Different Antifungal Activity of Anabaena sp., Ecklonia sp., and Jania sp. against Botrytis cinerea
Abstract
:1. Introduction
2. Results
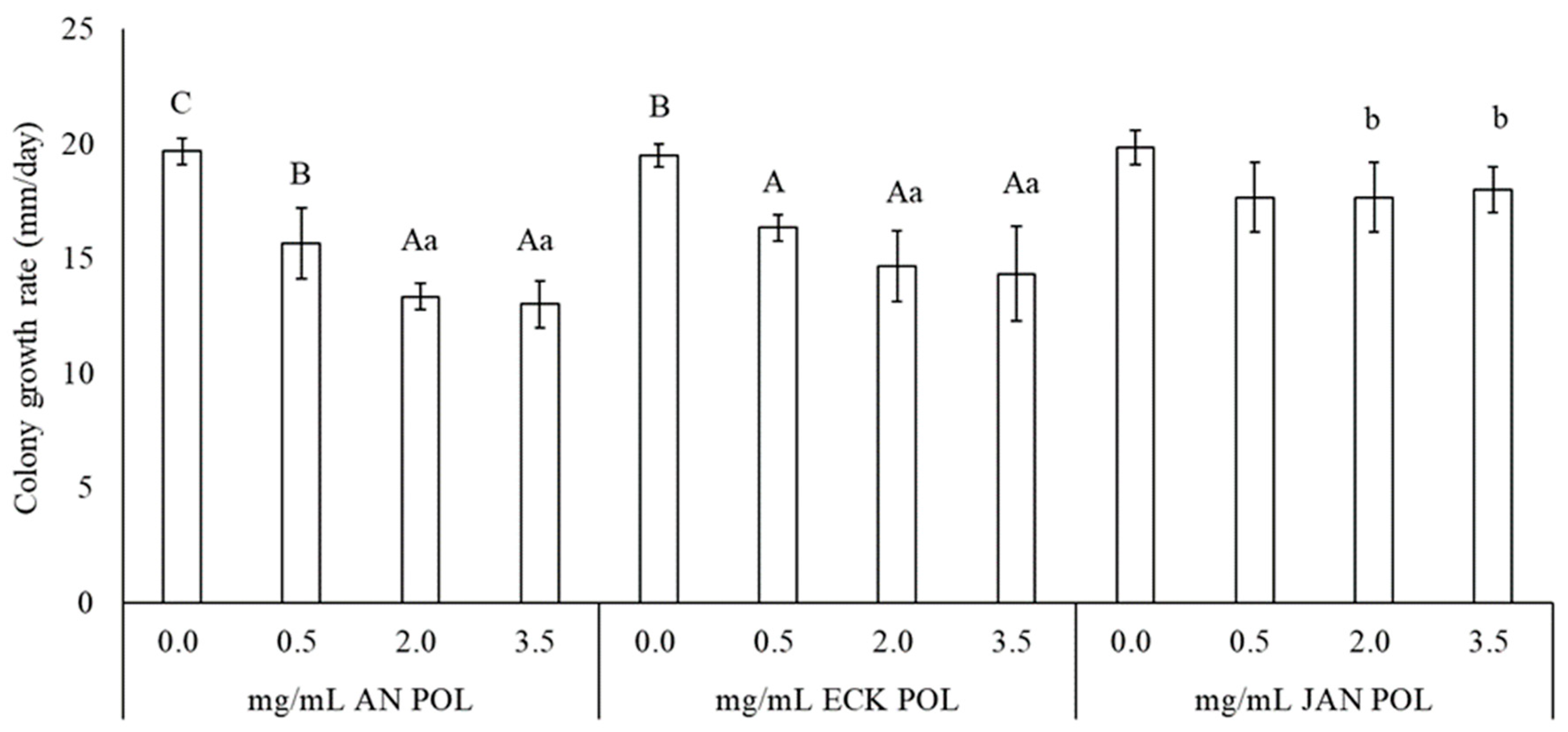
2.1. Antifungal Activity of Water Extract (WE) and Polysaccharides (POL)
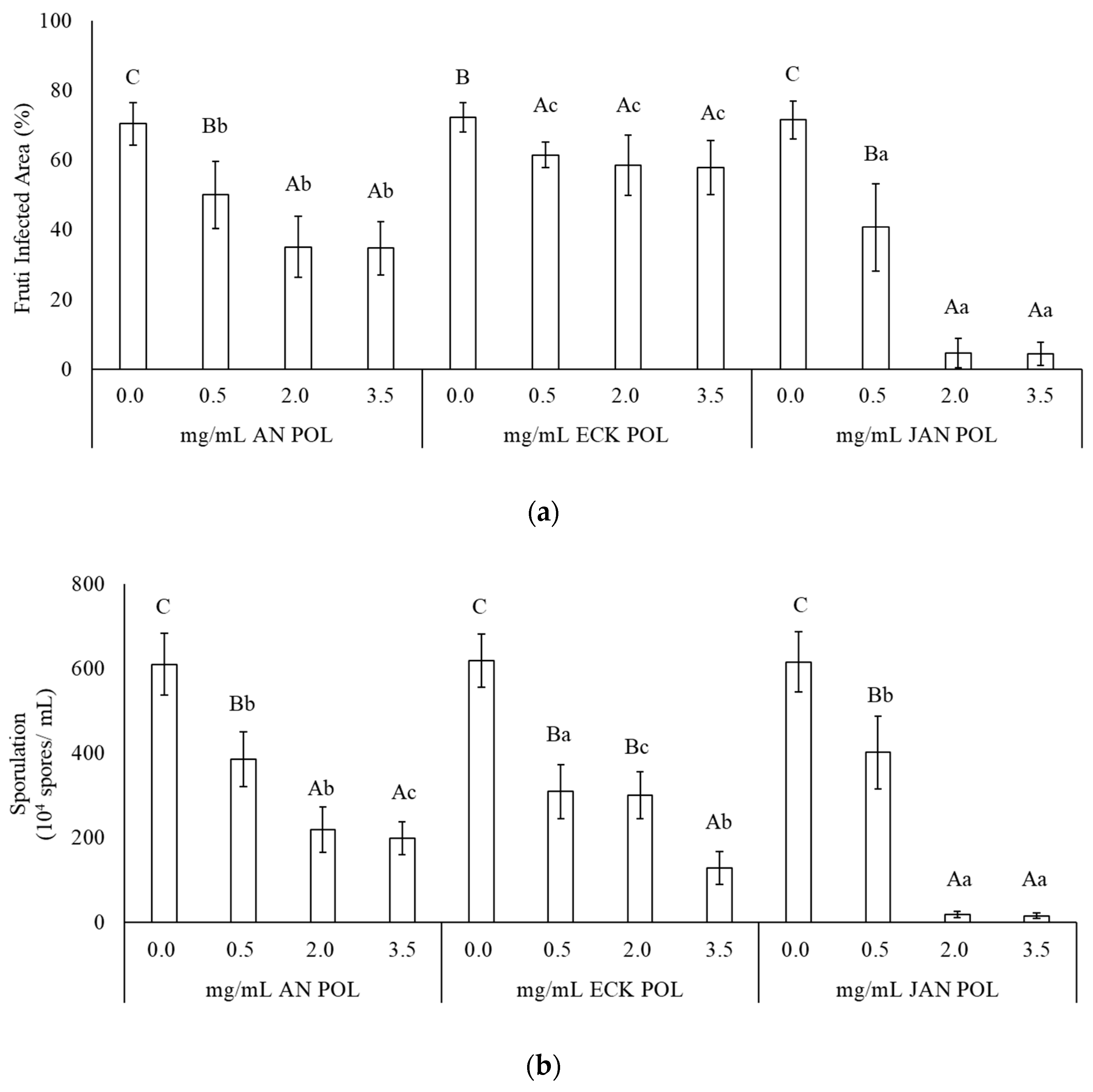
2.2. Effect of POL against B. cinerea on Strawberry Fruits
3. Discussion
4. Materials and Methods
4.1. Preparation of Water Extract, WE, Extraction of Polysaccharides, POL, and Pathogen
4.2. Antifungal Activity of WE on B. cinerea Colony Growth
4.3. Antifungal Activity of POL on B. cinerea Colony Growth, Spore Germination, and Colony Forming Units, CFUs
4.4. Effect of Polysaccharides against B. cinerea on Strawberry Fruits
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dallagnol, L.J.; Ferreira, L.V.; Araujo-Filho, J.A.; Camargo, L.E.A.; de Castro-Moretti, F.R. Gray mold caused by Botryotinia fuckeliana on edible pods of pea in Brazil. Plant Dis. 2013, 98, 569–570. [Google Scholar] [CrossRef] [PubMed]
- Michailides, T.J.; Elmer, P.A.G. Botrytis gray mold of kiwifruit caused by Botrytis cinerea in the United States and New Zealand. Plant Dis. 2007, 84, 208–223. [Google Scholar] [CrossRef] [PubMed]
- Ciliberti, N.; Fermaud, M.; Roudet, J.; Rossi, V. Environmental conditions affect Botrytis cinerea infection of mature grape berries more than the strain or transposon genotype. Phytopathology 2015, 105, 1090–1096. [Google Scholar] [CrossRef]
- Erper, I.; Celik, H.; Turkkan, M.; Cebi Kilicoglu, M. First report of Botrytis cinerea on golden berry. Australas. Plant Dis. Notes 2015, 10. [Google Scholar] [CrossRef]
- Rodríguez, A.; Acosta, A.; Rodríguez, C. Fungicide resistance of Botrytis cinerea in tomato greenhouses in the Canary Islands and effectiveness of non-chemical treatments against gray mold. World J. Microbiol. Biotechnol. 2014, 30, 2397–3406. [Google Scholar] [CrossRef]
- Blanco, C.; De Los Santos, B.; Romero, F. Relationship between concentrations of Botrytis cinerea conidia in air, environmental conditions, and the incidence of grey mould in strawberry flowers and fruits. Eur. J. Plant Pathol. 2006, 114, 415–425. [Google Scholar] [CrossRef]
- Rizvi, M.A.; Shameel, M. Studies on the bioactivity and elementology of marine algae from the coast of Karachi, Pakistan. Phyther. Res. 2004, 18, 865–872. [Google Scholar] [CrossRef]
