New Diterpenes with a Fused 6-5-6-6 Ring System Isolated from the Marine Sponge-Derived Fungus Trichoderma harzianum
Abstract
1. Introduction
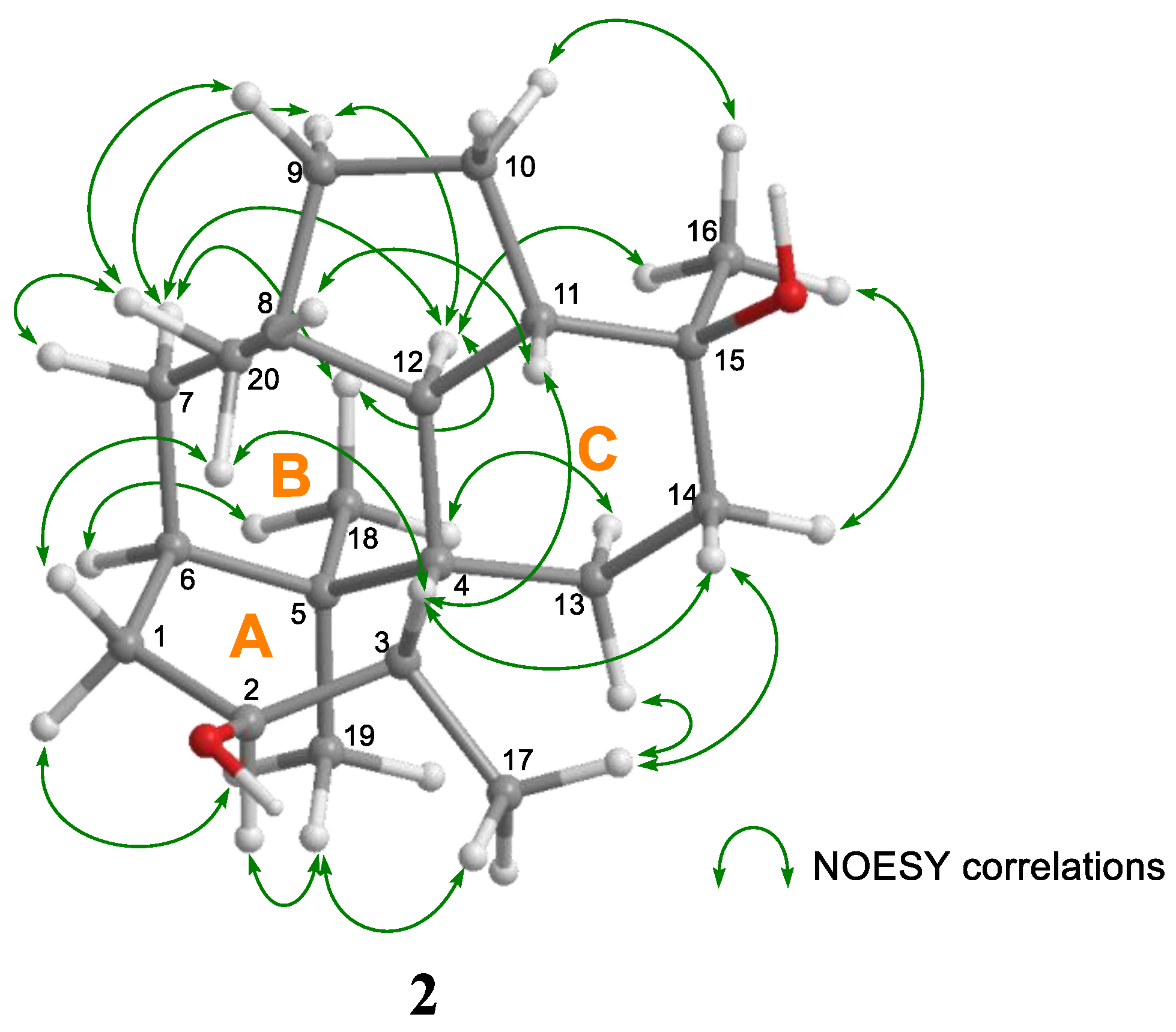
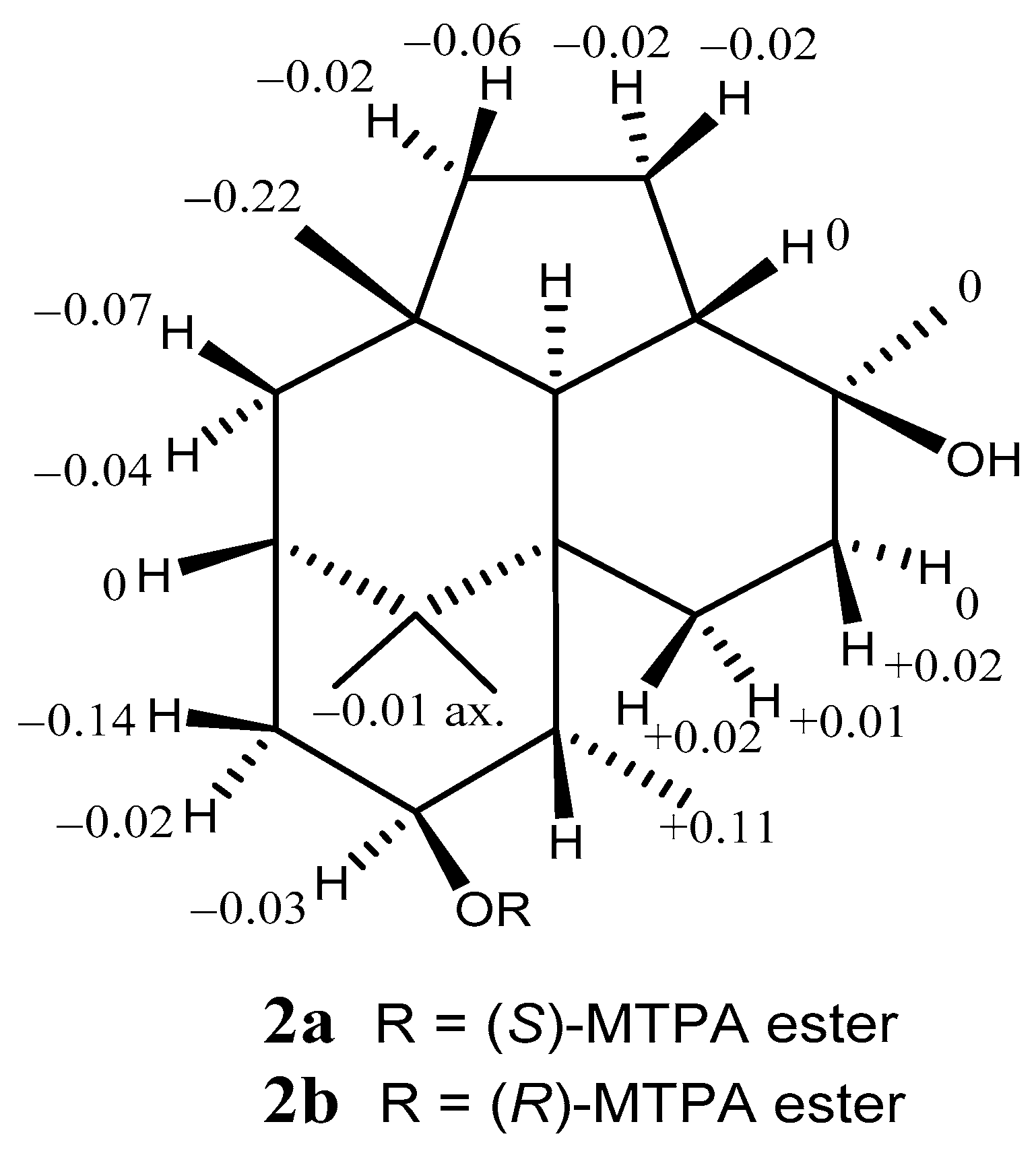
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Material
3.3. Culturing and Isolation of Metabolites
3.4. Formation of Dibenzoate of 1
3.5. Formation of the (S)- and (R)-MTPA Esters of 2
3.6. Assay for Cytotoxicity
3.7. The Origin of the Cell Lines
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nicoletti, R.; Vinale, F. Bioactive Compounds from Marine-Derived Aspergillus, Penicillium, Talaromyces and Trichoderma Species. Mar. Drugs 2018, 16, 408. [Google Scholar] [CrossRef] [PubMed]
- Imhoff, J. Natural Products from Marine Fungi—Still an Underrepresented Resource. Mar. Drugs 2016, 14, 19. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2017, 34, 235–294. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural 369 products. Nat. Prod. Rep. 2018, 35, 8–53. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Iritani, M.; Ohishi, H.; Tanaka, K.; Minoura, K.; Doi, M.; Numata, A. Pericosines, antitumour metabolites from the sea hare-derived fungus Periconia byssoides. Structures and biological activities. Org. Biomol. Chem. 2007, 5, 3979–3986. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Kitada, H.; Kajimoto, T.; Numata, A.; Tanaka, R. The relationship between the CD Cotton effect and the absolute configuration of FD-838 and its seven stereoisomers. J. Org. Chem. 2010, 75, 4146–4153. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Kikuchi, T.; Tanaka, R. Altercrasin A, a novel decalin derivative with spirotetramic acid, produced by a sea urchin-derived Alternaria sp. Tetrahedron Lett. 2015, 56, 1229–1232. [Google Scholar] [CrossRef]
