High-Intensity Interval Training Versus Moderate-Intensity Continuous Training in Middle-Aged and Older Patients with Type 2 Diabetes: A Randomized Controlled Crossover Trial of the Acute Effects of Treadmill Walking on Glycemic Control
Abstract
1. Introduction
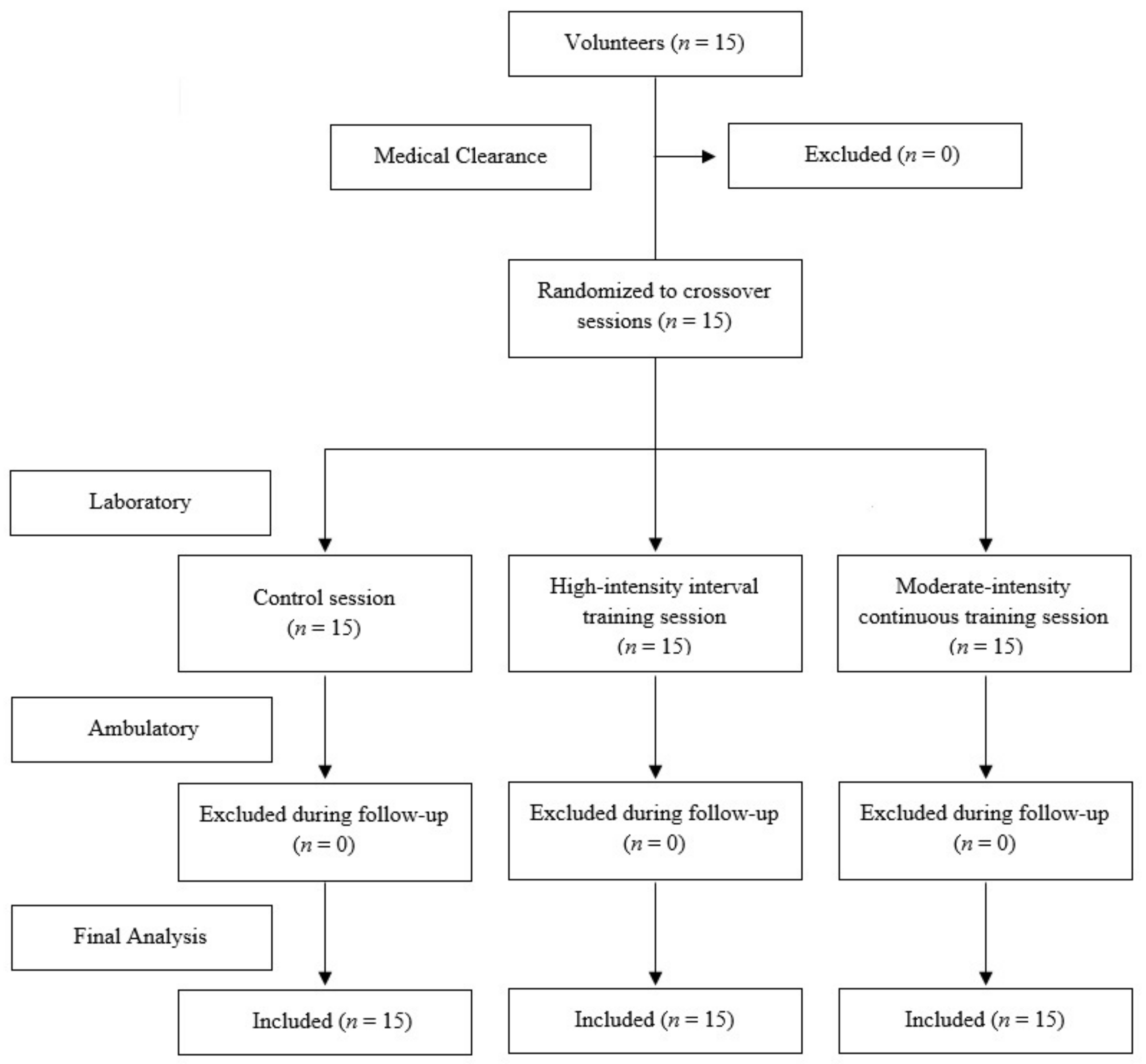
2. Materials and Methods
2.1. Study Design
2.2. Study Participants
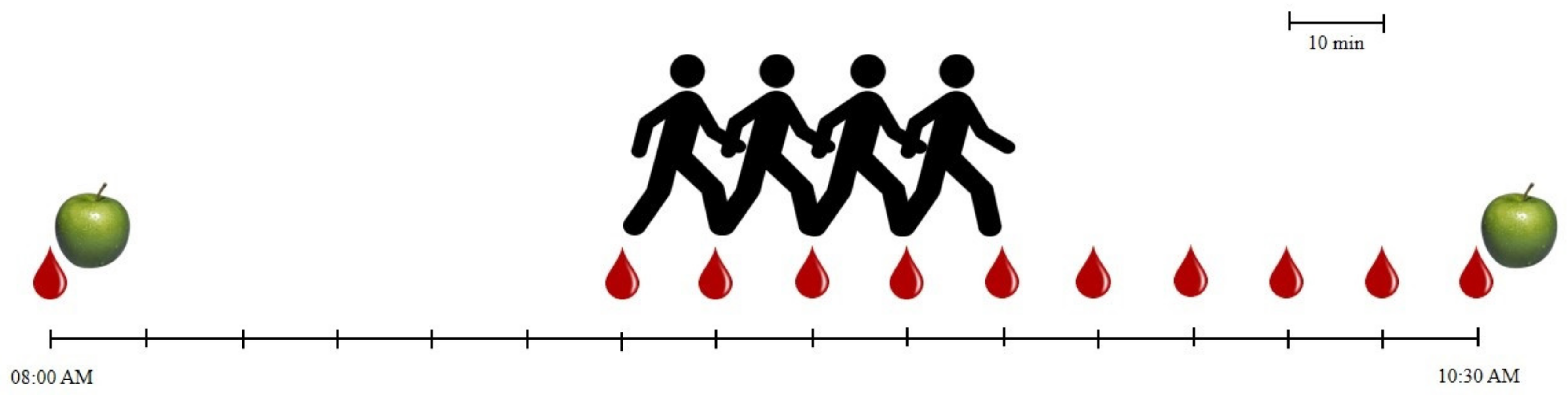
2.3. Laboratory Procedures
2.3.1. Preliminary Laboratory Procedures Adaptation
2.3.2. Baseline Period
2.3.3. Exercise Protocols
2.3.4. Control Session
2.3.5. Recovery Period
2.4. Ambulatory Procedures
2.5. Statistical Analysis
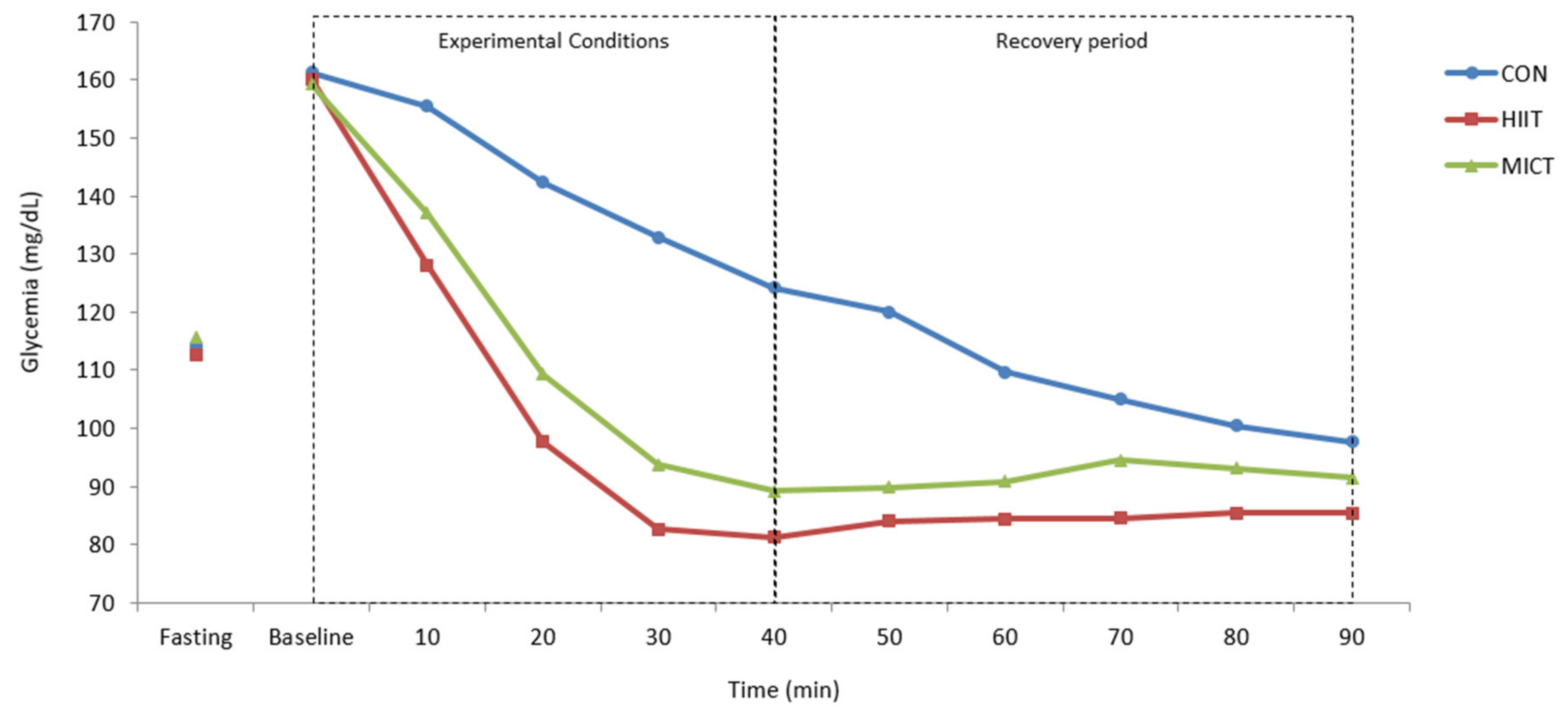
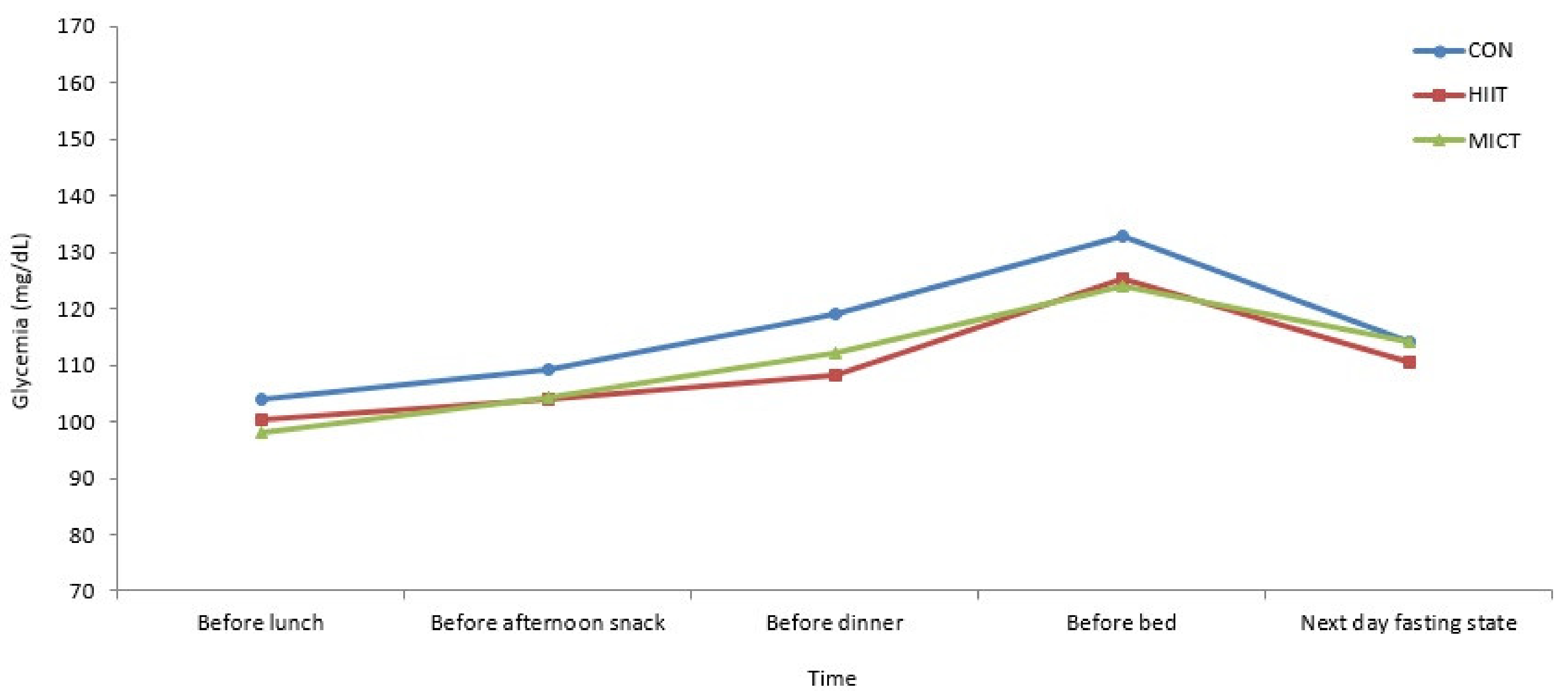
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas—Eighth Edition; International Diabetes Federation: Brussels, Belgium, 2017. [Google Scholar]
- World Health Organization. Global Report on Diabetes; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Mendes, R.; Sousa, N.; Almeida, A.; Subtil, P.; Guedes-Marques, F.; Reis, V.M.; Themudo-Barata, J.L. Exercise prescription for patients with type 2 diabetes—A synthesis of international recommendations: Narrative review. Br. J. Sports Med. 2016, 50, 1379–1381. [Google Scholar] [CrossRef] [PubMed]
- Colberg, S.R.; Sigal, R.J.; Yardley, J.E.; Riddell, M.C.; Dunstan, D.W.; Dempsey, P.C.; Horton, E.S.; Castorino, K.; Tate, D.F. Physical Activity/Exercise and Diabetes: A Position Statement of the American Diabetes Association. Diabetes Care 2016, 39, 2065–2079. [Google Scholar] [CrossRef] [PubMed]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.-M.; Nieman, D.C.; Swain, D.P. Quantity and Quality of Exercise for Developing and Maintaining Cardiorespiratory, Musculoskeletal, and Neuromotor Fitness in Apparently Healthy Adults: Guidance for Prescribing Exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef] [PubMed]
- Inzucchi, S.E.; Bergenstal, R.M.; Buse, J.B.; Diamant, M.; Ferrannini, E.; Nauck, M.; Peters, A.L.; Tsapas, A.; Wender, R.; Matthews, D.R. Management of Hyperglycemia in Type 2 Diabetes, 2015: A Patient-Centered Approach: Update to a Position Statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015, 38, 140–149. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation. Global Guideline for Managing Older People with Type 2 Diabetes; International Diabetes Federation: Brussels, Belgium, 2013. [Google Scholar]