- Righini, H.; Roberti, R.; Baraldi, E. Use of algae in strawberry management. J. Appl. Phycol. 2018, 30, 3551–3564. [Google Scholar] [CrossRef]
- Roberti, R.; Galletti, S.; Burzi, P.L.; Righini, H.; Cetrullo, S.; Perez, C. Induction of defense responses in zucchini (Cucurbita pepo) by Anabaena sp. water extract. Biol. Control 2015, 82, 61–68. [Google Scholar] [CrossRef]
- Sivakumar, S.R. Antibacterial potential of white crystalline solid from red algae Porteiria hornemanii against the plant pathogenic bacteria. African, J. Agric. Res. 2014, 2, 174–183. [Google Scholar]
- Jiménez, E.; Dorta, F.; Medina, C.; Ramírez, A.; Ramírez, I.; Peña-Cortés, H. Anti-phytopathogenic activities of macro-algae extracts. Mar. Drugs 2011, 9, 739–756. [Google Scholar] [CrossRef] [PubMed]
- Arunkumar, K.; Sivakumar, S.R.; Rengasamy, R. Review on bioactive potential in seaweeds (Marine Macroalgae): A special emphasis on bioactivity of seaweeds against plant pathogens. Asian J. Plant Sci. 2010, 9, 227–240. [Google Scholar] [CrossRef]
- Kumar, C.S.; Sarada, D.V.L.; Rengasamy, R. Seaweed extracts control the leaf spot disease of the medicinal plant Gymnema sylvestre. Indian J. Sci. Technol. 2008, 3, 1–5. [Google Scholar]
- De Corato, U.; Salimbeni, R.; De Pretis, A.; Avella, N.; Patruno, G. Antifungal activity of crude extracts from brown and red seaweeds by a supercritical carbon dioxide technique against fruit postharvest fungal diseases. Postharvest Biol. Technol. 2017, 131, 16–30. [Google Scholar] [CrossRef]
- Esserti, S.; Smaili, A.; Rifai, L.A.; Koussa, T.; Makroum, K.; Belfaiza, M.; Kabil, E.M.; Faize, L.; Burgos, L.; Alburquerque, N.; et al. Protective effect of three brown seaweed extracts against fungal and bacterial diseases of tomato. J. Appl. Phycol. 2017, 29, 1081–1093. [Google Scholar] [CrossRef]
- Jayaraman, J.; Norrie, J.; Punja, Z.K. Commercial extract from the brown seaweed Ascophyllum nodosum reduces fungal diseases in greenhouse cucumber. J. Appl. Phycol. 2011, 23, 353–361. [Google Scholar] [CrossRef]
- Agarwal, P.; Patel, K.; Das, A.K.; Ghosh, A.; Agarwal, P.K. Insights into the role of seaweed Kappaphycus alvarezii sap towards phytohormone signalling and regulating defense responsive genes in Lycopersicon esculentum. J. Appl. Phycol. 2016, 28, 2529–2537. [Google Scholar] [CrossRef]