- Yamada, T.; Tanaka, A.; Nehira, T.; Nishii, T.; Kikuchi, T. Altercrasins A–E, decalin derivatives, from a sea-urchin-derived Alternaria sp.: Isolation and structural analysis including stereochemistry. Mar. Drugs 2019, 17, 218. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Mizutani, Y.; Umebayashi, Y.; Inno, N.; Kawashima, M.; Kikuchi, T.; Tanaka, R. A novel ketoaldehyde decalin derivative, produced by a marine sponge-derived Trichoderma harzianum. Tetrahedron Lett. 2014, 55, 662–664. [Google Scholar] [CrossRef]
- Yamada, T.; Umebayashi, Y.; Kawashima, M.; Sugiura, Y.; Kikuchi, T.; Tanaka, R. Determination of the chemical structures of tandyukisins B–D, isolated from a marine sponge-derived fungus. Mar. Drugs 2015, 13, 3231–3240. [Google Scholar] [CrossRef] [PubMed]
- Suzue, M.; Kikuchi, T.; Tanaka, R.; Yamada, T. Tandyukisins E and F, novel cytotoxic decalin derivatives isolated from a marine sponge-derived fungus. Tetrahedron Lett. 2016, 57, 5070–5073. [Google Scholar] [CrossRef]
- Yamada, T.; Suzue, M.; Arai, T.; Kikuchi, T.; Tanaka, R. Trichodermanins C–E, new diterpenes with a fused 6-5-6-6 ring system produced by a marine sponge-derived fungus. Mar. Drugs 2017, 15, 169. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.X.; Zheng, C.J.; Li, W.C.; Huang, F.; Qin, L.P. Trichodermanin A, a novel diterpenoid from endophytic fungus culture. J. Nat. Med. 2011, 65, 381–384. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Izumi, N.; Ui, H.; Sueki, A.; Masuma, R.; Nonaka, K.; Hirose, T.; Sunazuka, T.; Nagai, T.; Yamada, H.; et al. Wickerols A and B: Novel anti-influenza virus diterpenes produced by Trichoderma atroviride FKI-3849. Tetrahedron 2012, 68, 9267–9271. [Google Scholar] [CrossRef]