- American Diabetes Association. 6. Glycemic targets: Standards of Medical Care in Diabetes—2019. Diabetes Care 2019, 42 (Suppl. 1), S61–S70. [Google Scholar] [CrossRef] [PubMed]
- Zanuso, S.; Jimenez, A.; Pugliese, G.; Corigliano, G.; Balducci, S. Exercise for the management of type 2 diabetes: A review of the evidence. Acta Diabetol. 2009, 47, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Haddad, E.; Wells, G.A.; Sigal, R.J.; Kenny, G.P. Meta-analysis of the effect of structured exercise training on cardiorespiratory fitness in Type 2 diabetes mellitus. Diabetologia 2003, 46, 1071–1081. [Google Scholar] [CrossRef]
- Jung, J.Y.; Han, K.A.; Ahn, H.J.; Kwon, H.R.; Lee, J.H.; Park, K.S.; Min, K.W. Effects of Aerobic Exercise Intensity on Abdominal and Thigh Adipose Tissue and Skeletal Muscle Attenuation in Overweight Women with Type 2 Diabetes Mellitus. Diabetes Metab. J. 2012, 36, 211–221. [Google Scholar] [CrossRef]
- Terada, T.; Friesen, A.; Chahal, B.S.; Bell, G.J.; McCargar, L.J.; Boulé, N.G. Feasibility and preliminary efficacy of high intensity interval training in type 2 diabetes. Diabetes Res. Clin. Pract. 2013, 99, 120–129. [Google Scholar] [CrossRef]
- Mitranun, W.; Deerochanawong, C.; Tanaka, H.; Suksom, D. Continuous vs interval training on glycemic control and macro- and microvascular reactivity in type 2 diabetic patients. Scand. J. Med. Sci. Sports 2014, 24, e69–e76. [Google Scholar] [CrossRef]
- Santiago, É.; Delevatti, R.S.; Bracht, C.G.; Netto, N.; Lisboa, S.C.; Vieira, A.F.; Costa, R.R.; Hübner, A.; Fossati, M.A.; Kruel, L.F.M. Acute glycemic and pressure responses of continuous and interval aerobic exercise in patients with type 2 diabetes. Clin. Exp. Hypertens. 2017, 40, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, S.; Thoma, C.; Houghton, D.; Trenell, M.I. High-intensity interval training: A review of its impact on glucose control and cardiometabolic health. Diabetologia 2017, 60, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Gibala, M.J.; Little, J.P.; Macdonald, M.J.; Hawley, J.A. Physiological adaptations to low-volume, high-intensity interval training in health and disease. J. Physiol. 