- Jaki, B.; Zerbe, O.; Heilmann, J.; Sticher, O. Two novel cyclic peptides with antifungal activity from the cyanobacterium Tolypothrix byssoidea (EAWAG 195). J. Nat. Prod. 2001, 64, 154–158. [Google Scholar] [CrossRef]
- Vera, J.; Castro, J.; Gonzalez, A.; Moenne, A. Seaweed polysaccharides and derived oligosaccharides stimulate defense responses and protection against pathogens in plants. Mar. Drugs 2011, 9, 2514–2525. [Google Scholar] [CrossRef]
- Pierre, G.; Sopena, V.; Juin, C.; Mastouri, A.; Graber, M.; Maugard, T. Antibacterial activity of a sulfated galactan extracted from the marine alga Chaetomorpha aerea against Staphylococcus aureus. Biotechnol. Bioprocess Eng. 2011, 16, 937–945. [Google Scholar] [CrossRef]
- Marudhupandi, T.; Thipparamalai Thangappan Ajith, K. Antibacterial effect of fucoidan from Sargassum wightii against the chosen human bacterial pathogens. Int. Curr. Pharm. J. 2013, 2, 156–158. [Google Scholar] [CrossRef]
- Jun, J.-Y.; Jung, M.-J.; Jeong, I.-H.; Yamazaki, K.; Kawai, Y.; Kim, B.-M. Antimicrobial and antibiofilm activities of sulfated polysaccharides from marine algae against dental plaque bacteria. Mar. Drugs 2018, 16, 301. [Google Scholar] [CrossRef]
- Pérez, M.J.; Falqué, E.; Domínguez, H. Antimicrobial action of compounds from marine seaweed. Mar. Drugs 2016, 14, 52. [Google Scholar] [CrossRef]
- Rioux, L.E.; Turgeon, S.L.; Beaulieu, M. Characterization of polysaccharides extracted from brown seaweeds. Carbohydr. Polym. 2007, 69, 530–537. [Google Scholar] [CrossRef]
- Liu, M.; Wang, G.; Xiao, L.; Xu, A.; Liu, X.; Xu, P.; Lin, X. Bis(2,3-dibromo-4,5-dihydroxybenzyl) ether, a marine algae derived bromophenol, inhibits the growth of Botrytis cinerea and interacts with DNA molecules. Mar. Drugs 2014, 12, 3838–3851. [Google Scholar] [CrossRef]
- Pugh, N.; Samir, A.R.; Hala, N.E.; Mahmoud, A.E.; David, S.P. Isolation of three high molecular weight polysaccharide preparations with potent immunostimulatory activity from Spirulina platensis, Aphanizomenon flos-aquae and Chlorella pyrenoidosa. Planta Med. 2001, 67, 737–742. [Google Scholar] [CrossRef]
