Synthesis and Biological Evaluation of Marine-Inspired Benzothiazole Derivatives as Retinoid X Receptor-α Antagonists with Anti-Cancer Activities
Abstract
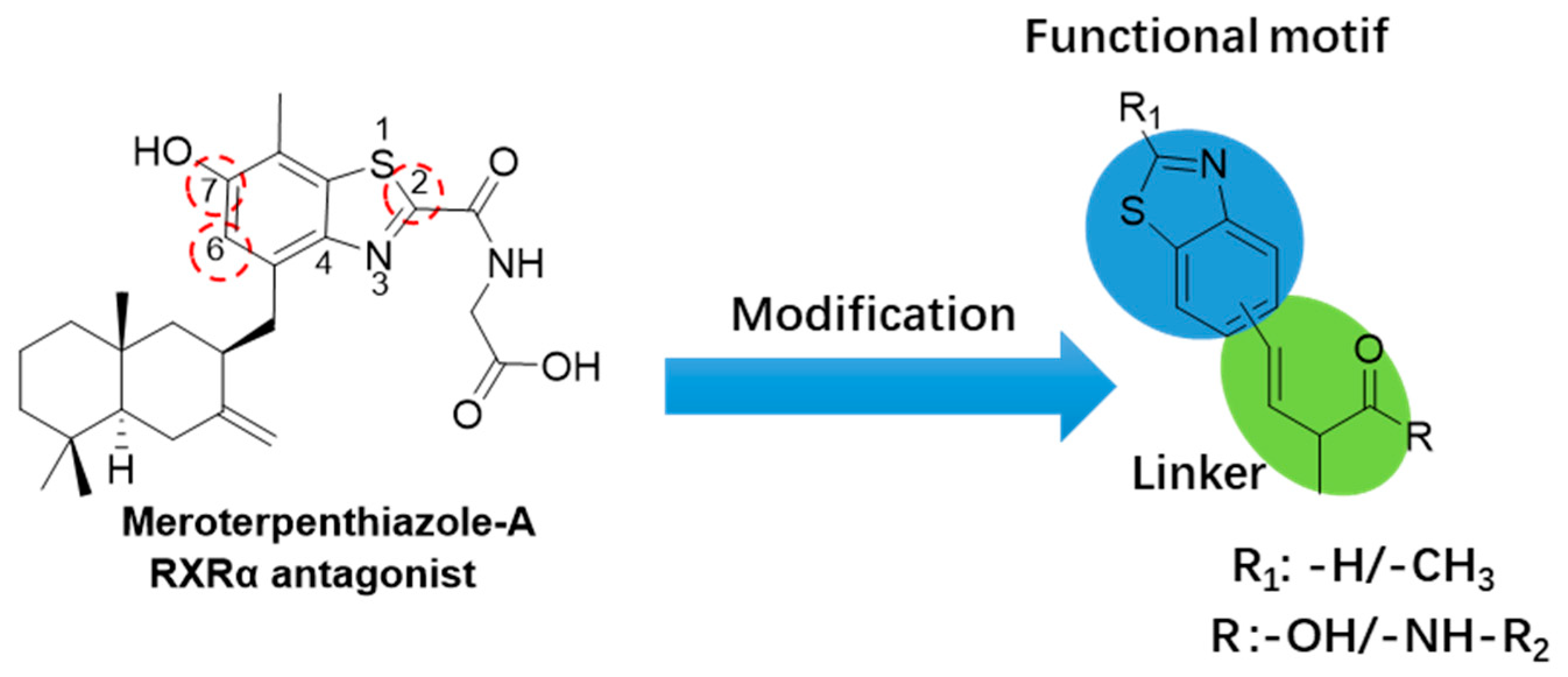
1. Introduction
2. Results and Discussion
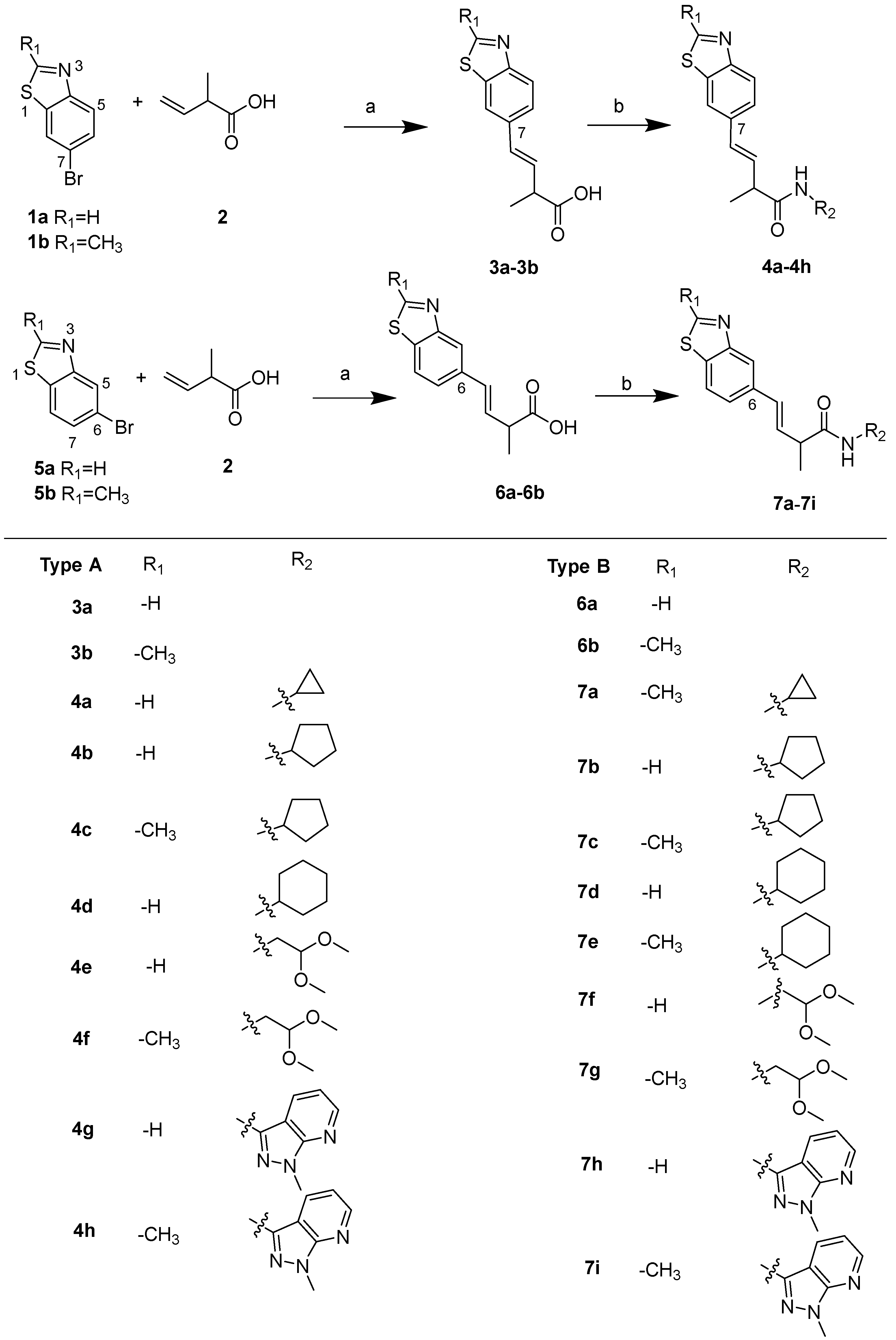
2.1. Synthesis of Benzothiazole Derivatives
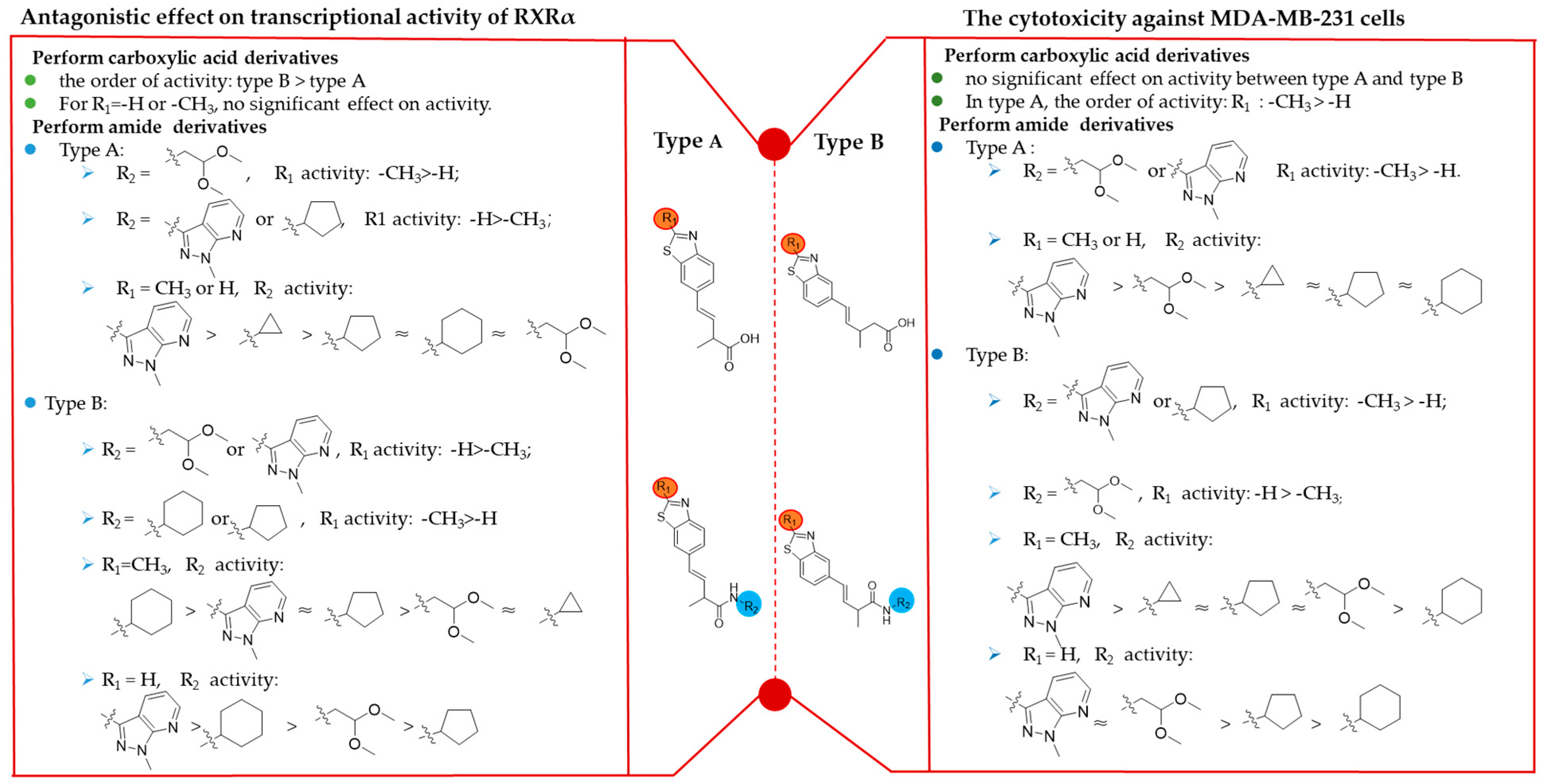
2.2. Biological Evaluation and SAR Analysis of Benzothiazole Derivatives
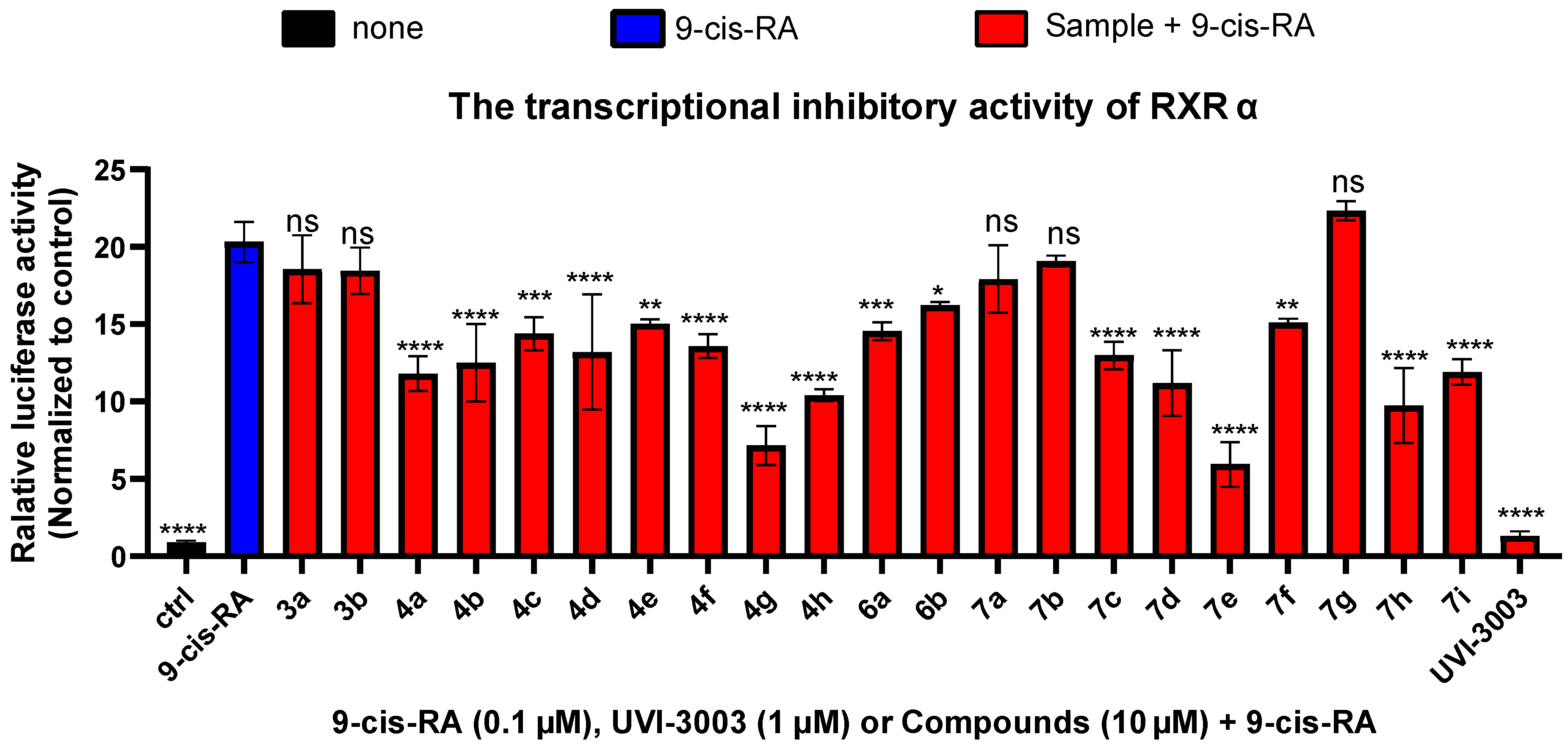
2.2.1. RXRα Transcriptional Activity
2.2.2. In Vitro Anti-Cancer Activity
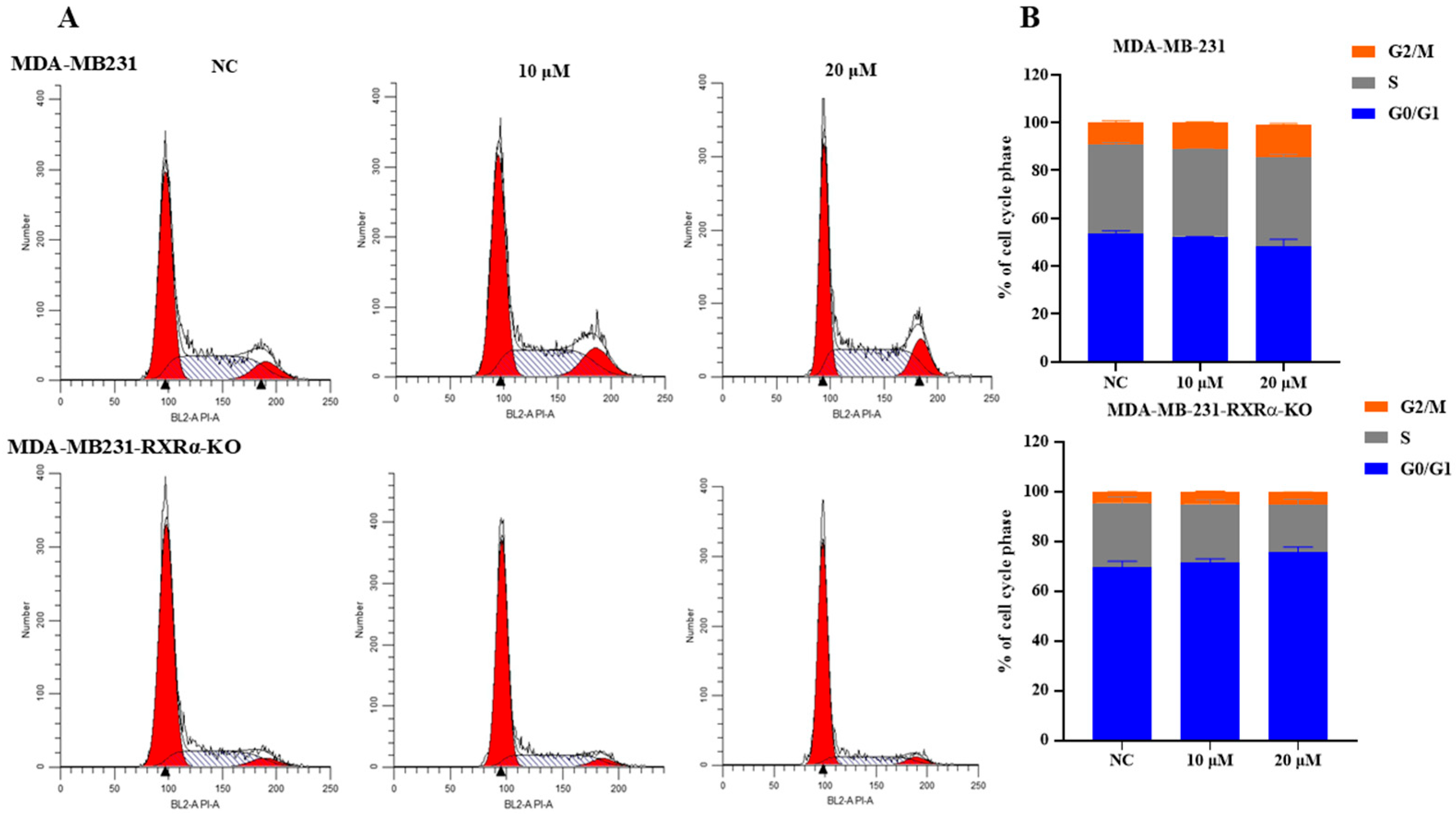
2.3. Compound 7i Inhibits the Proliferation of MDA-MB-231 by Inducing Cell Cycle Arrest at the G2/M Phase
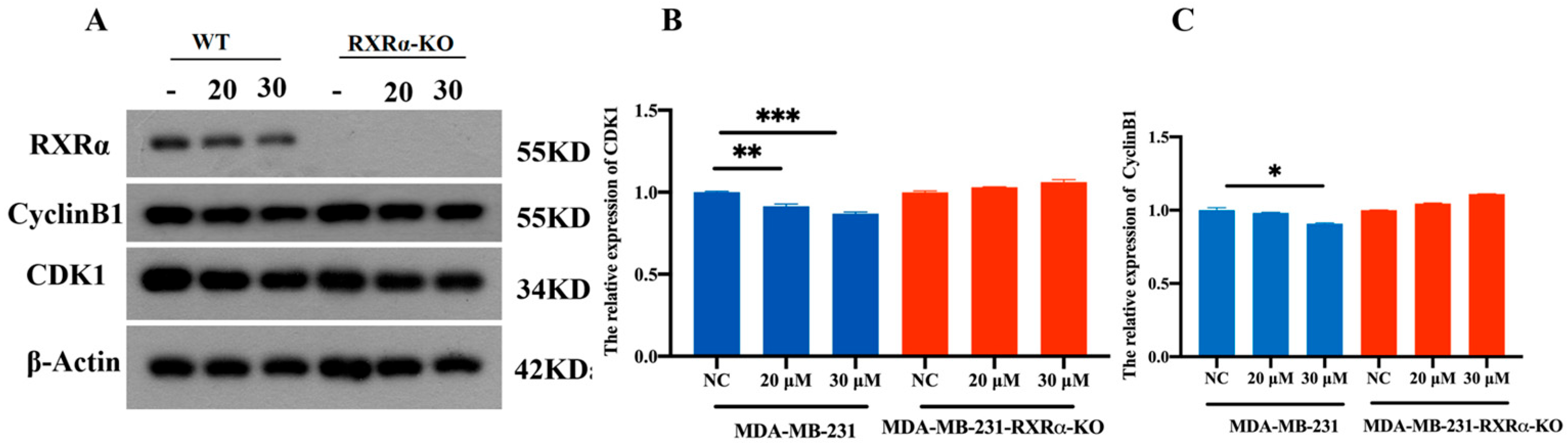
2.4. Detection of G2/M Phase Protein-Related Expression Levels by Western Blot
2.5. CETSA Confirms the Binding of Compound 7i to RXRα
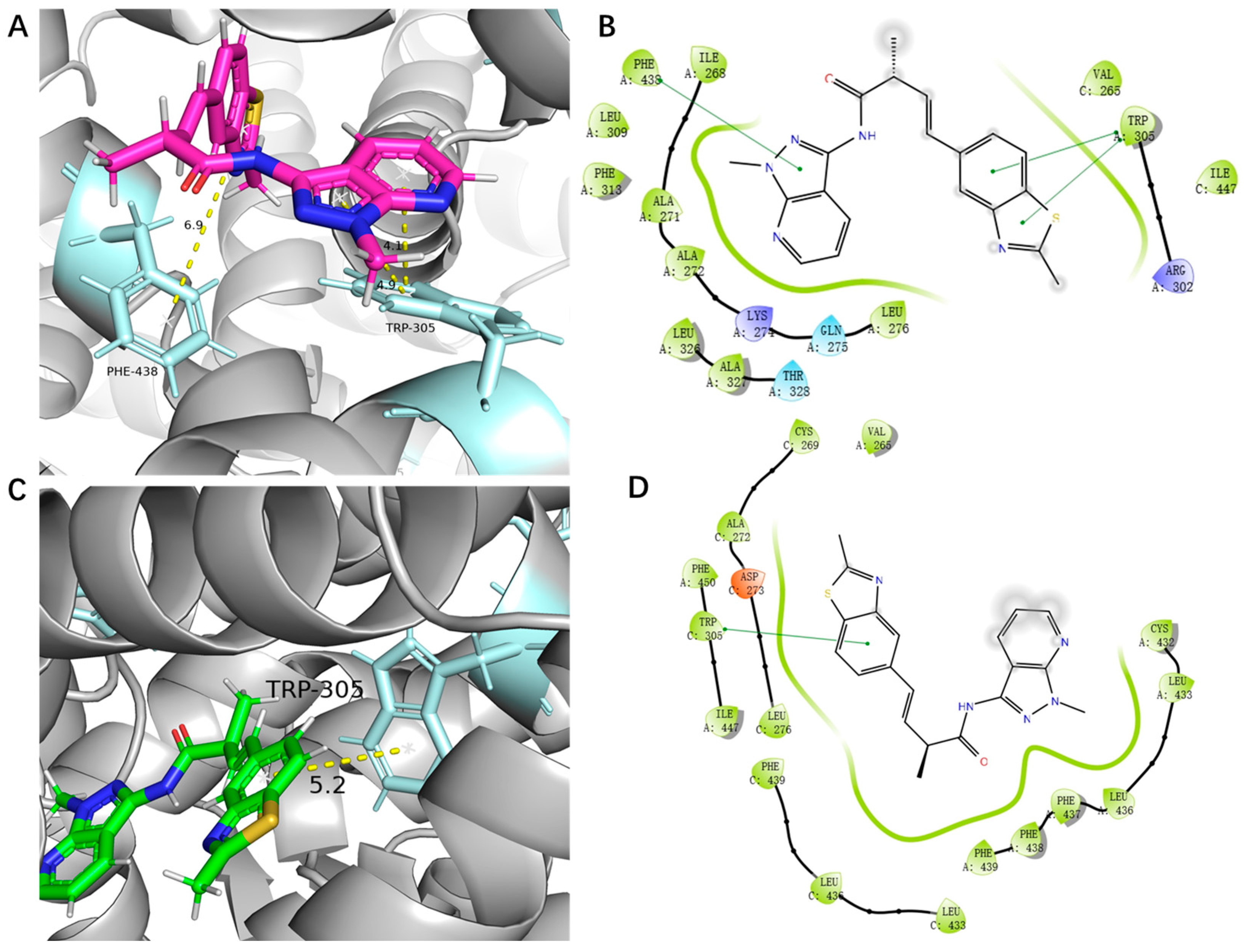
2.6. Molecular Docking
3. Materials and Methods
3.1. Instruments
3.2. Chemical Synthesis
3.2.1. General Procedure for Carboxylic Acid Benzothiazole Derivatives (3a–3b and 6a–6b)
(E)-4-(benzo[d]thiazol-6-yl)-2-methylbut-3-enoic Acid (3a)
(E)-2-methyl-4-(2-methylbenzo[d]thiazol-6-yl)-3-enoic Acid (3b)
(E)-4-(benzo[d]thiazol-5-yl)-2-methylbut-3-enoic Acid (6a)
