Activity Improvement and Vital Amino Acid Identification on the Marine-Derived Quorum Quenching Enzyme MomL by Protein Engineering
Abstract
1. Introduction
2. Results
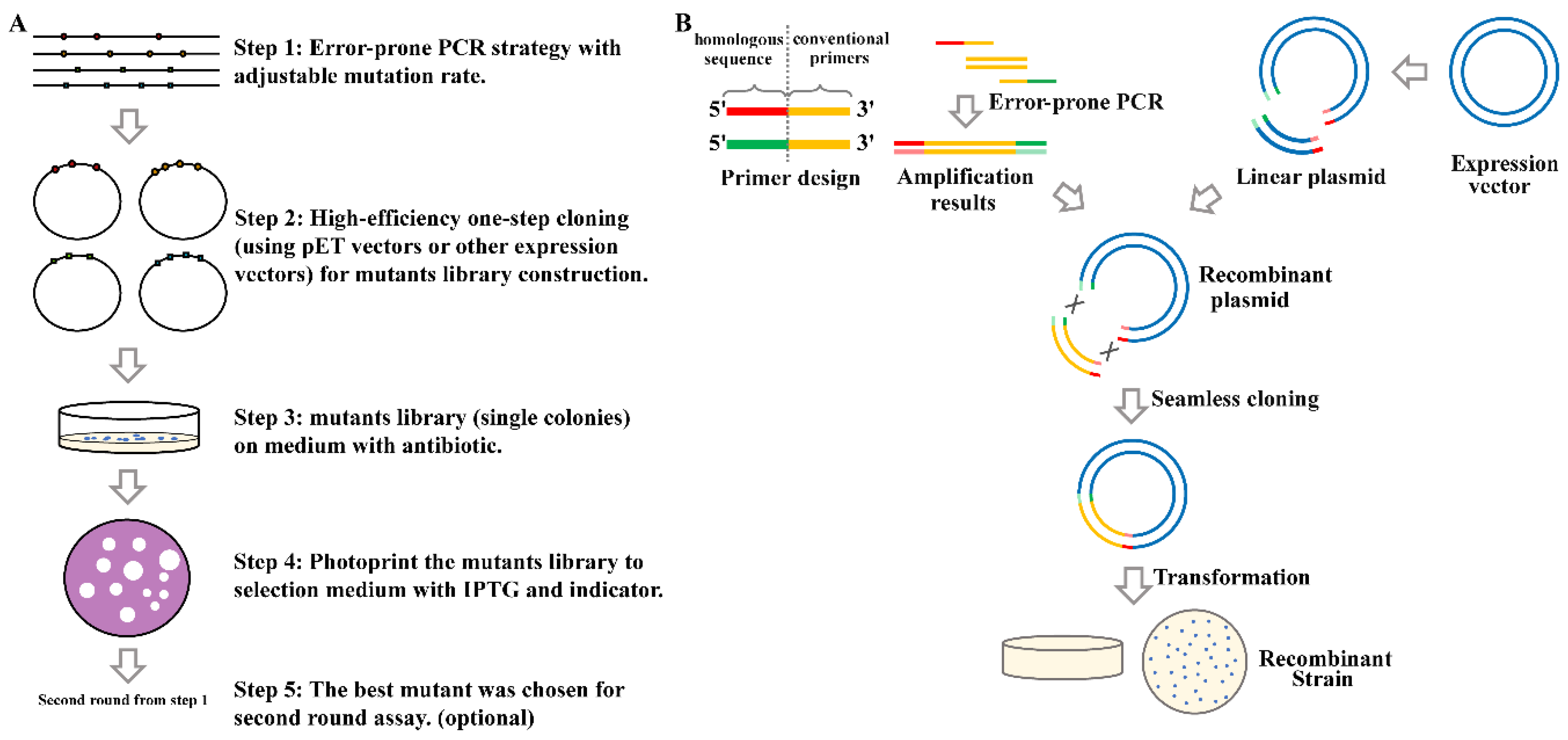
2.1. Overview of the High-Efficiency Strategy of Constructing and Screening a Random Mutagenesis Library
2.2. Error-Prone Polymerase Chain Reaction (EpPCR) Condition Optimization with Suitable Mutation Efficiency
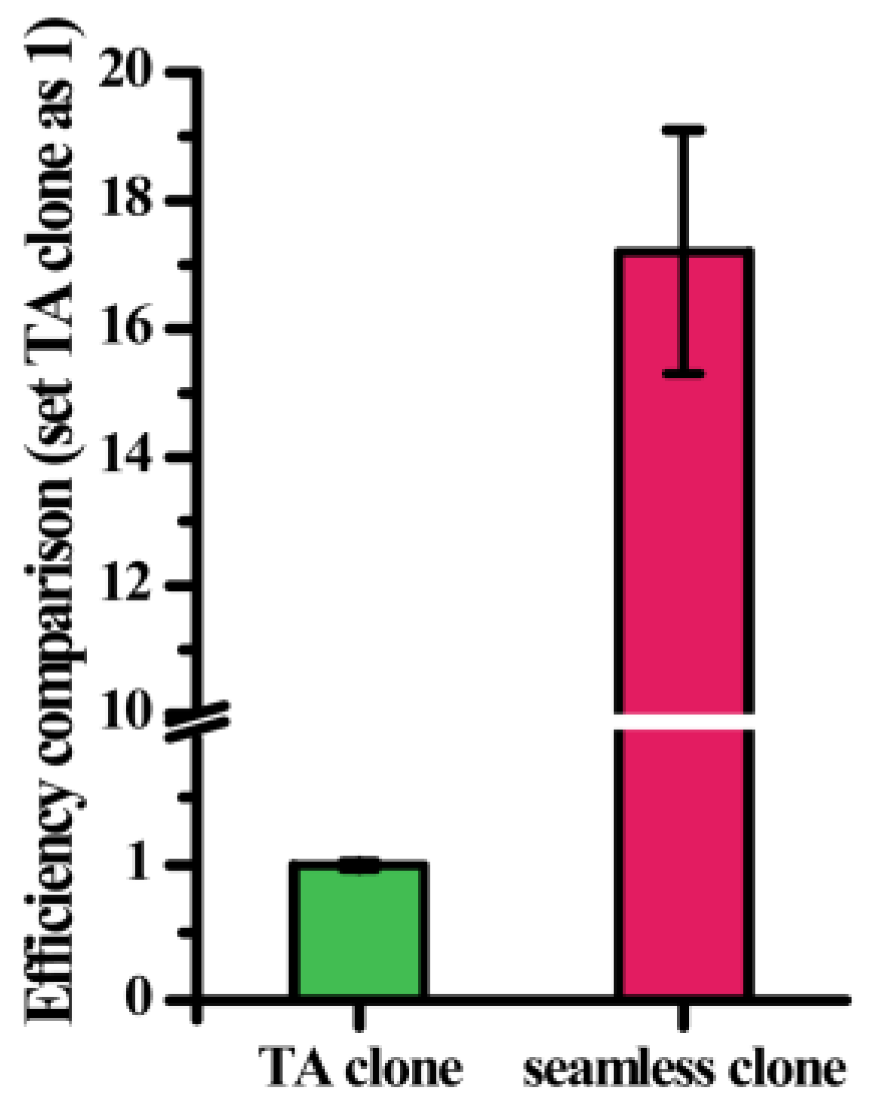
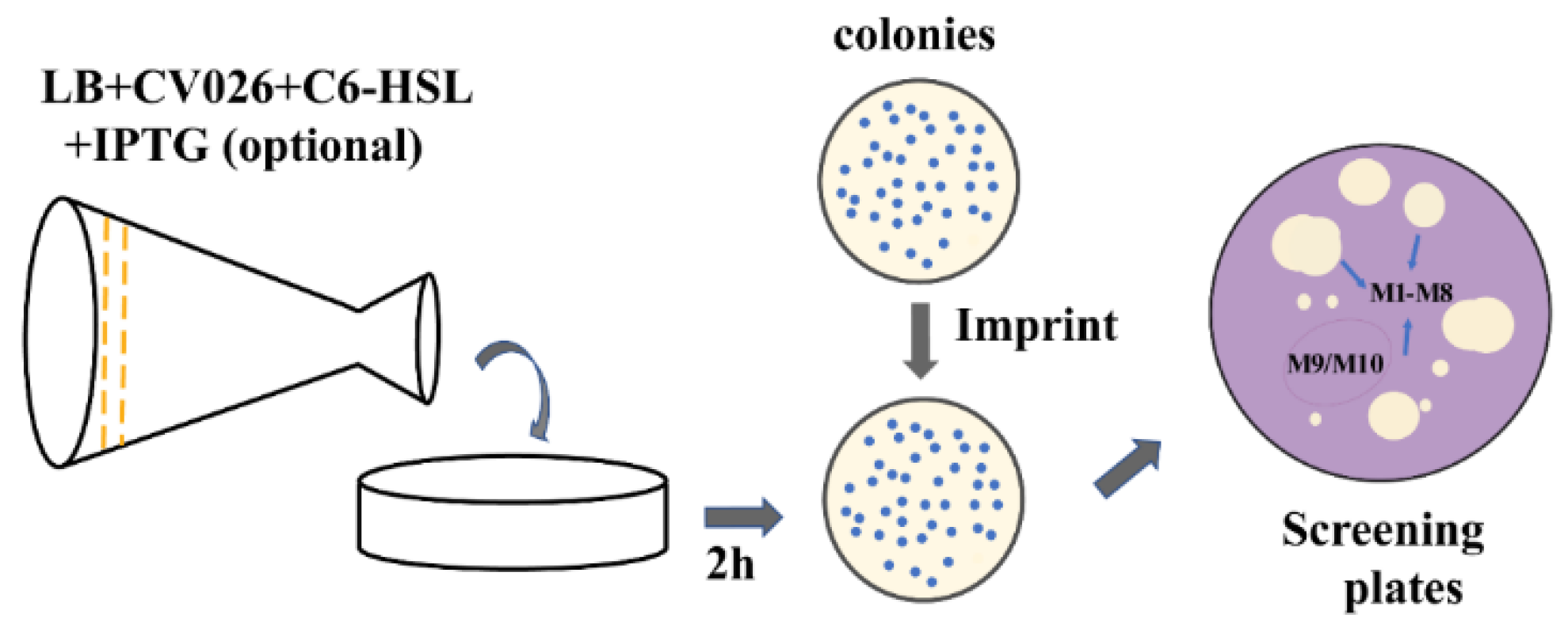
2.3. Screening of a Mutation Library Based on a Seamless and EpPCR Strategy
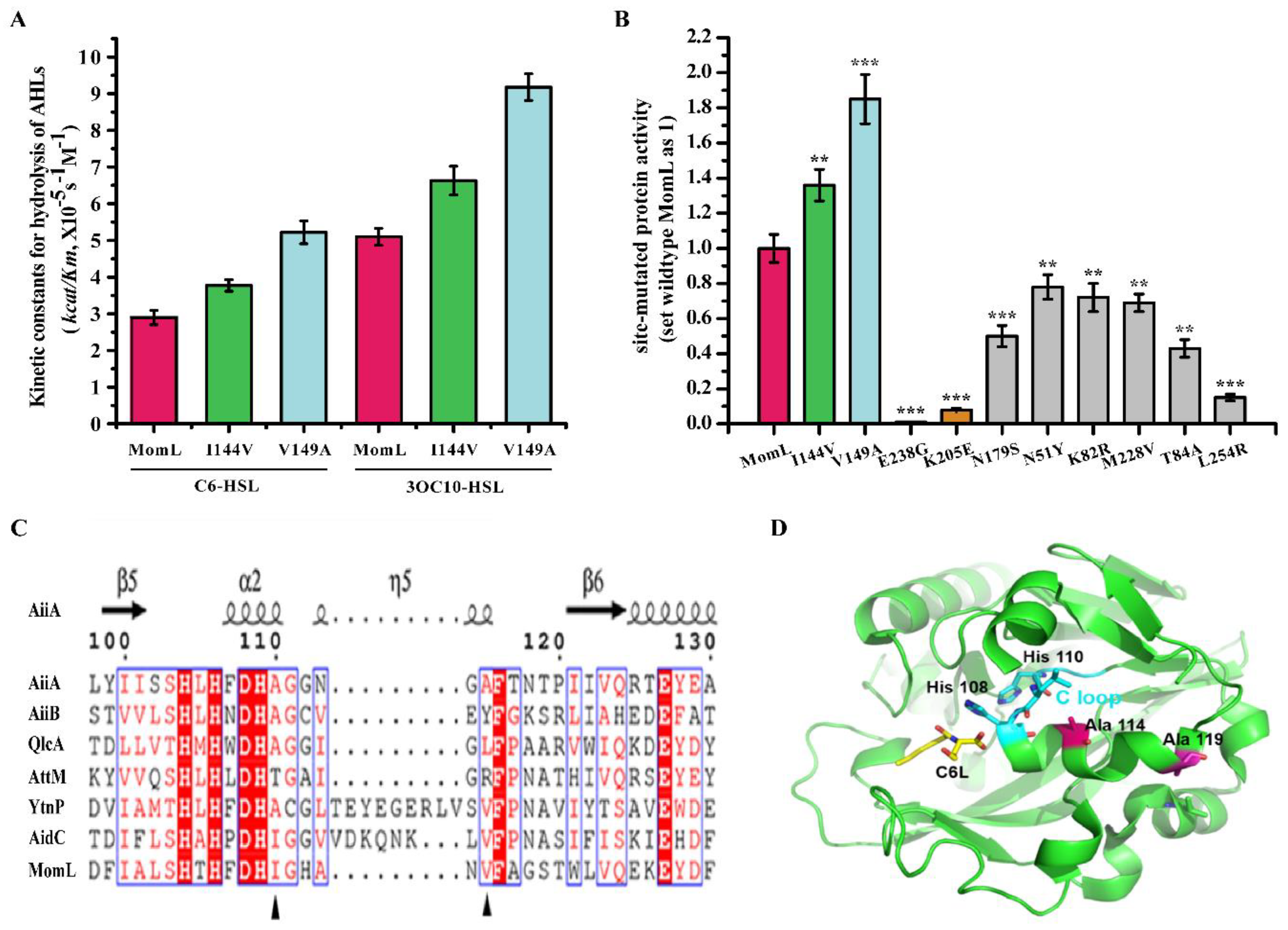
2.4. Analysis of Amino Acids in Mutant Proteins
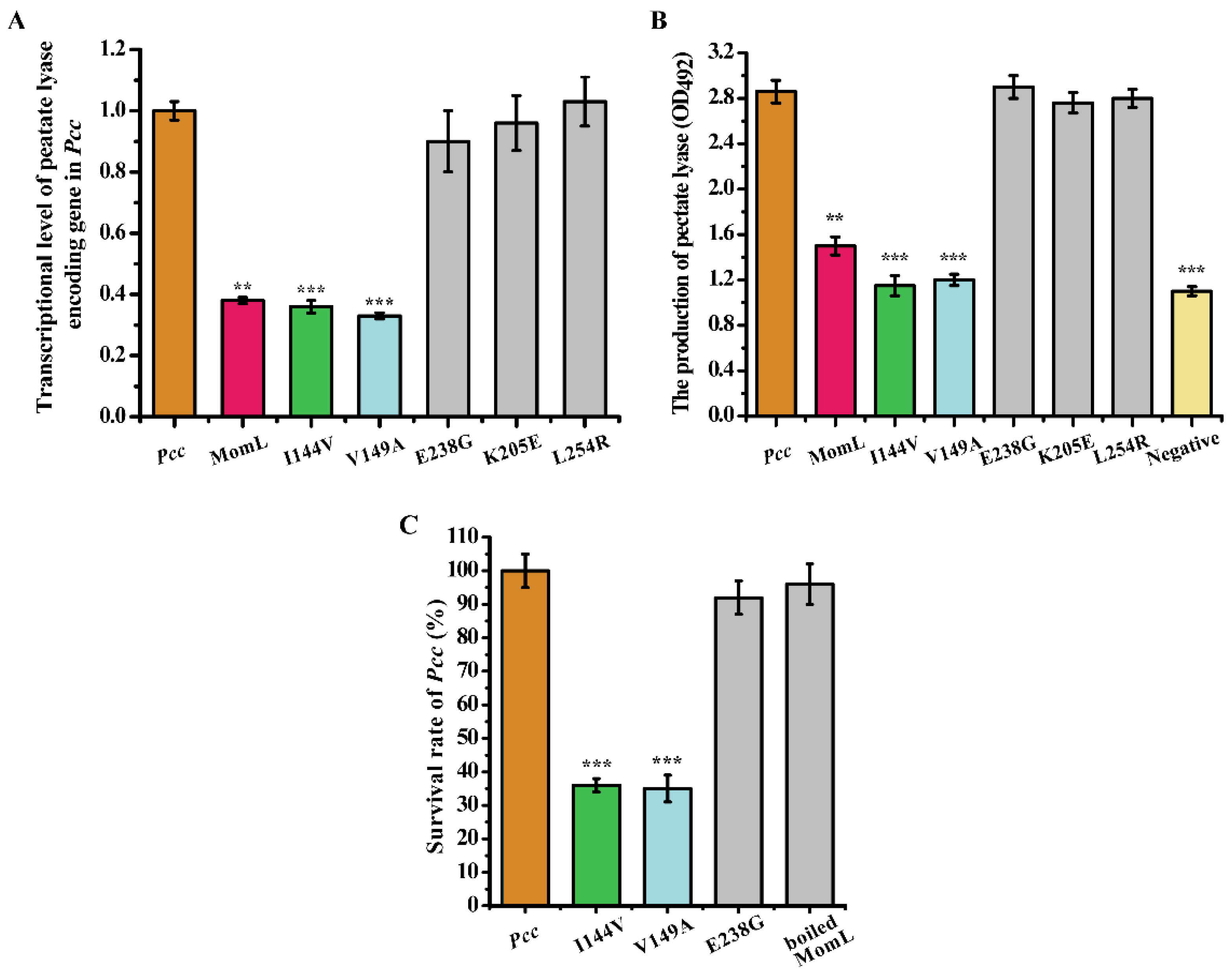
2.5. The Effect of Mutant Proteins on the Virulence Factors and Survival of Pectobacterium Carotovorum Subsp. Carotovorum (Pcc)
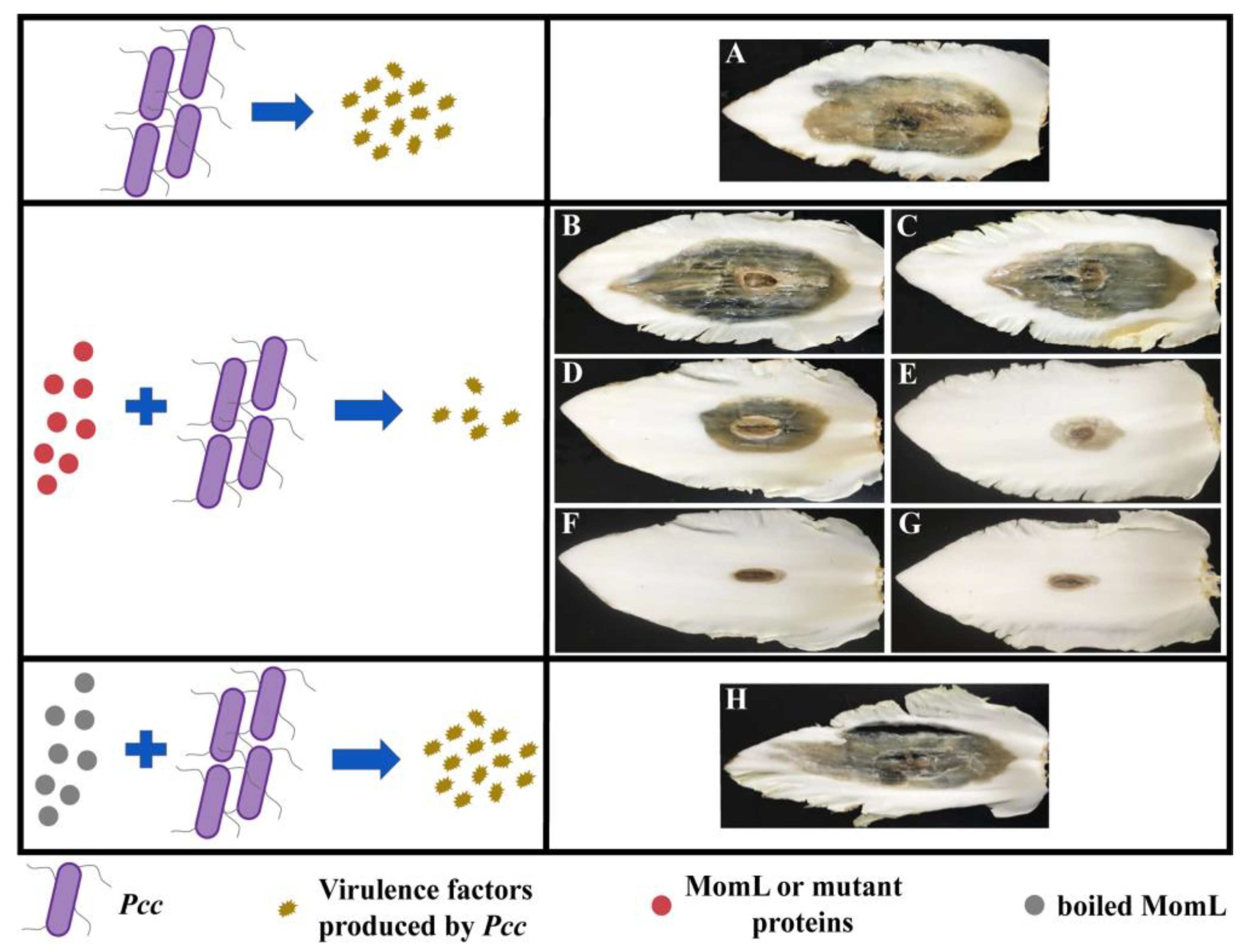
2.6. Effects of MomL and Mutant Proteins on Pcc Infection of Chinese Cabbage
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains, Plasmids, Media, Growth Conditions, and Chemicals
4.2. Random Mutant Library Construction and Identification of High-Activity Mutants
4.3. High-Throughput Screening of High-Activity Mutants
4.4. Expression and Purification of Mutant Proteins
4.5. N-Acyl Homoserine Lactone (AHL) Lactonases Activity Assay
4.6. Site-Directed Mutagenesis of Moml
4.7. Kinetic Assay of Moml and Mutant Proteins Activities
4.8. Effects of MomL and Mutant Proteins on the Pathogenicity of Pcc
4.9. Pcc Survival Rate Assay
4.10. Pcc Infection Experiment
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mion, S.; Rémy, B.; Plener, L.; Chabrière, É.; Daudé, D. Quorum sensing and quorum quenching: How to disrupt bacterial communication to inhibit virulence? Med. Sci. (Paris) 2019, 35, 31–38. [Google Scholar] [CrossRef]