- Hayashi, K.; Hayashi, T.; Kojima, I. A natural sulfated polysaccharide, calcium spirulan, isolated from Spirulina platensis: In vitro and ex vivo evaluation of anti-herpes simplex virus and anti-human immunodeficiency virus activities. AIDS Res. Hum. Retroviruses 1996, 12, 1463–1471. [Google Scholar] [CrossRef]
- Hahn, M.G.; Darvill, A.G.; Albersheim, P. Host-Pathogen Interactions: XIX. the endogenous elicitor, a fragment of a plant cell wall polysaccharide that elicits phytoalexin accumulation in soybeans. Plant Physiol. 2008, 68, 1161–1169. [Google Scholar] [CrossRef]
- Stadnik, M.J.; Freitas, M.B. de Algal polysaccharides as source of plant resistance inducers. Trop. Plant Pathol. 2014, 39, 111–118. [Google Scholar] [CrossRef]
- Jaulneau, V.; Lafitte, C.; Jacquet, C.; Fournier, S.; Salamagne, S.; Briand, X.; Esquerré-Tugayé, M.-T.; Dumas, B. Ulvan, a sulfated polysaccharide from green algae, activates plant immunity through the jasmonic acid signaling pathway. J. Biomed. Biotechnol. 2010, 2010, 525291. [Google Scholar] [CrossRef]
- Zhao, G.; Zhao, J.; Peng, L.; Zou, L.; Wang, J.; Zhong, L.; Xiang, D. Effects of yeast polysaccharide on growth and flavonoid accumulation in Fagopyrum tataricum sprout cultures. Molecules 2012, 17, 11335–11345. [Google Scholar] [CrossRef]
- Roberti, R.; Righini, H.; Reyes, C.P.; Roberti, R.; Righini, H.; Reyes, C.P. Activity of seaweed and cyanobacteria water extracts against Podosphaera xanthii on zucchini. Ital. J. Mycol. 2016, 45. [Google Scholar] [CrossRef]
- Khallil, A.M.; Daghman, I.M.; Fady, A.A. Antifungal potential in crude extracts of five selected brown seaweeds collected from the western Libya coast. J. Microbiol. Mod. Tech. 2015, 1, 103. [Google Scholar]
- Ibraheem, I.B.; Hamed, S.M.; Abd elrhman, A.A.; Mohamed Farag, F.; Abdel-Raouf, N. Antimicrobial activities of some brown macroalgae against some soil borne plant pathogens and in vivo management of Solanum melongena root diseases. Aust. J. Basic Appl. Sci. J. Basic Appl. Sci. 2017, 11, 157–168. [Google Scholar]
- Mabrouk, S.S.; El-Shayeb, N.M.A.; El-Refai, A.H.; Sallam, L.A.R.; Hamdy, A.A. Inhibitory activities of some marine algae on aflatoxin accumulation. Appl. Microbiol. Biotechnol. 1985, 22, 152–155. [Google Scholar] [CrossRef]
- Eom, S.H.; Kim, Y.M.; Kim, S.K. Antimicrobial effect of phlorotannins from marine brown algae. Food Chem. Toxicol. 2012, 50, 3251–3255. [Google Scholar] [CrossRef]