- Ohtani, I.; Kusumi, T.; Kashman, Y.; Kakisawa, H. High-field FT NMR application of Mosher’s method. The absolute configurations of marine terpenoids. J. Am. Chem. Soc. 1991, 113, 4092–4096. [Google Scholar] [CrossRef]
- Harada, N.; Nakanishi, K. Circular Dichroic Spectroscopy–Exciton Coupling in Organic Stereochemistry; University Science Books: Sausalito, CA, USA, 1983. [Google Scholar]
- Sun, Z.H.; Gu, J.; Ye, W.; Wen, L.X.; Lin, Q.B.; Li, S.N.; Che, Y.C.; Li, H.H.; Zhang, W.M. Geospallins A–C: New thiodiketopiperazines with inhibitory activity against angiotensin-converting enzyme from a deep-Sea-derived Fungus Geosmithia pallida FS140. Mar. Drugs 2018, 16, 464. [Google Scholar] [CrossRef] [PubMed]
- Miki, I.; Ishihara, N.; Ohtoshi, M.; Kase, H. Simple colorimetric cell-cell adhesion assay using MTT-stained leukemia cells. J. Immiunol. Methods 1993, 164, 155–261. [Google Scholar] [CrossRef]




| Position | 1 | 2 | 3 | 4 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| δHa | δC | δHa | δC | δHa | δC | δHa | δC | |||||||||
| 1α | 2.54 | ddd | 36.5 | (t) | ||||||||||||
| 1β | 4.11 | d | 80.4 | (d) | 1.68 | m | 4.20 | dd | 72.6 | (d) | 4.24 | dd | 72.1 | (d) | ||
| 2α | 3.88 | dd | 83.7 | (d) | 4.30 | ddd | 74.2 | (d) | 2.66 | ddd | 41.8 | (t) | 2.74 | ddd | 36.9 | (t) |
| 2β | 1.45 | m | 1.64 | ddd | ||||||||||||
| 3 | 1.88 | qd | 36.6 | (d) | 1.98 | qd | 37.9 | (d) | 2.10 | m | 26.7 | (d) | 1.88 | dddd | 34.0 | (d) |
