Marine Indole Alkaloids—Isolation, Structure and Bioactivities
Abstract
1. Introduction
2. Marine Microorganisms
2.1. Marine-Sourced Bacteria
2.1.1. Sediment-Sourced Bacteria
2.1.2. Sponge-Sourced Bacteria
2.1.3. Miscellaneous
2.2. Marine-Sourced Fungi
2.2.1. Sediment-Sourced Fungi
2.2.2. Coral-Sourced Fungi
2.2.3. Mollusk-Sourced Fungi
2.2.4. Mangrove-Sourced Fungi
2.2.5. Alga-Sourced Fungi
2.2.6. Sponge-Sourced Fungi
2.2.7. Miscellaneous
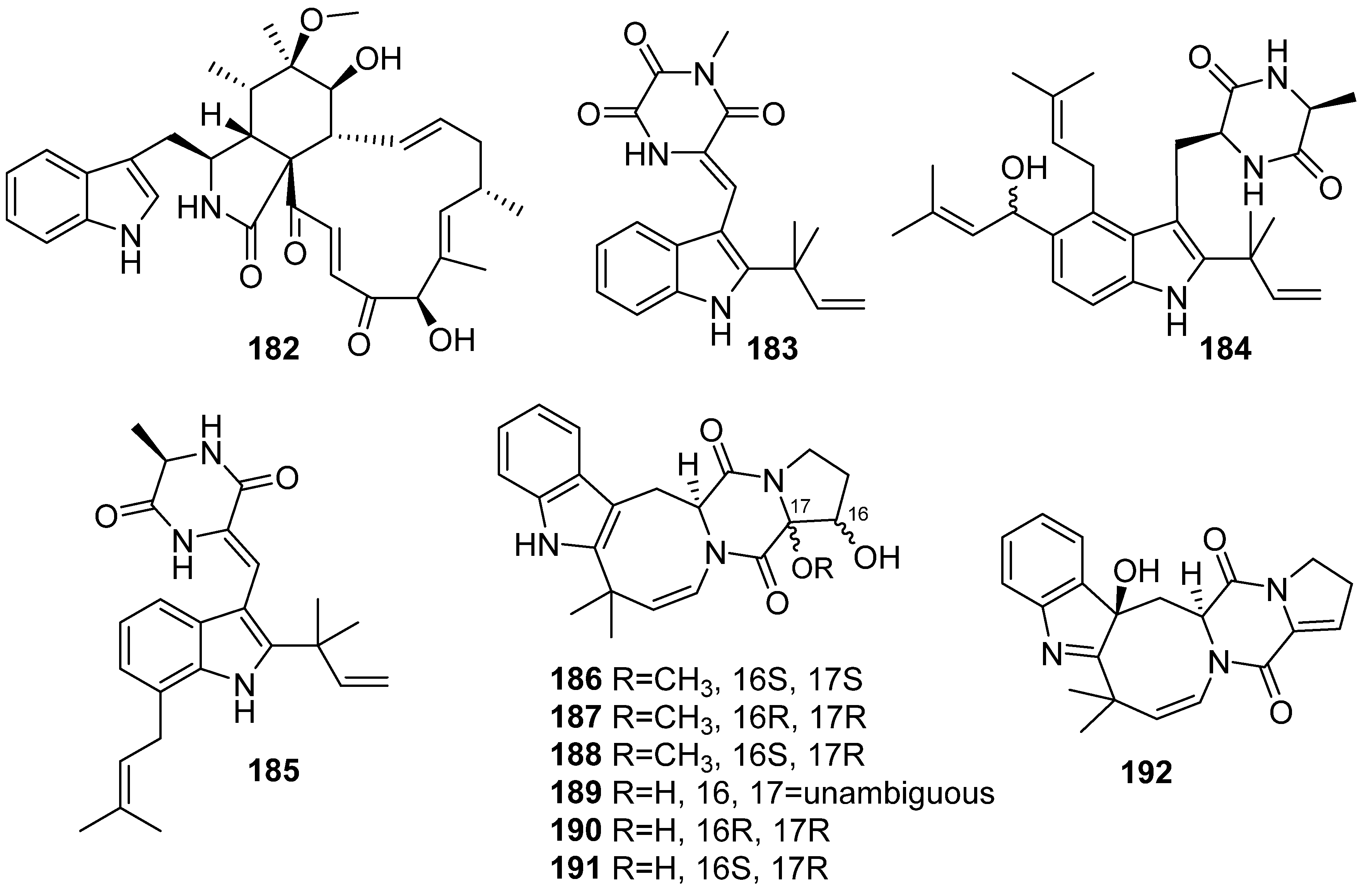
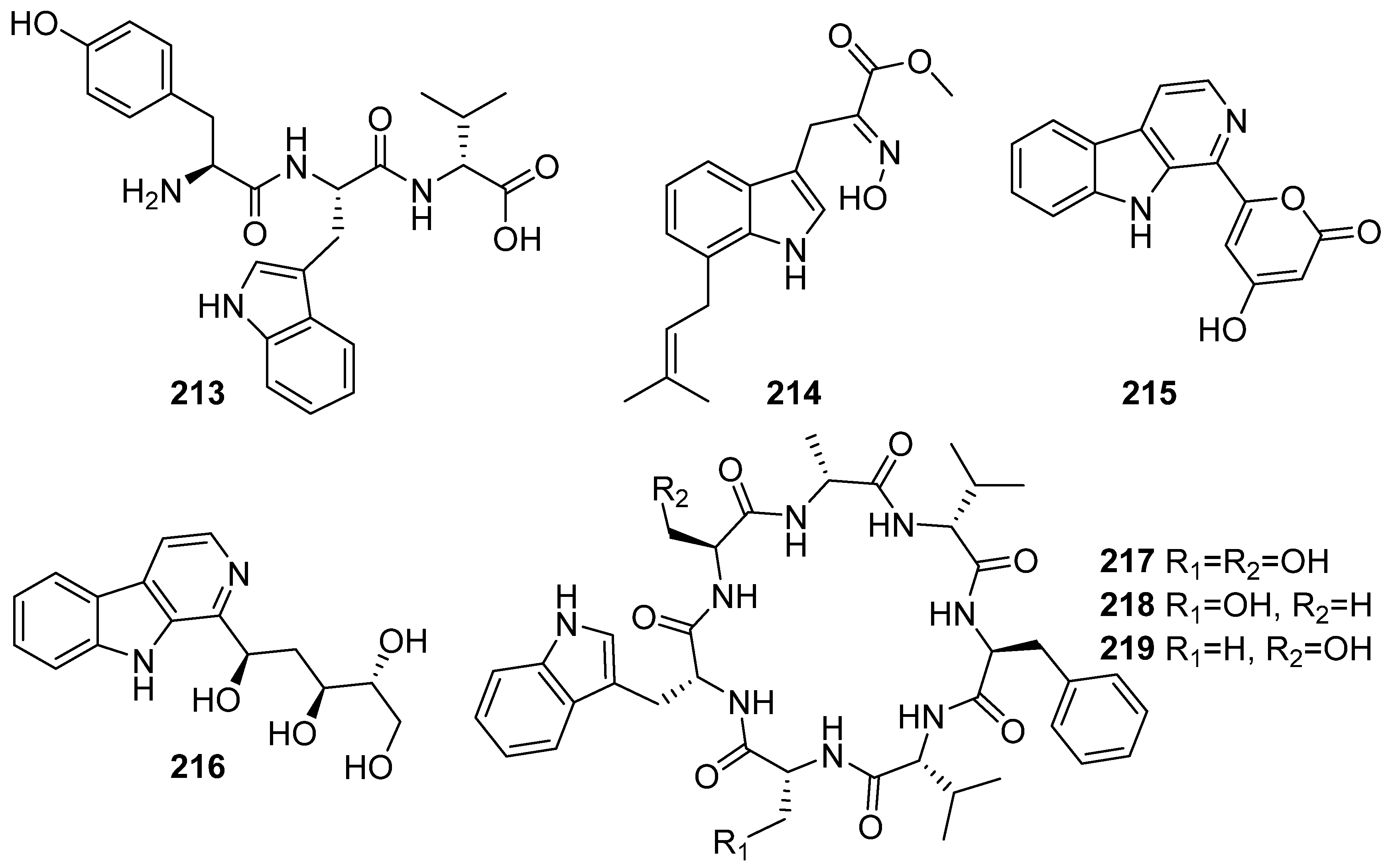
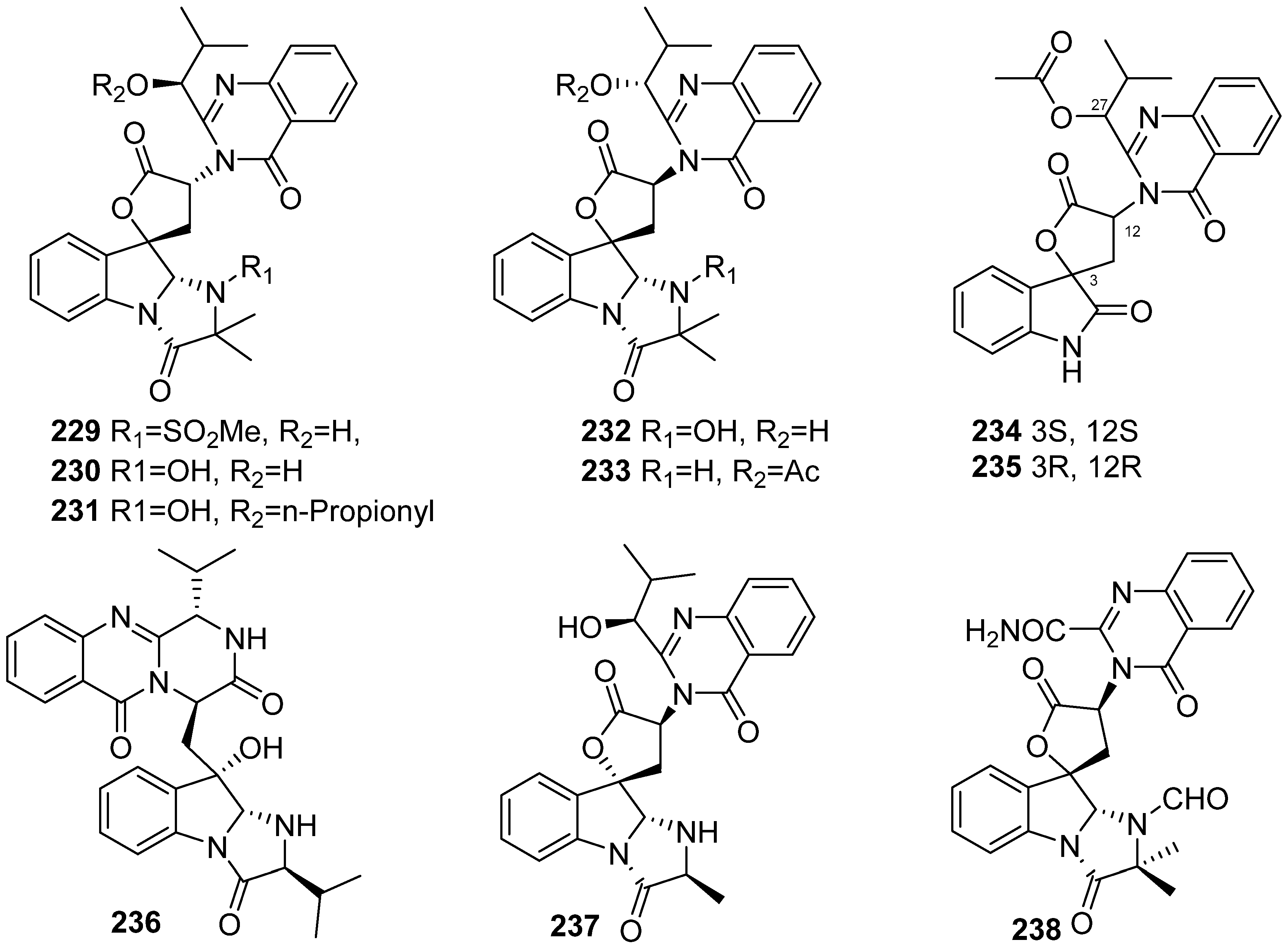
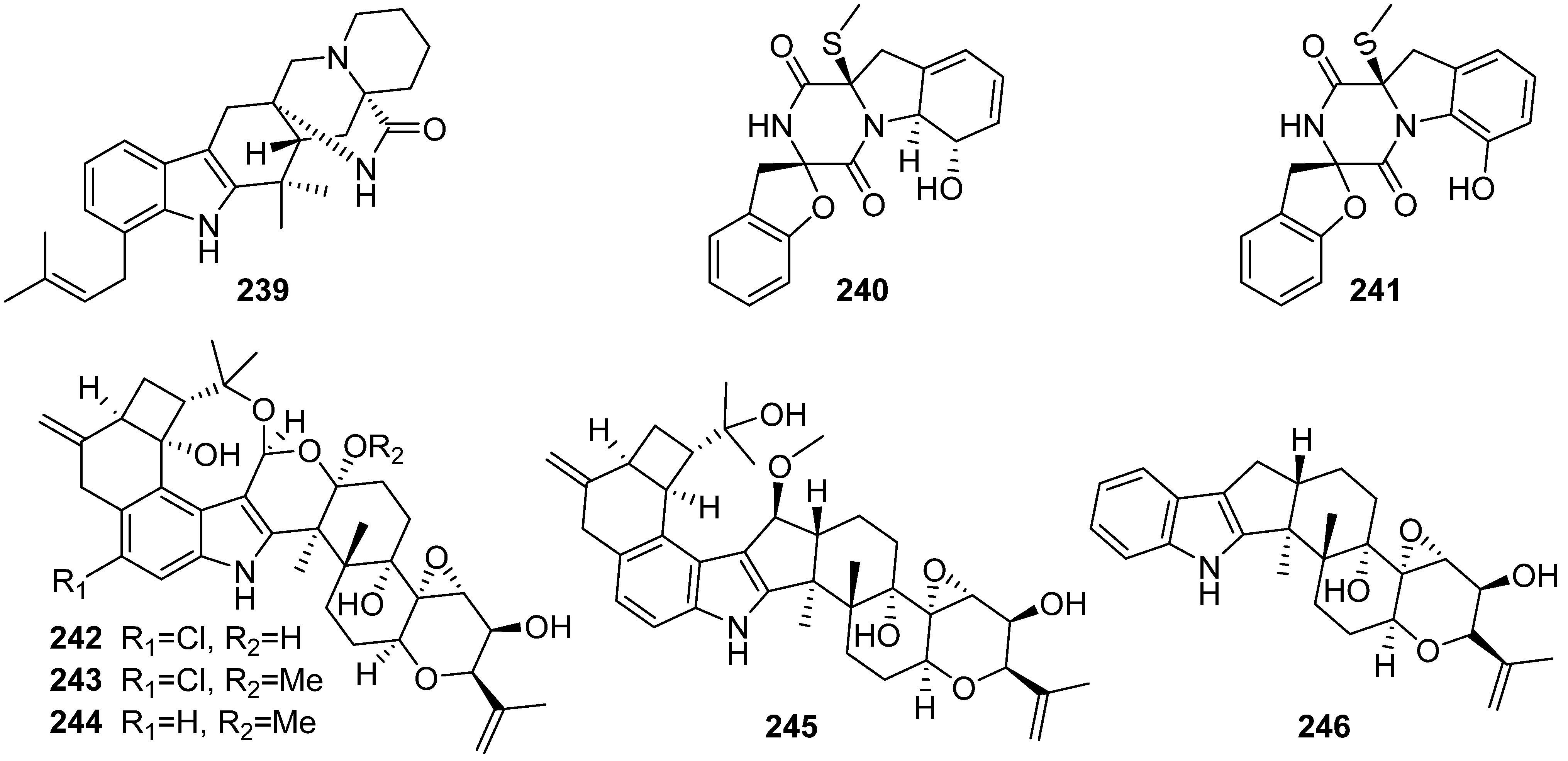
3. Marine Invertebrates
3.1. Sponges
3.2. Cnidarians
3.3. Bryozoans, Tunicates and Molluscs
4. Marine Plants
4.1. Cyanobacteria
4.2. Red Algae
4.3. Mangrove
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ame, P.; Clw, S.; Burke, M.D. The natural productome. Proc. Natl. Acad. Sci. USA 2017, 114, 5564–5566. [Google Scholar]
- Pye, C.R.; Bertin, M.J.; Lokey, R.S.; Gerwick, W.H.; Linington, R.G. Retrospective analysis of natural products provides insights for future discovery trends. Proc. Natl. Acad. Sci. USA 2017, 114, 201614680. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, T.; Reker, D.; Schneider, P.; Schneider, G. Counting on natural products for drug design. Nat. Chem. 2016, 8, 531. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.E.; Pond, C.D.; Pierce, E.; Harmer, Z.P.; Kwan, J.; Zachariah, M.M.; Harper, M.K.; Wyche, T.P.; Matainaho, T.K.; Bugni, T.S. Accessing chemical diversity from the uncultivated symbionts of small marine animals. Nat. Chem. Biol. 2018, 14, 179. [Google Scholar] [CrossRef] [PubMed]
- Abdelmohsen, U.R.; Balasubramanian, S.; Oelschlaeger, T.A.; Grkovic, T.; Pham, N.B.; Quinn, R.J.; Hentschel, U. Potential of marine natural products against drug-resistant fungal, viral, and parasitic infections. Lancet Infect. Dis. 2016, 17, e30. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, J.; Luo, L.; Gao, Y.; Bao, H.; Li, P.; Zhang, H. Research progress of indole compounds with potential antidiabetic activity. Eur. J. Med. Chem. 2021, 223, 113665. [Google Scholar] [CrossRef]
- Chauhan, M.; Saxena, A.; Saha, B. An insight in anti-malarial potential of indole scaffold: A review. Eur. J. Med. Chem. 2021, 218, 113400. [Google Scholar] [CrossRef]
- Kochanowska-Karamyan, A.J.; Hamann, M.T. Marine Indole Alkaloids: Potential New Drug Leads for the Control of Depression and Anxiety. Chem. Rev. 2010, 110, 4489–4497. [Google Scholar] [CrossRef]
- Han, Y.; Dong, W.; Guo, Q.; Li, X.; Huang, L. The importance of indole and azaindole scaffold in the development of antitumor agents. Eur. J. Med. Chem. 2020, 203, 112506. [Google Scholar] [CrossRef]
- Jia, Y.; Wen, X.; Gong, Y.; Wang, X. Current scenario of indole derivatives with potential anti-drug-resistant cancer activity. Eur. J. Med. Chem. 2020, 200, 112359. [Google Scholar] [CrossRef]
- Fiore, A.; Murray, P.J. Tryptophan and indole metabolism in immune regulation. Curr. Opin. Immunol. 2021, 70, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Netz, N.; Opatz, T. Marine Indole Alkaloids. Mar. Drugs 2015, 13, 4814–4914. [Google Scholar] [CrossRef]
- Xiong, Z.Q.; Liu, Q.X.; Pan, Z.L.; Zhao, N.; Feng, Z.X.; Wang, Y. Diversity and bioprospecting of culturable actinomycetes from marine sediment of the Yellow Sea, China. Arch. Microbiol. 2015, 197, 299–309. [Google Scholar] [CrossRef]
- Anjum, K.; Kaleem, S.; Yi, W.; Zheng, G.; Lian, X.; Zhang, Z. Novel Antimicrobial Indolepyrazines A and B from the Marine-Associated Acinetobacter sp. ZZ1275. Mar. Drugs 2019, 17, 89. [Google Scholar] [CrossRef]
- Yi, W.; Li, Q.; Song, T.; Chen, L.; Li, X.-C.; Zhang, Z.; Lian, X.-Y. Isolation, structure elucidation, and antibacterial evaluation of the metabolites produced by the marine-sourced Streptomyces sp. ZZ820. Tetrahedron 2019, 75, 1186–1193. [Google Scholar] [CrossRef]
- da Silva, A.B.; Pinto, F.C.L.; Silveira, E.R.; Costa-Lotufo, L.V.; Costa, W.S.; Ayala, A.P.; Canuto, K.M.; Barros, A.B.; Araújo, A.J.; Marinho Filho, J.D.B.; et al. 4-Hydroxy-pyran-2-one and 3-hydroxy-N-methyl-2-oxindole derivatives of Salinispora arenicola from Brazilian marine sediments. Fitoterapia 2019, 138, 104357. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Yang, J.; Yu, J.; Li, J.; Yuan, J.; Wong, N.K.; Ju, J. Chlorinated bis-indole alkaloids from deep-sea derived Streptomyces sp. SCSIO 11791 with antibacterial and cytotoxic activities. J. Antibiot. 2020, 73, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.N.; Zhang, H.J.; Li, J.Q.; Ding, W.J.; Ma, Z.J. Bioactive Indolocarbazoles from the Marine-Derived Streptomyces sp. DT-A61. J. Nat. Prod. 2018, 81, 949–956. [Google Scholar] [CrossRef]
- Zhou, B.; Qin, L.-L.; Ding, W.-J.; Ma, Z.-J. Cytotoxic indolocarbazoles alkaloids from the Streptomyces sp. A65. Tetrahedron 2018, 74, 726–730. [Google Scholar] [CrossRef]
- Qin, L.-L.; Zhou, B.; Ding, W.; Ma, Z. Bioactive metabolites from marine-derived Streptomyces sp. A68 and its Rifampicin resistant mutant strain R-M1. Phytochem. Lett. 2018, 23, 46–51. [Google Scholar] [CrossRef]
- Cheng, X.; Zhou, B.; Liu, H.; Huo, C.; Ding, W. One new indolocarbazole alkaloid from the Streptomyces sp. A22. Nat. Prod. Res. 2018, 32, 2583–2588. [Google Scholar] [CrossRef]
- Davies-Bolorunduro, O.F.; Adeleye, I.A.; Akinleye, M.O.; Wang, P.G. Anticancer potential of metabolic compounds from marine actinomycetes isolated from Lagos Lagoon sediment. J. Pharm. Anal. 2019, 9, 201–208. [Google Scholar] [CrossRef]
- Wang, C.; Monger, A.; Wang, L.; Fu, P.; Piyachaturawat, P.; Chairoungdua, A.; Zhu, W. Precursor-Directed Generation of Indolocarbazoles with Topoisomerase IIα Inhibitory Activity. Mar. Drugs 2018, 16, 168. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Tang, X.X.; Qin, D.; Yi, Z.W.; Fang, M.J.; Wu, Z.; Qiu, Y.K. Biosynthetic Functional Gene Analysis of Bis-Indole Metabolites from 25D7, a Clone Derived from a Deep-Sea Sediment Metagenomic Library. Mar. Drugs 2016, 14, 107. [Google Scholar] [CrossRef] [PubMed]
- Thi, Q.V.; Tran, V.H.; Mai, H.D.; Le, C.V.; Hong Mle, T.; Murphy, B.T.; Chau, V.M.; Pham, V.C. Secondary Metabolites from an Actinomycete from Vietnam’s East Sea. Nat. Prod. Commun. 2016, 11, 401–404. [Google Scholar] [CrossRef]
- Lorig-Roach, N.; Still, P.C.; Coppage, D.; Compton, J.E.; Crews, M.S.; Navarro, G.; Tenney, K.; Crews, P. Evaluating Nitrogen-Containing Biosynthetic Products Produced by Saltwater Culturing of Several California Littoral Zone Gram-Negative Bacteria. J. Nat. Prod. 2017, 80, 2304–2310. [Google Scholar] [CrossRef]