- De Cano, M.M.S.; de Mulé, M.C.; de Caire, G.Z.; de Halperin, D.R. Inhibition of Candida albicans and Staphylococcus aureus by phenolic compounds from the terrestrial cyanobacterium Nostoc muscorum. J. Appl. Phycol. 1990, 2, 79–81. [Google Scholar] [CrossRef]
- Frankmölle, W.P.; Larsen, L.K.; Caplan, F.R.; Patterson, G.M.L.; Knubel, G.; Levine, I.A.; Moore, R.E. Blue-green alga Anabaena laxa. I. Isolation and biological properties. J. Antibiot. (Tokyo) 1992, 45, 1451–1457. [Google Scholar] [CrossRef]
- Frankmölle, W.P.; Knubel, G.; Moore, R.E.; Patterson, G.M.L. Blue-green alga Anabaena laxa. II. Structures of laxaphycins a,b, d, and e. J. Antibiot. (Tokyo) 1992, 45, 1458–1466. [Google Scholar]
- Moon, S.S.; Lu Chen, J.; Moore, R.E.; Patterson, G.M.L. Calophycin, a fungicidal cyclic decapeptide from the terrestrial blue-green alga Calothrix fusca. J. Org. Chem. 1992, 57, 1097–1103. [Google Scholar] [CrossRef]
- Prasanna, R.; Nain, L.; Tripathi, R.; Gupta, V.; Chaudhary, V.; Middha, S.; Joshi, M.; Ancha, R.; Kaushik, B.D. Evaluation of fungicidal activity of extracellular filtrates of cyanobacteria—Possible role of hydrolytic enzymes. J. Basic Microbiol. 2008, 48, 186–194. [Google Scholar] [CrossRef]
- Radhakrishnan, B.; Prasanna, R.; Jaiswal, P.; Nayak, S.; Dureja, P. Modulation of biocidal activity of Calothrix sp. and Anabaena sp. by environmental factors. Biologia (Bratisl) 2009, 64, 881–889. [Google Scholar] [CrossRef]
- Manjunath, M.; Prasanna, R.; Nain, L.; Dureja, P.; Singh, R.; Kumar, A.; Jaggi, S.; Kaushik, B.D. Biocontrol potential of cyanobacterial metabolites against damping off disease caused by Pythium aphanidermatum in solanaceous vegetables. Arch. Phytopathol. Plant Prot. 2010, 43, 666–677. [Google Scholar] [CrossRef]
- Hoiczyk, E.; Hansel, A. Cyanobacterial cell walls: News from an unusual prokaryotic envelope. J. Bacteriol. 2000, 182, 1191–1199. [Google Scholar] [CrossRef]
- Shanura Fernando, I.; Asanka Sanjeewa, K.K.; Samarakoon, K.W.; Lee, W.W.; Kim, H.S.; Kim, E.A.; Gunasekara, U.K.D.S.S.; Abeytunga, D.T.U.; Nanayakkara, C.; de Silva, E.D.; et al. FTIR characterization and antioxidant activity of water soluble crude polysaccharides of Sri Lankan marine algae. Algae 2017, 32, 75–86. [Google Scholar] [CrossRef]
