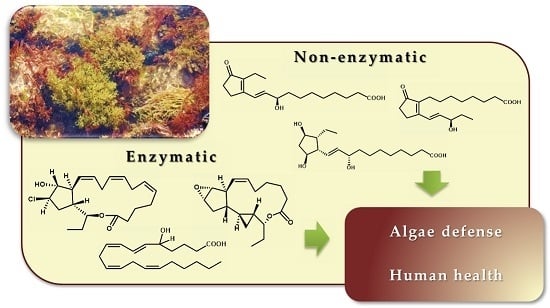
Biologically Active Oxylipins from Enzymatic and Nonenzymatic Routes in Macroalgae
Abstract
:1. Introduction
2. Oxylipin Biosynthesis in Macroalgae

2.1. Enzymatically-Derived Algal Oxylipins

2.1.1. Rhodophyta
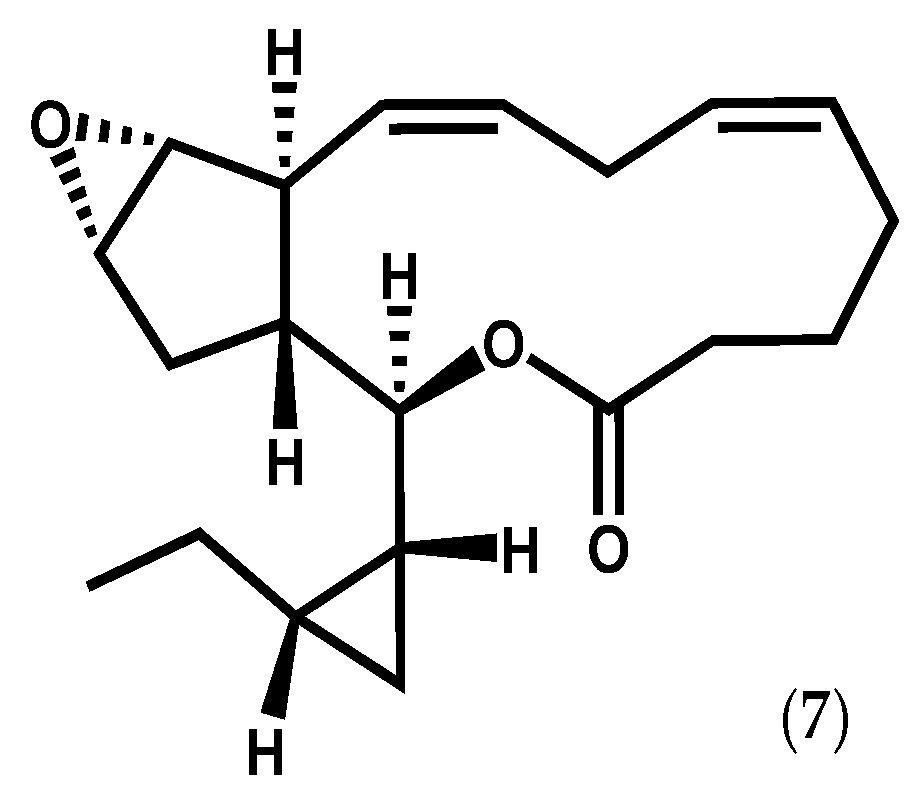
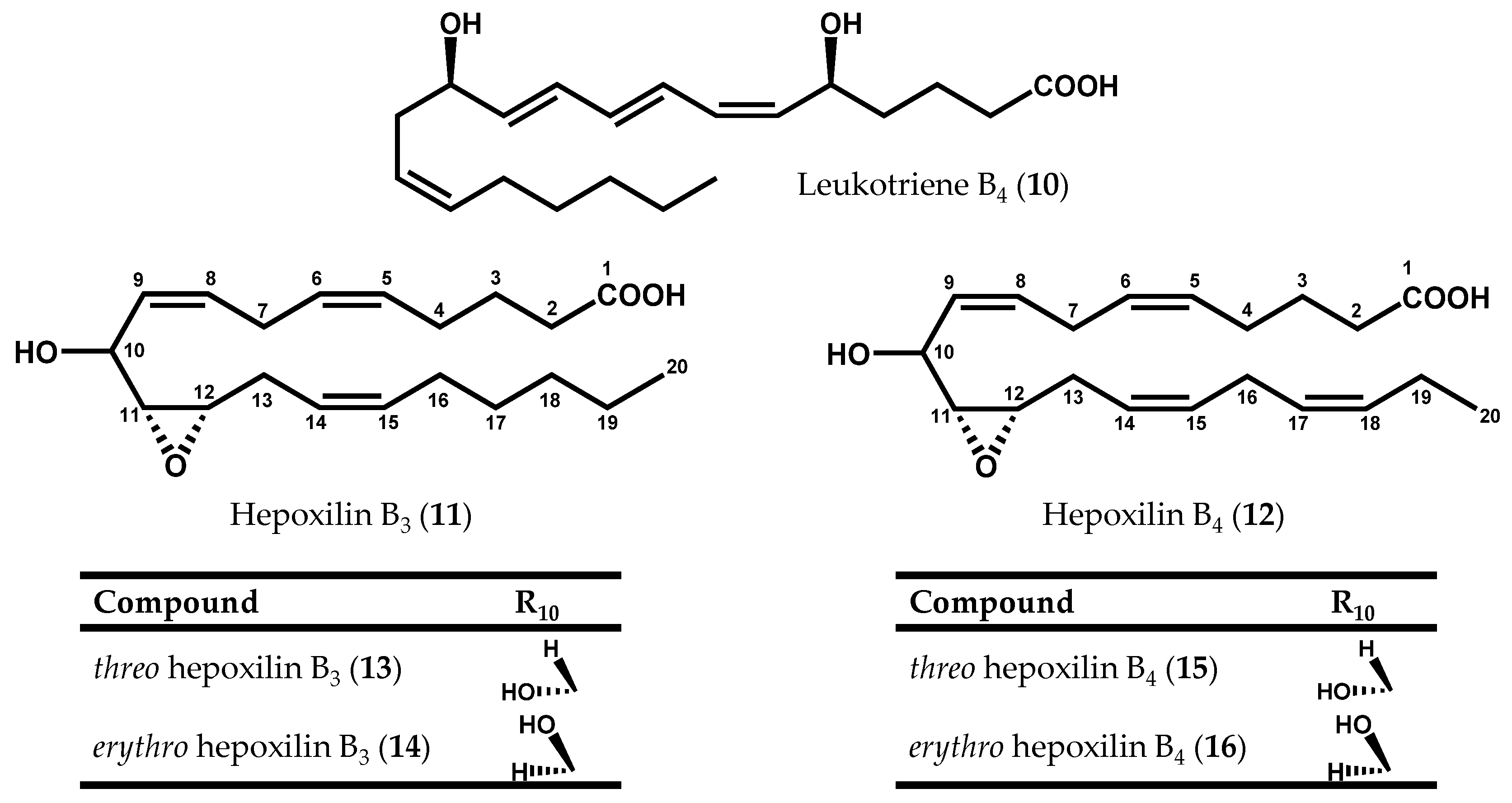
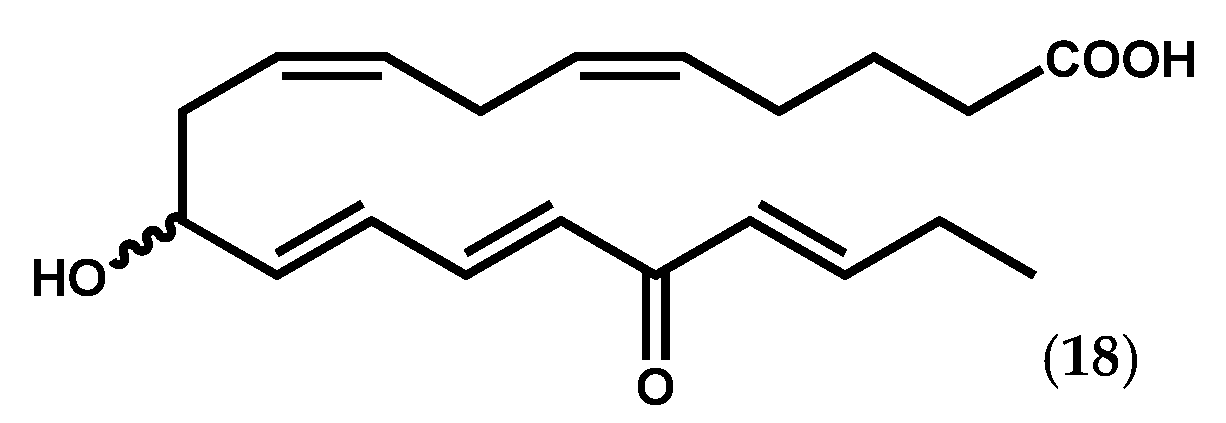
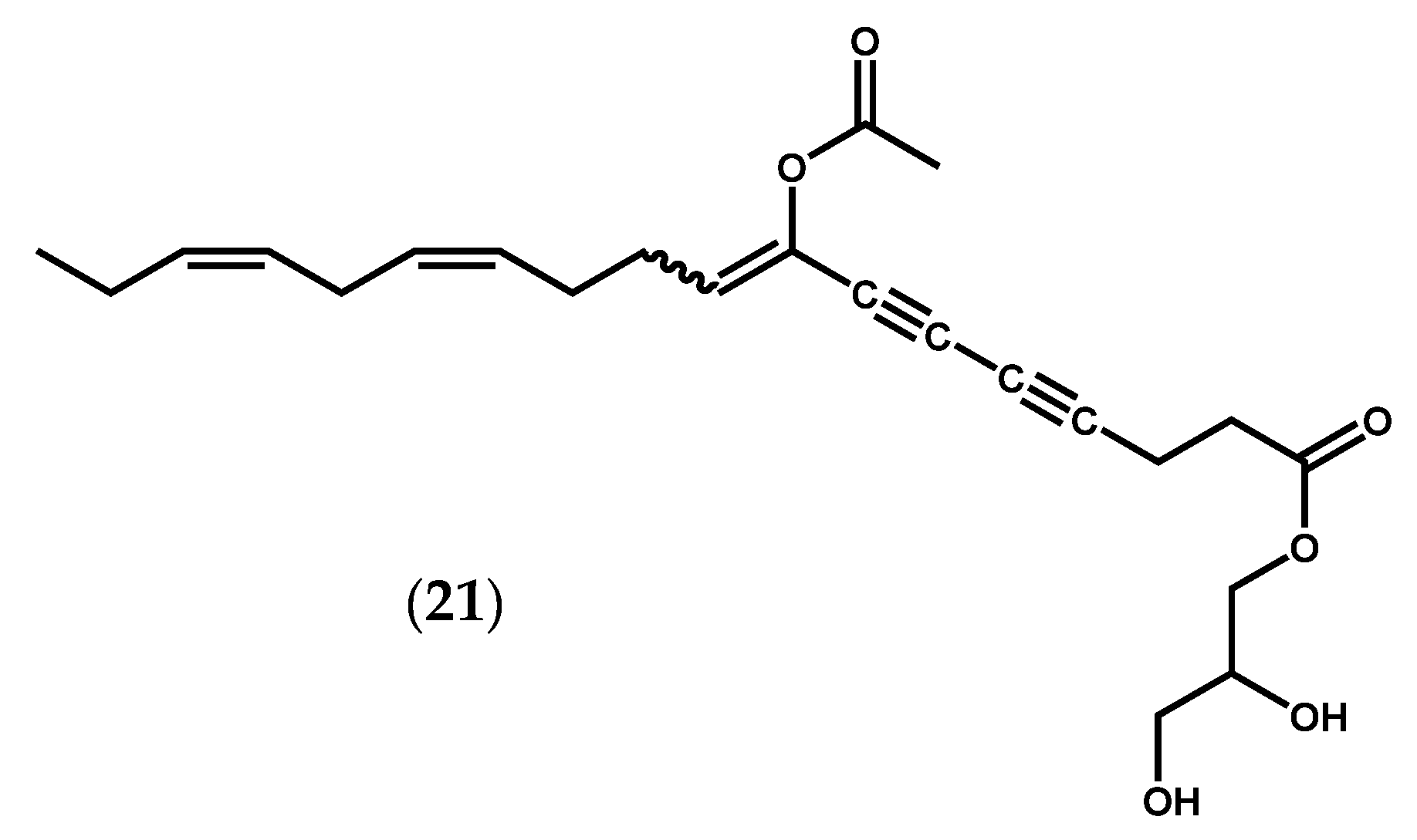
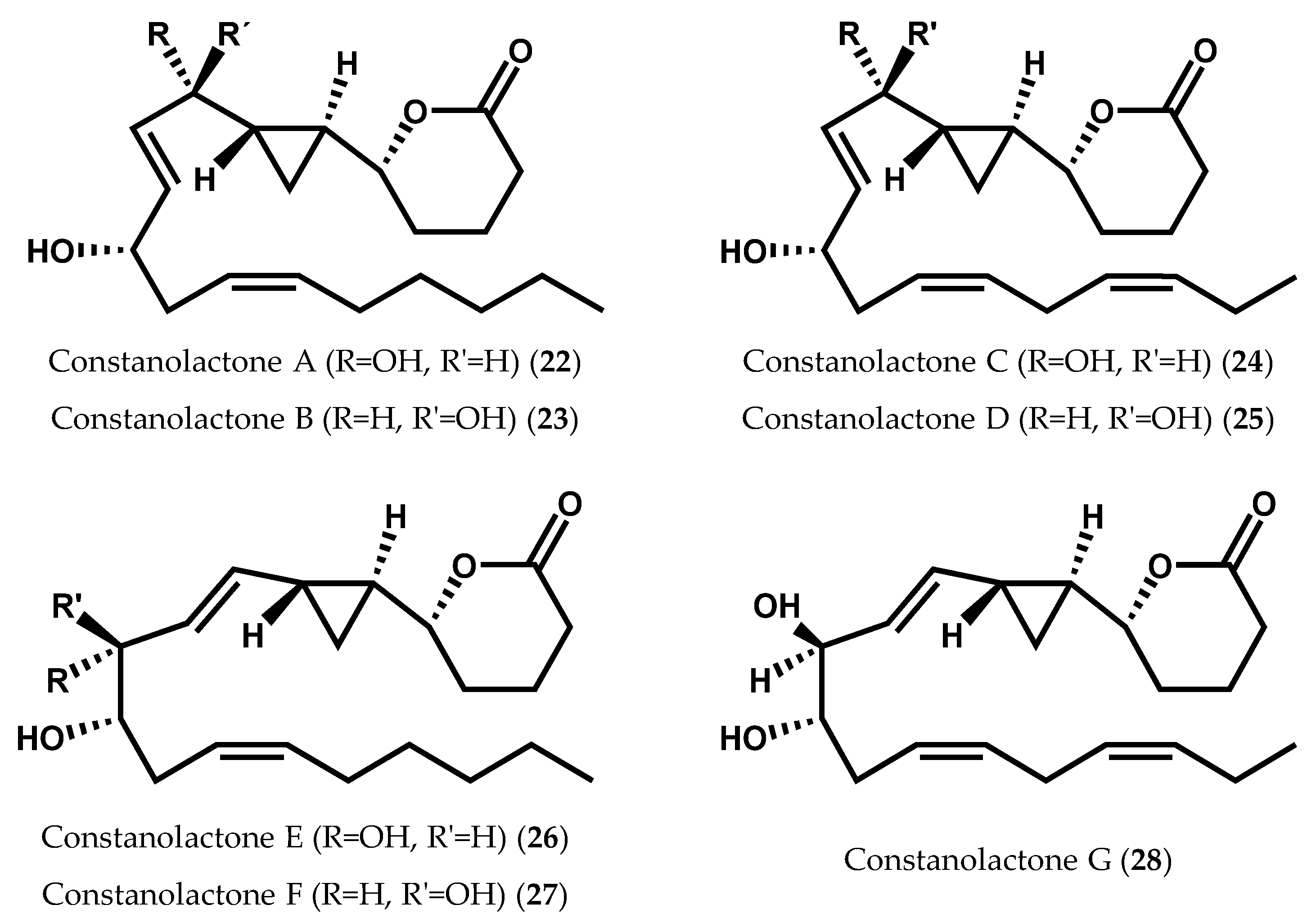
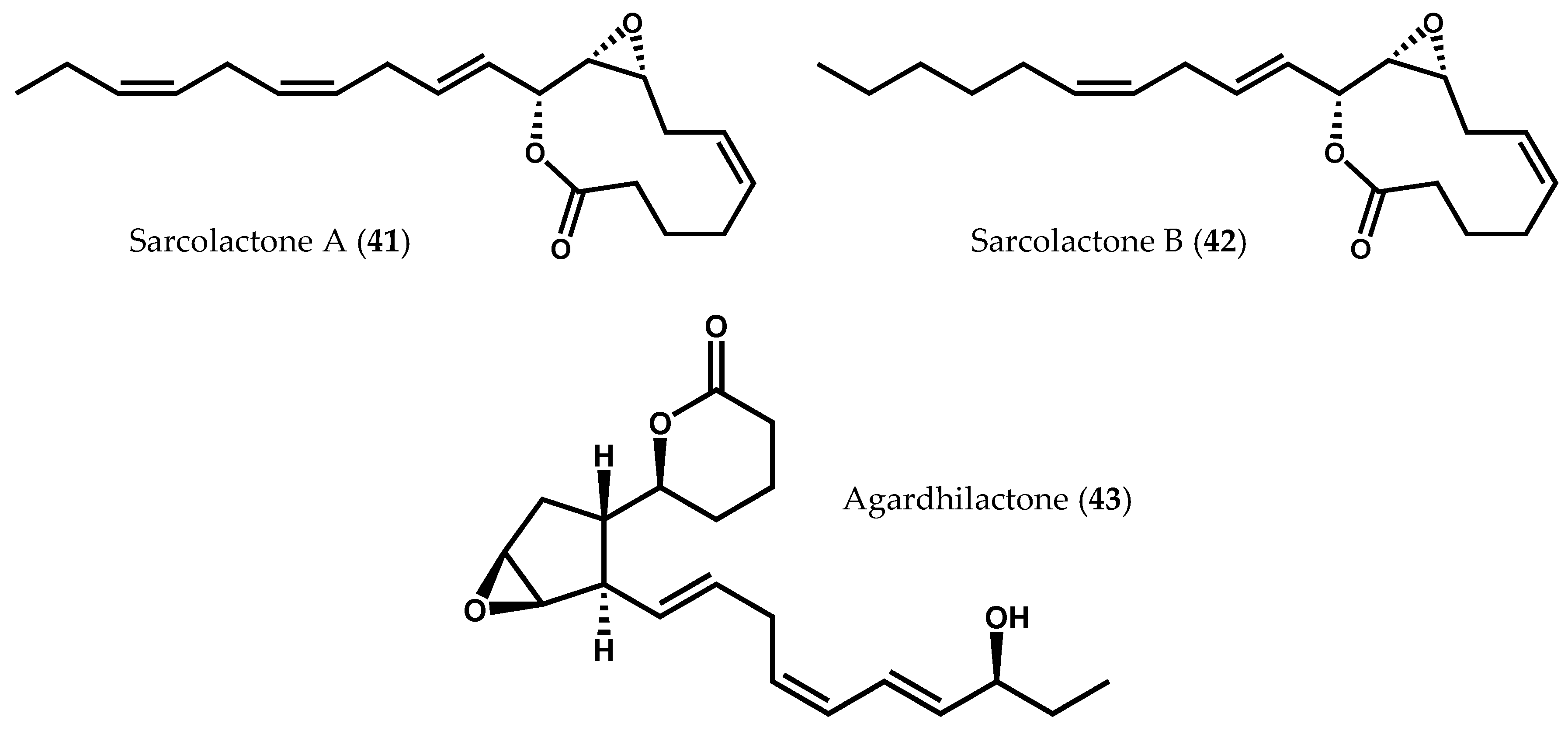
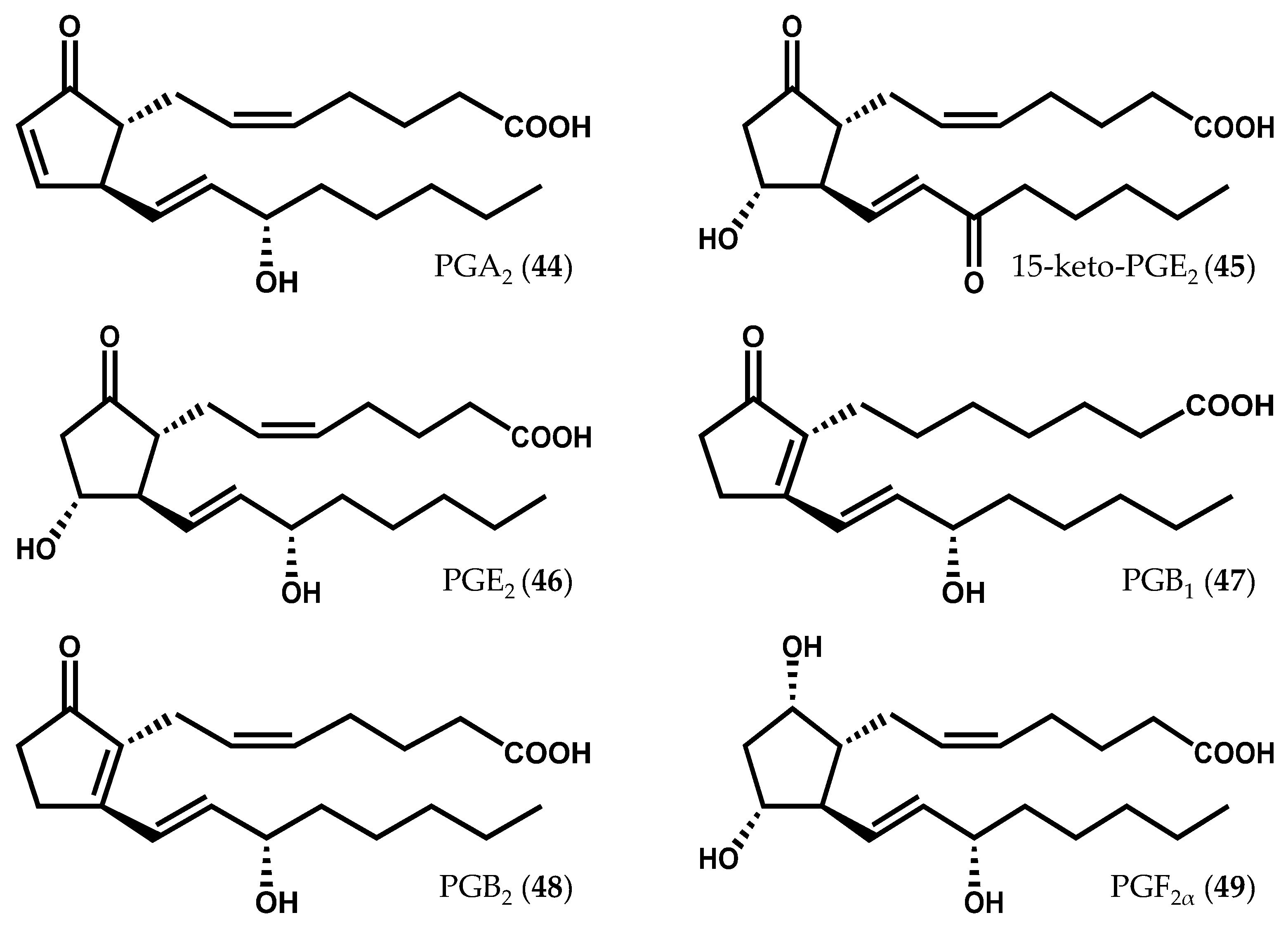
2.1.2. Ochrophyta
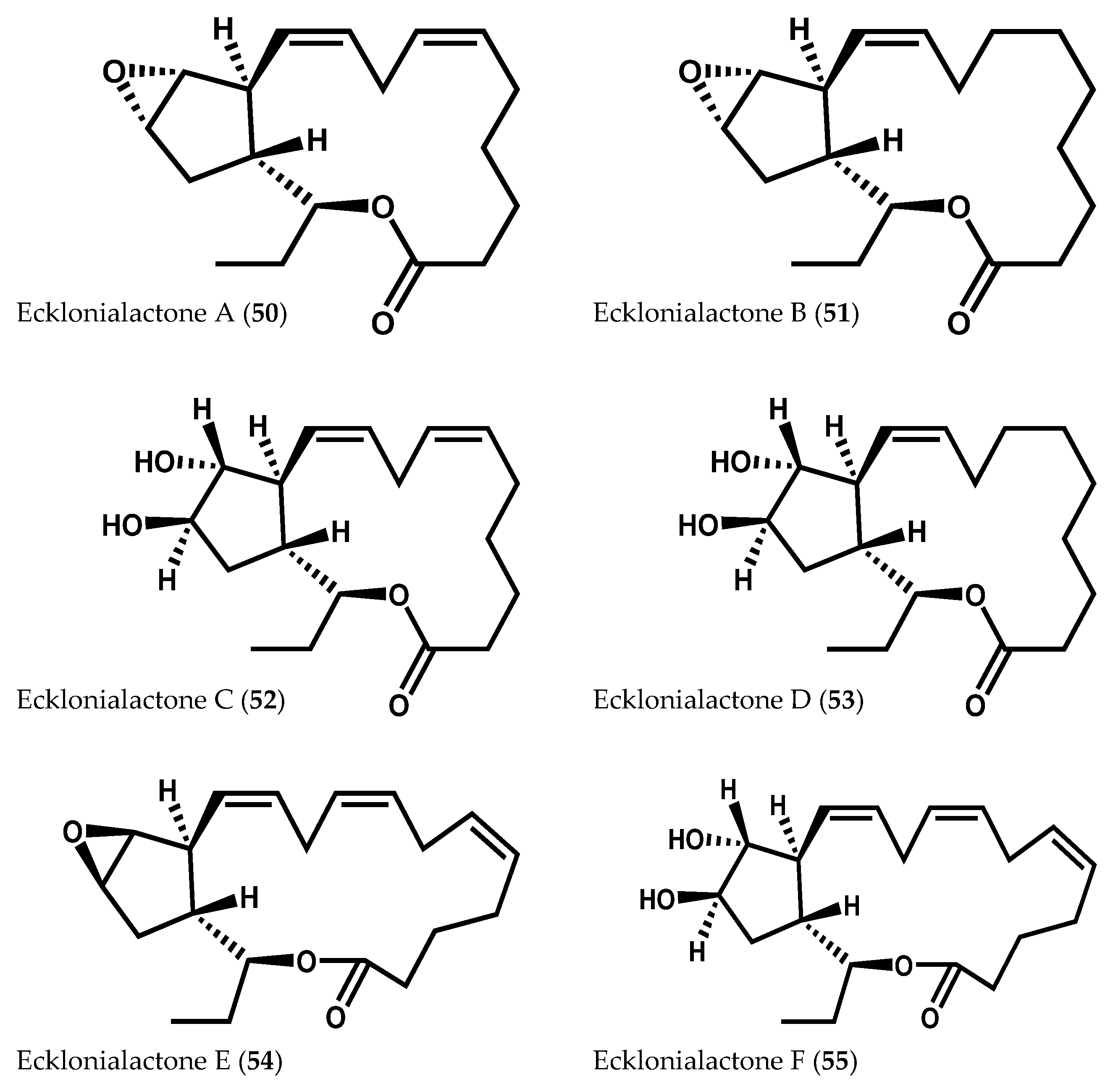
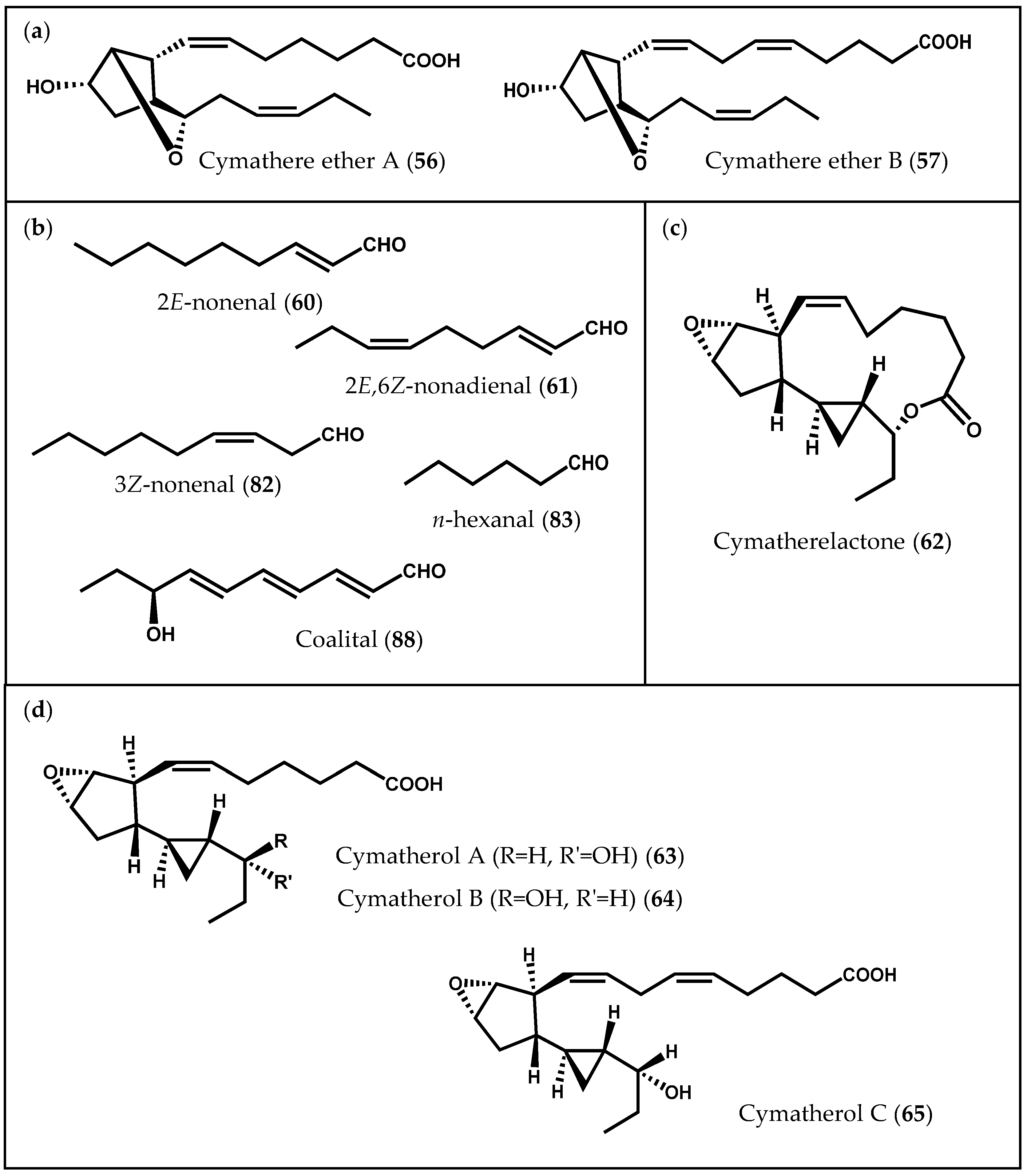
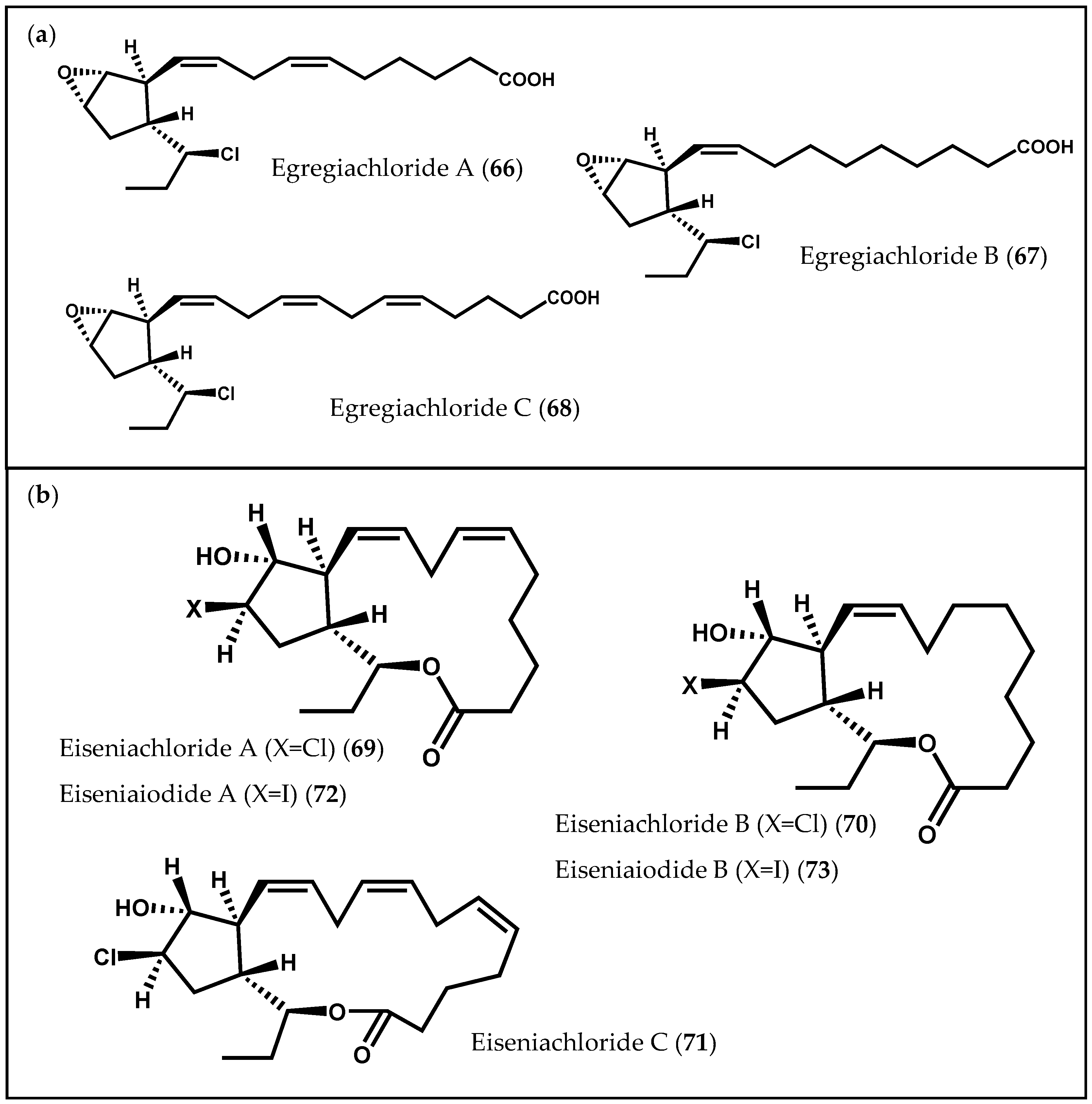
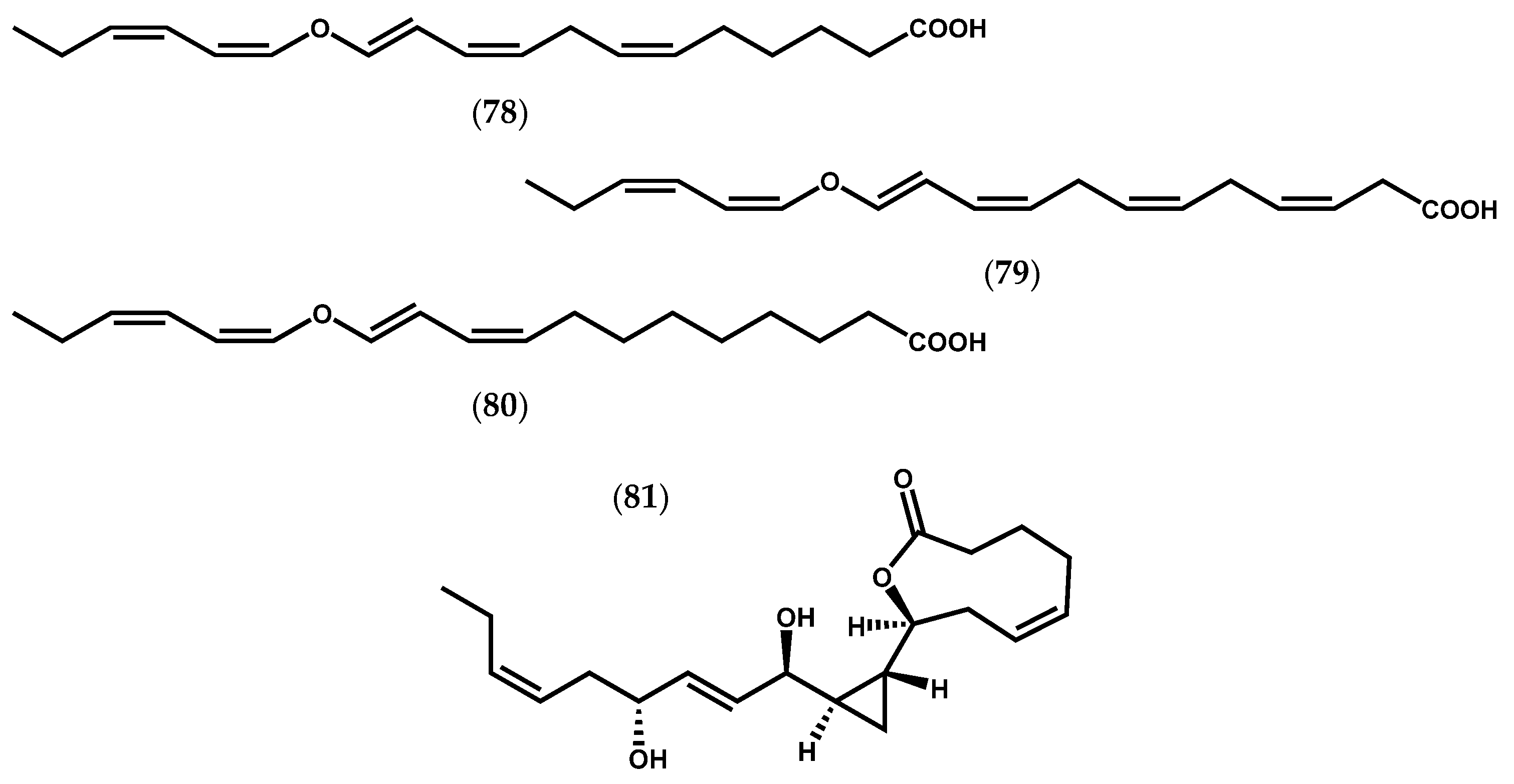
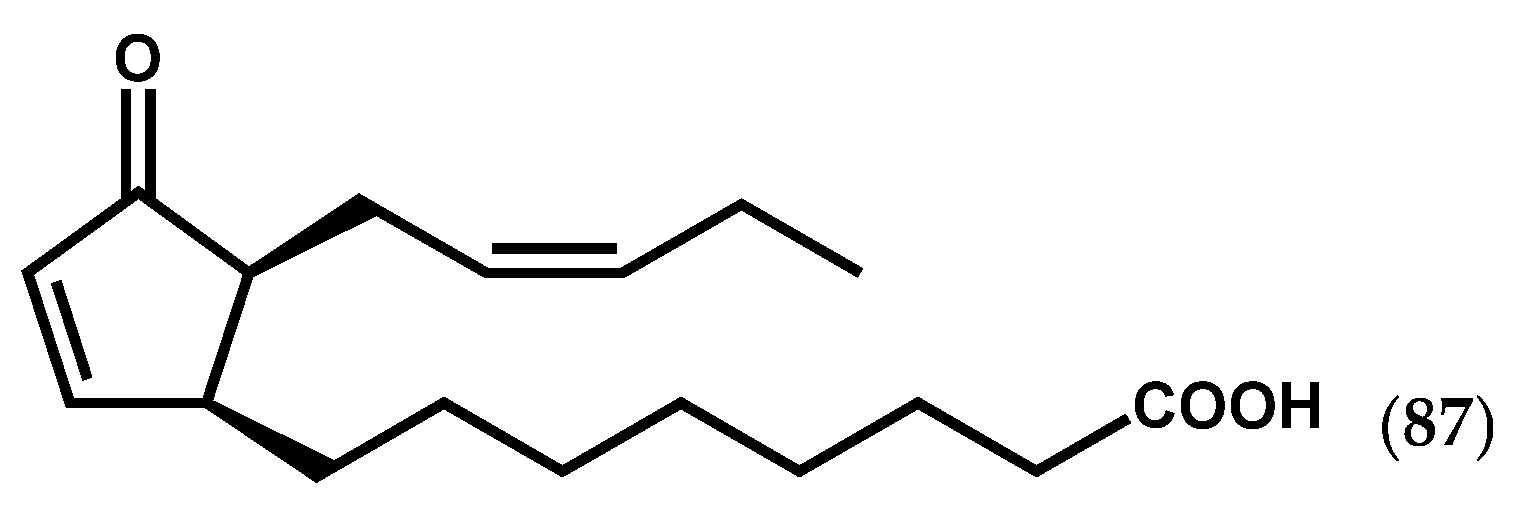
2.1.3. Chlorophyta
2.2. Nonenzymatically-Derived Algal Oxylipins: The Phytoprostanes
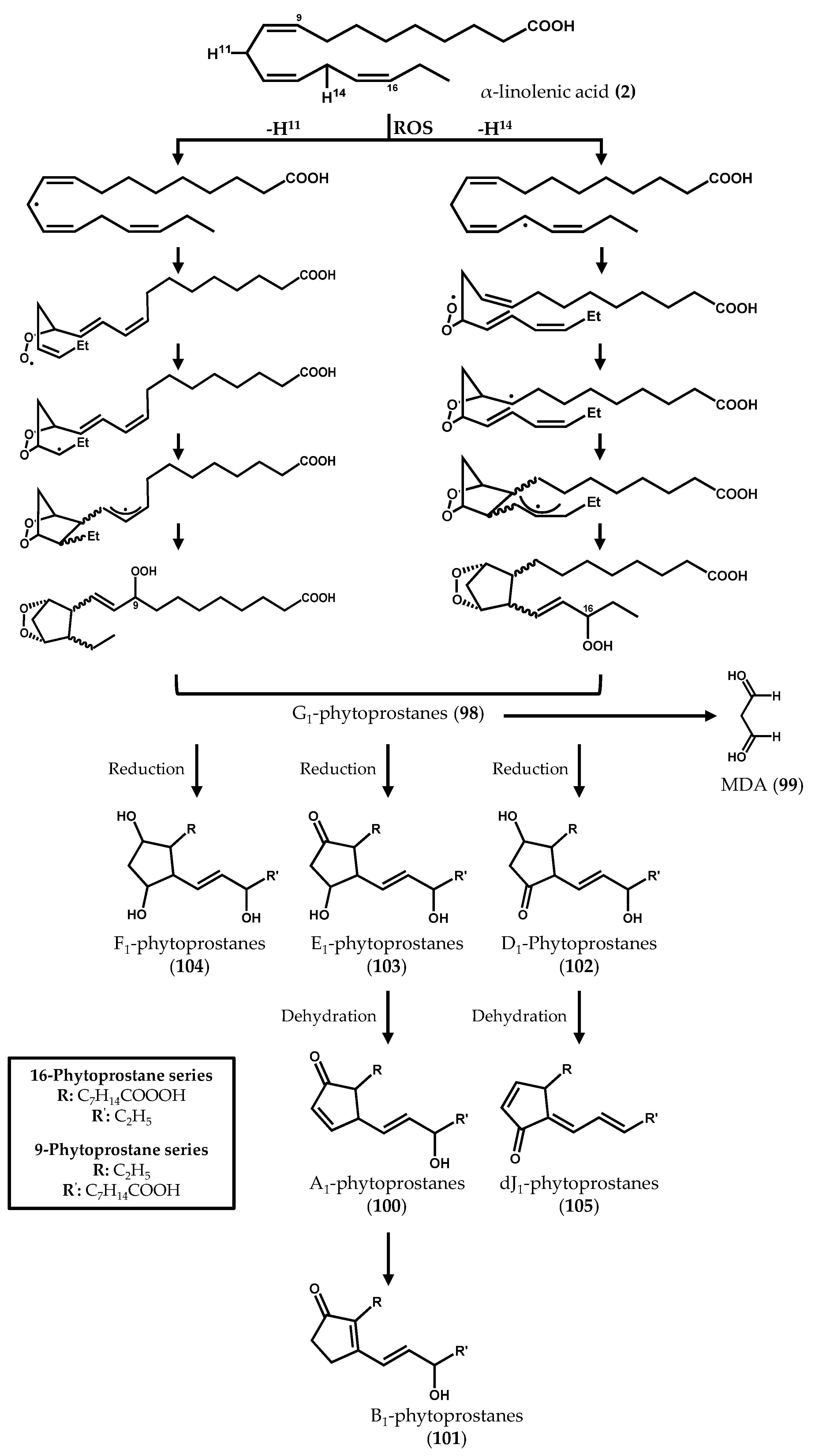
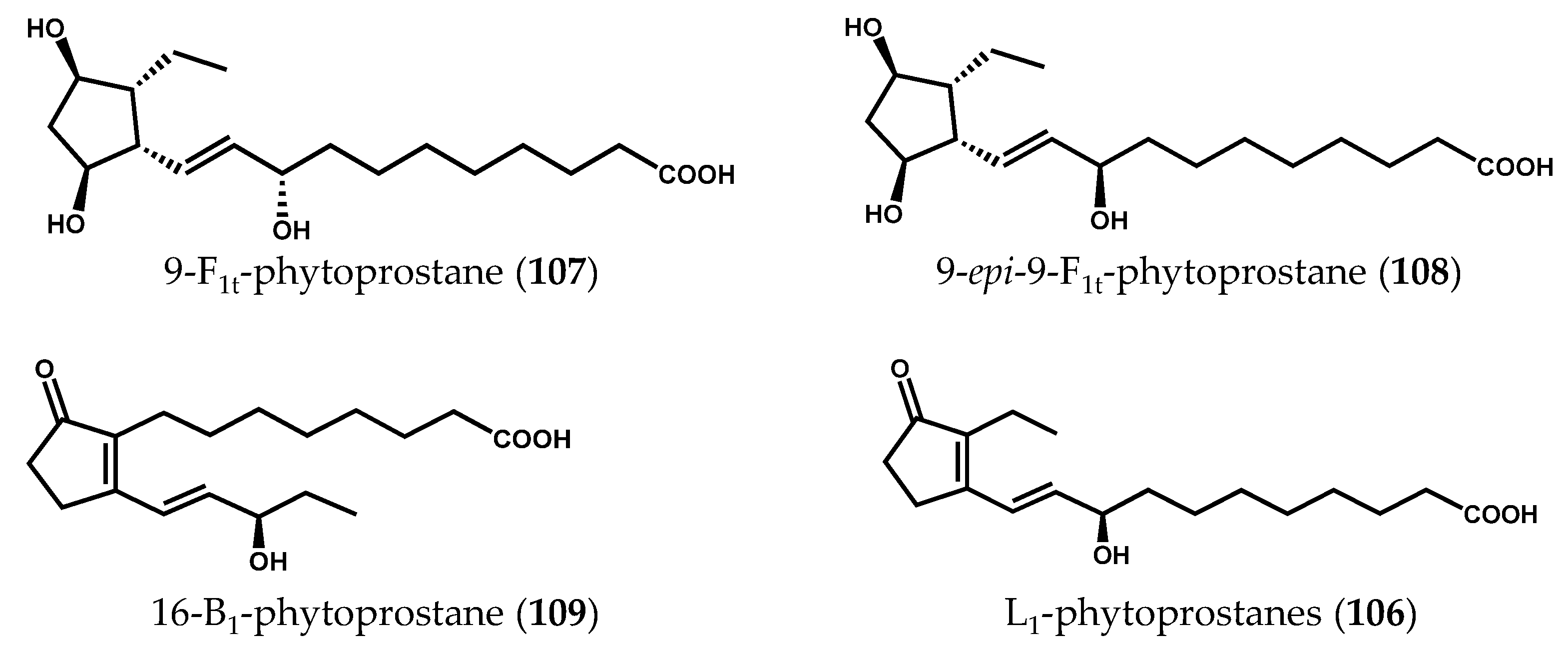
3. Conclusions
Acknowledgments
Conflicts of Interest
Abbreviations
| AOS/AOC | Allele oxide synthase/cyclase |
| COX | Cycloxygenase |
| DNMT | DNA methyl transferase |
| HDoHE | Hydroxydocosahexaenoic acid |
| HEK | Human embryonic kidney |
| HEPE | Hydroxyeicosapentaenoic acid |
| HETE | Hydroxyeicosatetraenoid acid |
| HODE | Hydroxyoctadecadienoic acid |
| HODTA | Hydroxyoctadecatetraenoic acid |
| HOTrE | Hydroxyoctadecatrienoic acid |
| HpETE | Hydroperoxyeicosatetraenoic acid |
| HPL | Hydroperoxide lyases |
| HpODE | Hydroperoxyoctadecadienoic acid |
| HpOTrE | Hydroperoxyoctadecatrienoic acid |
| IL | Interleukin |
| IMTA | Integrated multitrophic aquaculture |
| LOX | Lipoxygenases |
| MAPK | Mitogen-activated protein kinase |
| MDA: | Malondialdehyde |
| NF-κB | Nuclear factor-κB |
| NO | Nitric oxide |
| PDA | Phytodienoic acid |
| PGHS | Prostaglandin endoperoxide H synthase |
| PKC | Protein kinase C |
| PLA2 | Phospholipase A2 |
| PPAR | Peroxisome proliferator-activated receptor |
| PUA | Polyunsaturated aldehydes |
| PUFA | Polyunsaturated fatty acids |
| RBL | Rat basophil leukemia |
| RES | Reactive electrophile species |
| ROS | Reactive oxygen species |
| Th | T helper |
| TNF | Tumor necrosis factor |
| UHPLC-QqQ-MS/MS | Ultrahigh-performance liquid chromatography coupled to triple-quadrupole mass spectrometry |
References
- Mosblech, A.; Feussner, I.; Heilmann, I. Oxylipins: Structurally diverse metabolites from fatty acid oxidation. Plant Physiol. Biochem. 2009, 47, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Andreou, A.; Brodhun, F.; Feussner, I. Biosynthesis of oxylipins in non-mammals. Prog. Lipid Res. 2009, 48, 148–170. [Google Scholar] [CrossRef] [PubMed]
- Gerwick, W.H.; Singh, I.P. Structural diversity of marine oxylipins. In Lipid Biotechnology; Gardner, H.W., Kuo, T.M., Eds.; Marcel and Dekker: New York, NY, USA, 2002; pp. 249–275. [Google Scholar]
- Bleé, E. Impact of phyto-oxylipins in plant defense. Trends Plant Sci. 2002, 7, 315–322. [Google Scholar] [CrossRef]
- Kuhn, H.; Walther, M.; Kuban, R.J. Mammalian arachidonate 15-lipoxygenases structure, function, and biological implications. Prostaglandins Other Lipid Mediat. 2002, 68–69, 263–290. [Google Scholar] [CrossRef]
- Pohnert, G.; Boland, W. The oxylipin chemistry of attraction and defense in brown algae and diatoms. Nat. Prod. Rep. 2002, 19, 108–122. [Google Scholar] [PubMed]
- Prost, I.; Dhondt, S.; Rothe, G.; Vicente, J.; Rodriguez, M.J.; Kift, N.; Carbonne, F.; Griffiths, G.; Esquerré-Tugayé, M.T.; Rosahl, S.; et al. Evaluation of the antimicrobial activities of plant oxylipins supports their involvement in defense against pathogens. Plant Physiol. 2005, 139, 1902–1913. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, F. Pathogen-induced defense and innate immunity in macroalgae. Biol. Bull. 2007, 213, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Tsitsigiannis, D.I.; Keller, N.P. Oxylipins as developmental and host-fungal communication signals. Trends Microbiol. 2007, 15, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Kolomiets, M.V. Host-derived lipids and oxylipins are crucial signals in modulating mycotoxin production by fungi. Toxin Rev. 2009, 28, 79–88. [Google Scholar] [CrossRef]
- Cutignano, A.; Lamari, N.; d’ippolito, G.; Manzo, E.; Cimino, G.; Fontana, A. Lipoxygenase products in marine diatoms: A concise analytical method to explore the functional potential of oxylipins. J. Phycol. 2011, 47, 233–243. [Google Scholar] [CrossRef]