- Paulus, C.; Rebets, Y.; Tokovenko, B.; Nadmid, S.; Terekhova, L.P.; Myronovskyi, M.; Zotchev, S.B.; Rückert, C.; Braig, S.; Zahler, S.; et al. New natural products identified by combined genomics-metabolomics profiling of marine Streptomyces sp. MP131-18. Sci. Rep. 2017, 7, 42382. [Google Scholar] [CrossRef]
- Reynolds, K.A.; Luhavaya, H.; Li, J.; Dahesh, S.; Nizet, V.; Yamanaka, K.; Moore, B.S. Isolation and structure elucidation of lipopeptide antibiotic taromycin B from the activated taromycin biosynthetic gene cluster. J. Antibiot. 2018, 71, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-Y.; Wang, X.-Q.; Wang, Y.-M.; Geng, X.; Xu, X.-N.; Su, C.; Yang, Y.-L.; Tang, Y.-J.; Bai, F.-W.; Zhao, X.-Q. Genome mining of Streptomyces xinghaiensis NRRL B-24674T for the discovery of the gene cluster involved in anticomplement activities and detection of novel xiamycin analogs. Appl. Microbiol. Biotechnol. 2018, 102, 9549–9562. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Braun, D.R.; Rajski, S.R.; DeMaria, D.; Bugni, T.S. Enhypyrazinones A and B, Pyrazinone Natural Products from a Marine-Derived Myxobacterium Enhygromyxa sp. Mar. Drugs 2019, 17, 698. [Google Scholar] [CrossRef] [PubMed]
- Li, J.L.; Chen, D.; Huang, L.; Ni, M.; Zhao, Y.; Fan, H.; Bao, X. Antichlamydial Dimeric Indole Derivatives from Marine Actinomycete Rubrobacter radiotolerans. Planta Med. 2017, 83, 805–811. [Google Scholar] [CrossRef]
- Elsayed, Y.; Refaat, J.; Abdelmohsen, U.R.; Ahmed, S.; Fouad, M.A. Retraction Note to: Rhodozepinone, a new antitrypanosomal azepino-diindole alkaloid from the marine sponge-derived bacterium Rhodococcus sp. UA13. Med. Chem. Res. 2019, 28, 105. [Google Scholar] [CrossRef]
- Che, Q.; Qiao, L.; Han, X.; Liu, Y.; Wang, W.; Gu, Q.; Zhu, T.; Li, D. Anthranosides A-C, Anthranilate Derivatives from a Sponge-Derived Streptomyces sp. CMN-62. Org. Lett. 2018, 20, 5466–5469. [Google Scholar] [CrossRef]
- Kikuchi, S.; Okada, K.; Cho, Y.; Yoshida, S.; Kwon, E.; Yotsu-Yamashita, M.; Konoki, K. Isolation and structure determination of lysiformine from bacteria associated with marine sponge Halichondria okadai. Tetrahedron 2018, 74, 3742–3747. [Google Scholar] [CrossRef]
- El-Hawary, S.S.; Sayed, A.M.; Mohammed, R.; Khanfar, M.A.; Rateb, M.E.; Mohammed, T.A.; Hajjar, D.; Hassan, H.M.; Gulder, T.A.M.; Abdelmohsen, U.R. New Pim-1 Kinase Inhibitor from the Co-culture of Two Sponge-Associated Actinomycetes. Front. Chem. 2018, 6, 538. [Google Scholar] [CrossRef]
- Kim, M.C.; Cullum, R.; Machado, H.; Smith, A.J.; Yang, I.; Rodvold, J.J.; Fenical, W. Photopiperazines A–D, Photosensitive Interconverting Diketopiperazines with Significant and Selective Activity against U87 Glioblastoma Cells, from a Rare, Marine-Derived Actinomycete of the Family Streptomycetaceae. J. Nat. Prod. 2019, 82, 2262–2267. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Jin, J.; Yang, X.; Song, J.; Yu, J.; Geng, T.; Zhang, Z.; Ma, X.; Wang, G.; Xiao, H.; et al. Discovery of a Phenylamine-Incorporated Angucyclinone from Marine Streptomyces sp. PKU-MA00218 and Generation of Derivatives with Phenylamine Analogues. Org. Lett. 2019, 21, 2813–2817. [Google Scholar] [CrossRef]
- Baunach, M.; Ding, L.; Willing, K.; Hertweck, C. Bacterial Synthesis of Unusual Sulfonamide and Sulfone Antibiotics by Flavoenzyme-Mediated Sulfur Dioxide Capture. Angew. Chem. 2015, 54, 13279–13283. [Google Scholar] [CrossRef]
- Nair, V.; Schuhmann, I.; Anke, H.; Kelter, G.; Fiebig, H.H.; Helmke, E.; Laatsch, H. Marine Bacteria, XLVII—Psychrotolerant Bacteria from Extreme Antarctic Habitats as Producers of Rare Bis- and Trisindole Alkaloids. Planta Med. 2016, 82, 910–918. [Google Scholar] [CrossRef]
- Ding, L.; He, S.; Wu, W.; Jin, H.; Zhu, P.; Zhang, J.; Wang, T.; Yuan, Y.; Yan, X. Discovery and Structure-Based Optimization of 6-Bromotryptamine Derivatives as Potential 5-HT2A Receptor Antagonists. Molecules 2015, 20, 17675–17683. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.L.; Xia, J.M.; Su, R.Q.; Li, J.; Liu, Y.; Yang, X.W.; Yang, Q. Bacilsubteramide A, a new indole alkaloid, from the deep-sea-derived Bacillus subterraneus 11593. Nat. Prod. Res. 2018, 32, 2553–2557. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.M.S.; Rohée, C.; Fabre, T.; Batailler, N.; Sautel, F.; Carletti, I.; Nogues, S.; Suzuki, M.T.; Stien, D. Cytotoxic indole alkaloids from Pseudovibrio denitrificans BBCC725. Tetrahedron Lett. 2017, 58, 3172–3173. [Google Scholar] [CrossRef]
- Hodgkin, D.; Maslen, E.N.J.B.J. The X-ray analysis of the structure of cephalosporin C. Biochem. J. 1961, 79, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Abraham, E.; Newton, G.; Hale, C.J.B.j. Purification and some properties of cephalosporin N, a new penicillin. Biochem. J. 1954, 58, 94. [Google Scholar] [CrossRef]
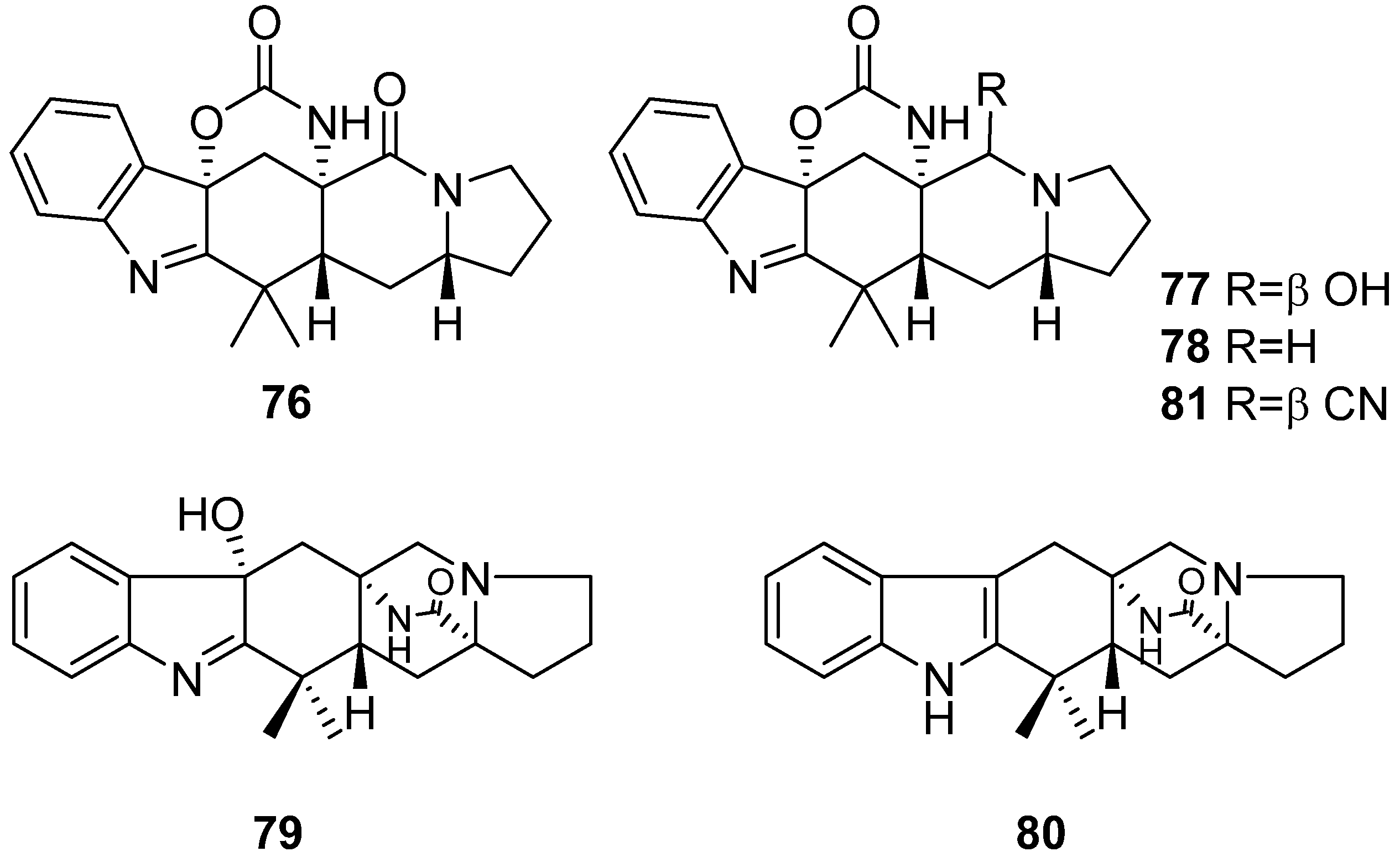
- Xu, X.; Zhang, X.; Nong, X.; Wei, X.; Qi, S. Oxindole alkaloids from the fungus Penicillium commune DFFSCS026 isolated from deep-sea-derived sediments. Tetrahedron 2015, 71, 610–615. [Google Scholar] [CrossRef]
- Song, F.; He, H.; Ma, R.; Xiao, X.; Wei, Q.; Wang, Q.; Ji, Z.; Dai, H.; Zhang, L.; Capon, R.J. Structure revision of the Penicillium alkaloids haenamindole and citreoindole. Tetrahedron Lett. 2016, 57, 3851–3852. [Google Scholar] [CrossRef]
- Kim, J.W.; Ko, S.-K.; Son, S.; Shin, K.-S.; Ryoo, I.-J.; Hong, Y.-S.; Oh, H.; Hwang, B.Y.; Hirota, H.; Takahashi, S.; et al. Haenamindole, an unusual diketopiperazine derivative from a marine-derived Penicillium sp. KCB12F005. Bioorg. Med. Chem. Lett. 2015, 25, 5398–5401. [Google Scholar] [CrossRef]
- Matsunaga, K.; Shizuri, Y.; Yamamura, S.; Kawai, K.; Furukawa, H. Isolation and structure of citreoindole, a new metabolite of Hybrid strain KO 0052 derived from Penicillium citreo-viride B. IFO 6200 and 4692. Tetrahedron Lett. 1991, 32, 6883–6884. [Google Scholar] [CrossRef]
- Wu, C.-J.; Li, C.-W.; Gao, H.; Huang, X.-J.; Cui, C.-B. Penicimutamides D–E: Two new prenylated indole alkaloids from a mutant of the marine-derived Penicillium purpurogenum G59. RSC Adv. 2017, 7, 24718–24722. [Google Scholar] [CrossRef]
- Li, C.-W.; Wu, C.-J.; Cui, C.-B.; Xu, L.-L.; Cao, F.; Zhu, H.-J. Penicimutamides A–C: Rare carbamate-containing alkaloids from a mutant of the marine-derived Penicillium purpurogenum G59. RSC Adv. 2016, 6, 73383–73387. [Google Scholar] [CrossRef]
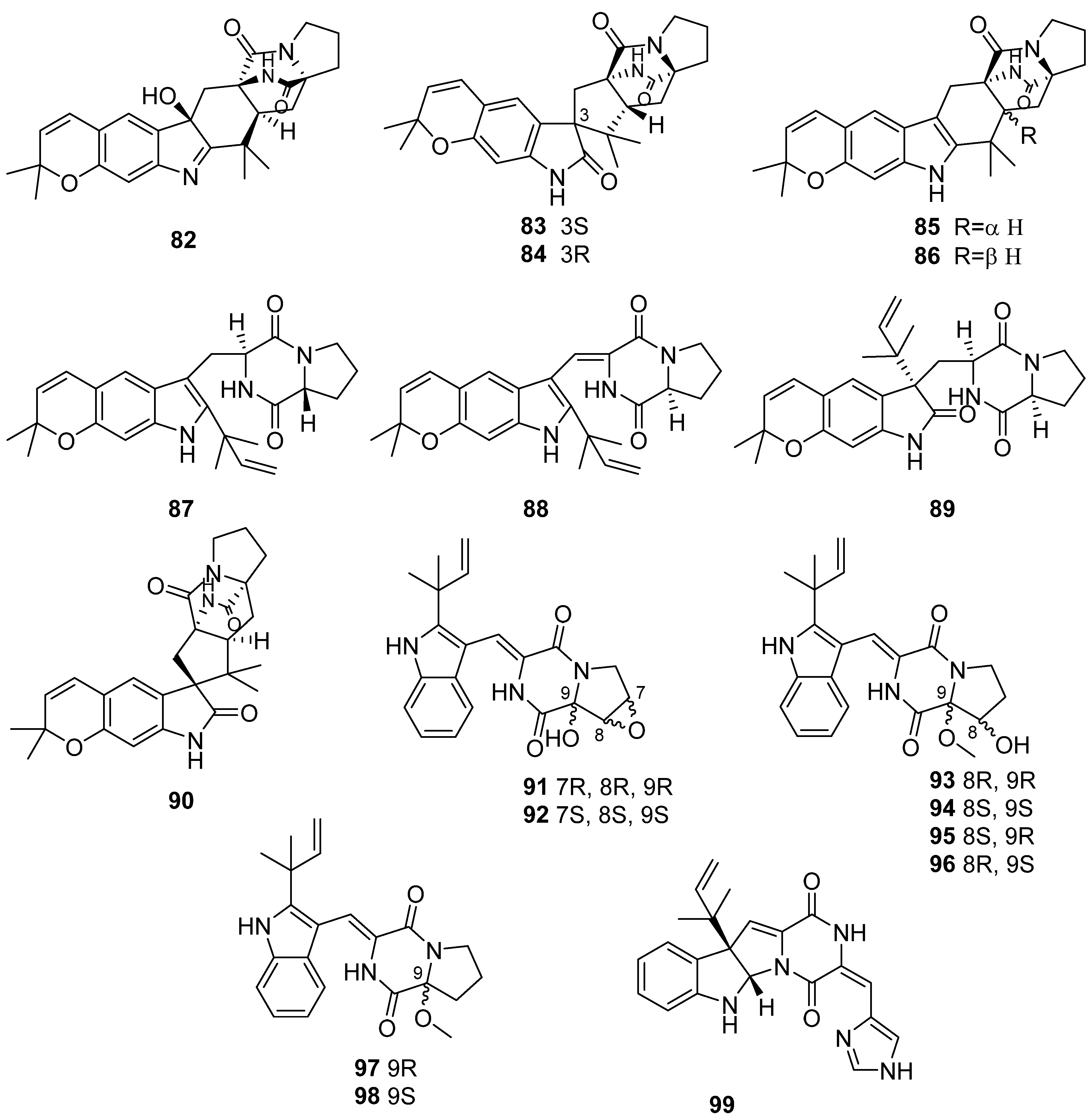
