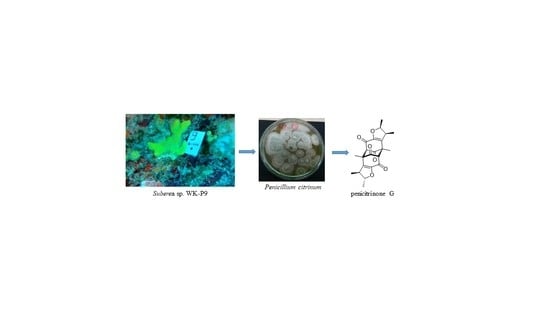
A New Citrinin Derivative from the Indonesian Marine Sponge-Associated Fungus Penicillium citrinum
Abstract
1. Introduction
2. Results
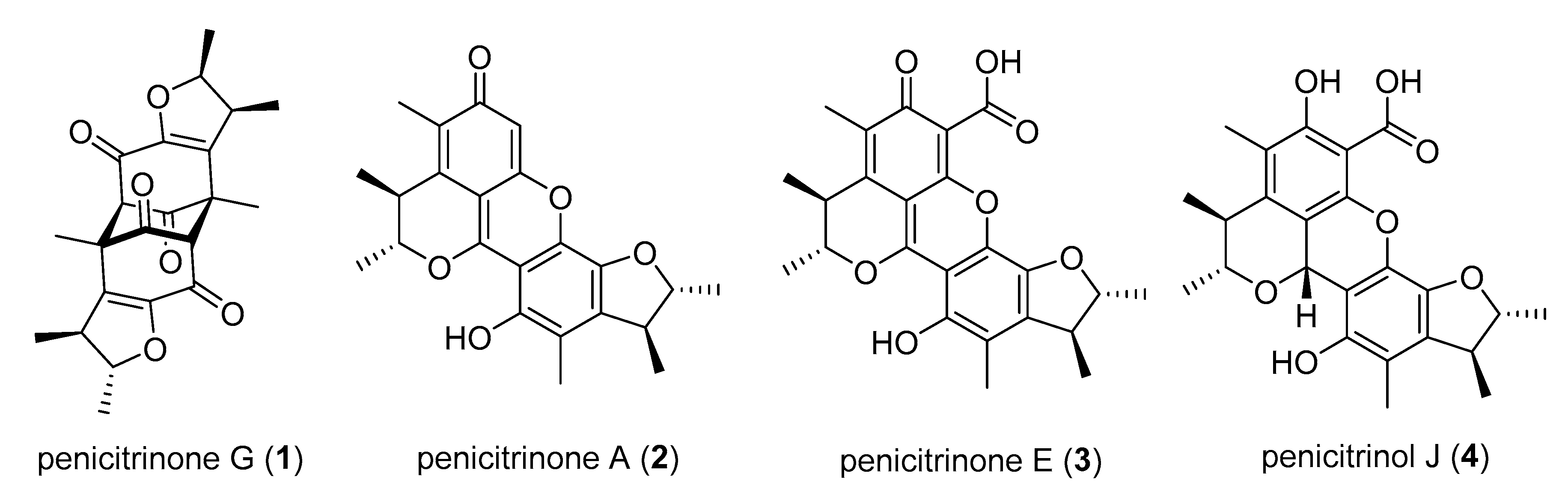
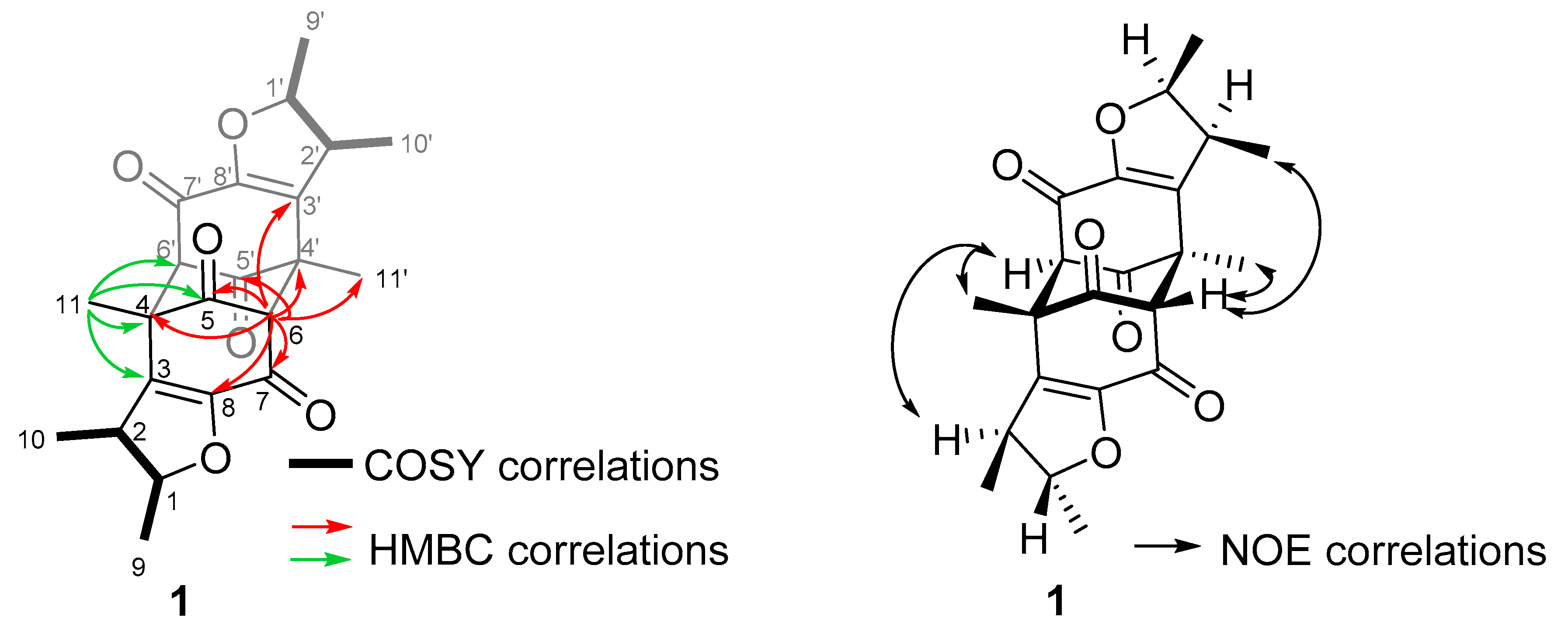
2.1. Sample Collection, Isolation, and Structure Elucidation
2.2. Bioactivity
3. Discussion
3.1. Bioactivity
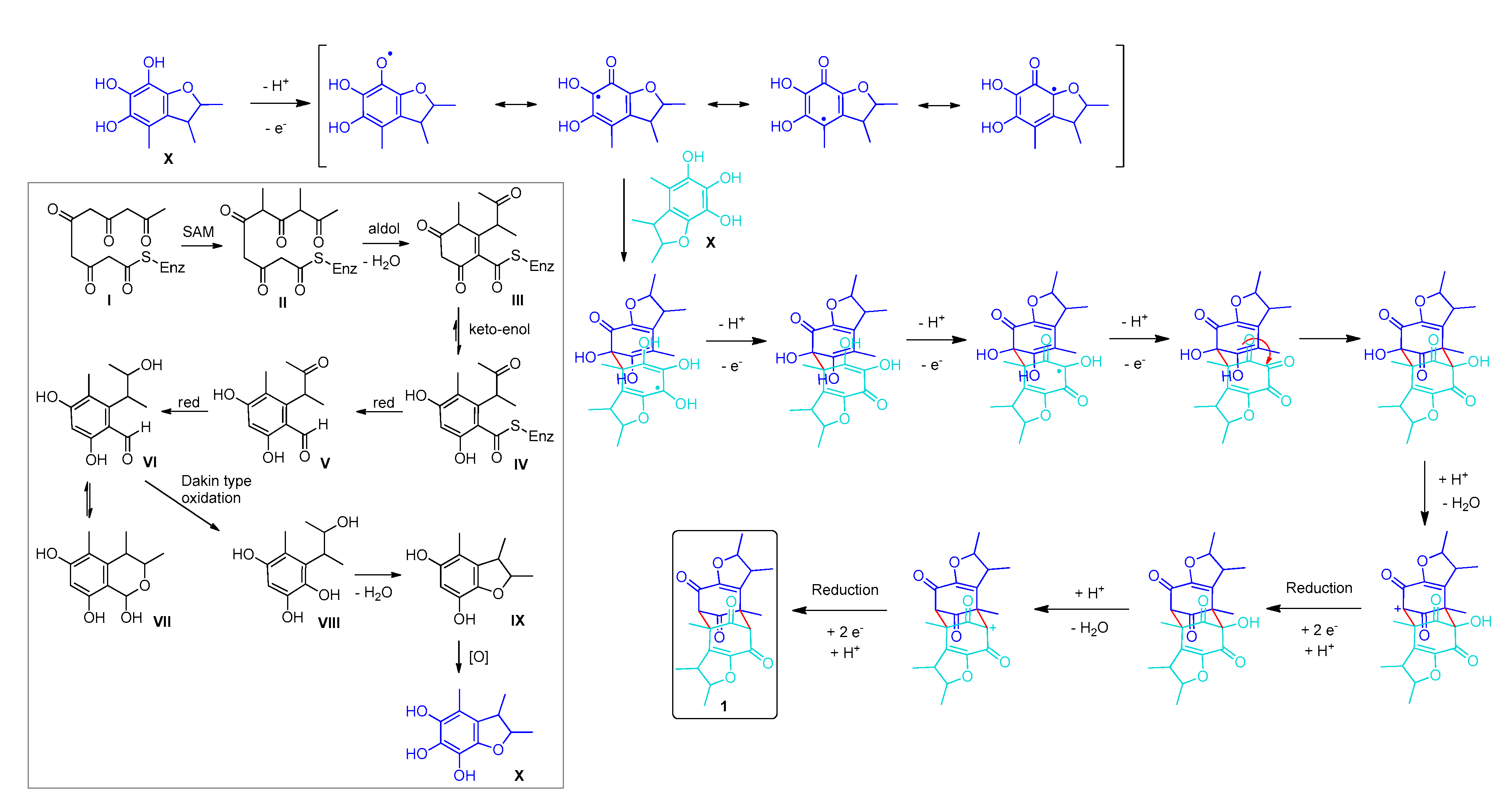
3.2. Biosynthesis
4. Materials and Methods
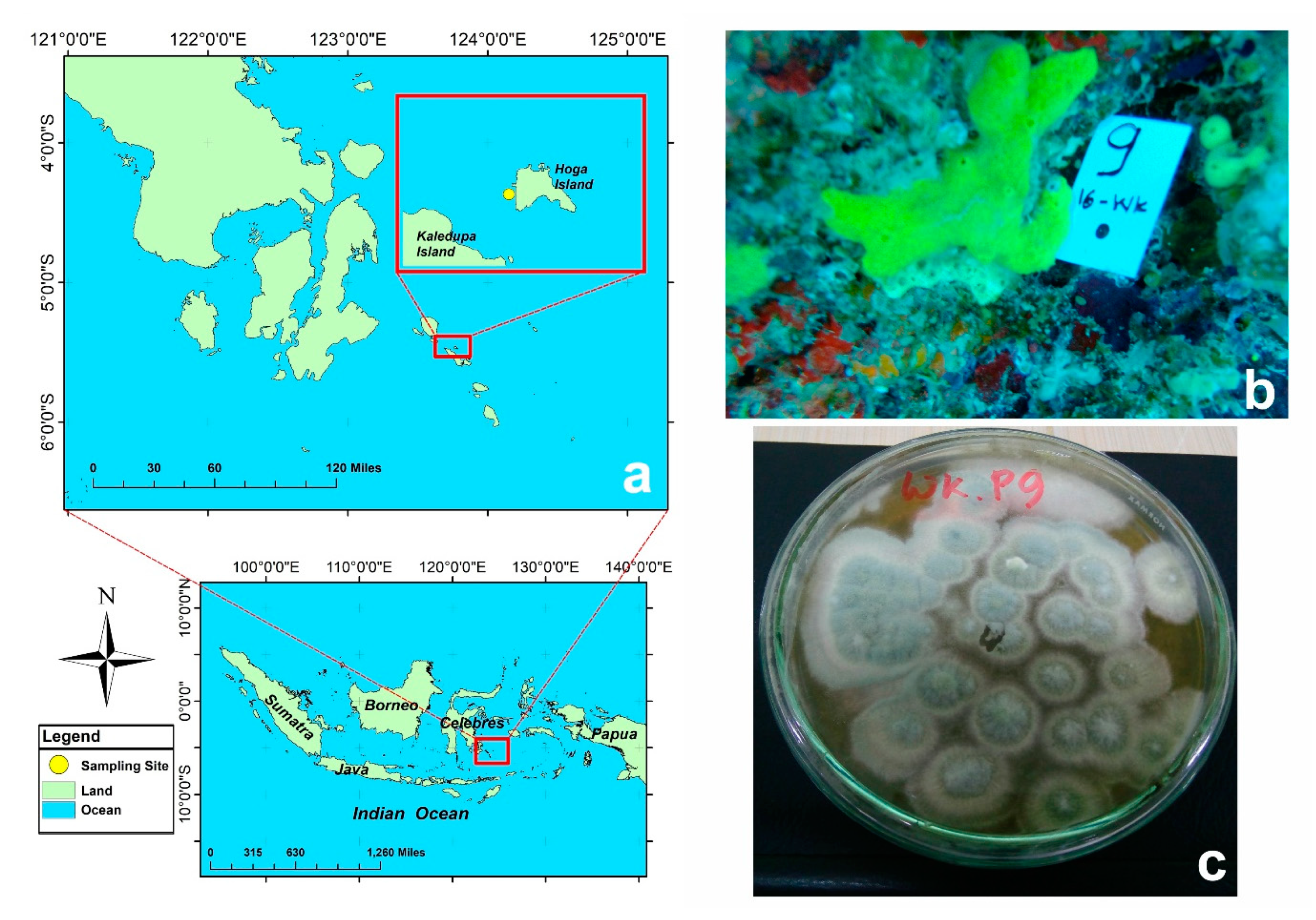
4.1. Sponge Collection
4.2. Fungal Isolation and Purification
4.3. Molecular Identification of the Fungus
4.4. Isolation and Structure Elucidation
4.5. Antibacterial Susceptibility Tests
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hu, G.P.; Yuan, J.; Sun, L.; She, Z.G.; Wu, J.H.; Lan, X.J.; Zhu, X.; Lin, Y.C.; Chen, S.P. Statistical Research on Marine Natural Products Based on Data Obtained between 1985 and 2008. Mar. Drugs 2011, 9, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Abad, M.J.; Bedoya, L.M.; Bermejo, P. Marine compounds and their antimicrobial activities. Fortamex 2011, 51, 1293–1306. [Google Scholar]
- Dyshlovoy, S.A.; Fedorov, S.N.; Shubina, L.K.; Kuzmich, A.S.; Bokemeyer, C.; Keller-Von Amsberg, G.; Honecker, F. Aaptamines from the marine sponge Aaptos sp. display anticancer activities in human cancer cell lines and modulate AP-1-, NF-κB-, and p53-dependent transcriptional activity in mouse JB6 Cl41 cells. Biomed. Res. Int. 2014, 2014, 469309. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Yang, F.; Sun, F.; Li, J.; Jiao, W.; Gan, J.; Hu, W.; Lin, H. Aaptamine Derivatives with Antifungal and Anti-HIV-1 Activities from the South China Sea Sponge Aaptos aaptos. Mar. Drugs 2014, 12, 6003–6013. [Google Scholar] [CrossRef]