- Ibañez, E.; Herrero, M.; Mendiola, J.; Castro-Puyana, M. Extraction and characterization of bioactive compounds with health benefits from marine resources: Macro and micro algae, cyanobacteria, and invertebrates. In Marine Bioactive Compounds: Sources, Characterization and Applications; Hayes, M., Ed.; Springer US: New York, NY, USA, 2012; pp. 55–98. [Google Scholar]
- Andrade, P.B.; Barbosa, M.; Matos, R.P.; Lopes, G.; Vinholes, J.; Mouga, T.; Valentão, P. Valuable compounds in macroalgae extracts. Food Chem. 2013, 138, 1819–1828. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Kumar, M.; Reddy, C.R.K.; Jha, B. Algal lipids, fatty acids and sterols. In Functional Ingredients from Algae for Foods and Nutraceuticals; Domínguez, H., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2013; pp. 87–134. [Google Scholar]
- Gerwick, W.H.; Bernart, M.W.; Moghaddam, M.F.; Jiang, Z.D.; Solem, M.L.; Nagle, D.G. Eicosanoids from the Rhodophyta: New metabolism in the algae. Hydrobiologia 1990, 204, 621–628. [Google Scholar] [CrossRef]
- Gerwick, W.H.; Moghaddam, M.; Hamberg, M. Oxylipin metabolism in the red alga Gracilariopsis lemaneiformis: Mechanism of formation of vicinal dihydroxy fatty acids. Arch. Biochem. Biophys. 1991, 290, 436–444. [Google Scholar] [CrossRef]
- Gerwick, W.H.; Proteau, P.J.; Nagle, D.G.; Wise, M.L.; Jiang, Z.D.; Bernart, M.W.; Hamberg, M. Biologically active oxylipins from seaweeds. Hydrobiologia 1993, 260, 653–665. [Google Scholar] [CrossRef]
- Proteau, P.J.; Gerwick, W.H. Divinyl ethers and hydroxy fatty acids from three species of Laminaria (brown algae). Lipids 1993, 28, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Todd, J.S.; Proteau, P.J.; Gerwick, W.H. The absolute configuration of ecklonialactones A, B, and E, novel oxylipins from brown algae of the genera Ecklonia and Egregia. J. Nat. Prod. 1994, 57, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Gerwick, W.H. Epoxy allylic carbocations as conceptual intermediates in the biogenesis of diverse marine oxylipins. Lipids 1996, 31, 1215–1231. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.D.; Gerwick, W.H. Novel oxylipins from the temperate red alga Polyneura latissima: Evidence for an arachidonate 9(S)-lipoxygenase. Lipids 1997, 32, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Kousaka, K.; Ogi, N.; Akazawa, Y.; Fujieda, M.; Yamamoto, Y.; Takada, Y.; Kimura, J. Novel oxylipin metabolites from the brown alga Eisenia bicyclis. J. Nat. Prod. 2003, 66, 1318–1323. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Proteau, P.J.; Byrum, T.; Gerwick, W.H. Cymatherelactone and cymatherols A–C, polycyclic oxylipins from the marine brown alga Cymathere triplicata. Phytochemistry 2012, 73, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Reddy, R.; Jha, B. Quantification of selected endogenous hydroxy-oxylipins from tropical marine macroalgae. Mar. Biotechnol. 2014, 16, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Ponce de León, I.; Hamberg, M.; Castresana, C. Oxylipins in moss development and defense. Front. Plant Sci. 2015, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bouarab, K.; Adas, F.; Gaquerel, E.; Kloareg, B.; Salaün, J.; Potin, P. The innate immunity of a marine red alga involves oxylipins from both the eicosanoid and octadecanoid pathways. Plant Physiol. 2004, 135, 1838–1848. [Google Scholar] [CrossRef] [PubMed]
- Lion, U.; Wiesemeier, T.; Weinberger, F.; Beltran, J.; Flores, V.; Faugeron, S.; Correa, J.; Pohnert, G. Phospholipases and galactolipases trigger oxylipin-mediated wound activated defence in the red alga Gracilaria chilensis against epiphytes. ChemBioChem 2006, 7, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Küpper, F.C.; Gaquerel, E.; Boneberg, E.; Morath, S.; Salaün, J.; Potin, P. Early events in the perception of lipopolysaccharides in the brown alga Laminaria digitata include an oxidative burst and activation of fatty acid oxidation cascades. J. Exp. Bot. 2006, 57, 1991–1999. [Google Scholar] [CrossRef] [PubMed]
