsharn Gill, Asgar Farahnaky, Oladipupo Odunayo Olatunde, and Tuyen Truong. 2022. "Enhancing the Biological Activities of Food Protein-Derived Peptides Using Non-Thermal Technologies: A Review" Foods 11, no. 13: 1823. https://doi.org/10.3390/foods11131823
APA StyleFadimu, G. J., Le, T. T., Gill, H., Farahnaky, A., Olatunde, O. O., & Truong, T. (2022). Enhancing the Biological Activities of Food Protein-Derived Peptides Using Non-Thermal Technologies: A Review. Foods, 11(13), 1823. https://doi.org/10.3390/foods11131823