| 4 | 41.2 | (s) | 40.8 | (s) | 38.9 | (s) | 38.9 | (s) | ||||||||
| 5 | 39.4 | (s) | 39.0 | (s) | 39.0 | (s) | 38.5 | (s) | ||||||||
| 6 | 1.50 | dd | 53.2 | (d) | 1.62 | m | 41.8 | (d) | 1.41 | dd | 52.2 | (d) | 1.45 | dd | 52.2 | (d) |
| 7α | 1.78 | dd | 40.9 | (t) | 1.71 | dd | 42.5 | (t) | 1.69 | dd | 41.3 | (t) | 1.74 | dd | 40.9 | (t) |
| 7β | 1.70 | dd | 1.56 | dd | 1.65 | dd | 1.68 | dd | ||||||||
| 8 | 39.6 | (s) | 39.7 | (s) | 38.8 | (s) | 39.2 | (s) | ||||||||
| 9α | 1.03 | m | 43.5 | (t) | 1.02 | m | 43.9 | (t) | 1.48 | m | 52.2 | (t) | 1.03 | m | 43.5 | (t) |
| 9β | 1.43 | m | 1.43 | m | 1.43 | m | ||||||||||
| 10α | 1.59 | m | 21.6 | (t) | 1.60 | m | 21.6 | (t) | 4.38 | ddd | 72.9 | (d) | 1.59 | m | 21.5 | (t) |
| 10β | 1.80 | m | 1.81 | m | 1.80 | m | ||||||||||
| 11 | 1.81 | dd | 44.2 | (d) | 1.85 | m | 44.4 | (d) | 1.85 | dd | 55.0 | (d) | 1.88 | ddd | 44.1 | (d) |
| 12 | 1.32 | d | 51.8 | (d) | 1.30 | d | 51.9 | (d) | 1.28 | d | 50.6 | (d) | 1.28 | d | 52.0 | (d) |
| 13α | 1.23 | ddd | 26.3 | (t) | 1.18 | ddd | 26.4 | (t) | 1.19 | ddd | 25.9 | (t) | 1.28 | m | 25.5 | (t) |
| 13β | 1.72 | ddd | 1.68 | m | 1.74 | ddd | 1.52 | m | ||||||||
| 14α | 1.64 | ddd | 41.1 | (t) | 1.62 | m | 41.2 | (t) | 1.60 | ddd | 41.0 | (t) | 1.67 | m | 40.87 | (t) |
| 14β | 1.46 | ddd | 1.46 | ddd | 1.50 | ddd | 1.50 | m | ||||||||
| 15 | 73.6 | (s) | 73.8 | (s) | 73.1 | (s) | 73.6 | (s) | ||||||||
| 16 | 1.18 | s | 20.5 | (q) | 1.18 | s | 20.4 | (q) | 1.22 | s | 21.5 | (q) | 1.18 | s | 20.5 | (q) |
| 17 | 1.23 | d | 20.0 | (q) | 1.19 | d | 20.4 | (q) | 1.09 | d | 22.4 | (q) | 3.60 | dd | 68.1 | (t) |
| 3.95 | dd | |||||||||||||||