- Miller, M.B.; Bassler, B.L. Quorum sensing in bacteria. Ann. Rev. Microbiol. 2000, 55, 165–199. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.; Winzer, K.; Chan, W.C.; Cámara, M. Look who’s talking: Communication and quorum sensing in the bacterial world. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2007, 362, 1119–1134. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.C.; Popat, R.; Diggle, S.P.; Brown, S.P. Targeting virulence: Can we make evolution-proof drugs? Nat. Rev. Microbiol. 2014, 12, 300. [Google Scholar] [CrossRef]
- Whiteley, M.; Diggle, S.P.; Greenberg, E.P. Progress in and promise of bacterial quorum sensing research. Nature 2017, 551, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.H.; Xu, J.L.; Li, X.Z.; Zhang, L.H. AiiA, an enzyme that inactivates the acylhomoserine lactone quorum-sensing signal and attenuates the virulence of Erwinia carotovora. Proc. Natl. Acad. Sci. USA 2000, 97, 3526–3531. [Google Scholar] [CrossRef] [PubMed]
- Fong, J.; Zhang, C.; Yang, R.; Boo, Z.Z.; Tan, S.K.; Nielsen, T.E.; Givskov, M.; Liu, X.W.; Bin, W.; Su, H. Combination Therapy Strategy of Quorum Quenching Enzyme and Quorum Sensing Inhibitor in Suppressing Multiple Quorum Sensing Pathways of P. aeruginosa. Sci. Rep. 2018, 8, 1155. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.Y.; Kim, M.H.; Bae, G.D.; Zhang, G.I.; Kim, Y.H.; Cho, B.C. Muricauda olearia sp. nov., isolated from crude-oil-contaminated seawater, and emended description of the genus Muricauda. Int. J. Syst. Evol. Microbiol. 2009, 59, 1856–1861. [Google Scholar] [CrossRef]
