Omacor Protects Normotensive and Hypertensive Rats Exposed to Continuous Light from Increased Risk to Malignant Cardiac Arrhythmias
Abstract
1. Introduction
2. Results
2.1. Effects of Treatment with Omacor® on Rat Systolic Blood Pressure and Biometric Parameters of Rats
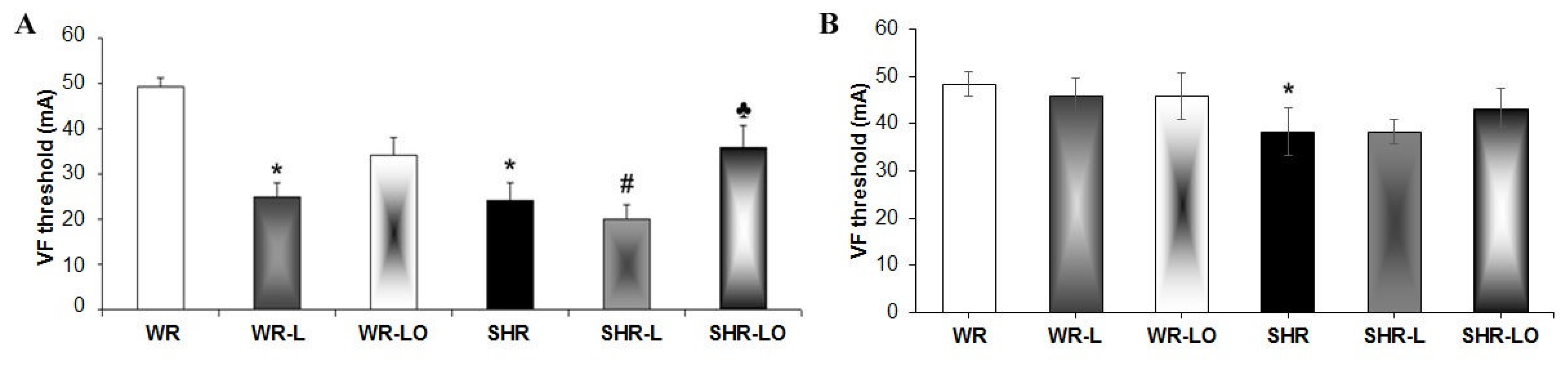
2.2. Effects of Treatment with Omacor® on Rat Heart Susceptibility to Electrically-Induced Sustained VF
2.3. Effects of Treatment with Omacor® on Myocardial Cx43 Gene Transcripts, Protein Levels, and Phosphorylation Status
2.4. Effect of Treatment with Omacor® on Myocardial PKCε and PKCδ Protein Levels
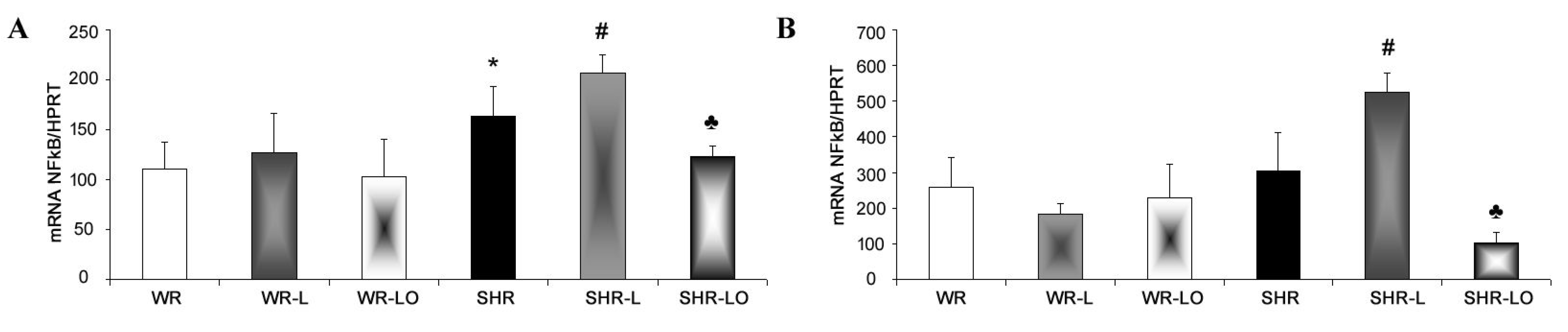
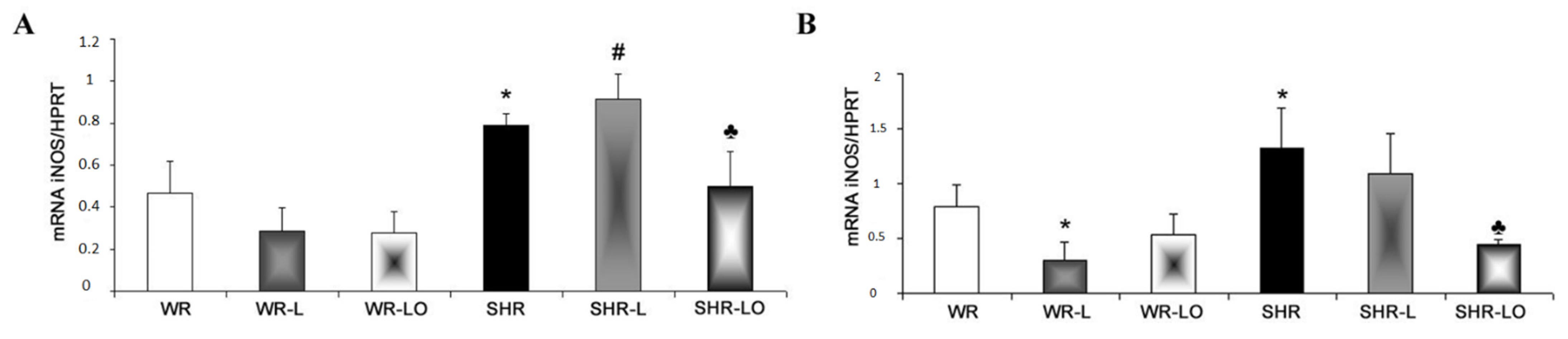
2.5. Effect of Treatment with Omacor® on NF-κB, iNOS and SERCA Gene Transripts
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Design
4.2. Examination of Vulnerability of the Heart to VF
4.3. Real-Time PCR for mRNA Expression of Cx43, NF-kB, iNOS, PPARy and SERCA
4.4. SDS-PAGE and Western Blot Analysis of Cx43, PKCε, and PKCδ Protein Levels
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Witte, K.; Grebmer, W.; Scalbert, E.; Delagrange, P.; Guardiola-Lemaître, B.; Lemmer, B. Effects of melatoninergic agonists on light-suppressed circadian rhythms in rats. Physiol. Behav. 1998, 65, 219–224. [Google Scholar] [CrossRef]
- Briaud, S.A.; Zhang, B.L.; Sannajust, F. Continuous light exposure and sympathectomy suppress circadian rhythm of blood pressure in rats. J. Cardiovasc. Pharmacol. Ther. 2004, 9, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.M.; Bar-Or, A.; Grossi, D.; Kashur, S.; Johannson, E.; Yie, S.M. Urinary 6-sulphatoxymelatonin, an index of pineal function in the rat. J. Pineal Res. 1991, 10, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Jeyaraj, D.; Haldar, S.M.; Wan, X.; McCauley, M.D.; Ripperger, J.A.; Hu, K.; Lu, Y.; Eapen, B.L.; Sharma, N.; Ficker, E.; et al. Circadian rhythms govern cardiac repolarization and arrhythmogenesis. Nature 2012, 483, 96–101. [Google Scholar] [CrossRef]