- Li, H.; Sun, W.; Deng, M.; Zhou, Q.; Wang, J.; Liu, J.; Chen, C.; Qi, C.; Luo, Z.; Xue, Y.; et al. Asperversiamides, Linearly Fused Prenylated Indole Alkaloids from the Marine-Derived Fungus Aspergillus versicolor. J. Org. Chem. 2018, 83, 8483–8492. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Li, Z.; Gao, J.; He, H.; Dai, H.; Xia, X.; Liu, C.; Zhang, L.; Song, F. New Diketopiperazines from a Marine-Derived Fungus Strain Aspergillus versicolor MF180151. Mar. Drugs 2019, 17, 262. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Wang, N.; Xie, C.-L.; Fan, Z.; Luo, Z.; Chen, H.-F.; Yang, X.-W. Roquefortine J, a novel roquefortine alkaloid, from the deep-sea-derived fungus Penicillium granulatum MCCC 3A00475. J. Antibiot. 2018, 71, 658–661. [Google Scholar] [CrossRef]
- Luo, M.; Zang, R.; Wang, X.; Chen, Z.; Song, X.; Ju, J.; Huang, H. Natural Hydroxamate-Containing Siderophore Acremonpeptides A–D and an Aluminum Complex of Acremonpeptide D from the Marine-Derived Acremonium persicinum SCSIO 115. J. Nat. Prod. 2019, 82, 2594–2600. [Google Scholar] [CrossRef]
- Zhang, Z.; Min, X.; Huang, J.; Zhong, Y.; Wu, Y.; Li, X.; Deng, Y.; Jiang, Z.; Shao, Z.; Zhang, L.; et al. Cytoglobosins H and I, New Antiproliferative Cytochalasans from Deep-Sea-Derived Fungus Chaetomium globosum. Mar. Drugs 2016, 14, 233. [Google Scholar] [CrossRef] [PubMed]
- Limbadri, S.; Luo, X.; Lin, X.; Liao, S.; Wang, J.; Zhou, X.; Yang, B.; Liu, Y. Bioactive Novel Indole Alkaloids and Steroids from Deep Sea-Derived Fungus Aspergillus fumigatus SCSIO 41012. Molecules 2018, 23, 2379. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.C.; Xu, L.L.; Yang, R.Y.; Yang, M.Y.; Hu, L.D.; Zhu, H.J.; Cao, F. Anti-Vibrio Indole-Diterpenoids and C-25 Epimeric Steroids From the Marine-Derived Fungus Penicillium janthinellum. Front. Chem. 2019, 7, 80. [Google Scholar] [CrossRef]
- Han, J.; Liu, M.; Jenkins, I.D.; Liu, X.; Zhang, L.; Quinn, R.J.; Feng, Y. Genome-Inspired Chemical Exploration of Marine Fungus Aspergillus fumigatus MF071. Mar. Drugs 2020, 18, 352. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, Y.; Li, S.; Hu, C.; Liu, H.; Zhang, W. Indole diketopiperazine alkaloids from the deep-sea-derived fungus Aspergillus sp. FS445. Nat. Prod. Res. 2021, 1–9. [Google Scholar] [CrossRef]
- Zhong, W.-M.; Wang, J.-F.; Shi, X.-F.; Wei, X.-Y.; Chen, Y.-C.; Zeng, Q.; Xiang, Y.; Chen, X.-Y.; Tian, X.-P.; Xiao, Z.-H.; et al. Eurotiumins A–E, Five New Alkaloids from the Marine-Derived Fungus Eurotium sp. SCSIO F452. Mar. Drugs 2018, 16, 136. [Google Scholar] [CrossRef]
- Zhong, W.; Wang, J.; Wei, X.; Fu, T.; Chen, Y.; Zeng, Q.; Huang, Z.; Huang, X.; Zhang, W.; Zhang, S.; et al. Three Pairs of New Spirocyclic Alkaloid Enantiomers From the Marine-Derived Fungus Eurotium sp. SCSIO F452. Front. Chem. 2019, 7, 350. [Google Scholar] [CrossRef]
- Zhong, W.; Wang, J.; Wei, X.; Chen, Y.; Fu, T.; Xiang, Y.; Huang, X.; Tian, X.; Xiao, Z.; Zhang, W.; et al. Variecolortins A–C, Three Pairs of Spirocyclic Diketopiperazine Enantiomers from the Marine-Derived Fungus Eurotium sp. SCSIO F452. Org. Lett. 2018, 20, 4593–4596. [Google Scholar] [CrossRef]
- Fukuda, T.; Nagai, K.; Kurihara, Y.; Kanamoto, A.; Tomoda, H. Graphiumins I and J, New Thiodiketopiperazines from the Marine-derived Fungus Graphium sp. OPMF00224. Nat. Prod. Sci. 2015, 21, 255–260. [Google Scholar] [CrossRef][Green Version]
- Fan, Z.; Sun, Z.-H.; Liu, Z.; Chen, Y.-C.; Liu, H.-X.; Li, H.-H.; Zhang, W.-M. Dichotocejpins A–C: New Diketopiperazines from a Deep-Sea-Derived Fungus Dichotomomyces cejpii FS110. Mar. Drugs 2016, 14, 164. [Google Scholar] [CrossRef]
- Yun, K.; Khong, T.T.; Leutou, A.S.; Kim, G.D.; Hong, J.; Lee, C.H.; Son, B.W. Cristazine, a New Cytotoxic Dioxopiperazine Alkaloid from the Mudflat-Sediment-Derived Fungus Chaetomium cristatum. Chem. Pharm. Bull. 2016, 64, 59–62. [Google Scholar] [CrossRef]
- Yu, G.; Wang, Y.; Yu, R.; Feng, Y.; Wang, L.; Che, Q.; Gu, Q.; Li, D.; Li, J.; Zhu, T. Chetracins E and F, cytotoxic epipolythiodioxopiperazines from the marine-derived fungus Acrostalagmus luteoalbus HDN13-530. RSC Adv. 2018, 8, 53–58. [Google Scholar] [CrossRef]
- Wakefield, J.; Hassan, H.M.; Jaspars, M.; Ebel, R.; Rateb, M.E. Dual Induction of New Microbial Secondary Metabolites by Fungal Bacterial Co-cultivation. Front. Microbiol. 2017, 8, 1284. [Google Scholar] [CrossRef]
- Afiyatullov, S.S.; Zhuravleva, O.I.; Antonov, A.S.; Berdyshev, D.V.; Pivkin, M.V.; Denisenko, V.A.; Popov, R.S.; Gerasimenko, A.V.; von Amsberg, G.; Dyshlovoy, S.A.; et al. Prenylated indole alkaloids from co-culture of marine-derived fungi Aspergillus sulphureus and Isaria felina. J. Antibiot. 2018, 71, 846–853. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, X.; Nong, X.; Wang, J.; Qi, S. Brevianamides and Mycophenolic Acid Derivatives from the Deep-Sea-Derived Fungus Penicillium brevicompactum DFFSCS025. Mar. Drugs 2017, 15, 43. [Google Scholar] [CrossRef]
- Yang, J.; Gong, L.; Guo, M.; Jiang, Y.; Ding, Y.; Wang, Z.; Xin, X.; An, F. Bioactive Indole Diketopiperazine Alkaloids from the Marine Endophytic Fungus Aspergillus sp. YJ191021. Mar. Drugs 2021, 19, 157. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Hao, X.; Li, S.; Jia, J.; Guan, Y.; Peng, Z.; Bi, H.; Xiao, C.; Cen, S.; et al. Broad-Spectrum Antiviral Natural Products from the Marine-Derived Penicillium sp. IMB17-046. Molecules 2019, 24, 2821. [Google Scholar] [CrossRef]
- Yang, B.; Tao, H.; Lin, X.; Wang, J.; Liao, S.; Dong, J.; Zhou, X.; Liu, Y. Prenylated indole alkaloids and chromone derivatives from the fungus Penicillium sp. SCSIO041218. Tetrahedron 2018, 74, 77–82. [Google Scholar] [CrossRef]
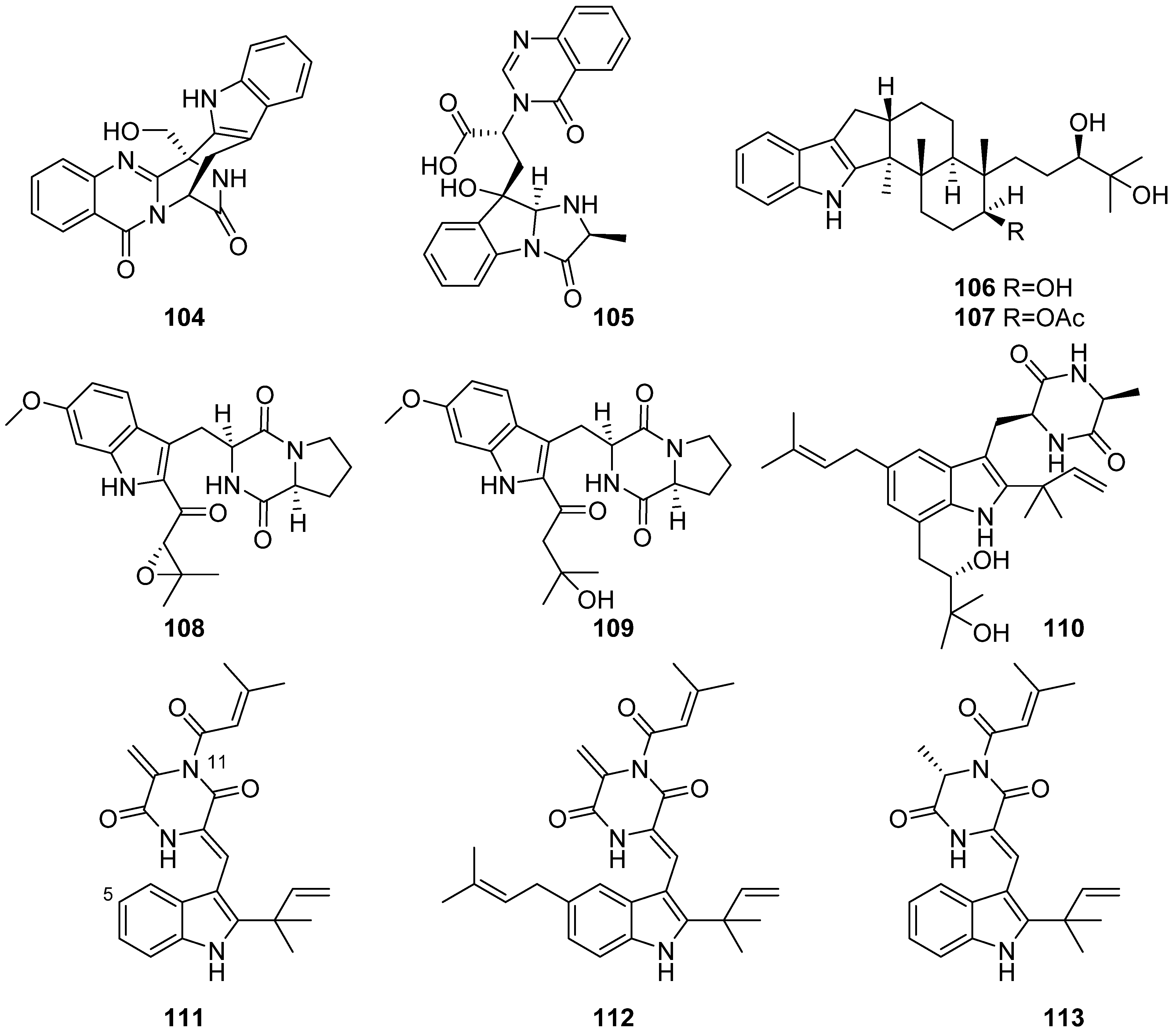
- Zheng, Y.-Y.; Shen, N.-X.; Liang, Z.-Y.; Shen, L.; Chen, M.; Wang, C.-Y. Paraherquamide J, a new prenylated indole alkaloid from the marine-derived fungus Penicillium janthinellum HK1-6. Nat. Prod. Res. 2020, 34, 378–384. [Google Scholar] [CrossRef]
- Li, J.; Hu, Y.; Hao, X.; Tan, J.; Li, F.; Qiao, X.; Chen, S.; Xiao, C.; Chen, M.; Peng, Z.; et al. Raistrickindole A, an Anti-HCV Oxazinoindole Alkaloid from Penicillium raistrickii IMB17-034. J. Nat. Prod. 2019, 82, 1391–1395. [Google Scholar] [CrossRef]
- Chen, Y.X.; Xu, M.Y.; Li, H.J.; Zeng, K.J.; Ma, W.Z.; Tian, G.B.; Xu, J.; Yang, D.P.; Lan, W.J. Diverse Secondary Metabolites from the Marine-Derived Fungus Dichotomomyces cejpii F31-1. Mar. Drugs 2017, 15, 339. [Google Scholar] [CrossRef]
- Huang, L.H.; Xu, M.Y.; Li, H.J.; Li, J.Q.; Chen, Y.X.; Ma, W.Z.; Li, Y.P.; Xu, J.; Yang, D.P.; Lan, W.J. Amino Acid-Directed Strategy for Inducing the Marine-Derived Fungus Scedosporium apiospermum F41-1 to Maximize Alkaloid Diversity. Org. Lett. 2017, 19, 4888–4891. [Google Scholar] [CrossRef]