- Lai, K.H.; Liu, Y.C.; Su, J.H.; El-Shazly, M.; Wu, C.F.; Du, Y.C.; Hsu, Y.M.; Yang, J.C.; Weng, M.K.; Chou, C.H.; et al. Antileukemic Scalarane Sesterterpenoids and Meroditerpenoid from Carteriospongia (Phyllospongia) sp., Induce Apoptosis via Dual Inhibitory Effects on Topoisomerase II and Hsp90. Sci. Rep. 2016, 6, 36170–36183. [Google Scholar] [CrossRef]
- Ito, T.; Nguyen, H.M.; Win, N.N.; Vo, H.Q.; Nguyen, H.T.; Moria, H. Three new sesquiterpene aminoquinones from a Vietnamese Spongia sp. and their biological activities. J. Nat. Med. 2018, 72, 298–303. [Google Scholar] [CrossRef]
- Trianto, A.; Hermawan, I.; Suzuka, T.; Tanaka, J. Two New Cytotoxic Candidaspongiolides from an Indonesian Sponge. ISRN Pharm. 2011, 2011, 852619. [Google Scholar] [CrossRef]
- Balansa, W.; Mettal, U.; Wuisan, Z.G.; Plubrukarn, A.; Ijong, F.G.; Liu, Y.; Schäberle, T.F. A New Sesquiterpenoid Aminoquinone from an Indonesian Marine Sponge. Mar. Drugs 2019, 17, 158. [Google Scholar] [CrossRef]
- Munro, M.H.G.; Blunt, J.W.; Dumdei, E.J.; Hickford, S.J.H.; Lill, R.E.; Li, S.; Battershill, C.N.; Duckworth, A.R. The discovery and development of marine compounds with pharmaceutical potential. J. Biotechnol. 1999, 35, 15–25. [Google Scholar] [CrossRef]
- Towle, M.J.; Salvato, K.A.; Budrow, J.; Wels, B.F.; Kuznetsov, G.; Aalfs, K.K.; Welsh, S.; Zheng, W.; Seletsky, B.M.; Palme, M.H.; et al. In vitro and in vivo anticancer activities of synthetic macrocyclic ketone analogues of halichondrin B. Cancer Res. 2001, 61, 1013–1021. [Google Scholar]