2012, 590, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Weston, K.S.; Wisløff, U.; Coombes, J.S. High-intensity interval training in patients with lifestyle-induced cardiometabolic disease: A systematic review and meta-analysis. Br. J. Sports Med. 2013, 48, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Cai, X.; Sun, Z.; Zügel, M.; Steinacker, J.M.; Schumann, U. Aerobic Interval Training and Cardiometabolic Health in Patients with Type 2 Diabetes: A Meta-Analysis. Front. Physiol. 2017, 8, 957. [Google Scholar] [CrossRef]
- Kessler, H.S.; Short, K.R.; Kessler, D.H.S.; Sisson, S.B. The Potential for High-Intensity Interval Training to Reduce Cardiometabolic Disease Risk. Sports Med. 2012, 42, 489–509. [Google Scholar] [CrossRef]
- Buchheit, M.; Laursen, P.B. High-Intensity Interval Training, Solutions to the Programming Puzzle. Sports Med. 2013, 43, 313–338. [Google Scholar] [CrossRef]
- Gibala, M. Molecular responses to high-intensity interval exercise. Appl. Physiol. Nutr. Metab. 2009, 34, 428–432. [Google Scholar] [CrossRef]
- Riebe, D.; Franklin, B.A.; Thompson, P.D.; Garber, C.E.; Whitfield, G.P.; Magal, M.; Pescatello, L.S. Updating ACSM’s Recommendations for Exercise Preparticipation Health Screening. Med. Sci. Sports Exerc. 2015, 47, 2473–2479. [Google Scholar] [CrossRef]
- Mendes, R.; Sousa, N.; Reis, V.; Themudo-Barata, J.L. Prevention of exercise-related injuries and adverse events in patients with type 2 diabetes. Postgrad. Med. J. 2013, 89, 715–721. [Google Scholar] [CrossRef]
- Wormgoor, S.G.; Dalleck, L.C.; Zinn, C.; Harris, N.K. Effects of High-Intensity Interval Training on People Living with Type 2 Diabetes: A Narrative Review. Can. J. Diabetes 2017, 41, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Hamasaki, H. Interval Exercise Therapy for Type 2 Diabetes. Curr. Diabetes Rev. 2018, 14, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Francois, M.E.; Little, J.P. Effectiveness and Safety of High-Intensity Interval Training in Patients with Type 2 Diabetes. Diabetes Spectr. 2015, 28, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Terada, T.; Wilson, B.J.; Myette-Côté, É.; Kuzik, N.; Bell, G.J.; McCargar, L.J.; Boulé, N.G.; Myette-Cόté, E. Targeting specific interstitial glycemic parameters with high-intensity interval exercise and fasted-state exercise in type 2 diabetes. Metabolism 2016, 65, 599–608. [Google Scholar] [CrossRef]