- Yuan, M.X.; Qiu, Y.; Ran, Y.Q.; Feng, G.K.; Deng, R.; Zhu, X.F.; Lan, W.J.; Li, H.J. Exploration of Indole Alkaloids from Marine Fungus Pseudallescheria boydii F44-1 Using an Amino Acid-Directed Strategy. Mar. Drugs 2019, 17, 77. [Google Scholar] [CrossRef]
- Li, C.-J.; Chen, P.-N.; Li, H.-J.; Mahmud, T.; Wu, D.-L.; Xu, J.; Lan, W.-J. Potential Antidiabetic Fumiquinazoline Alkaloids from the Marine-Derived Fungus Scedosporium apiospermum F41-1. J. Nat. Prod. 2020, 83, 1082–1091. [Google Scholar] [CrossRef]
- Liu, W.; Li, H.-J.; Xu, M.-Y.; Ju, Y.-C.; Wang, L.-Y.; Xu, J.; Yang, D.-P.; Lan, W.-J. Pseudellones A–C, Three Alkaloids from the Marine-Derived Fungus Pseudallescheria ellipsoidea F42-3. Org. Lett. 2015, 17, 5156–5159. [Google Scholar] [CrossRef]
- Lan, W.-J.; Wang, K.-T.; Xu, M.-Y.; Zhang, J.-J.; Lam, C.-K.; Zhong, G.-H.; Xu, J.; Yang, D.-P.; Li, H.-J.; Wang, L.-Y. Secondary metabolites with chemical diversity from the marine-derived fungus Pseudallescheria boydii F19-1 and their cytotoxic activity. RSC Adv. 2016, 6, 76206–76213. [Google Scholar] [CrossRef]
- Wang, K.T.; Xu, M.Y.; Liu, W.; Li, H.J.; Xu, J.; Yang, D.P.; Lan, W.J.; Wang, L.Y. Two Additional New Compounds from the Marine-Derived Fungus Pseudallescheria ellipsoidea F42-3. Molecules 2016, 21, 442. [Google Scholar] [CrossRef]
- Luo, X.W.; Gao, C.H.; Lu, H.M.; Wang, J.M.; Su, Z.Q.; Tao, H.M.; Zhou, X.F.; Yang, B.; Liu, Y.H. HPLC-DAD-Guided Isolation of Diversified Chaetoglobosins from the Coral-Associated Fungus Chaetomium globosum C2F17. Molecules 2020, 25, 1237. [Google Scholar] [CrossRef]
- Wei, X.; Feng, C.; Wang, S.Y.; Zhang, D.M.; Li, X.H.; Zhang, C.X. New Indole Diketopiperazine Alkaloids from Soft Coral-Associated Epiphytic Fungus Aspergillus sp. EGF 15-0-3. Chem. Biodivers. 2020, 17, e2000106. [Google Scholar] [CrossRef]
- Zhuravleva, O.I.; Antonov, A.S.; Trang, V.T.D.; Pivkin, M.V.; Khudyakova, Y.V.; Denisenko, V.A.; Popov, R.S.; Kim, N.Y.; Yurchenko, E.A.; Gerasimenko, A.V.; et al. New Deoxyisoaustamide Derivatives from the Coral-Derived Fungus Penicillium dimorphosporum KMM 4689. Mar. Drugs 2021, 19, 32. [Google Scholar] [CrossRef]
- Cheng, Z.; Lou, L.; Liu, D.; Li, X.; Proksch, P.; Yin, S.; Lin, W. Versiquinazolines A–K, Fumiquinazoline-Type Alkaloids from the Gorgonian-Derived Fungus Aspergillus versicolor LZD-14-1. J. Nat. Prod. 2016, 79, 2941–2952. [Google Scholar] [CrossRef]
- Cheng, Z.; Liu, D.; Cheng, W.; Proksch, P.; Lin, W. Versiquinazolines L–Q, new polycyclic alkaloids from the marine-derived fungus Aspergillus versicolor. RSC Adv. 2018, 8, 31427–31439. [Google Scholar] [CrossRef]
- Ma, X.; Nong, X.-H.; Ren, Z.; Wang, J.; Liang, X.; Wang, L.; Qi, S.-H. Antiviral peptides from marine gorgonian-derived fungus Aspergillus sp. SCSIO 41501. Tetrahedron Lett. 2017, 58, 1151–1155. [Google Scholar] [CrossRef]
- Liu, M.; Sun, W.; Wang, J.; He, Y.; Zhang, J.; Li, F.; Qi, C.; Zhu, H.; Xue, Y.; Hu, Z.; et al. Bioactive secondary metabolites from the marine-associated fungus Aspergillus terreus. Bioorg. Chem. 2018, 80, 525–530. [Google Scholar] [CrossRef]
- Ma, X.; Liang, X.; Huang, Z.-H.; Qi, S.-H. New alkaloids and isocoumarins from the marine gorgonian-derived fungus Aspergillus sp. SCSIO 41501. Nat. Prod. Res. 2020, 34, 1992–2000. [Google Scholar] [CrossRef]
- Hou, X.M.; Liang, T.M.; Guo, Z.Y.; Wang, C.Y.; Shao, C.L. Discovery, absolute assignments, and total synthesis of asperversiamides A-C and their potent activity against Mycobacterium marinum. Chem. Commun. 2019, 55, 1104–1107. [Google Scholar] [CrossRef]
- Kong, F.D.; Fan, P.; Zhou, L.M.; Ma, Q.Y.; Xie, Q.Y.; Zheng, H.Z.; Zheng, Z.H.; Zhang, R.S.; Yuan, J.Z.; Dai, H.F.; et al. Penerpenes A-D, Four Indole Terpenoids with Potent Protein Tyrosine Phosphatase Inhibitory Activity from the Marine-Derived Fungus Penicillium sp. KFD28. Org. Lett. 2019, 21, 4864–4867. [Google Scholar] [CrossRef]
- Zhou, L.M.; Kong, F.D.; Fan, P.; Ma, Q.Y.; Xie, Q.Y.; Li, J.H.; Zheng, H.Z.; Zheng, Z.H.; Yuan, J.Z.; Dai, H.F.; et al. Indole-Diterpenoids with Protein Tyrosine Phosphatase Inhibitory Activities from the Marine-Derived Fungus Penicillium sp. KFD28. J. Nat. Prod. 2019, 82, 2638–2644. [Google Scholar] [CrossRef]
- Chen, M.Y.; Xie, Q.Y.; Kong, F.D.; Ma, Q.Y.; Zhou, L.M.; Yuan, J.Z.; Dai, H.F.; Wu, Y.G.; Zhao, Y.X. Two new indole-diterpenoids from the marine-derived fungus Penicillium sp. KFD28. J. Asian Nat. Prod. Res. 2020, 23, 1030–1036. [Google Scholar] [CrossRef]
- Kong, F.D.; Zhang, S.L.; Zhou, S.Q.; Ma, Q.Y.; Xie, Q.Y.; Chen, J.P.; Li, J.H.; Zhou, L.M.; Yuan, J.Z.; Hu, Z.; et al. Quinazoline-Containing Indole Alkaloids from the Marine-Derived Fungus Aspergillus sp. HNMF114. J. Nat. Prod. 2019, 82, 3456–3463. [Google Scholar] [CrossRef]
- Liu, S.S.; Yang, L.; Kong, F.D.; Zhao, J.H.; Yao, L.; Yuchi, Z.G.; Ma, Q.Y.; Xie, Q.Y.; Zhou, L.M.; Guo, M.F.; et al. Three New Quinazoline-Containing Indole Alkaloids From the Marine-Derived Fungus Aspergillus sp. HNMF114. Front. Microbiol. 2021, 12, 680879. [Google Scholar] [CrossRef]
- Zhang, P.; Li, X.-M.; Liu, H.; Li, X.; Wang, B.-G. Two new alkaloids from Penicillium oxalicum EN-201, an endophytic fungus derived from the marine mangrove plant Rhizophora stylosa. Phytochem. Lett. 2015, 13, 160–164. [Google Scholar] [CrossRef]
- Meng, L.H.; Wang, C.Y.; Mándi, A.; Li, X.M.; Hu, X.Y.; Kassack, M.U.; Kurtán, T.; Wang, B.G. Three Diketopiperazine Alkaloids with Spirocyclic Skeletons and One Bisthiodiketopiperazine Derivative from the Mangrove-Derived Endophytic Fungus Penicillium brocae MA-231. Org. Lett. 2016, 18, 5304–5307. [Google Scholar] [CrossRef]
- Gao, S.S.; Li, X.M.; Williams, K.; Proksch, P.; Ji, N.Y.; Wang, B.G. Rhizovarins A-F, Indole-Diterpenes from the Mangrove-Derived Endophytic Fungus Mucor irregularis QEN-189. J. Nat. Prod. 2016, 79, 2066–2074. [Google Scholar] [CrossRef]
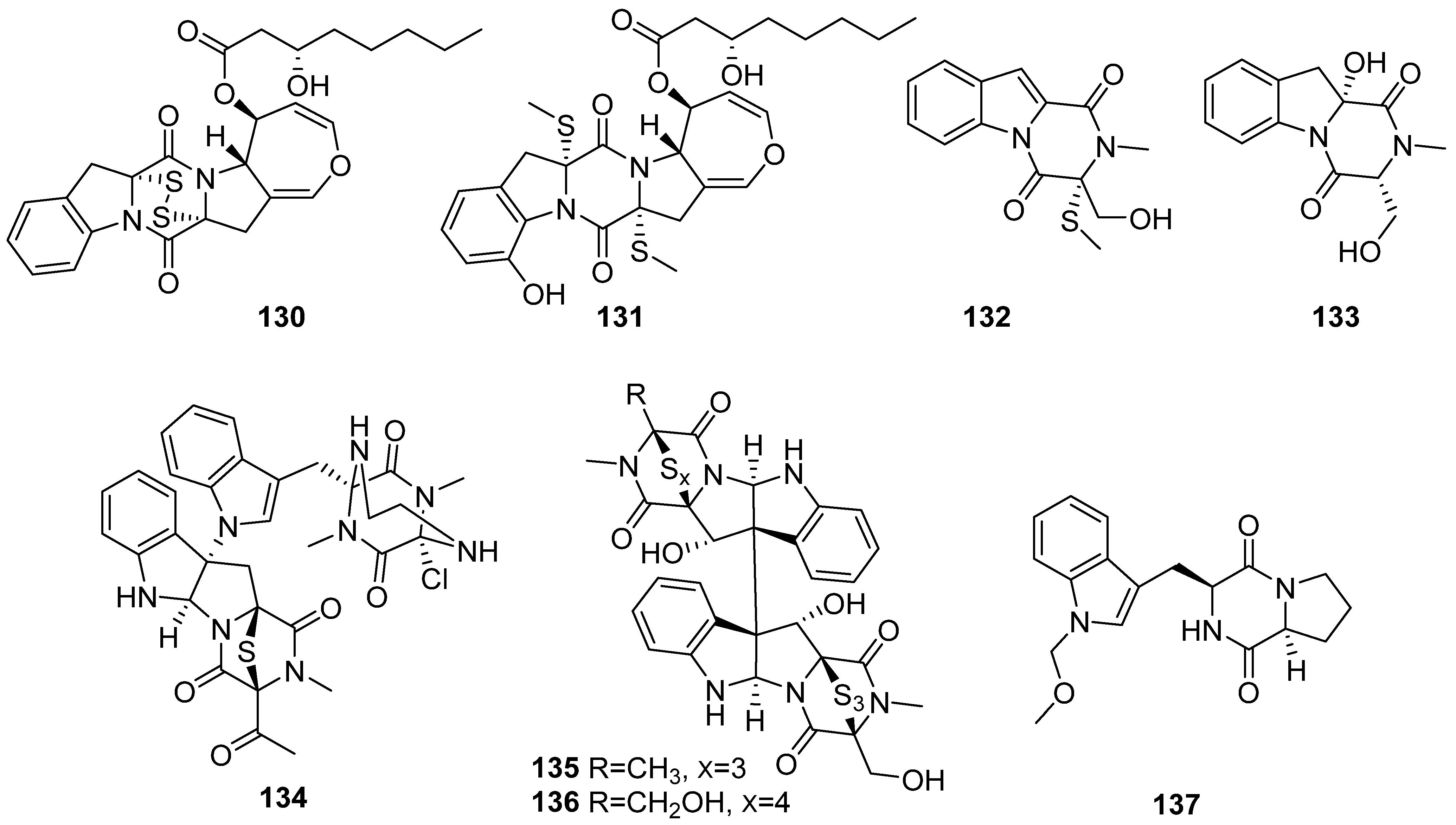
- Huang, S.; Chen, H.; Li, W.; Zhu, X.; Ding, W.; Li, C. Bioactive Chaetoglobosins from the Mangrove Endophytic Fungus Penicillium chrysogenum. Mar. Drugs 2016, 14, 172. [Google Scholar] [CrossRef]