- Johansen, H.W. Coralline Algae, a First Synthesis. In Coralline Algae, A First Synthesis; Taylor & Francis Group, Ed.; CRC Press, Inc.: Boca Raton, FL, USA, 2018; p. 699. [Google Scholar]
- Kantachumpoo, A.; Chirapart, A. Components and antimicrobial activity of polysaccharides extracted from thai brown seaweeds. Kasetsart, J. Nat. Sci. 2010, 44, 220–233. [Google Scholar]
- Asker, M.M.S.; Sahera, F.M.; Ali, F.M.; El-Sayed, O.H. Chemical structure and antiviral activity of water-soluble sulfated polysaccharides from Surgassum latifolium. J. Appl. Sci. Res. 2007, 3, 1178–1185. [Google Scholar]
- Skalicka-Woźniak, K.; Szypowski, J.; Łoś, R.; Siwulski, M.; Sobieralski, K.; Głowniak, K.; Malm, A. Evaluation of polysaccharides content in fruit bodies and their antimicrobial activity of four Ganoderma lucidum (W Curt.: Fr.) P. Karst. strains cultivated on different wood type substrates. Acta Soc. Bot. Pol. 2012, 81, 17–21. [Google Scholar] [CrossRef]
- Lipipun, V.; Nantawanit, N.; Pongsamart, S. Antimicrobial activity (in vitro) of polysaccharide gel from durian fruit-hulls. Songklanakarin, J. Sci. Tehcnol. 2002, 24, 31–38. [Google Scholar]
- Zaitseva, N. A Polysaccharide Extracted from Sphagnum Moss as Antifungal Agent in Archaeological Conservation. Ph.D. Thesis, Queen’s University, Kingston, ON, Canada, December 2009. [Google Scholar]
- Palma-Guerrero, J.; Jansson, H.B.; Salinas, J.; Lopez-Llorca, L.V. Effect of chitosan on hyphal growth and spore germination of plant pathogenic and biocontrol fungi. J. Appl. Microbiol. 2008, 104, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Hammond-Kosack, K.E.; Jones, J.D.G. Resistance gene-dependent plant defense responses. Plant Cell 1996, 8, 1773–1791. [Google Scholar] [CrossRef]
- Reymond, P.; Farmer, E.E. Jasmonate and salicylate as global signals for defense gene expression. Curr. Opin. Plant Biol. 1998, 1, 404–411. [Google Scholar] [CrossRef]
- Paulert, R.; Talamini, V.; Cassolato, J.E.F.; Duarte, M.E.R.; Noseda, M.D.; Smania, A.; Stadnik, M.J. Effects of sulfated polysaccharide and alcoholic extracts from green seaweed Ulva fasciata on anthracnose severity and growth of common bean (Phaseolus vulgaris L.). J. Plant Dis. Prot. 2009, 116, 263–270. [Google Scholar] [CrossRef]