(E)-2-methyl-4-(2-methylbenzo[d]thiazol-5-yl) but-3-enoic Acid (6b)
3.2.2. General Procedure for Amide Benzothiazole Derivatives (4a–4b and 7a–7i)
(E)-4-(benzo[d]thiazol-6-yl)-N-cyclopropyl-2-methylbut-3-enamide (4a)
(E)-4-(benzo[d]thiazol-6-yl)-N-cyclopentyl-2-methylbut-3-enamide (4b)
(E)-N-cyclopentyl-2-methyl-4- (2-methylbenzo[d]thiazol-6-yl) but-3-enamide (4c)
(E)-4-(benzo[d]thiazol-6-yl)-N-cyclohexyl-2-methylbut-3-enamide (4d)
(E)-4-(benzo[d]thiazol-6-yl)-N-(2,2-dimethoxyethyl)-2-methylbut-3-enamide (4e)
(E)-N-(2,2-dimethoxyethyl)-2-methyl-4-(2-methylbenzo[d]thiazol-6-yl) but-3-enamide (4f)
(E)-4-(benzo[d]thiazol-6-yl)-2-methyl-N-(1-methyl-1H-pyrazolo[3,4-b] pyridin-3-yl) but-3-enamide (4g)
(E)-4-(benzo[d]thiazol-6-yl)-2-methyl-N-(1-methyl-1H-pyrazolo[3,4-b] pyridin-3-yl) but-3-enamide (4h)
(E)-N-cyclopropyl-2-methyl-4- (2-methylbenzo[d]thiazol-5-yl) but-3-enamide (7a)
(E)-4-(benzo[d]thiazol-5-yl)-N-cyclopentyl-2-methylbut-3-enamide (7b)
(E)-N-cyclopentyl-2-methyl-4- (2-methylbenzo[d]thiazol-5-yl) but-3-enamide (7c)
(E)-4-(benzo[d]thiazol-5-yl)-N-cyclohexyl-2-methylbut-3-enamide (7d)
(E)-N-cyclohexyl-2-methyl-4- (2-methylbenzo[d]thiazol-5-yl) but-3-enamide (7e)
(E)-4-(benzo[d]thiazol-5-yl)-N-(2,2-dimethoxyethyl)-2-methylbut-3-enamide (7f)