- Molcan, L.; Teplan, M.; Vesela, A.; Zeman, M. The long-term effects of phase advance shifts of photoperiod on cardiovascular parameters as measured by radiotelemetry in rats. Physiol. Meas. 2013, 34, 1623–1632. [Google Scholar] [CrossRef]
- Schlingmann, F.; De Rijk, S.; Pereboom, W.; Remie, R. “Avoidance” as a behavioural parameter in the determination of distress amongst albino and pigmented rats at various light intensities. Anim. Technol. 1993, 44, 87. [Google Scholar]
- Matsuo, M.; Tsuji, K. Strain differences of the light-dark preference in inbred rats. Behav. Genet. 1989, 19, 457–466. [Google Scholar] [CrossRef]
- Fernando, H.A.; Chin, H.F.; Ton, S.H.; Abdul Kadir, K. Stress and its effects on glucose metabolism and 11 β-HSD activities in rats fed on a combination of high-fat and high-sucrose diet with glycyrrhizic acid. J. Diabetes Res. 2013, 2013, 190395. [Google Scholar] [CrossRef]
- Paulis, L.; Važan, R.; Šimko, F.; Pecháňová, O.; Styk, J.; Babál, P.; Janega, P. Morphological alterations and NO-synthase expression in the heart after continuous light exposure of rats. Physiol. Res. 2007, 56, S71. [Google Scholar]
- Simko, F.; Pechanova, O. Remodelling of the heart and vessels in experimental hypertension: Advances in protection. J. Hypertens. 2010, 28, S1–S6. [Google Scholar] [CrossRef]
- Važan, R.; Janega, P.; Hojná, S.; Zicha, J.; Šimko, F.; Pecháňová, O.; Styk, J.; Paulis, L. The effect of continuous light exposure of rats on cardiac response to ischemia-reperfusion and NO-synthase activity. Physiol. Res. 2007, 56, S63. [Google Scholar] [PubMed]