- Terada, T.; Friesen, A.; Chahal, B.S.; Bell, G.J.; McCargar, L.J.; Boulé, N.G. Exploring the Variability in Acute Glycemic Responses to Exercise in Type 2 Diabetes. J. Diabetes Res. 2013, 2013, 591574. [Google Scholar] [CrossRef]
- Karstoft, K.; Christensen, C.S.; Pedersen, B.K.; Solomon, T.P.J. The Acute Effects of Interval- Vs Continuous-Walking Exercise on Glycemic Control in Subjects with Type 2 Diabetes: A Crossover, Controlled Study. J. Clin. Endocrinol. Metab. 2014, 99, 3334–3342. [Google Scholar] [CrossRef]
- Marwick, T.H.; Hordern, M.D.; Miller, T.; Chyun, D.A.; Bertoni, A.G.; Blumenthal, R.S.; Philippides, G.; Rocchini, A. Exercise Training for Type 2 Diabetes Mellitus: Impact on Cardiovascular Risk: A Scientific Statement from the American Heart Association. Circulation 2009, 119, 3244–3262. [Google Scholar] [CrossRef]
- World Medical Association. Declaration of Helsinki. Ethical principles for medical research involving human subjects. J. Indian Med. Assoc. 2009, 107, 403–405. [Google Scholar]
- Freckmann, G.; Baumstark, A.; Jendrike, N.; Zschornack, E.; Kocher, S.; Tshiananga, J.; Heister, F.; Haug, C. System Accuracy Evaluation of 27 Blood Glucose Monitoring Systems According to DIN EN ISO 15197. Diabetes Technol. Ther. 2010, 12, 221–231. [Google Scholar] [CrossRef]
- Laguna Neto, D.; Robles, F.C.; Dias, F.G.; Pires, A.C. Analysis of fingerstick capillary glycemia versus alternative site: Results and patients’ preferences. Arq. Bras. Endocrinol. Metabol. 2009, 53, 344–347. [Google Scholar] [CrossRef]
- Belghazi, J.; El Feghali, R.N.; Moussalem, T.; Rejdych, M.; Asmar, R.G. Validation of four automatic devices for self-measurement of blood pressure according to the International Protocol of the European Society of Hypertension. Vasc. Health Risk Manag. 2007, 3, 389–400. [Google Scholar] [PubMed]
- O’Brien, E.; Asmar, R.; Beilin, L.; Imai, Y.; Mallion, J.-M.; Mancia, G.; Mengden, T.; Myers, M.; Padfield, P.; Palatini, P.; et al. European Society of Hypertension recommendations for conventional, ambulatory and home blood pressure measurement. J. Hypertens. 2003, 21, 821–848. [Google Scholar] [CrossRef] [PubMed]