(E)-N-(2,2-dimethoxyethyl)-2-methyl-4-(2-methylbenzo[d]thiazol-5-yl)-3-enamide (7g)
(E)-4-(benzo[d]thiazol-5-yl)-2-methyl-N-(1-methyl-1H-pyrazolo[3,4-b] pyridin-3-yl) but-3-enamide (7h)
(E)-2-methyl-N-(1-methyl-1H-pyrazolo[3,4-b] pyridin-3-yl)-4-(2-methylbenzo[d]-thiazol-5-yl) but-3-enamide (7i)
3.3. Dual-Luciferase Reporter Assay
3.4. MTT Assay
3.5. Cell Cycle Analysis
3.6. Western Blot
3.7. Cellular Thermal Shift Assay
3.8. Statistical Analysis
3.9. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Szanto, A.; Narkar, V.; Shen, Q.; Uray, I.P.; Davies, P.J.A.; Nagy, L. Retinoid X receptors: X-ploring their (patho)physiological functions. Cell Death Differ. 2004, 11, S126–S143. [Google Scholar] [CrossRef]
- Huang, J.; Powell, W.C.; Khodavirdi, A.C.; Wu, J.; Roy-Burman, P. Prostatic Intraepithelial Neoplasia in Mice with Conditional Disruption of the Retinoid X Receptor α Allele in the Prostate Epithelium. Cancer Res. 2002, 62, 4812–4819. [Google Scholar]
- Zhang, X.K.; Zhang, W.; Xu, D.; Chen, Z.; Zeng, Z.; Su, Y. Recent progress in the design and discovery of RXR modulators targeting alternate binding sites of the receptor. Curr. Top. Med. Chem. 2016, 17, 663–675. [Google Scholar]