| 18ax | 0.99 | s | 25.7 | (q) | 0.95 | s | 25.3 | (q) | 0.97 | s | 25.8 | (q) | 0.99 | s | 25.7 | (q) |
| 19eq | 1.04 | s | 25.2 | (q) | 0.96 | s | 25.4 | (q) | 1.14 | s | 24.9 | (q) | 1.13 | s | 25.5 | (q) |
| 20 | 0.98 | s | 19.8 | (q) | 1.13 | s | 20.3 | (q) | 1.17 | s | 21.4 | (q) | 0.92 | s | 19.5 | (q) |
| Compounds | Cell line P388 | Cell line HL-60 | Cell line L1210 |
|---|---|---|---|
| IC50 (μM) a | IC50 (μM) a | IC50 (μM) a | |
| 1 | 52.1 ± 1.3 | 59.8 ± 2.2 | 125.2 ± 4.3 |
| 2 | 58.9 ± 1.2 | 42.9 ± 3.0 | 41.5 ± 2.5 |
| 3 | >300 | >300 | >300 |
| 4 | >300 | 85.3 ± 2.1 | 73.2 ± 2.2 |
| DMSO (control) | >300 | >300 | >300 |
| 5-fluorouracil b | 3.9 ± 0.6 | 3.7 ± 0.1 | 4.2 ± 0.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamada, T.; Fujii, A.; Kikuchi, T. New Diterpenes with a Fused 6-5-6-6 Ring System Isolated from the Marine Sponge-Derived Fungus Trichoderma harzianum. Mar. Drugs 2019, 17, 480. https://doi.org/10.3390/md17080480
Yamada T, Fujii A, Kikuchi T. New Diterpenes with a Fused 6-5-6-6 Ring System Isolated from the Marine Sponge-Derived Fungus Trichoderma harzianum. Marine Drugs. 2019; 17(8):480. https://doi.org/10.3390/md17080480
Chicago/Turabian StyleYamada, Takeshi, Ayano Fujii, and Takashi Kikuchi. 2019. "New Diterpenes with a Fused 6-5-6-6 Ring System Isolated from the Marine Sponge-Derived Fungus Trichoderma harzianum" Marine Drugs 17, no. 8: 480. https://doi.org/10.3390/md17080480
APA StyleYamada, T., Fujii, A., & Kikuchi, T. (2019). New Diterpenes with a Fused 6-5-6-6 Ring System Isolated from the Marine Sponge-Derived Fungus Trichoderma harzianum. Marine Drugs, 17(8), 480. https://doi.org/10.3390/md17080480