- Zhu, X.; Zhou, D.; Liang, F.; Wu, Z.; She, Z.; Li, C. Penochalasin K, a new unusual chaetoglobosin from the mangrove endophytic fungus Penicillium chrysogenum V11 and its effective semi-synthesis. Fitoterapia 2017, 123, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Zhou, G.; Zhu, M.; Wang, W.; Zhu, T.; Gu, Q.; Li, D. Neosartoryadins A and B, Fumiquinazoline Alkaloids from a Mangrove-Derived Fungus Neosartorya udagawae HDN13-313. Org. Lett. 2016, 18, 244–247. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.J.; Bai, M.; Zhou, X.M.; Huang, G.L.; Shao, T.M.; Luo, Y.P.; Niu, Z.G.; Niu, Y.Y.; Chen, G.Y.; Han, C.R. Penicilindoles A-C, Cytotoxic Indole Diterpenes from the Mangrove-Derived Fungus Eupenicillium sp. HJ002. J. Nat. Prod. 2018, 81, 1045–1049. [Google Scholar] [CrossRef] [PubMed]
- May Zin, W.W.; Buttachon, S.; Dethoup, T.; Pereira, J.A.; Gales, L.; Inácio, Â.; Costa, P.M.; Lee, M.; Sekeroglu, N.; Silva, A.M.S.; et al. Antibacterial and antibiofilm activities of the metabolites isolated from the culture of the mangrove-derived endophytic fungus Eurotium chevalieri KUFA 0006. Phytochemistry 2017, 141, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Du, F.-Y.; Li, X.; Li, X.-M.; Zhu, L.-W.; Wang, B.-G. Indolediketopiperazine Alkaloids from Eurotium cristatum EN-220, an Endophytic Fungus Isolated from the Marine Alga Sargassum thunbergii. Mar. Drugs 2017, 15, 24. [Google Scholar] [CrossRef]
- Zhang, P.; Li, X.M.; Mao, X.X.; Mándi, A.; Kurtán, T.; Wang, B.G. Varioloid A, a new indolyl-6,10b-dihydro-5aH-[1]benzofuro[2,3-b]indole derivative from the marine alga-derived endophytic fungus Paecilomyces variotii EN-291. Beilstein J. Org. Chem. 2016, 12, 2012–2018, Correction in 2018, 14, 2394–2395. [Google Scholar] [CrossRef]
- Cao, J.; Li, X.M.; Meng, L.H.; Konuklugil, B.; Li, X.; Li, H.L.; Wang, B.G. Isolation and characterization of three pairs of indolediketopiperazine enantiomers containing infrequent N-methoxy substitution from the marine algal-derived endophytic fungus Acrostalagmus luteoalbus TK-43. Bioorg. Chem. 2019, 90, 103030. [Google Scholar] [CrossRef]
- Liu, W.; Wang, L.; Wang, B.; Xu, Y.; Zhu, G.; Lan, M.; Zhu, W.; Sun, K. Diketopiperazine and Diphenylether Derivatives from Marine Algae-Derived Aspergillus versicolor OUCMDZ-2738 by Epigenetic Activation. Mar. Drugs 2019, 17, 6. [Google Scholar] [CrossRef]
- Yang, S.-Q.; Li, X.-M.; Li, X.; Chi, L.-P.; Wang, B.-G. Two New Diketomorpholine Derivatives and a New Highly Conjugated Ergostane-Type Steroid from the Marine Algal-Derived Endophytic Fungus Aspergillus alabamensis EN-547. Mar. Drugs 2018, 16, 114. [Google Scholar] [CrossRef]
- Yurchenko, E.A.; Menchinskaya, E.S.; Pislyagin, E.A.; Trinh, P.T.; Ivanets, E.V.; Smetanina, O.F.; Yurchenko, A.N. Neuroprotective Activity of Some Marine Fungal Metabolites in the 6-Hydroxydopamin- and Paraquat-Induced Parkinson’s Disease Models. Mar. Drugs 2018, 16, 457. [Google Scholar] [CrossRef]
- Ma, X.; Peng, J.; Wu, G.; Zhu, T.; Li, G.; Gu, Q.; Li, D. Speradines B-D, oxygenated cyclopiazonic acid alkaloids from the sponge-derived fungus Aspergillus flavus MXH-X104. Tetrahedron 2015, 71, 3522–3527. [Google Scholar] [CrossRef]
- Harms, H.; Orlikova, B.; Ji, S.; Nesaei-Mosaferan, D.; König, G.M.; Diederich, M. Epipolythiodiketopiperazines from the Marine Derived Fungus Dichotomomyces cejpii with NF-κB Inhibitory Potential. Mar. Drugs 2015, 13, 4949–4966. [Google Scholar] [CrossRef]
- May Zin, W.W.; Buttachon, S.; Dethoup, T.; Fernandes, C.; Cravo, S.; Pinto, M.M.; Gales, L.; Pereira, J.A.; Silva, A.M.; Sekeroglu, N.; et al. New Cyclotetrapeptides and a New Diketopiperzine Derivative from the Marine Sponge-Associated Fungus Neosartorya glabra KUFA 0702. Mar. Drugs 2016, 14, 136. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.H.; Sohn, J.H.; Oh, H. Isolation and structure determination of a new diketopiperazine dimer from marine-derived fungus Aspergillus sp. SF-5280. Nat. Prod. Res. 2018, 32, 214–221. [Google Scholar] [CrossRef]
- Özkaya, F.C.; Ebrahim, W.; El-Neketi, M.; Tansel Tanrıkul, T.; Kalscheuer, R.; Müller, W.E.G.; Guo, Z.; Zou, K.; Liu, Z.; Proksch, P. Induction of new metabolites from sponge-associated fungus Aspergillus carneus by OSMAC approach. Fitoterapia 2018, 131, 9–14. [Google Scholar] [CrossRef]
- Buttachon, S.; Ramos, A.A.; Inácio, Â.; Dethoup, T.; Gales, L.; Lee, M.; Costa, P.M.; Silva, A.M.S.; Sekeroglu, N.; Rocha, E.; et al. Bis-Indolyl Benzenoids, Hydroxypyrrolidine Derivatives and Other Constituents from Cultures of the Marine Sponge-Associated Fungus Aspergillus candidus KUFA0062. Mar. Drugs 2018, 16, 119. [Google Scholar] [CrossRef]
- Liu, J.; Gu, B.; Yang, L.; Yang, F.; Lin, H. New Anti-inflammatory Cyclopeptides From a Sponge-Derived Fungus Aspergillus violaceofuscus. Front. Chem. 2018, 6, 226. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.; Ling, J.; Liu, Y.; Chen, X.; Tian, X.; Meng, D.; Pan, H.; Hu, J.; Wang, N. Characterization and abolishment of the cyclopiazonic acids produced by Aspergillus oryzae HMP-F28. Biosci. Biotechnol. Biochem. 2018, 82, 1832–1839. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.-W.; Lin, Y.; Lu, Y.-J.; Zhou, X.-F.; Liu, Y.-H. Peptides and polyketides isolated from the marine sponge-derived fungus Aspergillus terreus SCSIO 41008. Chin. J. Nat. Med. 2019, 17, 149–154. [Google Scholar] [CrossRef]
- Guo, C.; Wang, P.; Lin, X.; Salendra, L.; Kong, F.; Liao, S.; Yang, B.; Zhou, X.; Wang, J.; Liu, Y. Phloroglucinol heterodimers and bis-indolyl alkaloids from the sponge-derived fungus Aspergillus sp. SCSIO 41018. Org. Chem. Front. 2019, 6, 3053–3059. [Google Scholar] [CrossRef]
- Elsebai, M.F.; Schoeder, C.T.; Muller, C.E. Fintiamin: A diketopiperazine from the marine sponge-derived fungus Eurotium sp. Arch. Der Pharm. 2021, 354, e2100206. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.Y.; Meng, L.H.; Li, X.; Yang, S.Q.; Li, X.M.; Wang, B.G. Three New Indole Diterpenoids from the Sea-Anemone-Derived Fungus Penicillium sp. AS-79. Mar. Drugs 2017, 15, 137. [Google Scholar] [CrossRef]
- Ivanets, E.V.; Yurchenko, A.N.; Smetanina, O.F.; Rasin, A.B.; Zhuravleva, O.I.; Pivkin, M.V.; Popov, R.S.; von Amsberg, G.; Afiyatullov, S.S.; Dyshlovoy, S.A. Asperindoles A–D and a p-Terphenyl Derivative from the Ascidian-Derived Fungus Aspergillus sp. KMM 4676. Mar. Drugs 2018, 16, 232. [Google Scholar] [CrossRef]
- Liu, L.; Xu, W.; Li, S.; Chen, M.; Cheng, Y.; Yuan, W.; Cheng, Z.; Li, Q. Penicindopene A, a new indole diterpene from the deep-sea fungus Penicillium sp. YPCMAC1. Nat. Prod. Res. 2019, 33, 2988–2994. [Google Scholar] [CrossRef]
- Zhou, R.; Liao, X.; Li, H.; Li, J.; Feng, P.; Zhao, B.; Xu, S. Isolation and Synthesis of Misszrtine A: A Novel Indole Alkaloid From Marine Sponge-Associated Aspergillus sp. SCSIO XWS03F03. Front. Chem. 2018, 6, 212. [Google Scholar] [CrossRef]