- Gaquerel, E.; Hervé, C.; Labrière, C.; Boyen, C.; Potin, P.; Salaün, J. Evidence for oxylipin synthesis and induction of a new polyunsaturated fatty acid hydroxylase activity in Chondrus crispus in response to methyl jasmonates. Biochim. Biophys. Acta 2007, 1771, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Küpper, F.C.; Gaquerel, E.; Cosse, A.; Adas, F.; Peters, A.F.; Müller, D.G.; Kloareg, B.; Salaün, J.; Potin, P. Free fatty acids and methyl jasmonate trigger defense reactions in Laminaria digitata. Plant. Cell Physiol. 2009, 50, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Nylund, G.M.; Weinberger, F.; Rempt, M.; Pohnert, G. Metabolomic assessment of induced and activated chemical defence in the invasive red alga Gracilaria vermiculophylla. PLoS ONE 2011, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, F.; Lion, U.; Delage, L.; Kloareg, B.; Potin, P.; Beltrán, J.; Flores, V.; Faugeron, S.; Correa, J.; Pohnert, G. Up-regulation of lipoxygenase, phospholipase, and oxylipin-production in the induced chemical defense of the red alga Gracilaria chilensis against epiphytes. J. Chem. Ecol. 2011, 37, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Rempt, M.; Weinberger, F.; Grosser, K.; Pohnert, G. Conserved and species-specific oxylipin pathways in the wound-activated chemical defense of the noninvasive red alga Gracilaria chilensis and the invasive Gracilaria vermiculophylla. Beilstein J. Org. Chem. 2012, 8, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Zambounis, A.; Gaquerel, E.; Strittmatter, M.; Salaün, J.; Potin, P.; Küpper, F.C. Prostaglandin A2 triggers a strong oxidative burst in Laminaria: A novel defense inducer in brown algae. Algae 2012, 27, 21–32. [Google Scholar] [CrossRef]
- Potin, P. Oxidative burst and related responses in biotic interactions of algae. In Algal Chemical Ecology; Amsler, C.D., Ed.; Springer: Berlin, Germany, 2008; pp. 245–271. [Google Scholar]
- Wang, X.; Chen, H.; Chen, J.; Luo, Q.; Xu, J.; Yan, X. Response of Pyropia haitanensis to agaro-oligosaccharides evidenced mainly by the activation of the eicosanoid pathway. J. Appl. Phycol. 2013, 25, 1895–1902. [Google Scholar] [CrossRef]
- Howe, G.A.; Schilmiller, A.L. Oxylipin metabolism in response to stress. Curr. Opin. Plant Biol. 2002, 5, 230–236. [Google Scholar] [CrossRef]
- Funk, C.D. Prostaglandins and leukotrienes: Advances in eicosanoid biology. Science 2001, 294, 1871–1875. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Kumari, P.; gupta, V.; Anisha, P.A.; Reddy, C.R.K.; Jha, B. Differential responses to cadmium induced oxidative stress in marine macroalga Ulva lactuca (Ulvales, Chlorophyta). Biometals 2010, 23, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Ritter, A.; Goulitquer, S.; Salaün, J.; Tonon, T.; Correa, J.A.; Potin, P. Copper stress induces biosynthesis of octadecanoid and eicosanoid oxygenated derivatives in the brown algal kelp Laminaria digitata. New Phytol. 2008, 180, 809–821. [Google Scholar] [CrossRef] [PubMed]
- Contreras, L.; Mella, D.; Moenne, A.; Correa, J.A. Differential responses to copper-induced oxidative stress in the marine macroalgae Lessonia nigrescens and Scytosiphon lomentaria (Phaeophyceae). Aquat. Toxicol. 2009, 94, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Ritter, A.; Dittami, S.M.; Goulitquer, S.; Correa, J.A.; Boyen, C.; Potin, P.; Tonon, T. Transcriptomic and metabolomic analysis of copper stress acclimation in Ectocarpus siliculosus highlights signaling and tolerance mechanisms in brown algae. BMC Plant Biol. 2014, 14, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Gupta, V.; Trivedi, N.; Kumari, P.; Bijo, A.J.; Reddy, C.R.K.; Jha, B. Desiccation induced oxidative stress and its biochemical responses in intertidal red alga Gracilaria corticata (Gracilariales, Rhodophyta). Environ. Exp. Bot. 2011, 72, 194–201. [Google Scholar] [CrossRef]
- Contreras-Porcia, L.; Thomas, D.; Flores, V.; Correa, J.A. Tolerance to oxidative stress induced by desiccation in Porphyra columbina (Bangiales, Rhodophyta). J. Exp. Bot. 2011, 62, 1815–1829. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Kumari, P.; Gupta, V.; Reddy, C.R.K.; Jha, B. Biochemical responses of red algae Gracilaria corticata (Gracilariales, Rhodophyta) to salinity induced oxidative stress. J. Exp. Mar. Biol. Ecol. 2010, 391, 27–34. [Google Scholar] [CrossRef]