- Bell, J. Functional roles of sponges. Estuar. Coast Shelf Sci. 2008, 79, 341–353. [Google Scholar] [CrossRef]
- Swathi, J.; Narendra, K.; Sowjanya, K.M.; Satya, A.K. Marine fungal metabolites as a rich source of bioactive compounds. Afr. J. Biochem. Res. 2013, 7, 184–196. [Google Scholar] [CrossRef]
- Hasan, S.; Ansari, M.I.; Ahmad, A.; Mishra, M. Major bioactive metabolites from marine fungi: A Review. Bioinformation 2015, 11, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Setyowati, E.P.; Pratiwi, S.U.T.; Purwantiningsih; Purwantini, I. In-vitro cytotoxicity and apoptosis mechanism of ethyl acetate extract from Trichoderma reesei strain TV221 associated with marine sponge: Stylissa flabelliformis. J. Appl. Pharm. Sci. 2018, 8, 151–157. [Google Scholar]
- Jadulco, R.; Brauers, G.; Edrada, R.A.; Ebel, R.; Wray, V.; Sudarsono, S.; Proksch, P. New Metabolites from Sponge-Derived Fungi Curvularia lunata and Cladosporium herbarum. J. Nat. Prod. 2002, 65, 730–733. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.H.; Jang, S.; Heo, Y.M.; Min, M.; Lee, H.; Lee, Y.M.; Lee, H.; Kim, J.J. Investigation of marine-derived fungal diversity and their exploitable biological activities. Mar. Drugs 2015, 13, 4137–4155. [Google Scholar] [CrossRef] [PubMed]
- Prompanya, C.; Dethoup, T.; Bessa, L.J.; Pinto, M.M.M.; Gales, L.; Costa, P.M.; Silva, A.M.S.; Kijjoa, A. New isocoumarin derivatives and meroterpenoids from the marine sponge-associated fungus Aspergillus similanensis sp. Nov. KUFA 0013. Mar. Drugs 2014, 12, 5160–5173. [Google Scholar] [CrossRef]