- Karvonen, J.; Karvonen, D.J.; Vuorimaa, T. Heart Rate and Exercise Intensity during Sports Activities. Sports Med. 1988, 5, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.; Arena, R.; Franklin, B.; Pina, I.; Kraus, W.E.; McInnis, K.; Balady, G.J. Recommendations for clinical exercise laboratories: A scientific statement from the american heart association. Circulation 2009, 119, 3144–3161. [Google Scholar] [CrossRef]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef]
- Sawka, M.N.; Burke, L.M.; Eichner, E.R.; Maughan, R.J.; Montain, S.J.; Stachenfeld, N.S. American College of Sports Medicine position stand. Exercise and fluid replacement. Med. Sci. Sports Exerc. 2007, 39, 377–390. [Google Scholar]
- International Hypoglycaemia Study Group. Glucose Concentrations of Less Than 3.0 mmol/L (54 mg/dL) Should Be Reported in Clinical Trials: A Joint Position Statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2017, 40, 155–157. [Google Scholar] [CrossRef]
- World Health Organization. Global Recommendations on Physical Activity for Health; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Fritz, T.; Rosenqvist, U. Walking for exercise? Immediate effect on blood glucose levels in type 2 diabetes. Scand. J. Prim. Health Care 2001, 19, 31–33. [Google Scholar]
- Kato, M.; Goto, A.; Tanaka, T.; Sasaki, S.; Igata, A.; Noda, M. Effects of walking on medical cost: A quantitative evaluation by simulation focusing on diabetes. J. Diabetes Investig. 2013, 4, 667–672. [Google Scholar] [CrossRef]
- Ainsworth, B.E.; Haskell, W.L.; Herrmann, S.D.; Meckes, N.; Bassett, D.R., Jr.; Tudor-Locke, C.; Greer, J.L.; Vezina, J.; Whitt-Glover, M.C.; Leon, A.S. 2011 Compendium of Physical Activities: A second update of codes and MET values. Med. Sci. Sports Exerc. 2011, 43, 1575–1581. [Google Scholar] [CrossRef]
- Karstoft, K.; Winding, K.; Knudsen, S.H.; Nielsen, J.S.; Thomsen, C.; Pedersen, B.K.; Solomon, T.P. The Effects of Free-Living Interval-Walking Training on Glycemic Control, Body Composition, and Physical Fitness in Type 2 Diabetes Patients: A randomized, controlled trial. Diabetes Care 2013, 36, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.; Sousa, N.; Reis, V.M.; Themudo-Barata, J.L. Implementing Low-Cost, Community-Based Exercise Programs for Middle-Aged and Older Patients with Type 2 Diabetes: What Are the Benefits for Glycemic Control and Cardiovascular Risk? Int. J. Environ. Res. Public Health 2017, 14, 1057. [Google Scholar] [CrossRef]