- Kumar, M.; Trivedi, N.; Reddy, C.R.K.; Jha, B. Toxic effects of imidazolium ionic liquids on the green seaweed Ulva lactuca: Oxidative stress and DNA damage. Chem. Res. Toxicol. 2011, 24, 1882–1890. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Kumar, M.; Reddy, C.R.K.; Jha, B. Nitrate and phosphate regimes induced lipidomic and biochemical changes in the intertidal macroalga Ulva lactuca (Ulvophyceae, Chlorophyta). Pant Cell Physiol. 2014, 55, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Feussner, I.; Wasternack, C. The lipoxygenase pathway. Annu. Rev. Plant Biol. 2002, 53, 275–97. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Qian, F.; Yang, R.; Chen, J.; Luo, Q.; Chen, H.; Yan, X. A lipoxygenase from red alga Pyropia haitanensis, a unique enzyme catalyzing the free radical reactions of polyunsaturated fatty acids with triple ethylenic bonds. PLoS ONE 2015, 10, e0117351. [Google Scholar] [CrossRef] [PubMed]
- Kuo, J.-M.; Hwang, A.; Yeh, D. Purification, substrate specificity, and products of a Ca2+-stimulating lipoxygenase from sea algae (Ulva lactuca). J. Agric. Food Chem. 1997, 45, 2055–2060. [Google Scholar] [CrossRef]
- Guschina, I.A.; Harwood, J.L. Lipids and lipid metabolism in eukaryotic algae. Prog. Lipid Res. 2006, 45, 160–186. [Google Scholar] [CrossRef] [PubMed]
- Nagle, D.G.; Gerwick, W.H. Isolation and structure of constanolactones A and B, new cyclopropyl hydroxy-eicosanoids from the temperate red alga Constantinea simplex. Tetrahedron Lett. 1990, 31, 2995–2998. [Google Scholar] [CrossRef]
- Stonik, V.; Stonick, I. Low-molecular-weight metabolites from diatoms: Structures, biological roles and biosynthesis. Mar. Drugs 2015, 13, 3672–3709. [Google Scholar] [CrossRef] [PubMed]
- Cardellina, J.H.; Moore, R.E. Malyngic acid, a new fatty acid from Lyngbya majuscula. Tetrahedron 1980, 36, 993–996. [Google Scholar] [CrossRef]
- Pollio, A.; Della Greca, M.; Monaco, P.; Pinto, G.; Previtera, L. Lipid composition of the acidophilic alga Dunaliella. acidophila (Volvocales, Chlorophyta) I. Nonpolar lipids. Biochim. Biophys. Acta 1988, 963, 53–60. [Google Scholar] [CrossRef]
- Murakami, N.; Shirahashi, H.; Nagatsu, A.; Sakakibara, J. Two unsaturated 9R-hydroxy fatty acids from the cyanobacterium Anabaena flos-aquae f. flos-aquae. Lipids 1992, 27, 776–778. [Google Scholar] [CrossRef]
- Mundt, S.; Kreitlow, S.; Jansen, R. Fatty acids with antibacterial activity from the cyanobacterium Oscillatoria redekei HUB 051. J. Appl. Phycol. 2003, 15, 263–267. [Google Scholar] [CrossRef]
- Lang, I.; Feussner, I. Oxylipin formation in Nostoc punctiforme (PCC73102). Phytochemistry 2007, 68, 1120–1127. [Google Scholar] [CrossRef] [PubMed]
- Lang, I.; Göbel, C.; Porzel, A.; Heilmann, I.; Feussner, I. A lipoxygenase with linoleate diol synthase activity from Nostoc sp. PCC 7120. Biochem. J. 2008, 410, 347–357. [Google Scholar] [CrossRef] [PubMed]
- De Los Reyes, C.; Ávila-Román, J.; Ortega, M.J.; de la Jara, A.; García-Mauriño, S.; Motilva, V.; Zubía, E. Oxylipins from the microalgae Chlamydomonas debaryana and Nannochloropsis gaditana and their activity as TNF-α inhibitors. Phytochemistry 2014, 102, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.Y.; Reith, M.E. Isolation of a gametophyte-specific cDNA encoding a lipoxygenase from the red alga Porphyra purpurea. Mol. Mar. Biol. Biotechnol. 1994, 3, 206–209. [Google Scholar] [PubMed]
- Chen, H.; Zhu, Z.; Chen, J.; Yang, R.; Luo, Q.; Xu, J.; Shan, H.; Yan, X. A multifunctional lipoxygenase from Pyropia. haitanensis—The cloned and functioned complex eukaryotic algae oxylipin pathway enzyme. Algal Res. 2015, 12, 316–327. [Google Scholar] [CrossRef]
- Potin, P.; Bouarab, K.; Salaün, J.; Pohnert, G.; Kloareg, B. Biotic interactions of marine algae. Curr. Opin. Plant Biol. 2002, 5, 308–317. [Google Scholar]
- Higgs, M.D.; Mulheirn, L.J. Hybridalactone, an unusual fatty acid metabolite from the red alga Laurencia hybrida (Rhodophyta, Rhodomelaceae). Tetrahedron 1981, 37, 4259–4262. [Google Scholar] [CrossRef]
- Corey, E.J.; De, B.; Ponder, J.W.; Berg, J.M. The stereochemistry and biosynthesis of hybridalactone, an eicosanoid from Laurencia hybrida. Tetrahedron Lett. 1984, 25, 1015–1018. [Google Scholar] [CrossRef]
- Hickmann, V.; Kondoh, A.; Gabor, B.; Alcarazo, M.; Fürstner, A. Catalysis-based and protecting-group-free total syntheses of the marine oxylipins hybridalactone and the ecklonialactones A, B, and C. J. Am. Chem. Soc. 2011, 133, 13471–13480. [Google Scholar] [CrossRef] [PubMed]
- Corey, E.J.; De, B. Total synthesis and stereochemistry of hybridalactone. J. Am. Chem. Soc. 1984, 106, 2735–2736. [Google Scholar] [CrossRef]