- Ratnaweera, P.B.; Williams, D.E.; Dilip de Silva, E.; Andersen, R.J. Antibacterial metabolites from the Sri Lankan Antibacterial metabolites from the Sri Lankan demosponge-derived fungus, Aspergillus flavipes. Curr. Sci. 2016, 111, 1473–1479. [Google Scholar] [CrossRef]
- Kumla, D.; Aung, T.S.; Buttachon, S.; Dethoup, T.; Gales, L.; Pereira, J.A.; Inacio, A.; Costa, P.M.; Lee, M.; Sekeroglu, N.; et al. A New Dihydrochromone Dimer and Other Secondary Metabolites from Cultures of the Marine Sponge-Associated Fungi Neosartorya fennelliae KUFA 0811 and Neosartorya tsunodae KUFC 9217. Mar. Drugs 2017, 15, 375. [Google Scholar] [CrossRef]
- Kassem, K.; Madeja, E. The Coral Triangle; John Beaufoy Publishing: Oxford, UK, 2014; pp. 1–208. [Google Scholar]
- Caras, T.; Pasternak, Z. Long-term environmental impact of coral mining at the Wakatobi marine park, Indonesia. Ocean Coast. Manag. 2009, 52, 539–544. [Google Scholar] [CrossRef]
- Clifton, J.; Unsworth, R.K.F.; Smith, D.J. Marine Research and Conservation in the Coral Triangle: The Wakatobi National Park; Nova Science Publishers: Hauppauge, NY, USA, 2010; pp. 1–258. [Google Scholar]
- Tapilatu, Y.H. Status of Drug Discovery Research Based on Marine Organisms from Eastern Indonesia. Procedia Chem. 2015, 14, 484–492. [Google Scholar] [CrossRef]
- Clark, B.R.; Capon, R.J.; Lacey, E.; Tennant, S.; Gill, J.H. Citrinin revisited: From monomers to dimers and beyond. Org. Biomol. Chem. 2006, 4, 1520–1528. [Google Scholar] [CrossRef] [PubMed]
- Wakana, D.; Hosoe, T.; Itabashi, T.; Okada, K.; Takaki, G.M.C.; Yaguchi, T.; Fukushima, K.; Kawai, K.I. New citrinin derivatives isolated from Penicillium citrinum. J. Nat. Med. 2006, 60, 279–284. [Google Scholar] [CrossRef]