- Sharma, H.S.S.; Fleming, C.; Selby, C.; Rao, J.R.; Martin, T. Plant biostimulants: A review on the processing of macroalgae and use of extracts for crop management to reduce abiotic and biotic stresses. J. Appl. Phycol. 2014, 26, 465–490. [Google Scholar] [CrossRef]
- Klarzynski, O.; Plesse, B.; Joubert, J.-M.; Yvin, J.-C.; Kopp, M.; Kloareg, B.; Fritig, B. Linear β-1,3 glucans are elicitors of defense responses in tobacco. Plant Physiol. 2000, 124, 1027–1037. [Google Scholar] [CrossRef]
- Ben Salah, I.; Aghrouss, S.; Douira, A.; Aissam, S.; El Alaoui-Talibi, Z.; Filali-Maltouf, A.; El Modafar, C. Seaweed polysaccharides as bio-elicitors of natural defenses in olive trees against Verticillium wilt of olive. J. Plant Interact. 2018, 13, 248–255. [Google Scholar] [CrossRef]
- Mercier, L.; Lafitte, C.; Borderies, G.; Briand, X.; Esquerré-Tugayé, M.T.; Fournier, J. The algal polysaccharide carrageenans can act as an elicitor of plant defense. New Phytol. 2001, 149, 43–51. [Google Scholar] [CrossRef]
- Ghannam, A.; Abbas, A.; Alek, H.; Al-Waari, Z.; Al-Ktaifani, M. Enhancement of local plant immunity against tobacco mosaic virus infection after treatment with sulphated-carrageenan from red alga (Hypnea musciformis). Physiol. Mol. Plant Pathol. 2013, 84, 19–27. [Google Scholar] [CrossRef]
- Zheng, Y.; Sheng, J.; Zhao, R.; Zhang, J.; Lv, S.; Liu, L.; Shen, L. Preharvest l -arginine treatment induced postharvest disease resistance to Botrysis cinerea in tomato fruits. J. Agric. Food Chem. 2011, 59, 6543–6549. [Google Scholar] [CrossRef]
- Feliziani, E.; Landi, L.; Romanazzi, G. Preharvest treatments with chitosan and other alternatives to conventional fungicides to control postharvest decay of strawberry. Carbohydr. Polym. 2015, 132, 111–117. [Google Scholar] [CrossRef]
- Yao, H.; Tian, S. Effects of pre- and post-harvest application of salicylic acid or methyl jasmonate on inducing disease resistance of sweet cherry fruit in storage. Postharvest Biol. Technol. 2005, 35, 253–262. [Google Scholar] [CrossRef]
- Abdala Díaz, R.T.; Chabrillón, M.; Cabello-Pasini, A.; Gómez-Pinchetti, J.L.; Figueroa, F.L. Characterization of polysaccharides from Hypnea spinella (Gigartinales) and Halopithys incurva (Ceramiales) and their effect on RAW 264.7 macrophage activity. J. Appl. Phycol. 2010, 23, 523–528. [Google Scholar] [CrossRef]