- Ota, K.; Sugata, N.; Ohshiro, Y.; Kawashima, E.; Miyaoka, H. Total synthesis of marine eicosanoid (−)-hybridalactone. Chemistry 2012, 18, 13531–13537. [Google Scholar] [CrossRef] [PubMed]
- Bernart, M.; Gerwick, W.H. Isolation of 12-(S)-HEPE from the red marine alga Murrayella periclados and revision of structure of an acyclic icosanoid from Laurencia hybrida. Implications to the biosynthesis of the marine prostanoid hybridalactone. Tetrahedron Lett. 1988, 29, 2015–2018. [Google Scholar] [CrossRef]
- Moghaddam, M.F.; Gerwick, W.H.; Ballantine, D.L. Discovery of 12-(S)-hydroxy-5,8,10,14-icosatetraenoic acid [12-(S)-HETE] in the tropical red alga Platysiphonia miniata. Prostaglandins 1989, 37, 303–308. [Google Scholar] [CrossRef]
- Jiang, Z.D.; Gerwick, W.H. Eicosanoids and other hydroxylated fatty acids from the marine alga Gracilariopsis lemaneiformis. Phytochemistry 1991, 30, 1187–1190. [Google Scholar] [CrossRef]
- Bernart, M.W.; Gerwick, W.H. Eicosanoids from the tropical red alga Murrayella periclados. Phytochemistry 1994, 36, 1233–1240. [Google Scholar] [CrossRef]
- Moghaddam, M.F.; Gerwick, W.H.; Ballantine, D.L. Discovery of the mammalian insulin release modulator, hepoxilin B3, from the tropical red algae Platysiphonia miniata and Cottoniella filamentosa. J. Biol. Chem. 1990, 265, 6126–6130. [Google Scholar] [PubMed]
- Antón, R.; Camacho, M.; Puig, L.; Vila, L. Hepoxilin B3 and its enzymatically formed derivative trioxilin B3 are incorporated into phospholipids in psoriatic lesions. J. Investig. Dermatol. 2002, 118, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Pace-Asciak, C.R. Arachidonic acid epoxides. Demonstration through [18O] oxygen studies of an intramolecular transfer of the terminal hydroxyl group of (12S)-hydroperoxyeicosa-5,8,10,14-tetraenoic acid to form hydroxyepoxides. J. Biol. Chem. 1984, 259, 8332–8337. [Google Scholar] [PubMed]
- Muñoz-Garcia, A.; Thomas, C.P.; Keeney, D.S.; Zheng, Y.; Brash, A.R. The importance of the lipoxygenase-hepoxilin pathway in the mammalian epidermal barrier. Biochim. Biophys. Acta 2014, 1841, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.; Gerwick, W.H. Two new icosapentaenoic acids from the temperate red seaweed Ptilota filicina J. Agardh. Lipids 1987, 22, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.; Gerwick, W.H. Ptilodene, a novel icosanoid inhibitor of 5-lipoxygenase and Na+/K+ ATPase from the red marine alga Ptilota filicina J. Agardh. Tetrahedron Lett. 1988, 29, 1505–1506. [Google Scholar] [CrossRef]
- Solem, M.L.; Jiang, Z.D.; Gerwick, W.H. Three new and bioactive icosanoids from the temperate red marine alga Farlowia mollis. Lipids 1989, 24, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Hamberg, M.; Gerwick, W.H. Biosynthesis of vicinal dihydroxy fatty acids in the red alga Gracilariopsis lemaneiformis: Identification of a sodium-dependent 12-lipoxygenase and a hydroperoxide isomerase. Arch. Biochem. Biophys. 1993, 305, 115–122. [Google Scholar] [CrossRef] [PubMed]
- McPhail, K.L.; France, D.; Cornell-Kennon, S.; Gerwick, W.H. Peyssonenynes A and B, novel enediyne oxylipins with DNA methyl transferase inhibitory activity from the red marine alga Peyssonnelia caulifera. J. Nat. Prod. 2004, 67, 1010–1013. [Google Scholar] [CrossRef] [PubMed]
- García-Domínguez, P.; Lepore, I.; Erb, C.; Gronemeyer, H.; Altucci, L.; Álvarez, R.; de Lera, A.R. Total synthesis of the proposed structures of the DNA methyl transferase inhibitors peyssonenynes, and structural revision of peyssonenyne B. Org. Biomol. Chem. 2011, 9, 6979–6987. [Google Scholar] [CrossRef] [PubMed]
- García-Domínguez, P.; Álvarez, R.; de Lera, A.R. Survey of synthetic approaches to natural (peyssonenynes) and unnatural acetoxyenediynes. Eur. J. Org. Chem. 2012, 2012, 4762–4782. [Google Scholar] [CrossRef]
- Nagle, D.G.; Gerwick, W.H. Structure and stereochemistry of constanolactones A–G, lactonized cyclopropyl oxylipins from the red marine alga Constantinea simplex. J. Org. Chem. 1994, 59, 7227–7237. [Google Scholar] [CrossRef]
- Ziboh, V.A.; Miller, C.C.; Cho, Y. Metabolism of polyunsaturated fatty acids by skin epidermal enzymes: Generation of anti-inflammatory and antiproliferative metabolites. Am. J. Clin. Nutr. 2000, 71, 361S–366S. [Google Scholar] [PubMed]