- Tang, K.; Su, Y.; Brackman, G.; Cui, F.; Zhang, Y.; Shi, X.; Coenye, T.; Zhang, X.H. MomL, a novel marine-derived N-acyl homoserine lactonase from Muricauda olearia. Appl. Environ. Microbiol. 2015, 81, 774–782. [Google Scholar] [CrossRef]
- Mayer, C.; Romero, M.; Muras, A.; Otero, A. Aii20J, a wide-spectrum thermostable N-acylhomoserine lactonase from the marine bacterium Tenacibaculum sp. 20J, can quench AHL-mediated acid resistance in Escherichia coli. Appl. Microbiol. Biotechnol. 2015, 99, 9523–9539. [Google Scholar] [CrossRef]
- Liu, D.; Momb, J.; Thomas, P.W.; Moulin, A.; Petsko, G.A.; Fast, W.; Ringe, D. Mechanism of the quorum-quenching lactonase (AiiA) from Bacillus thuringiensis. 1. Product-bound structures. Biochemistry 2008, 47, 7706–7714. [Google Scholar] [CrossRef] [PubMed]
- Momb, J.; Wang, C.; Liu, D.; Thomas, P.W.; Petsko, G.A.; Guo, H.; Ringe, D.; Fast, W. Mechanism of the quorum-quenching lactonase (AiiA) from Bacillus thuringiensis. 2. Substrate modeling and active site mutations. Biochemistry 2008, 47, 7715–7725. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, J.; Tang, K.; Yu, M.; Coenye, T.; Zhang, X.H. Genome analysis of Flaviramulus ichthyoenteri Th78T in the family Flavobacteriaceae: Insights into its quorum quenching property and potential roles in fish intestine. BMC Gen. 2015, 16, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.H.; Wang, L.H.; Xu, J.L.; Zhang, H.B.; Zhang, X.F.; Zhang, L.H. Quenching quorum-sensing-dependent bacterial infection by an N-acyl homoserine lactonase. Nature 2001, 411, 813–817. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.G.; Atkinson, S.; Mathee, K.; Sam, C.K.; Chhabra, S.R.; Cámara, M.; Koh, C.L.; Williams, P. Characterization of N-acylhomoserine lactone-degrading bacteria associated with the Zingiber officinale (ginger) rhizosphere: Co-existence of quorum quenching and quorum sensing in Acinetobacter and Burkholderia. BMC Microbiol. 2011, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.W.; Koh, C.L.; Sam, C.K.; Yin, W.F.; Chan, K.G. Complete genome sequence of Burkholderia sp. Strain GG4, a betaproteobacterium that reduces 3-oxo-N-acylhomoserine lactones and produces different N-acylhomoserine lactones. J. Bacteriol. 2012, 194, 6317. [Google Scholar] [CrossRef] [PubMed]
- Uroz, S.; Chhabra, S.R.; Cámara, M.; Williams, P.; Oger, P.; Dessaux, Y. N-Acylhomoserine lactone quorum-sensing molecules are modified and degraded by Rhodococcus erythropolis W2 by both amidolytic and novel oxidoreductase activities. Microbiology 2005, 151, 3313–3322. [Google Scholar] [CrossRef]
- Gooding, J.R.; May, A.L.; Hilliard, K.R.; Campagna, S.R. Establishing a quantitative definition of quorum sensing provides insight into the information content of the autoinducer signals in Vibrio harveyi and Escherichia coli. Biochemistry 2010, 49, 5621–5623. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.; Cui, X.; Zhang, X.H. A novel stress response mechanism, triggered by indole, involved in quorum quenching enzyme MomL and iron-sulfur cluster in Muricauda olearia Th120. Sci. Rep. 2017, 7, 4252. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Feng, T.; Du, R.; Tian, X.; Wang, Y.; Zhang, X.-H. Heterologous Expression of the Marine-Derived Quorum Quenching Enzyme MomL Can Expand the Antibacterial Spectrum of Bacillus brevis. Mar. Drugs 2019, 17, 128. [Google Scholar] [CrossRef]