- Morris Quevedo, H.J.; Martínez Manrique, C.; Abdala Díaz, R.; Cobas Pupo, G. Evidencias preliminares de la actividad inmunomoduladora de la fracción polisacárida de origen marino Pc-1. Rev. Cubana Oncol. 2000, 16, 171–176. [Google Scholar]
- Álvarez-Gómez, F.; Korbee, N.; Figueroa, F.L. Analysis of antioxidant capacity and bioactive compounds in marine macroalgal and lichenic extracts using different solvents and evaluation methods. Cienc. Mar. 2016, 42, 271–288. [Google Scholar]
| Concentration (mg/mL) WE Treatment | 0.0 | 2.5 | 5.0 | 10.0 | Mean |
|---|---|---|---|---|---|
| Colony Growth Rate (mm/day) | |||||
| AN | 18.3 ± 0.6 | 16.3 ± 0.3 | 16.0 ± 1.3 | 16.2 ± 0.3 | 16.7 ± 1.2 A |
| ECK | 18.2 ± 0.8 | 18.2 ± 0.3 | 16.8 ± 0.8 | 16.5 ± 0.5 | 17.4 ± 0.9 A |
| JAN | 18.7 ± 0.6 | 17.5 ± 0.5 | 16.8 ± 0.3 | 16.8 ± 0.8 | 17.5 ± 0.9 A |
| Mean | 18.4 ± 0.6 c | 17.3 ± 0.9 b | 16.6 ± 0.9 a | 16.5 ± 0.6 a | |
| Treatment | EC50 POL (mg/mL) | ||
|---|---|---|---|
| Spore Germination | CFUs | CFU Colony Growth | |
| AN POL | 0.058 (0.027–0.127) | 0.553 (0.255–1.202) | 1.064 (0.665–1.703) |
| ECK POL | 0.096 (0.048–0.195) | 1.201 (0.584–5.392) | 2.087 (1.627–2.676) |
| JAN POL | 0.202 (0.118–0.346) | nd a | nd a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Righini, H.; Baraldi, E.; García Fernández, Y.; Martel Quintana, A.; Roberti, R. Different Antifungal Activity of Anabaena sp., Ecklonia sp., and Jania sp. against Botrytis cinerea. Mar. Drugs 2019, 17, 299. https://doi.org/10.3390/md17050299
Righini H, Baraldi E, García Fernández Y, Martel Quintana A, Roberti R. Different Antifungal Activity of Anabaena sp., Ecklonia sp., and Jania sp. against Botrytis cinerea. Marine Drugs. 2019; 17(5):299. https://doi.org/10.3390/md17050299
Chicago/Turabian StyleRighini, Hillary, Elena Baraldi, Yolanda García Fernández, Antera Martel Quintana, and Roberta Roberti. 2019. "Different Antifungal Activity of Anabaena sp., Ecklonia sp., and Jania sp. against Botrytis cinerea" Marine Drugs 17, no. 5: 299. https://doi.org/10.3390/md17050299
APA StyleRighini, H., Baraldi, E., García Fernández, Y., Martel Quintana, A., & Roberti, R. (2019). Different Antifungal Activity of Anabaena sp., Ecklonia sp., and Jania sp. against Botrytis cinerea. Marine Drugs, 17(5), 299. https://doi.org/10.3390/md17050299