- Hamberg, M.; Gerwick, W.H.; Åsen, P.A. Linoleic acid metabolism in the red alga Lithothamnion corallioides: Biosynthesis of 11(R)-hydroxy-9(Z),12(Z)-octadecadienoic acid. Lipids 1992, 27, 487–493. [Google Scholar] [CrossRef]
- Gerwick, W.H.; Åsen, P.; Hamberg, M. Biosynthesis of 13R-hydroxyarachidonic acid, an unusual oxylipin from the red alga Lithothamnion corallioides. Phytochemistry 1993, 34, 1029–1033. [Google Scholar] [CrossRef]
- Kumari, P.; Reddy, C.R.; Jha, B. Methyl jasmonate-induced lipidomic and biochemical alterations in the intertidal macroalga Gracilaria dura (Gracilariaceae, Rhodophyta). Plant Cell Physiol. 2015, 56, 1877–1889. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.D.; Ketchum, S.O.; Gerwick, W.H. 5-Lipoxygenase-derived oxylipins from the red alga Rhodymenia pertusa. Phytochemistry 2000, 53, 129–133. [Google Scholar] [CrossRef]
- Proteau, P.J. Oxylipins from Temperate Marine Algae and a Photoprotective Sheath Pigment from Blue-Green Algae. Ph.D. Thesis, Oregon State University, Corvallis, OR, USA, 13 August 1993. [Google Scholar]
- Graber, M.A.; Gerwick, W.H.; Cheney, D.P. The isolation and characterization of agardhilactone, a novel oxylipin from the marine red alga Agardhiella subulata. Tetrahedron Lett. 1996, 37, 4635–4638. [Google Scholar] [CrossRef]
- Gregson, R.P.; Marwood, J.F.; Quinn, R.J. The occurrence of prostaglandins PGE2 and PGF2α in a plant—The red alga Gracilaria Lichenoides. Tetrahedron Lett. 1979, 20, 4505–4506. [Google Scholar] [CrossRef]
- Fusetani, N.; Hashimoto, K. Prostaglandin E2—A candidate for causative agent of ogonori poisoning. Bull. Jpn. Soc. Sci. 1984, 50, 465–469. [Google Scholar] [CrossRef]
- Imbs, A.B.; Vologodskaya, A.V.; Nevshupova, N.V.; Khotimchenko, S.V.; Titlyanov, E.A. Response of prostaglandin content in the red alga Gracilaria verrucosa to season and solar irradiance. Phytochemistry 2001, 58, 1067–1072. [Google Scholar] [CrossRef]
- Dang, T.H.; Lee, H.J.; Yoo, E.S.; Hong, J.; Choi, J.S.; Jung, J.H. The occurrence of 15-keto-prostaglandins in the red alga Gracilaria verrucosa. Arch. Pharm. Res. 2010, 33, 1325–1329. [Google Scholar] [CrossRef] [PubMed]
- Varvas, K.; Koljak, R.; Järving, I.; Pehka, T.; Samel, N. Endoperoxide pathway in prostaglandin biosynthesis in the soft coral Gersemia fruticosa. Tetrahedron Lett. 1994, 35, 8267–8270. [Google Scholar] [CrossRef]
- Valmsen, K.; Boeglin, W.E.; Jarving, I.; Schneider, C.; Varvas, K.; Brash, A.R.; Samel, N. Structural and functional comparison of 15S- and 15R-specific cyclooxygenases from the coral Plexaura homomalla. Eur. J. Biochem. 2004, 271, 3533–3538. [Google Scholar] [CrossRef] [PubMed]
- Järving, R.; Järving, I.; Kurg, R.; Brash, A.R.; Samel, N. On the evolutionary origin of cyclooxygenase (COX) isozymes: Characterization of marine invertebrate COX genes points to independent duplication events in vertebrate and invertebrate lineages. J. Biol. Chem. 2004, 279, 13624–13633. [Google Scholar] [CrossRef] [PubMed]
- Varvas, K.; Kurg, R.; Hansen, K.; Järving, R.; Järving, I.; Valmsen, K.; Lõhelaid, H.; Samel, N. Direct evidence of the cyclooxygenase pathway of prostaglandin synthesis in arthropods: Genetic and biochemical characterization of two crustacean cyclooxygenases. Insect Biochem. Mol. Biol. 2009, 39, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Kanamoto, H.; Takemura, M.; Ohyama, K. Identification of a cyclooxygenases gene from the red alga Gracilaria vermiculophylla and bioconversion of arachidonic acid to PGF2α in engineered Escherichia coli. Appl. Microbiol. Biotechnol. 2011, 91, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Varvas, K.; Kasvandik, S.; Hansen, K.; Järving, I.; Morell, I.; Samel, N. Structural and catalytic insights into the algal prostaglandin H synthase reveal atypical features of the first non-animal cyclooxygenase. Biochim. Biophys. Acta. 2013, 1831, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Kurata, K.; Taniguchi, K.; Shiraishi, K.; Hayama, N.; Tanaka, I.; Suzuki, M. Ecklonialactone-A and B, two unusual metabolites from the brown alga Ecklonia stolonifera Okamura. Chem. Lett. 1989, 267–270. [Google Scholar] [CrossRef]
- Kurata, K.; Taniguchi, K.; Shiraishi, K.; Suzuki, M. Ecklonialactones-C–F from the brown alga Ecklonia stolonifera. Phytochemistry 1993, 33, 155–159. [Google Scholar] [CrossRef]