- Severs, N.J. Gap junction remodeling and cardiac arrhythmogenesis: Cause or coincidence? J. Cell. Mol. Med. 2001, 5, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Gutstein, D.E.; Morley, G.E.; Tamaddon, H.; Vaidya, D.; Schneider, M.D.; Chen, J.; Chien, K.R.; Stuhlmann, H.; Fishman, G.I. Conduction slowing and sudden arrhythmic death in mice with cardiac-restricted inactivation of connexin43. Circ. Res. 2001, 88, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Tribulova, N.; Bacova, B.S.; Benova, T.; Viczenczova, C. Can we protect from malignant arrhythmias by modulation of cardiac cell-to-cell coupling? J. Electrocardiol. 2015, 48, 434–440. [Google Scholar] [CrossRef]
- Andelova, K.; Benova, T.E.; Bacova, B.S.; Sykora, M.; Prado, N.J.; Diez, E.R.; Hlivak, P.; Tribulova, N. Cardiac connexin-43 hemichannels and pannexin1 channels: Provocative antiarrhythmic targets. Int. J. Mol. Sci. 2021, 22, 260. [Google Scholar] [CrossRef] [PubMed]
- Baum, J.R.; Dolmatova, E.; Tan, A.; Duffy, H.S. Omega 3 fatty acid inhibition of inflammatory cytokine-mediated Connexin43 regulation in the heart. Front. Physiol. 2012, 3, 272. [Google Scholar] [CrossRef]
- Kirca, M.; Kleinbongard, P.; Soetkamp, D.; Heger, J.; Csonka, C.; Ferdinandy, P.; Schulz, R. Interaction between Connexin 43 and nitric oxide synthase in mice heart mitochondria. J. Cell. Mol. Med. 2015, 19, 815–825. [Google Scholar] [CrossRef]
- Landstrom, A.P.; Dobrev, D.; Wehrens, X.H.T. Calcium Signaling and Cardiac Arrhythmias. Circ. Res. 2017, 120, 1969–1993. [Google Scholar] [CrossRef]
- Sovari, A.A.; Bonini, M.G.; Dudley, S.C. Effective antioxidant therapy for the management of arrhythmia. Expert Rev. Cardiovasc. Ther. 2011, 9, 797–800. [Google Scholar] [CrossRef]
- Tribulova, N.; Bacova, B.S.; Benova, T.E.; Knezl, V.; Barancik, M.; Slezak, J. Omega-3 index and anti-arrhythmic potential of omega-3 PUFAs. Nutrients 2017, 9, 1191. [Google Scholar] [CrossRef]
- Pronova, BioPharma. Available online: https://www.norwayexports.no/listing/pronova-biopharma-norge-as/ (accessed on 20 November 2021).