- Wang, M.L.; Lu, C.H.; Xu, Q.Y.; Song, S.Y.; Hu, Z.Y.; Zheng, Z.H. Four new citrinin derivatives from a marine-derived Penicillium sp. fungal strain. Molecules 2013, 18, 5723–5735. [Google Scholar] [CrossRef]
- Yang, S.Q.; Li, X.M.; Li, X.; Li, H.L.; Meng, L.H.; Wang, B.G. New citrinin analogues produced by coculture of the marine algal-derived endophytic fungal strains Aspergillus sydowii EN-534 and Penicillium citrinum EN-535. Phytochem. Lett. 2018, 25, 191–195. [Google Scholar] [CrossRef]
- Heider, E.M.; Harper, J.K.; Grant, D.M.; Hoffman, A.; Dugan, F.; Tomere, D.P.; O’Neill, K.L. Exploring unusual antioxidant activity in a benzoic acid derivative: A proposed mechanism for citrinin. Tetrahedron 2006, 62, 1199–1208. [Google Scholar] [CrossRef]
- Vanacloig-Pedros, E.; Proft, M.; Pascual-Ahuir, A. Different Toxicity Mechanisms for Citrinin and Ochratoxin A Revealed by Transcriptomic Analysis in Yeast. Toxins 2016, 8, 273. [Google Scholar] [CrossRef]
- Haraguchi, H.; Hashimoto, K.; Shibata, K.; Taniguchi, M.; Oi, S. Mechanism of Antifungal Action of Citrinin. Agric. Biol. Chem. 1987, 51, 1373–1378. [Google Scholar]
- Xu, L.-L.; Cao, F.; Tian, S.-S.; Zhu, H.-J. Alkaloids and Polyketides from the Soil Fungus Aspergillus terreus and Their Antibacterial Activities. Chem. Nat. Compd. 2017, 53, 1212–1215. [Google Scholar] [CrossRef]
- Carter, R.H.; Garson, M.J.; Staunton, J. Biosynthesis of citrinin: Incorporation studies with advanced precursors. J. Chem. Soc. Chem. Commun. 1979, 23, 1097–1098. [Google Scholar] [CrossRef]