- Yan, W.; Zhao, S.S.; Ye, Y.H.; Zhang, Y.Y.; Zhang, Y.; Xu, J.Y.; Yin, S.M.; Tan, R.X. Generation of Indoles with Agrochemical Significance through Biotransformation by Chaetomium globosum. J. Nat. Prod. 2019, 82, 2132–2137. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Geng, C.; Zhang, X.W.; Zhu, H.J.; Shao, C.L.; Cao, F.; Wang, C.Y. Discovery of Bioactive Indole-Diketopiperazines from the Marine-Derived Fungus Penicillium brasilianum Aided by Genomic Information. Mar. Drugs 2019, 17, 514. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhang, M.; Li, H.; Wang, R.; Hou, H.; Li, X.; Liu, K.; Chen, H. New Prenylated Indole Homodimeric and Pteridine Alkaloids from the Marine-Derived Fungus Aspergillus austroafricanus Y32-2. Mar. Drugs 2021, 19, 98. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, S.; Ding, W.; Qiu, F.; Xu, J. Asperginine, an Unprecedented Alkaloid from the Marine-derived Fungus Aspergillus sp. Nat. Prod. Commun. 2015, 10, 1363–1364. [Google Scholar] [CrossRef]
- Xu, J.; Hu, Q.; Ding, W.; Wang, P.; Di, Y. New asymmetrical bispyrrolidinoindoline diketopiperazines from the marine fungus Aspergillus sp. DX4H. Nat. Prod. Res. 2018, 32, 815–820. [Google Scholar] [CrossRef]
- Kwon, J.; Lee, H.; Ko, W.; Kim, D.-C.; Kim, K.-W.; Kwon, H.C.; Guo, Y.; Sohn, J.H.; Yim, J.H.; Kim, Y.-C.; et al. Chemical constituents isolated from Antarctic marine-derived Aspergillus sp. SF-5976 and their anti-inflammatory effects in LPS-stimulated RAW 264.7 and BV2 cells. Tetrahedron 2017, 73, 3905–3912. [Google Scholar] [CrossRef]
- Takahashi, K.; Sakai, K.; Fukasawa, W.; Nagano, Y.; Sakaguchi, S.O.; Lima, A.O.; Pellizari, V.H.; Iwatsuki, M.; Takishita, K.; Yoshida, T.; et al. Quellenin, a new anti-Saprolegnia compound isolated from the deep-sea fungus, Aspergillus sp. YK-76. J. Antibiot. 2018, 71, 741–744. [Google Scholar] [CrossRef] [PubMed]
- Kubota, T.; Nakamura, K.; Sakai, K.; Fromont, J.; Gonoi, T.; Kobayashi, J.i. Hyrtinadines C and D, New Azepinoindole-Type Alkaloids from a Marine Sponge Hyrtios sp. Chem. Pharm. Bull. 2016, 64, 975–978. [Google Scholar] [CrossRef]
- Takahashi, H.; Kurimoto, S.-i.; Kobayashi, J.i.; Kubota, T. Ishigadine A, a new canthin-6-one alkaloid from an Okinawan marine sponge Hyrtios sp. Tetrahedron Lett. 2018, 59, 4500–4502. [Google Scholar] [CrossRef]
- Shady, N.H.; Abdelmohsen, U.R.; Ahmed, S.; Fouad, M.; Kamel, M.S.J.J.P.P. Phytochemical and biological investigation of the red sea marine sponge Hyrtios sp. J. Pharmacogn. Phytochem. 2017, 6, 241–246. [Google Scholar]
- Shady, N.H.; Fouad, M.A.; Ahmed, S.; Pimentel-Elardo, S.M.; Nodwell, J.R.; Kamel, M.S.; Abdelmohsen, U.R. A new antitrypanosomal alkaloid from the Red Sea marine sponge Hyrtios sp. J. Antibiot. 2018, 71, 1036–1039. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-I.; Lee, Y.-J.; Won, H.; Oh, K.-B.; Lee, H.-S. Indole Alkaloids from Tropical Sponge Hyrtios sp. as Isocitrate Lyase Inhibitors. Nat. Prod. Commun. 2018, 13, 1934578X1801300608. [Google Scholar] [CrossRef]
- Bagalagel, A.A.; Bogari, H.A.; Ahmed, S.A.; Diri, R.M.; Elhady, S.S.J.H. New Bromoindole Alkaloid Isolated from the Marine Sponge Hyrtios erectus. Heterocycles 2018, 96, 749–756. [Google Scholar]
- Wang, Q.; Tang, X.L.; Luo, X.C.; de Voog, N.J.; Li, P.L.; Li, G.Q. Aplysinopsin-type and Bromotyrosine-derived Alkaloids from the South China Sea Sponge Fascaplysinopsis reticulata. Sci. Rep. 2019, 9, 2248. [Google Scholar] [CrossRef]
- Campos, P.E.; Pichon, E.; Moriou, C.; Clerc, P.; Trépos, R.; Frederich, M.; De Voogd, N.; Hellio, C.; Gauvin-Bialecki, A.; Al-Mourabit, A. New Antimalarial and Antimicrobial Tryptamine Derivatives from the Marine Sponge Fascaplysinopsis reticulata. Mar. Drugs 2019, 17, 167. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Mohamed, G.A. Ingenines C and D, new cytotoxic pyrimidine-β-carboline alkaloids from the Indonesian sponge Acanthostrongylophora ingens. Phytochem. Lett. 2016, 18, 168–171. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Mohamed, G.A. Ingenine E, a new cytotoxic β-carboline alkaloid from the Indonesian sponge Acanthostrongylophora ingens. J. Asian Nat. Prod. Res. 2017, 19, 504–509. [Google Scholar] [CrossRef]
- Ibrahim, S.; Mohamed, G.; Al Haidari, R.; El-Kholy, A.; Zayed, M. Ingenine F: A New Cytotoxic Tetrahydro Carboline Alkaloid from the Indonesian Marine Sponge Acanthostrongylophora ingens. Pharmacogn. Mag. 2018, 14, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.K.; Riswanto, R.; Won, T.H.; Kim, H.; Elya, B.; Sim, C.J.; Oh, D.C.; Oh, K.B.; Shin, J. Manzamine Alkaloids from an Acanthostrongylophora sp. Sponge. J. Nat. Prod. 2017, 80, 1575–1583. [Google Scholar] [CrossRef] [PubMed]
- Okada, M.; Sugita, T.; Wong, C.P.; Wakimoto, T.; Abe, I. Identification of Pyridinium with Three Indole Moieties as an Antimicrobial Agent. J. Nat. Prod. 2017, 80, 1205–1209. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.-L.; Yang, X.-L. Indole Derivatives Produced by the Metagenome Genes of the Escherichia coli-Harboring Marine Sponge Discodermia calyx. Molecules 2017, 22, 681. [Google Scholar] [CrossRef]
- Yang, I.; Choi, H.; Nam, S.-J.; Kang, H. Two Indole-Alkaloids from a Korean Marine Sponge Spongia sp. Bull. Korean Chem. Soc. 2015, 36, 2120–2123. [Google Scholar] [CrossRef]
- Al-Massarani, S.M.; El-Gamal, A.A.; Al-Said, M.S.; Abdel-Kader, M.S.; Ashour, A.E.; Kumar, A.; Abdel-Mageed, W.M.; Al-Rehaily, A.J.; Ghabbour, H.A.; Fun, H.K. Studies on the Red Sea Sponge Haliclona sp. for its Chemical and Cytotoxic Properties. Pharmacogn. Mag. 2016, 12, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Hitora, Y.; Takada, K.; Ise, Y.; Okada, S.; Matsunaga, S. Dragmacidins G and H, Bisindole Alkaloids Tethered by a Guanidino Ethylthiopyrazine Moiety, from a Lipastrotethya sp. Marine Sponge. J. Nat. Prod. 2016, 79, 2973–2976. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Feng, Y.; Murtaza, M.; Wood, S.; Mellick, G.; Hooper, J.N.; Quinn, R.J. A Grand Challenge: Unbiased Phenotypic Function of Metabolites from Jaspis splendens against Parkinson’s Disease. J. Nat. Prod. 2016, 79, 353–361. [Google Scholar] [CrossRef]
- Ebada, S.S.; Müller, W.E.G.; Lin, W.; Proksch, P. New Acyclic Cytotoxic Jasplakinolide Derivative from the Marine Sponge Jaspis splendens. Mar. Drugs 2019, 17, 100. [Google Scholar] [CrossRef]
- Jamison, M.T.; Molinski, T.F. Jamaicensamide A, a Peptide Containing β-Amino-α-keto and Thiazole-Homologated η-Amino Acid Residues from the Sponge Plakina jamaicensis. J. Nat. Prod. 2016, 79, 2243–2249. [Google Scholar] [CrossRef]