- Younk, L.M.; Mikeladze, M.; Tate, D.; Davis, S.N. Exercise-related hypoglycemia in diabetes mellitus. Expert Rev. Endocrinol. Metab. 2011, 6, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Esteban, I.; Mataix, A.; Segura, M.A.; I Figuls, M.R.; Moher, D. Metformin monotherapy for type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2005. [Google Scholar] [CrossRef]
- Goldstein, B.J.; Feinglos, M.N.; Lunceford, J.K.; Johnson, J.; Williams-Herman, D.E. Effect of Initial Combination Therapy with Sitagliptin, a Dipeptidyl Peptidase-4 Inhibitor, and Metformin on Glycemic Control in Patients with Type 2 Diabetes. Diabetes Care 2007, 30, 1979–1987. [Google Scholar] [CrossRef]
- Guarino, E.; Nigi, L.; Patti, A.; Fondelli, C.; Dotta, F. Combination therapy with metformin plus vildagliptin in type 2 diabetes mellitus. Expert Opin. Pharmacother. 2012, 13, 1377–1384. [Google Scholar] [CrossRef]
- Ribisl, P.M.; Lang, W.; Jaramillo, S.A.; Jakicic, J.M.; Stewart, K.J.; Bahnson, J.; Bright, R.; Curtis, J.F.; Crow, R.S.; Soberman, J.E. Exercise Capacity and Cardiovascular/Metabolic Characteristics of Overweight and Obese Individuals with Type 2 Diabetes: The Look AHEAD clinical trial. Diabetes Care 2007, 30, 2679–2684. [Google Scholar] [CrossRef]
- Harrell, R.M.; Orzeck, E.A. Coding Guidelines for Continuous Glucose Monitoring. Endocr. Pract. 2010, 16, 151–154. [Google Scholar] [CrossRef]
- Klonoff, D.C. Continuous glucose monitoring: Roadmap for 21st century diabetes therapy. Diabetes Care 2005, 28, 1231–1239. [Google Scholar] [CrossRef]
- Tudor-Locke, C.E.; Bell, R.C.; Myers, A.M.; Harris, S.B.; Lauzon, N.; Rodger, N.W. Pedometer-determined ambulatory activity in individuals with type 2 diabetes. Diabetes Res. Clin. Pract. 2002, 55, 191–199. [Google Scholar] [CrossRef]
- Bjørgaas, M.; Vik, J.T.; Saeterhaug, A.; Langlo, L.; Sakshaug, T.; Mohus, R.M.; Grill, V.; Sæterhaug, A. Relationship between pedometer-registered activity, aerobic capacity and self-reported activity and fitness in patients with type 2 diabetes. Diabetes Obes. Metab. 2005, 7, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Strycker, L.A.; Duncan, S.C.; Chaumeton, N.R.; Duncan, T.E.; Toobert, D.J. Reliability of pedometer data in samples of youth and older women. Int. J. Behav. Nutr. Phys. Act. 2007, 4, 4. [Google Scholar] [CrossRef] [PubMed]