- Bhatnagar, D.; Hussain, F. Omega-3 fatty acid ethyl esters (Omacor®) for the treatment of hypertriglyceridemia. Future Lipidol. 2007, 2, 263–270. [Google Scholar] [CrossRef][Green Version]
- Kar, S. Omacor and omega-3 fatty acids for treatment of coronary artery disease and the pleiotropic effects. Am. J. Ther. 2014, 21, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Bacova, B.S.; Radosinska, J.; Wallukat, G.; Barancik, M.; Wallukat, A.; Knezl, V.; Sykora, M.; Paulis, L.; Tribulova, N. Suppression of β1-adrenoceptor autoantibodies is involved in the antiarrhythmic effects of omega-3 fatty acids in male and female hypertensive rats. Int. J. Mol. Sci. 2020, 21, 526. [Google Scholar] [CrossRef] [PubMed]
- Tribulova, N.; Knezl, V.; Bacova, B.S.; Benova, T.E.; Viczenczova, C.; Gonçalvesova, E.; Slezak, J. Disordered myocardial Ca2+ homeostasis results in substructural alterations that may promote occurrence of malignant arrhythmias. Physiol. Res. 2016, 65, S139–S148. [Google Scholar] [CrossRef]
- Tribulová, N.; Dupont, E.; Soukup, T.; Okruhlicová, L.; Severs, N.J. Sex differences in connexin-43 expression in left ventricles of aging rats. Physiol. Res. 2005, 54, 705–708. [Google Scholar]
- Stauffer, B.L.; Sobus, R.D.; Sucharov, C.C. Sex differences in cardiomyocyte connexin43 expression. J. Cardiovasc. Pharmacol. 2011, 58, 32–39. [Google Scholar] [CrossRef]
- Gellert, S.; Schuchardt, J.P.; Hahn, A. Low long chain omega-3 fatty acid status in middle-aged women. Prostaglandins Leukot. Essent. Fat. Acids 2017, 117, 54–59. [Google Scholar] [CrossRef]
- Bačová, B.; Seč, P.; Radošinská, J.; Čertík, M.; Vachulová, A.; Tribulová, N. Lower Omega-3 index is a marker of increased propensity of hypertensive rat heart to malignant arrhythmias. Physiol. Res. 2013, 62, 201–208. [Google Scholar] [CrossRef]
- Lin, H.; Ogawa, K.; Imanaga, I.; Tribulova, N. Remodeling of connexin 43 in the diabetic rat heart. Mol. Cell. Biochem. 2006, 290, 69–78. [Google Scholar] [CrossRef]
- Lin, H.; Mitasikova, M.; Dlugosova, K.; Okruhlicova, L.; Imanaga, I.; Ogawa, K.; Weismann, P.; Tribulova, N.; Republic, S. Thyroid hormones suppress ε-pkc signalling, down-regulate connexin-43 and increase lethal arrhythmia susceptibility in non-diabetic and diabetic rat hearts. J. Physiol. Pharmacol. 2008, 59, 271–285. [Google Scholar]
- Bacova, B.S.; Viczenczova, C.; Andelova, K.; Sykora, M.; Chaudagar, K.; Barancik, M.; Adamcova, M.; Knezl, V.; Benova, T.E.; Weismann, P.; et al. Antiarrhythmic effects of melatonin and omega-3 are linked with protection of myocardial cx43 topology and suppression of fibrosis in catecholamine stressed normotensive and hypertensive rats. Antioxidants 2020, 9, 546. [Google Scholar] [CrossRef] [PubMed]