- He, Y.; Cox, R.J. The molecular steps of citrinin biosynthesis in fungi. Chem. Sci. 2016, 7, 2119–2127. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.J.; Du, L.; Hao, P.F.; Lin, Z.J.; Gu, Q.Q. Citrinal A, a novel tricyclic derivative of citrinin, from an algicolous fungus Penicillium sp. i-1-1. Chin. Chem. Lett. 2009, 20, 917–920. [Google Scholar] [CrossRef]
- Ellerbrock, P. Biomimetic Synthesis of Polyketides: Dibefurin and Epicolactone and Synthetic Studies Toward Gracilin Terpenoids. Ph.D. Thesis, Ludwig-Maximilians-Universität München, München, Germany, 2015; p. 57. [Google Scholar]
- Guo, W.; Li, D.; Peng, J.; Zhu, T.; Gu, Q.; Li, D. Penicitols A−C and Penixanacid A from the Mangrove-Derived Penicillium chrysogenum HDN11-24. J. Nat. Prod. 2015, 78, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-H.; Shaw, C.-Y.; Chen, C.-C.; Tsai, Y.-C. 2,3,4-Trimethyl-5,7-dihydroxy-2,3-dihydrobenzofuran, a Novel Antioxidant, from Penicillium citrinum F5. J. Nat. Prod. 2002, 65, 740–741. [Google Scholar] [CrossRef]
- Hooper, J.N.A.; Van Soest, R.W.M. Systema Porifera: A Guide to the Classification of Sponges; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2002; pp. 1–1099. [Google Scholar]
- Kjer, J.; Debbab, A.; Aly, A.H.; Proksch, P. Methods for isolation of marine-derived endophytic fungi and their bioactive secondary products. Nat. Protoc. 2010, 5, 479–490. [Google Scholar] [CrossRef]
- Sabdaningsih, A.; Cristianawati, O.; Sibero, M.T.; Nuryadi, H.; Radjasa, O.K.; Sabdono, A.; Trianto, A. Screening Antibacterial Agent from Crude Extract of Marine- Derived Fungi Associated with Soft Corals against MDR- Staphylococcus haemolyticus. IOP Conf. Ser. Earth Environ. Sci. 2017, 55, 1–8. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