- Harris, T.J.; Owen, C.G.; Victor, C.R.; Adams, R.; Ekelund, U.; Cook, D.G. A comparison of questionnaire, accelerometer, and pedometer: Measures in older people. Med. Sci. Sports Exerc. 2009, 41, 1392–1402. [Google Scholar] [CrossRef] [PubMed]
- Dube, M.-C.; Tremblay, A.; Lavoie, C.; Weisnagel, S.J. Effect of exercise on food consumption and appetite sensations in subjects with diabetes. Appetite 2013, 71, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Karstoft, K.; Winding, K.; Knudsen, S.H.; James, N.G.; Scheel, M.M.; Olesen, J.; Holst, J.J.; Pedersen, B.K.; Solomon, T.P.J. Mechanisms behind the superior effects of interval vs continuous training on glycaemic control in individuals with type 2 diabetes: A randomised controlled trial. Diabetologia 2014, 57, 2081–2093. [Google Scholar] [CrossRef] [PubMed]
- Maillard, F.; Rousset, S.; Pereira, B.; Traore, A.; Del Amaze, P.D.P.; Boirie, Y.; Duclos, M.; Boisseau, N. High-intensity interval training reduces abdominal fat mass in postmenopausal women with type 2 diabetes. Diabetes Metab. 2016, 42, 433–441. [Google Scholar] [CrossRef]
- Støa, E.M.; Meling, S.; Nyhus, L.-K.; Strømstad, G.; Mangerud, K.M.; Helgerud, J.; Bratland-Sanda, S.; Støren, Ø. High-intensity aerobic interval training improves aerobic fitness and HbA1c among persons diagnosed with type 2 diabetes. Eur. J. Appl. Physiol. 2017, 117, 455–467. [Google Scholar] [CrossRef]
| Variable | Mean ± Standard Deviation |
|---|---|
| Age (years) | 60.25 ± 3.14 |
| Diabetes duration (years) | 5.33 ± 2.31 |
| Glycated hemoglobin (%) | 7.03 ± 0.33 |
| Clinical systolic blood pressure (mmHg) | 123.33 ± 10.47 |
| Clinical diastolic blood pressure (mmHg) | 74.25 ± 8.13 |
| Body mass index (kg/m2) | 29.57 ± 4.61 |
| Oral antidiabetic agents | n = 15 (100.00%) |
| Metformin only | n = 6 (40.00%) |
| Metformin + Sitagliptin | n = 5 (33.33%) |
| Metformin + Vildagliptin | n = 4 (26.67%) |
| Time | CON | HIIT | MICT |
|---|---|---|---|
| Fasting state | 114.25 ± 24.65 | 112.67 ± 21.98 | 115.75 ± 21.84 |
| Baseline | 161.25 ± 26.89 | 160.17 ± 30.90 | 159.25 ± 24.62 |
| 10 min | 155.50 ± 33.38 | 128.08 ± 29.36 | 137.00 ± 32.99 |
| 20 min | 142.42 ± 31.62 | 97.75 ± 25.55 | 109.25 ± 27.56 |
| 30 min | 132.92 ± 31.43 | 82.75 ± 21.65 | 93.75 ± 25.90 |
| 40 min | 124.17 ± 29.94 | 81.33 ± 18.00 | 89.25 ± 20.82 |
| 50 min | 120.08 ± 29.54 | 84.08 ± 14.43 | 89.92 ± 15.07 |