- Kiran, M.D.; Adikesavan, N.V.; Cirioni, O.; Giacometti, A.; Silvestri, C.; Scalise, G.; Ghiselli, R.; Saba, V.; Orlando, F.; Shoham, M.; et al. Discovery of a quorum-sensing inhibitor of drug-resistant staphylococcal infections by structure-based virtual screening. Mol. Pharmacol. 2008, 73, 1578–1586. [Google Scholar] [CrossRef] [PubMed]
- Roy, V.; Fernandes, R.; Tsao, C.; Bentley, W. Cross Species Quorum Quenching Using a Native AI-2 Processing Enzyme. ACS Chem. Biol. 2010, 5, 223. [Google Scholar] [CrossRef]
- Corbett, M.; Virtue, S.; Bell, K.; Birch, P.; Burr, T.; Hyman, L.; Lilley, K.; Poock, S.; Toth, I.; Salmond, G. Identification of a new quorum-sensing-controlled virulence factor in Erwinia carotovora subsp. atroseptica secreted via the type II targeting pathway. Mol. Plant Microbe Interact. 2005, 18, 334–342. [Google Scholar] [CrossRef]
- Burr, T.; Barnard, A.M.L.; Corbett, M.J.; Pemberton, C.L.; Simpson, N.J.L.; Salmond, G.P.C. Identification of the central quorum sensing regulator of virulence in the enteric phytopathogen, Erwinia carotovora: The VirR repressor. Mol. Microbiol. 2006, 59, 113–125. [Google Scholar] [CrossRef]
- Lee, D.H.; Lim, J.A.; Lee, J.; Roh, E.; Jung, K.; Choi, M.; Oh, C.; Ryu, S.; Yun, J.; Heu, S. Characterization of genes required for the pathogenicity of Pectobacterium carotovorum subsp. carotovorum Pcc21 in Chinese cabbage. Microbiology 2013, 159, 1487–1496. [Google Scholar] [CrossRef]
- Kotoujansky, A. Molecular Genetics of Pathogenesis by Soft-Rot Erwinias. Annu. Rev. Phytopathol. 1987, 25, 405–430. [Google Scholar] [CrossRef]
- Jafra, S.; Jalink, H.; Schoor, R.V.D.; Wolf, J.M.V.D. Pectobacterium carotovorum subsp. carotovorum Strains Show Diversity in Production of and Response to N-acyl Homoserine Lactones. J. Phytopathol. 2010, 154, 729–739. [Google Scholar] [CrossRef]