- Yan, Z.; Chong, S.; Lin, H.; Yang, Q.; Wang, X.; Zhang, W.; Zhang, X.; Zeng, Z.; Su, Y. Design, synthesis and biological evaluation of tetrazole-containing RXRα ligands as anticancer agents. Eur. J. Med. Chem. 2019, 164, 562–575. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, T.; He, F.; Zhong, Y.; Wang, S.; Tang, Z.; Qiu, Y.; Wu, Z.; Fang, M. Discovery of bipyridine amide derivatives targeting pRXRα-PLK1 interaction for anticancer therapy. Eur. J. Med. Chem. 2023, 254, 115341. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Zhou, Y.; Tu, X.; Ye, X.; Xu, L.; Xiao, Z.; Wang, Q.; Wang, X.; Du, M.; Chen, Z.; et al. Centrosomal Localization of RXRα Promotes PLK1 Activation and Mitotic Progression and Constitutes a Tumor Vulnerability. Dev. Cell 2020, 55, 707–722. [Google Scholar] [CrossRef]
- Wang, X.; Sun, Z.; Yuan, R.; Zhang, W.; Shen, Y.; Yin, A.; Li, Y.; Ji, Q.; Wang, X.; Li, Y.; et al. K-80003 Inhibition of Macrophage Apoptosis and Necrotic Core Development in Atherosclerotic Vulnerable Plaques. Cardiovasc. Drugs Ther. 2021, 36, 1061–1073. [Google Scholar] [CrossRef]
- Sumit; Kumar, A.; Mishra, A.K. Advancement in Pharmacological Activities of Benzothiazole and its Derivatives: An Up to Date Review. Mini-Rev. Med. Chem. 2021, 21, 314–335. [Google Scholar] [CrossRef] [PubMed]