| Position | 13C | 1H | HMBC | |
|---|---|---|---|---|
| 1 | 89.2 | (CH) | 4.43 qd (6.4, 3.6) | C-3, C-8, C-9 |
| 2 | 45.3 | (CH) | 2.99 qd (6.7, 3.6) | C-10, C-3, C-8 |
| 3 | 140.0 | (Cq) | ||
| 4 | 55.6 | (Cq) | ||
| 5 | 199.5 | (Cq) | ||
| 6 | 73.1 | (CH) | 3.63 s | C-4, C-5, C-7, C-8, C-3′, C-4′, C-5′, C-11′ |
| 7 | 184.7 | (Cq) | ||
| 8 | 150.3 | (Cq) | ||
| 9 | 20.6 | (CH3) | 1.24 d (6.4) | C-1, C-2 |
| 10 | 20.3 | (CH3) | 1.22 d (6.7) | C-1, C-2, C-3 |
| 11 | 16.1 | (CH3) | 1.325 s | C-3, C-4, C-5, C-6′, C-8 |
| 1′ | 88.3 | (CH) | 4.37 p (6.4) | C-3′, C-8′, C-10′ |
| 2′ | 47.1 | (CH) | 2.91 p (7.0) | C-1′, C-3′, C-8′, C-9′ |
| 3′ | 139.2 | (Cq) | ||
| 4′ | 55.1 | (Cq) | ||
| 5′ | 199.0 | (Cq) | ||
| 6′ | 71.9 | (CH) | 3.71 s | C-3, C-4, C-5, C-11, C-4′, C-5′, C-7′, C-8′ |
| 7′ | 184.2 | (Cq) | ||
| 8′ | 150.8 | (Cq) | ||
| 9′ | 20.7 | (CH3) | 1.30 d (6.4) | C-1′, C-2′ |
| 10′ | 19.3 | (CH3) | 1.36 d (7.0) | C-1′, C-2′, C-3′ |
| 11′ | 14.5 | (CH3) | 1.332 s | C-6, C-3′, C-4′, C-5′, C-8′ |
| Microorganisms Tested | MIC (µg/mL) a | |||
|---|---|---|---|---|
| Penicitrinone G (1) | Penicitrinone A (2) | Penicitrinone E (3) | Penicitrinol J (4) | |
| Bacillus megaterium DSM32 | >64 | >64 | >64 | 16 |
| Bacillus subtilis JH642 | >64 | >64 | >64 | 16 |
| Bacillus subtilis DSM10 | >64 | >64 | >64 | 64 |
| Micrococcus luteus ATCC4698 | >64 | >64 | >64 | >64 |
| Mycobacterium smegmatis ATCC607 | >64 | 32 | >64 | 32 |