| 60 min | 109.75 ± 26.54 | 84.50 ± 11.00 | 90.92 ± 16.17 |
| 70 min | 105.00 ± 25.86 | 84.58 ± 9.89 | 94.58 ± 14.96 |
| 80 min | 100.42 ± 22.98 | 85.42 ± 9.78 | 93.17 ± 14.94 |
| 90 min | 97.75 ± 25.06 | 85.50 ± 11.01 | 91.50 ± 14.52 |
| Before lunch | 104.00 ± 28.19 | 100.42 ± 15.36 | 98.00 ± 11.95 |
| Before afternoon snack | 109.17 ± 28.60 | 103.92 ± 19.25 | 104.42 ± 23.13 |
| Before dinner | 119.00 ± 19.48 | 108.17 ± 14.08 | 112.08 ± 24.95 |
| Before bed | 132.92 ± 39.35 | 125.33 ± 26.17 | 123.83 ± 39.19 |
| Next day fasting state | 114.08 ± 24.27 | 110.50 ± 17.73 | 114.00 ± 22.31 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendes, R.; Sousa, N.; Themudo-Barata, J.L.; Reis, V.M. High-Intensity Interval Training Versus Moderate-Intensity Continuous Training in Middle-Aged and Older Patients with Type 2 Diabetes: A Randomized Controlled Crossover Trial of the Acute Effects of Treadmill Walking on Glycemic Control. Int. J. Environ. Res. Public Health 2019, 16, 4163. https://doi.org/10.3390/ijerph16214163
Mendes R, Sousa N, Themudo-Barata JL, Reis VM. High-Intensity Interval Training Versus Moderate-Intensity Continuous Training in Middle-Aged and Older Patients with Type 2 Diabetes: A Randomized Controlled Crossover Trial of the Acute Effects of Treadmill Walking on Glycemic Control. International Journal of Environmental Research and Public Health. 2019; 16(21):4163. https://doi.org/10.3390/ijerph16214163
Chicago/Turabian StyleMendes, Romeu, Nelson Sousa, José Luís Themudo-Barata, and Victor Machado Reis. 2019. "High-Intensity Interval Training Versus Moderate-Intensity Continuous Training in Middle-Aged and Older Patients with Type 2 Diabetes: A Randomized Controlled Crossover Trial of the Acute Effects of Treadmill Walking on Glycemic Control" International Journal of Environmental Research and Public Health 16, no. 21: 4163. https://doi.org/10.3390/ijerph16214163
APA StyleMendes, R., Sousa, N., Themudo-Barata, J. L., & Reis, V. M. (2019). High-Intensity Interval Training Versus Moderate-Intensity Continuous Training in Middle-Aged and Older Patients with Type 2 Diabetes: A Randomized Controlled Crossover Trial of the Acute Effects of Treadmill Walking on Glycemic Control. International Journal of Environmental Research and Public Health, 16(21), 4163. https://doi.org/10.3390/ijerph16214163