- Chu, P.-L.; Feng, Y.-M.; Long, Z.-Q.; Xiao, W.-L.; Ji, J.; Zhou, X.; Qi, P.-Y.; Zhang, T.-H.; Zhang, H.; Liu, L.-W.; et al. Novel Benzothiazole Derivatives as Potential Anti-Quorum Sensing Agents for Managing Plant Bacterial Diseases: Synthesis, Antibacterial Activity Assessment, and SAR Study. J. Agric. Food Chem. 2023, 71, 6525–6540. [Google Scholar] [CrossRef]
- Azzam, R.A.; Elboshi, H.A.; Elgemeie, G.H. Novel Synthesis and Antiviral Evaluation of New Benzothiazole-Bearing N-Sulfonamide 2-Pyridone Derivatives as USP7 Enzyme Inhibitors. ACS Omega 2020, 5, 30023–30036. [Google Scholar] [CrossRef]
- Azzam, R.A.; Gad, N.M.; Elgemeie, G.H. Novel Thiophene Thioglycosides Substituted with the Benzothiazole Moiety: Synthesis, Characterization, Antiviral and Anticancer Evaluations, and NS3/4A and USP7 Enzyme Inhibitions. ACS Omega 2022, 7, 35656–35667. [Google Scholar] [CrossRef]
- Sluter, M.N.; Bhuniya, R.; Yuan, X.; Ramaraju, A.; Chen, Y.; Yu, Y.; Parmar, K.R.; Temrikar, Z.H.; Srivastava, A.; Meibohm, B.; et al. Novel, Brain-Permeable, Cross-Species Benzothiazole Inhibitors of Microsomal Prostaglandin E Synthase-1 (mPGES-1) Dampen Neuroinflammation In Vitro and In Vivo. ACS Pharmacol. Transl. Sci. 2023, 6, 587–599. [Google Scholar] [CrossRef]