- Proteau, P.J.; Gerwick, W.H. Cymathere ethers A and B: Bicyclic oxylipins from the marine brown alga Cymathere triplicata. Tetrahedron Lett. 1992, 33, 4393–4396. [Google Scholar] [CrossRef]
- Proteau, P.J.; Rossi, J.V.; Gerwick, W.H. Absolute stereochemistry of neohalicholactone from the brown alga Laminaria sinclairii. J. Nat. Prod. 1994, 57, 1717–1719. [Google Scholar] [CrossRef] [PubMed]
- Todd, J.S.; Proteau, P.J.; Gerwick, W.H. Egregiachlorides A–C: New chlorinated oxylipins from the marine brown alga Egregia menziesii. Tetrahedron Lett. 1993, 34, 7689–7692. [Google Scholar] [CrossRef]
- Shureiqi, I.; Jiang, W.; Zuo, X.; Wu, Y.; Stimmel, J.B.; Leesnitzer, L.M.; Morris, J.S.; Fan, H.-Z.; Fischer, S.M.; Lippman, S.M. The 15-lipoxygenase-1 product 13-S-hydroxyoctadecadienoic acid down-regulates PPAR-δ to induce apoptosis in colorectal cancer cells. Proc. Natl. Acad. Sci. USA 2003, 100, 9968–9973. [Google Scholar] [CrossRef] [PubMed]
- Vang, K.; Ziboh, V.A. 15-Lipoxygenase metabolites of γ-linolenic acid/eicosapentaenoic acid suppress growth and arachidonic acid metabolism in human prostatic adenocarcinoma cells: Possible implications of dietary fatty acids. Prostaglandins Leukot. Essent. Fatty Acids 2005, 72, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Boonprab, K.; Matsui, K.; Akakabe, Y.; Yotsukura, N.; Kajiwara, T. Hydroperoxy-arachidonic acid mediated n-hexanal and (Z)-3- and (E)-2-nonenal formation in Laminaria angustata. Phytochemistry 2003, 63, 669–678. [Google Scholar] [CrossRef]
- Boonprab, K.; Matsui, K.; Akakabe, Y.; Yotsukura, N.; Kajiwara, T. Arachidonic acid conversion by lipoxygenase in the brown alga, Laminaria angustata. Kasetsart J. Nat. Sci. 2004, 38, 72–77. [Google Scholar]
- Cock, J.M.; Sterck, L.; Rouzé, P.; Scornet, D.; Allen, A.E.; Amoutzias, G.; Anthouard, V.; Artiguenave, F.; Aury, J.M.; Badger, J.H.; et al. The Ectocarpus genome and the independent evolution of multicellularity in brown algae. Nature 2010, 465, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Rorrer, G.L.; Yoo, H.D.; Huang, Y.M.; Hayden, C.; Gerwick, W.H. Production of hydroxy fatty acids by cell suspension cultures of the marine brown alga Laminaria saccharina. Phytochemistry 1997, 46, 871–877. [Google Scholar] [CrossRef]
- Arterburn, L.M.; Barclay, W.; Dangi, B.; Flatt, J.; Lee, J.; Vinjamoori, D.; Elswik, M.V. Oxylipins from Polyunsaturated Fatty Acids and Methods of Making and Using the Same. WO Patent 2008/103753 A2, 28 August 2008. [Google Scholar]
- Arterburn, L.M.; Barclay, W.; Dangi, B.; Flatt, J.; Lee, J.; Vinjamoori, D. Oxylipins from Stearidonic Acid and Gamma-Linolenic Acid and Methods of Making and Using Same. U.S. Patent 2011/0112191 A1, 12 May 2011. [Google Scholar]
- Dangi, B. Methods for Preparation of Oxylipins. U.S. Patent 2011/0027841 A1, 3 February 2011. [Google Scholar]
- Bernart, M.W.; Whatley, G.G.; Gerwick, W.H. Unprecedented oxylipins from the marine green alga Acrosiphonia coalita. J. Nat. Prod. 1993, 56, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Kuo, J.-M.; Hwang, A.; Hsu, H.H.; Pan, B.S. Preliminary identification of lipoxygenase in algae (Enteromorpha intestinalis) for aroma formation. J. Agric. Food Chem. 1996, 44, 2073–2077. [Google Scholar] [CrossRef]
- Akakabe, Y.; Matsui, K.; Kajiwara, T. Enantioselective formation of (R)-9-HPODE and (R)-9-HPOTrE in marine green alga Ulva conglobata. Bioorg. Med. Chem. 2002, 10, 3171–3173. [Google Scholar] [CrossRef]
- Akakabe, Y.; Matsui, K.; Kajiwara, T. 2,4-Decadienals are produced via (R)-11-HPITE from arachidonic acid in marine green alga Ulva conglobata. Bioorg. Med. Chem. 2003, 11, 3607–3609. [Google Scholar] [CrossRef]
- Tsai, C.J.; Li, W.F.; Pan, B.S. Characterization and immobilization of marine algal 11-lipoxygenase from Ulva fasciata. J. Am. Oil Chem. Soc. 2008, 85, 731–737. [Google Scholar] [CrossRef]
- Mueller, M.J. Archetype signals in plants: The phytoprostanes. Curr. Opin. Plant Biol. 2004, 7, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Farmer, E.E.; Mueller, M.J. ROS-mediated lipid peroxidation and RES-activated signaling. Annu. Rev. Plant Biol. 2013, 64, 429–450. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.; Collado-González, J.; Andrade, P.B.; Ferreres, F.; Valentão, P.; Galano, J.-M.; Durand, T.; Gil-Izquierdo, Á. Nonenzymatic α-linolenic acid derivatives from the sea: Macroalgae as novel sources of phytoprostanes. J. Agric. Food Chem. 2015, 63, 6466–6474. [Google Scholar] [CrossRef] [PubMed]