| Listeria monocytogenes DSM20600 | >64 | >64 | >64 | >64 |
| Staphylococcus aureus ATCC25923 | >64 | >64 | >64 | 64 |
| Escherichia coli K12 | >64 | >64 | >64 | >64 |
| Candida albicans FH2173 | >64 | >64 | >64 | >64 |
| Aspergillus flavus ATCC9170 | >64 | >64 | >64 | >64 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabdaningsih, A.; Liu, Y.; Mettal, U.; Heep, J.; Riyanti; Wang, L.; Cristianawati, O.; Nuryadi, H.; Triandala Sibero, M.; Marner, M.; et al. A New Citrinin Derivative from the Indonesian Marine Sponge-Associated Fungus Penicillium citrinum. Mar. Drugs 2020, 18, 227. https://doi.org/10.3390/md18040227
Sabdaningsih A, Liu Y, Mettal U, Heep J, Riyanti, Wang L, Cristianawati O, Nuryadi H, Triandala Sibero M, Marner M, et al. A New Citrinin Derivative from the Indonesian Marine Sponge-Associated Fungus Penicillium citrinum. Marine Drugs. 2020; 18(4):227. https://doi.org/10.3390/md18040227
Chicago/Turabian StyleSabdaningsih, Aninditia, Yang Liu, Ute Mettal, John Heep, Riyanti, Lei Wang, Olvi Cristianawati, Handung Nuryadi, Mada Triandala Sibero, Michael Marner, and et al. 2020. "A New Citrinin Derivative from the Indonesian Marine Sponge-Associated Fungus Penicillium citrinum" Marine Drugs 18, no. 4: 227. https://doi.org/10.3390/md18040227
APA StyleSabdaningsih, A., Liu, Y., Mettal, U., Heep, J., Riyanti, Wang, L., Cristianawati, O., Nuryadi, H., Triandala Sibero, M., Marner, M., Radjasa, O. K., Sabdono, A., Trianto, A., & Schäberle, T. F. (2020). A New Citrinin Derivative from the Indonesian Marine Sponge-Associated Fungus Penicillium citrinum. Marine Drugs, 18(4), 227. https://doi.org/10.3390/md18040227