- Morales-Garcia, J.A.; Salado, I.G.; Sanz-San Cristobal, M.; Gil, C.; Pérez-Castillo, A.; Martínez, A.; Pérez, D.I. Biological and Pharmacological Characterization of Benzothiazole-Based CK-1δ Inhibitors in Models of Parkinson’s Disease. ACS Omega 2017, 2, 5215–5220. [Google Scholar] [CrossRef]
- Xie, C.-L.; Zhang, D.; Guo, K.-Q.; Yan, Q.-X.; Zou, Z.-B.; He, Z.-H.; Wu, Z.; Zhang, X.-K.; Chen, H.-F.; Yang, X.-W. Meroterpenthiazole A, a unique meroterpenoid from the deep-sea-derived Penicillium allii-sativi, significantly inhibited retinoid X receptor (RXR)-α transcriptional effect. Chin. Chem. Lett. 2022, 33, 2057–2059. [Google Scholar] [CrossRef]
- Liu, W.-T.; Chen, C.; Lu, I.C.; Kuo, S.-C.; Lee, K.-H.; Chen, T.-L.; Song, T.-S.; Lu, Y.-L.; Gean, P.-W.; Hour, M.-J. MJ-66 induces malignant glioma cells G2/M phase arrest and mitotic catastrophe through regulation of cyclin B1/Cdk1 complex. Neuropharmacology 2014, 86, 219–227. [Google Scholar] [CrossRef]
- Su, C.C.; Lin, J.G.; Chen, G.W.; Lin, W.C.; Chung, J.G. Down-regulation of Cdc25c, CDK1 and Cyclin B1 and Up-regulation of Wee1 by Curcumin Promotes Human Colon Cancer Colo 205 Cell Entry into G2/M-phase of Cell Cycle. Cancer Genom. Proteom. 2006, 3, 55–61. [Google Scholar]