- Olsen, E.K.; Hansen, E.; Moodie, L.M.K.; Isaksson, J.; Sepčić, K.; Cergolj, M.; Svenson, J.; Andersen, J.H. Marine AchE inhibitors isolated from Geodia barretti: Natural compounds and their synthetic analogs. Org. Biomol. Chem. 2016, 14, 1629–1640. [Google Scholar] [CrossRef] [PubMed]
- Di, X.; Rouger, C.; Hardardottir, I.; Freysdottir, J.; Molinski, T.F.; Tasdemir, D.; Omarsdottir, S. 6-Bromoindole Derivatives from the Icelandic Marine Sponge Geodia barretti: Isolation and Anti-Inflammatory Activity. Mar. Drugs 2018, 16, 437. [Google Scholar] [CrossRef]
- Liu, H.B.; Lauro, G.; O‘Connor, R.D.; Lohith, K.; Kelly, M.; Colin, P.; Bifulco, G.; Bewley, C.A. Tulongicin, an Antibacterial Tri-Indole Alkaloid from a Deep-Water Topsentia sp. Sponge. J. Nat. Prod. 2017, 80, 2556–2560. [Google Scholar] [CrossRef] [PubMed]
- Lorig-Roach, N.; Hamkins-Indik, F.; Johnson, T.A.; Tenney, K.; Valeriote, F.A.; Crews, P. The potential of achiral sponge-derived and synthetic bromoindoles as selective cytotoxins against PANC-1 tumor cells. Tetrahedron 2018, 74, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Cruz, P.G.; Martínez Leal, J.F.; Daranas, A.H.; Pérez, M.; Cuevas, C. On the Mechanism of Action of Dragmacidins I and J, Two New Representatives of a New Class of Protein Phosphatase 1 and 2A Inhibitors. ACS Omega 2018, 3, 3760–3767. [Google Scholar] [CrossRef]
- Salib, M.N.; Molinski, T.F. Six Trikentrin-like Cyclopentanoindoles from Trikentrion flabelliforme. Absolute Structural Assignment by NMR and ECD. J. Org. Chem. 2018, 83, 1278–1286. [Google Scholar] [CrossRef]
- Jennings, L.K.; Khan, N.M.D.; Kaur, N.; Rodrigues, D.; Morrow, C.; Boyd, A.; Thomas, O.P. Brominated Bisindole Alkaloids from the Celtic Sea Sponge Spongosorites calcicola. Molecules 2019, 24, 3890. [Google Scholar] [CrossRef]
- Ragini, K.; Piggott, A.M.; Karuso, P. Bisindole Alkaloids from a New Zealand Deep-Sea Marine Sponge Lamellomorpha strongylata. Mar. Drugs 2019, 17, 683. [Google Scholar] [CrossRef]
- Guzii, A.G.; Makarieva, T.N.; Denisenko, V.A.; Gerasimenko, A.V.; Udovenko, A.A.; Popov, R.S.; Dmitrenok, P.S.; Golotin, V.A.; Fedorov, S.N.; Grebnev, B.B.; et al. Guitarrins A-E and Aluminumguitarrin A: 5-Azaindoles from the Northwestern Pacific Marine Sponge Guitarra fimbriata. J. Nat. Prod. 2019, 82, 1704–1709. [Google Scholar] [CrossRef]
- El-Hawary, S.S.; Sayed, A.M.; Mohammed, R.; Hassan, H.M.; Rateb, M.E.; Amin, E.; Mohammed, T.A.; El-Mesery, M.; Bin Muhsinah, A.; Alsayari, A.; et al. Bioactive Brominated Oxindole Alkaloids from the Red Sea Sponge Callyspongia siphonella. Mar. Drugs 2019, 17, 465. [Google Scholar] [CrossRef] [PubMed]
- Ki, D.W.; Kodama, T.; El-Desoky, A.H.; Wong, C.P.; Nguyen, H.M.; Do, K.M.; Thai, Q.M.; Ton Nu, L.H.; Morita, H. Chemical Constituents of the Vietnamese Marine Sponge Gelliodes sp. and Their Cytotoxic Activities. Chem. Biodivers. 2020, 17, e2000303. [Google Scholar] [CrossRef] [PubMed]
- Moosmann, P.; Taniguchi, T.; Furihata, K.; Utsumi, H.; Ise, Y.; Morii, Y.; Yamawaki, N.; Takatani, T.; Arakawa, O.; Okada, S.; et al. Myrindole A, an Antimicrobial Bis-indole from a Marine Sponge Myrmekioderma sp. Org. Lett. 2021, 23, 3477–3480. [Google Scholar] [CrossRef] [PubMed]
- Ovenden, S.P.; Capon, R.J. Echinosulfonic acids A-C and echinosulfone A: Novel bromoindole sulfonic acids and a sulfone from a southern australian marine sponge, Echinodictyum. J. Nat. Prod. 1999, 62, 1246–1249. [Google Scholar] [CrossRef]
- Rubnov, S.; Chevallier, C.; Thoison, O.; Debitus, C.; Laprevote, O.; Guénard, D.; Sévenet, T. Echinosulfonic acid D: An ESI MSn evaluation of a new cytotoxic alkaloid from the New-Caledonian sponge Psammoclemma sp. Nat. Prod. Res. 2005, 19, 75–79. [Google Scholar] [CrossRef]
- Holland, D.C.; Kiefel, M.J.; Carroll, A.R. Structure Revisions of the Sponge-Derived Dibrominated Bis-indole Alkaloids, Echinosulfone A and the Echinosulfonic Acids A to D. J. Org. Chem. 2020, 85, 3490–3496. [Google Scholar] [CrossRef]
- Neupane, P.; Salim, A.A.; Capon, R.J. Structure revision of the rare sponge metabolite echinosulfone A, and biosynthetically related echinosulfonic acids A–D. Tetrahedron Lett. 2020, 61, 151651. [Google Scholar] [CrossRef]
- Sala, S.; Nealon, G.L.; Sobolev, A.N.; Fromont, J.; Gomez, O.; Flematti, G.R. Structure Reassignment of Echinosulfone A and the Echinosulfonic Acids A–D Supported by Single-Crystal X-ray Diffraction and Density Functional Theory Analysis. J. Nat. Prod. 2020, 83, 105–110. [Google Scholar] [CrossRef]
- Hanif, N.; Yamada, K.; Kitamura, M.; Kawazoe, Y.; de Voogd, N.J.; Uemura, D. New Indole Alkaloids from the Sponge Plakortis sp. Chem. Nat. Compd. 2015, 51, 1130–1133. [Google Scholar] [CrossRef]
- Kubota, T.; Nakamura, K.; Kurimoto, S.I.; Sakai, K.; Fromont, J.; Gonoi, T.; Kobayashi, J. Zamamidine D, a Manzamine Alkaloid from an Okinawan Amphimedon sp. Marine Sponge. J. Nat. Prod. 2017, 80, 1196–1199. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.D.; Cartner, L.K.; Bokesch, H.R.; Henrich, C.J.; Wang, X.W.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P.; O’Keefe, B.R.; Gustafson, K.R. NMR characterization of rearranged staurosporine aglycone analogues from the marine sponge Damiria sp. Magn. Reson. Chem. 2021, 59, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Taufa, T.; Gordon, R.M.A.; Hashmi, M.A.; Hira, K.; Miller, J.H.; Lein, M.; Fromont, J.; Northcote, P.T.; Keyzers, R.A. Pyrroloquinoline derivatives from a Tongan specimen of the marine sponge Strongylodesma tongaensis. Tetrahedron Lett. 2019, 60, 1825–1829. [Google Scholar] [CrossRef]
- Tabudravu, J.N.; Pellissier, L.; Smith, A.J.; Subko, K.; Autréau, C.; Feussner, K.; Hardy, D.; Butler, D.; Kidd, R.; Milton, E.J.; et al. LC-HRMS-Database Screening Metrics for Rapid Prioritization of Samples to Accelerate the Discovery of Structurally New Natural Products. J. Nat. Prod. 2019, 82, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Miguel-Gordo, M.; Gegunde, S.; Calabro, K.; Jennings, L.K.; Alfonso, A.; Genta-Jouve, G.; Vacelet, J.; Botana, L.M.; Thomas, O.P. Bromotryptamine and Bromotyramine Derivatives from the Tropical Southwestern Pacific Sponge Narrabeena nigra. Mar. Drugs 2019, 17, 319. [Google Scholar] [CrossRef]
- Khushi, S.; Nahar, L.; Salim, A.A.; Capon, R.J. Trachycladindoles H-M: Molecular Networking Guided Exploration of a Library of Southern Australian Marine Sponges. Aust. J. Chem. 2020, 73, 338–343. [Google Scholar] [CrossRef]
- Knestrick, M.A.; Wilson, N.G.; Roth, A.; Adams, J.H.; Baker, B.J. Friomaramide, a Highly Modified Linear Hexapeptide from an Antarctic Sponge, Inhibits Plasmodium falciparum Liver-Stage Development. J. Nat. Prod. 2019, 82, 2354–2358. [Google Scholar] [CrossRef]