- Lane, M.D.; Seelig, B. Advances in the directed evolution of proteins. Curr. Opin. Chem. Biol. 2014, 22, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.H.; Lengyel, J.A.; Langridge, J. Evolution of a Second Gene for β -Galactosidase in Escherichia coli. Proc. Natl. Acad. Sci. USA 1973, 70, 1841–1845. [Google Scholar] [CrossRef]
- Kan, S.B.J.; Lewis, R.D.; Chen, K.; Arnold, F.H. Directed evolution of cytochrome c for carbon-silicon bond formation: Bringing silicon to life. Science 2016, 354, 1048–1051. [Google Scholar] [CrossRef]
- Kan, S.B.J.; Huang, X.Y.; Gumulya, Y.; Chen, K.; Arnold, F.H. Genetically programmed chiral organoborane synthesis. Nature 2017, 552, 132. [Google Scholar] [CrossRef] [PubMed]
- Drummond, D.A.; Iverson, B.L.; Georgiou, G.; Arnold, F.H. Why high-error-rate random mutagenesis libraries are enriched in functional and improved proteins. J. Mol. Biol. 2005, 350, 806–816. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, A. Role of magnesium in genomic stability. Mut. Res. 2001, 475, 113–121. [Google Scholar] [CrossRef]
- Romero, M.; Martin-Cuadrado, A.B.; Otero, A. Determination of Whether Quorum Quenching Is a Common Activity in Marine Bacteria by Analysis of Cultivable Bacteria and Metagenomic Sequences. Appl. Environ. Microbiol. 2012, 78, 6345. [Google Scholar] [CrossRef] [PubMed]
- Romero, M.; Diggle, S.P.; Heeb, S.; Cámara, M.; Otero, A. Quorum quenching activity in Anabaena sp. PCC 7120: Identification of AiiC, a novel AHL-acylase. FEMS Microbiol. Lett. 2008, 280, 73–80. [Google Scholar] [CrossRef]