- Dai, L.; Prabhu, N.; Yu, L.Y.; Bacanu, S.; Ramos, A.D.; Nordlund, P. Horizontal Cell Biology: Monitoring Global Changes of Protein Interaction States with the Proteome-Wide Cellular Thermal Shift Assay (CETSA). Annu. Rev. Biochem. 2019, 88, 383–408. [Google Scholar] [CrossRef]
- Wu, J.; Wang, W.; Leung, C.-H. Computational strategies for PROTAC drug discovery. Acta Mater. Medica 2023, 2, 42–53. [Google Scholar] [CrossRef]
- Ren, C.-X.; Gao, M.-Y.; Li, N.; Tang, C.; Chu, G.-H.; Yusuf, A.; Xiao, L.-X.; Yang, Z.-Q.; Guan, T.-Z. Identification and mechanism elucidation of medicative diet for food therapy XQCSY in NAFLD prevention: An integrative in silico study. Food Med. Homol. 2024, 1, 9420015. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Z.-G.; Aleshin, A.E.; Chen, F.; Chen, J.; Jiang, F.; Alitongbieke, G.; Zeng, Z.; Ma, Y.; Huang, M.; et al. Sulindac-Derived RXRα Modulators Inhibit Cancer Cell Growth by Binding to a Novel Site. Chem. Biol. 2014, 21, 596–607. [Google Scholar] [CrossRef]
- Yu, S.; Zhou, X.; Tong, X. Acid/Base-Tuned Asymmetric Reductive Heck and Denitrogenative Heck Reactions of In Situ-Formed α,β-Unsaturated Hydrazone. ACS Catal. 2024, 15, 72–80. [Google Scholar] [CrossRef]
- Bai, P.; Huang, X.-F.; Xu, G.-D.; Huang, Z.-Z. Cascade C–H Functionalization/Amidation Reaction for Synthesis of Azepinone Derivatives. Org. Lett. 2016, 18, 3058–3061. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.; Peng, M.; Yang, R.; Wang, G.; Chen, J.; Ding, R.; Sun, C.; Tian, W.; Chen, H. Synthesis and Biological Evaluation of Marine-Inspired Benzothiazole Derivatives as Retinoid X Receptor-α Antagonists with Anti-Cancer Activities. Mar. Drugs 2025, 23, 368. https://doi.org/10.3390/md23090368
Lin Y, Peng M, Yang R, Wang G, Chen J, Ding R, Sun C, Tian W, Chen H. Synthesis and Biological Evaluation of Marine-Inspired Benzothiazole Derivatives as Retinoid X Receptor-α Antagonists with Anti-Cancer Activities. Marine Drugs. 2025; 23(9):368. https://doi.org/10.3390/md23090368
Chicago/Turabian StyleLin, Yingting, Ming Peng, Renjing Yang, Guanghui Wang, Junjie Chen, Rong Ding, Cuiling Sun, Wenjing Tian, and Haifeng Chen. 2025. "Synthesis and Biological Evaluation of Marine-Inspired Benzothiazole Derivatives as Retinoid X Receptor-α Antagonists with Anti-Cancer Activities" Marine Drugs 23, no. 9: 368. https://doi.org/10.3390/md23090368
APA StyleLin, Y., Peng, M., Yang, R., Wang, G., Chen, J., Ding, R., Sun, C., Tian, W., & Chen, H. (2025). Synthesis and Biological Evaluation of Marine-Inspired Benzothiazole Derivatives as Retinoid X Receptor-α Antagonists with Anti-Cancer Activities. Marine Drugs, 23(9), 368. https://doi.org/10.3390/md23090368