- Hahn, D.; Kim, H.; Yang, I.; Chin, J.; Hwang, H.; Won, D.H.; Lee, B.; Nam, S.J.; Ekins, M.; Choi, H.; et al. The Halicylindramides, Farnesoid X Receptor Antagonizing Depsipeptides from a Petrosia sp. Marine Sponge Collected in Korea. J. Nat. Prod. 2016, 79, 499–506. [Google Scholar] [CrossRef]
- Bewley, C.A.; Debitus, C.; Faulkner, D.J. Microsclerodermins A and B. Antifungal Cyclic Peptides from the Lithistid Sponge Microscleroderma sp. J. Am. Chem. Soc. 1994, 116, 7631–7636. [Google Scholar] [CrossRef]
- Zhang, X.; Jacob, M.R.; Rao, R.R.; Wang, Y.-H.; Agarwal, A.K.; Newman, D.J.; Khan, I.A.; Clark, A.M.; Li, X.-C. Antifungal cyclic peptides from the marine sponge Microscleroderma herdmani. Res. Rep. Med. Chem. 2012, 2, 7–14. [Google Scholar] [CrossRef][Green Version]
- Melikhova, E.Y.; Pullin, R.D.; Winter, C.; Donohoe, T.J. Dehydromicrosclerodermin B and Microsclerodermin J: Total Synthesis and Structural Revision. Angew. Chem. 2016, 55, 9753–9757. [Google Scholar] [CrossRef] [PubMed]
- Golantsov, N.E.; Festa, A.A.; Varlamov, A.V.; Voskressensky, L.G. Revision of the Structure and Total Synthesis of Topsentin C. Synthesis 2017, 49, 2562–2574. [Google Scholar] [CrossRef]
- Shaker, K.H.; Göhl, M.; Müller, T.; Seifert, K. Indole Alkaloids from the Sea Anemone Heteractis aurora and Homarine from Octopus cyanea. Chem. Biodivers. 2015, 12, 1746–1755. [Google Scholar] [CrossRef] [PubMed]
- Guzii, A.G.; Makarieva, T.N.; Fedorov, S.N.; Denisenko, V.A.; Dmitrenok, P.S.; Kuzmich, A.S.; Krasokhin, V.B.; Lee, H.S.; Lee, Y.J.; Stonik, V.A. Gramine-derived Bromo-alkaloids Activating NF—kB-dependent Transcription from the Marine Hydroid Abietinaria abietina. Nat. Prod. Commun. 2016, 11, 1263–1265. [Google Scholar] [PubMed]
- Hansen, K.; Andersen, J.H.; Bayer, A.; Pandey, S.K.; Lorentzen, M.; Jørgensen, K.B.; Sydnes, M.O.; Guttormsen, Y.; Baumann, M.; Koch, U.; et al. Kinase Chemodiversity from the Arctic: The Breitfussins. J. Med. Chem. 2019, 62, 10167–10181. [Google Scholar] [CrossRef]
- Hansen, K.; Isaksson, J.; Bayer, A.; Johansen, J.A.; Andersen, J.H.; Hansen, E. Securamine Derivatives from the Arctic Bryozoan Securiflustra securifrons. J. Nat. Prod. 2017, 80, 3276–3283. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, L.A.; Clavico, E.E.; Parra, L.L.; Berlinck, R.G.; Ferreira, A.G.; Paul, V.J.; Pereira, R.C. Evaluation of chemical defense and chemical diversity in the exotic bryozoan Amathia verticillata. J. Braz. Chem. Soc. 2017, 28, 435–442. [Google Scholar] [CrossRef]
- Kleks, G.; Holland, D.C.; Kennedy, E.K.; Avery, V.M.; Carroll, A.R. Antiplasmodial Alkaloids from the Australian Bryozoan Amathia lamourouxi. J. Nat. Prod. 2020, 83, 3435–3444. [Google Scholar] [CrossRef]
- Maltseva, A.L.; Kotenko, O.N.; Kutyumov, V.A.; Matvienko, D.A.; Shavarda, A.L.; Winson, M.K.; Ostrovsky, A.N. Novel brominated metabolites from Bryozoa: A functional analysis. Nat. Prod. Res. 2017, 31, 1840–1848. [Google Scholar] [CrossRef]
- Hahn, D.; Kim, G.J.; Choi, H.; Kang, H. A Novel Bromoindole Alkaloid from a Korean Colonial Tunicate Didemnum sp. Nat. Prod. Sci. 2015, 21, 278–281. [Google Scholar] [CrossRef]
- Tran, T.D.; Pham, N.B.; Ekins, M.; Hooper, J.N.A.; Quinn, R.J. Isolation and Total Synthesis of Stolonines A–C, Unique Taurine Amides from the Australian Marine Tunicate Cnemidocarpa stolonifera. Mar. Drugs 2015, 13, 4556–4575. [Google Scholar] [CrossRef]
- Goudou, F.; Petit, P.; Moriou, C.; Gros, O.; Al-Mourabit, A. Orbicularisine: A Spiro-Indolothiazine Isolated from Gills of the Tropical Bivalve Codakia orbicularis. J. Nat. Prod. 2017, 80, 1693–1696. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-S.; Cheng, Y.-B.; Lin, Y.-C.; Liaw, C.-C.; Chang, J.-Y.; Kuo, Y.-H.; Shen, Y.-C. Nitrogen-Containing Diterpenoids, Sesquiterpenoids, and Nor-Diterpenoids from Cespitularia taeniata. Mar. Drugs 2015, 13, 5796–5814. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, K.; Iwasaki, A.; Sumimoto, S.; Sano, T.; Hitomi, Y.; Ohno, O.; Suenaga, K. Croissamide, a proline-rich cyclic peptide with an N-prenylated tryptophan from a marine cyanobacterium Symploca sp. Tetrahedron Lett. 2018, 59, 3806–3809. [Google Scholar] [CrossRef]
- Phyo, M.Y.; Ding, C.Y.G.; Goh, H.C.; Goh, J.X.; Ong, J.F.M.; Chan, S.H.; Yung, P.Y.M.; Candra, H.; Tan, L.T. Trikoramide A, a Prenylated Cyanobactin from the Marine Cyanobacterium Symploca hydnoides. J. Nat. Prod. 2019, 82, 3482–3488. [Google Scholar] [CrossRef]
- Woolner, V.H.; Jones, C.M.; Field, J.J.; Fadzilah, N.H.; Munkacsi, A.B.; Miller, J.H.; Keyzers, R.A.; Northcote, P.T. Polyhalogenated Indoles from the Red Alga Rhodophyllis membranacea: The First Isolation of Bromo-Chloro-Iodo Secondary Metabolites. J. Nat. Prod. 2016, 79, 463–469. [Google Scholar] [CrossRef]
- Li, M.C.; Sun, W.S.; Cheng, W.; Liu, D.; Liang, H.; Zhang, Q.Y.; Lin, W.H. Four new minor brominated indole related alkaloids with antibacterial activities from Laurencia similis. Bioorg. Med. Chem. Lett. 2016, 26, 3590–3593. [Google Scholar] [CrossRef]
- Cai, Y.S.; Sun, J.Z.; Tang, Q.Q.; Fan, F.; Guo, Y.W. Acanthiline A, a pyrido[1,2-a]indole alkaloid from Chinese mangrove Acanthus ilicifolius. J. Asian Nat. Prod. Res. 2018, 20, 1088–1092. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, Z.L.; Wang, Y.; Tian, L.; Pei, Y.H.; Hua, H.M. A new alkaloid from the marine-derived fungus Hypocrea virens. Nat. Prod. Res. 2011, 25, 1596–1599. [Google Scholar] [CrossRef]
- Green, M.T.; Peczkowski, G.R.; Al-Ani, A.J.; Benjamin, S.L.; Simpkins, N.S.; Jones, A.M. Total synthesis and structural revision of a mangrove alkaloid. RSC Adv. 2017, 7, 48754–48758. [Google Scholar] [CrossRef]

















Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Chen, S.; Yang, F.; Dong, S. Marine Indole Alkaloids—Isolation, Structure and Bioactivities. Mar. Drugs 2021, 19, 658. https://doi.org/10.3390/md19120658
Hu Y, Chen S, Yang F, Dong S. Marine Indole Alkaloids—Isolation, Structure and Bioactivities. Marine Drugs. 2021; 19(12):658. https://doi.org/10.3390/md19120658
Chicago/Turabian StyleHu, Yong, Siling Chen, Fang Yang, and Shuai Dong. 2021. "Marine Indole Alkaloids—Isolation, Structure and Bioactivities" Marine Drugs 19, no. 12: 658. https://doi.org/10.3390/md19120658
APA StyleHu, Y., Chen, S., Yang, F., & Dong, S. (2021). Marine Indole Alkaloids—Isolation, Structure and Bioactivities. Marine Drugs, 19(12), 658. https://doi.org/10.3390/md19120658