- Wei, H.; Lin, Y.; Yi, S.; Liu, P.; Jie, S.; Shao, Z.; Liu, Z. QsdH, a Novel AHL Lactonase in the RND-Type Inner Membrane of Marine Pseudoalteromonas byunsanensis Strain 1A01261. PLoS ONE 2012, 7, e46587. [Google Scholar]
- Kim, J.H.; Choi, D.C.; Yeon, K.M.; Kim, S.R.; Lee, C.H. Enzyme-immobilized nanofiltration membrane to mitigate biofouling based on quorum quenching. Environ. Sci. Technol. 2011, 45, 1601–1607. [Google Scholar] [CrossRef]
- Torres, M.; Rubio-Portillo, E.; Anton, J.; Ramos-Espla, A.A.; Quesada, E.; Llamas, I. Selection of the N-Acylhomoserine Lactone-Degrading Bacterium Alteromonas stellipolaris PQQ-42 and of Its Potential for Biocontrol in Aquaculture. Front. Microbiol. 2016, 7, 646. [Google Scholar] [CrossRef]
- Torres, M.; Dessaux, Y.; Llamas, I. Saline Environments as a Source of Potential Quorum Sensing Disruptors to Control Bacterial Infections: A Review. Mar. Drugs 2019, 17, 191. [Google Scholar] [CrossRef] [PubMed]
- Ruff, A.J.; Dennig, A.; Schwaneberg, U. To get what we aim for—Progress in diversity generation methods. FEBS J. 2013, 280, 2961–2978. [Google Scholar] [CrossRef] [PubMed]
- Pennypacker, B.W.; Dickey, R.S.; Nelson, P.E. A histological comparison of the response of a chrysanthemum cultivar susceptible to Erwinia chrysanthemi and Erwinia subsp. carotovora. Phytopathology 1981, 71. [Google Scholar]
- Mcclean, K.H.; Winson, M.K.; Fish, L.; Taylor, A.; Chhabra, S.R.; Camara, M.; Daykin, M.; Lamb, J.H.; Swift, S.; Bycroft, B.W.; et al. Quorum sensing and Chromobacterium violaceum: Exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology 1997, 143, 3703. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Wang, Y.; Yin, C.; Zhang, X.H. LaaA, a novel high-active alkalophilic alpha-amylase from deep-sea bacterium Luteimonas abyssi XH031 T. En. Microbial. Technol. 2016, 90, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Primrose, S.B.; Twyman, R.M. Principles of Gene Manipulation and Genomics, 7th ed.; Blackwell: Oxford, UK, 2006. [Google Scholar]