- Collado-González, J.; Durand, T.; Ferreres, F.; Medina, S.; Torrecillas, A.; Gil-Izquierdo, Á. Phytoprostanes. Lipid Technol. 2015, 27, 127–130. [Google Scholar] [CrossRef]
- Durand, T.; Bultel-Poncé, V.; Guy, A.; Berger, S.; Mueller, M.J.; Galano, J.-M. New bioactive oxylipins formed by non-enzymatic free radical-catalyzed pathways: The phytoprostanes. Lipids 2009, 44, 875–888. [Google Scholar] [CrossRef] [PubMed]
- Jahn, U.; Galano, J.-M.; Durand, T. A cautionary note on the correct structure assignment of phytoprostanes and the emergence of a new prostane ring system. Prostaglandins Leukot. Essent. Fatty Acids 2010, 82, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Thoma, I.; Loeffler, C.; Sinha, A.; Gupta, M.; Krischke, M.; Steffan, B.; Roitsch, T.; Mueller, M. Cyclopentenone isoprostanes induced by reactive oxygen species trigger defense gene activation and phytoalexin accumulation in plants. Plant. J. 2003, 34, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Loeffler, C.; Berger, S.; Guy, A.; Durand, T.; Bringmann, G.; Dreyer, M.; von Rad, U.; Durner, J.; Mueller, M. B1-phytoprostanes trigger plant defense and detoxification responses. Plant Physiol. 2005, 137, 328–340. [Google Scholar] [CrossRef] [PubMed]
- Savchenko, T.; Zastrijnaja, O.; Klimov, V. Oxylipins and plant abiotic stress resistance. Biochemistry (Moscow) 2014, 79, 362–375. [Google Scholar] [CrossRef] [PubMed]
- Thoma, I.; Krischke, M.; Loeffler, C.; Mueller, M. The isoprostanoid pathway in plants. Chem. Phys. Lipids 2004, 128, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Traidl-Hoffmann, C.; Mariani, V.; Hochrein, H.; Karg, K.; Wagner, H.; Ring, J.; Mueller, M.J.; Jakob, T.; Behrendt, H. Pollen associated phytoprostanes inhibit dendritic cell interleukin-l2 production and augment T helper type 2 cell polarization. J. Exp. Med. 2005, 201, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Gutermuth, J.; Bewersdorff, M.; Traidl-Hoffmann, C.; Ring, J.; Mueller, M.J.; Behrendt, H.; Jakob, T. Immunomodulatory effects of aqueous birch pollen extracts and phytoprostanes on primary immune responses in vivo. J. Allergy Clin. Immunol. 2007, 120, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Gilles, S.; Mariani, V.; Bryce, M.; Mueller, M.J.; Ring, J.; Jakob, T.; Pastore, S.; Behrendt, H.; Traidl-Hoffmann, C. Pollen-derived E1-phytoprostanes signal via PPAR-gamma and NF-kappaB-dependent mechanisms. J. Immunol. 2009, 182, 6653–6658. [Google Scholar] [CrossRef] [PubMed]
- Gilles, S.; Beck, I.; Lange, S.; Ring, J.; Behrendt, H.; Traidl-Hoffmann, C. Non-allergenic factors from pollen modulate T helper cell instructing notch ligands on dendritic cells. World Allergy Organ. J. 2015, 8. [Google Scholar] [CrossRef] [PubMed]
- Karg, K.; Dirsch, V.; Vollmar, A.M.; Cracowski, J.-L.; Laporte, F.; Mueller, M.J. Biologically active oxidized lipids (phytoprostanes) in the plant diet and parenteral lipid nutrition. Free Radic. Res. 2007, 41, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Minghetti, L.; Salvi, R.; Lavinia Salvatori, M.; Ajmone-Cat, M.A.; de Nuccio, C.; Visentin, S.; Bultel-Poncé, V.; Oger, C.; Guy, A.; Galano, J.-M.; et al. Nonenzymatic oxygenated metabolites of α-linolenic acid B1- and L1-phytoprostanes protect immature neurons from oxidant injury and promote differentiation of oligodendrocyte progenitors through PPAR-γ activation. Free Radic. Biol. Med. 2014, 73, 41–50. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC–BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbosa, M.; Valentão, P.; Andrade, P.B. Biologically Active Oxylipins from Enzymatic and Nonenzymatic Routes in Macroalgae. Mar. Drugs 2016, 14, 23. https://doi.org/10.3390/md14010023
Barbosa M, Valentão P, Andrade PB. Biologically Active Oxylipins from Enzymatic and Nonenzymatic Routes in Macroalgae. Marine Drugs. 2016; 14(1):23. https://doi.org/10.3390/md14010023
Chicago/Turabian StyleBarbosa, Mariana, Patrícia Valentão, and Paula B. Andrade. 2016. "Biologically Active Oxylipins from Enzymatic and Nonenzymatic Routes in Macroalgae" Marine Drugs 14, no. 1: 23. https://doi.org/10.3390/md14010023
APA StyleBarbosa, M., Valentão, P., & Andrade, P. B. (2016). Biologically Active Oxylipins from Enzymatic and Nonenzymatic Routes in Macroalgae. Marine Drugs, 14(1), 23. https://doi.org/10.3390/md14010023