- Albert, C.M.; Cook, N.R.; Pester, J.; Moorthy, M.V.; Ridge, C.; Danik, J.S.; Gencer, B.; Siddiqi, H.K.; Ng, C.; Gibson, H.; et al. Effect of Marine Omega-3 Fatty Acid and Vitamin D Supplementation on Incident Atrial Fibrillation: A Randomized Clinical Trial. JAMA 2021, 325, 1061–1073. [Google Scholar] [CrossRef] [PubMed]
- Benova, T.E.; Viczenczova, C.; Bacova, B.S.; Knezl, V.; Dosenko, V.; Rauchova, H.; Zeman, M.; Reiter, R.J.; Tribulova, N. Obesity-associated alterations in cardiac connexin-43 and PKC signaling are attenuated by melatonin and omega-3 fatty acids in female rats. Mol. Cell. Biochem. 2019, 454, 191–202. [Google Scholar] [CrossRef] [PubMed]





| Male | WR | WR-L | WR-LO | SHR | SHR-L | SHR-LO |
| SBP (mmHg) | 108 ± 21 | 129 ± 12 * | 117 ± 13 | 175 ± 6 * | 201 ± 19 # | 181 ± 12 ♣ |
| HW (mg) | 763 ± 74 | 789 ± 71 | 801 ± 57 | 931 ± 107 * | 954 ± 81 | 896 ± 131 |
| HW/BW | 2.7 ± 0.1 | 2.9 ± 0.2 * | 2.9 ± 0.2 | 4.1 ± 0.1 * | 4.2 ± 0.1 | 4 ± 0.2 ♣ |
| LVW (mg) | 559 ± 49 | 566 ± 43 | 573 ± 41 | 690 ± 93 * | 717 ± 53 | 679 ± 105 |
| LVW/BW | 2 ± 0.1 | 2.1 ± 0.2 | 2.1 ± 0.2 | 3.1 ± 0.1 * | 3.2 ± 0.1 # | 3.0 ± 0.2 ♣ |
| Female | WR | WR-L | WR-LO | SHR | SHR-L | SHR-LO |
| SBP (mmHg) | 109.7 ± 13 | 123.6 ± 19 | 106.1 ± 16 | 154 ± 19 * | 170.6 ± 26 | 150.6 ± 9 |
| HW (mg) | 609 ± 23 | 571 ± 43 | 586 ± 31 | 671 ± 87 | 670 ± 35 | 622 ± 32 ♣ |
| HW/BW | 3.1 ± 0.1 | 3.2 ± 0.2 | 3.1 ± 0.1 | 4.4 ± 0.1 * | 4.4 ± 0.1 | 4.6 ± 0.2 ♣ |
| LVW (mg) | 427 ± 24 | 419 ± 43 | 431 ± 14 | 495 ± 57 * | 490 ± 25 | 466 ± 30 |
| LVW/BW | 2.2 ± 0.1 | 2.3 ± 0.2 | 2.3 ± 0.1 | 3.3 ± 0.1 * | 3.2 ± 0.1 | 3.5 ± 0.2 ♣ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Egan Benova, T.; Viczenczova, C.; Szeiffova Bacova, B.; Zurmanova, J.; Knezl, V.; Andelova, K.; Tribulova, N. Omacor Protects Normotensive and Hypertensive Rats Exposed to Continuous Light from Increased Risk to Malignant Cardiac Arrhythmias. Mar. Drugs 2021, 19, 659. https://doi.org/10.3390/md19120659
Egan Benova T, Viczenczova C, Szeiffova Bacova B, Zurmanova J, Knezl V, Andelova K, Tribulova N. Omacor Protects Normotensive and Hypertensive Rats Exposed to Continuous Light from Increased Risk to Malignant Cardiac Arrhythmias. Marine Drugs. 2021; 19(12):659. https://doi.org/10.3390/md19120659
Chicago/Turabian StyleEgan Benova, Tamara, Csilla Viczenczova, Barbara Szeiffova Bacova, Jitka Zurmanova, Vladimir Knezl, Katarina Andelova, and Narcis Tribulova. 2021. "Omacor Protects Normotensive and Hypertensive Rats Exposed to Continuous Light from Increased Risk to Malignant Cardiac Arrhythmias" Marine Drugs 19, no. 12: 659. https://doi.org/10.3390/md19120659
APA StyleEgan Benova, T., Viczenczova, C., Szeiffova Bacova, B., Zurmanova, J., Knezl, V., Andelova, K., & Tribulova, N. (2021). Omacor Protects Normotensive and Hypertensive Rats Exposed to Continuous Light from Increased Risk to Malignant Cardiac Arrhythmias. Marine Drugs, 19(12), 659. https://doi.org/10.3390/md19120659