- Chapman, E.; Wong, C.H. A pH sensitive colorometric assay for the high-Throughput screening of enzyme inhibitors and substrates: A case study using kinases. Bioorg. Med. Chem. 2002, 10, 551–555. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Lin, J.; Zhang, Y.; Zhang, J.; Feng, T.; Li, H.; Wang, X.; Sun, Q.; Zhang, X.; Wang, Y. Activity Improvement and Vital Amino Acid Identification on the Marine-Derived Quorum Quenching Enzyme MomL by Protein Engineering. Mar. Drugs 2019, 17, 300. https://doi.org/10.3390/md17050300
Wang J, Lin J, Zhang Y, Zhang J, Feng T, Li H, Wang X, Sun Q, Zhang X, Wang Y. Activity Improvement and Vital Amino Acid Identification on the Marine-Derived Quorum Quenching Enzyme MomL by Protein Engineering. Marine Drugs. 2019; 17(5):300. https://doi.org/10.3390/md17050300
Chicago/Turabian StyleWang, Jiayi, Jing Lin, Yunhui Zhang, Jingjing Zhang, Tao Feng, Hui Li, Xianghong Wang, Qingyang Sun, Xiaohua Zhang, and Yan Wang. 2019. "Activity Improvement and Vital Amino Acid Identification on the Marine-Derived Quorum Quenching Enzyme MomL by Protein Engineering" Marine Drugs 17, no. 5: 300. https://doi.org/10.3390/md17050300
APA StyleWang, J., Lin, J., Zhang, Y., Zhang, J., Feng, T., Li, H., Wang, X., Sun, Q., Zhang, X., & Wang, Y. (2019). Activity Improvement and Vital Amino Acid Identification on the Marine-Derived Quorum Quenching Enzyme MomL by Protein Engineering. Marine Drugs, 17(5), 300. https://doi.org/10.3390/md17050300




