Myocardical Infarction in Young Adults: Revisiting Risk Factors and Atherothrombotic Pathways
Abstract
1. Introduction
2. Epidemiology of Premature Myocardic Infarctions
3. Global Incidence and Prevalence
4. Traditional Risk Factors
4.1. Dyslipidemia
4.2. Hypertension
4.3. Smoking
4.4. Obesity
4.5. Diabetes Mellitus
5. Emerging Risk Factors
5.1. Recreational Drug Use
5.2. Systemic Inflammation and Autoimmune Disease
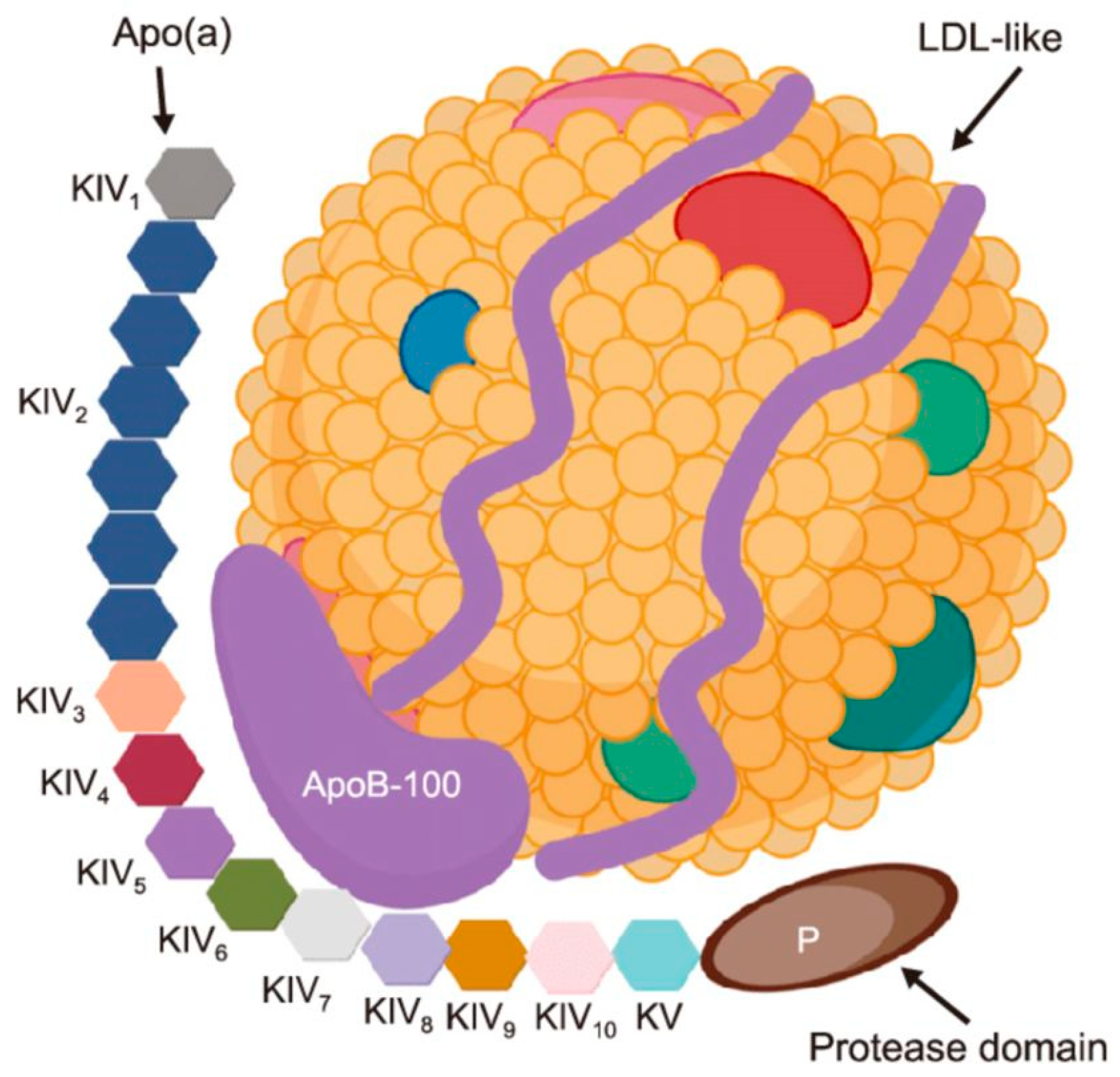
5.3. Hereditary Conditions: Hypercholesterolemia and Lipoprotein(A)
5.4. Psychosocial Factors: Stress, Depressions, Burnout
5.5. Endothelial Dysfunctions and the Microbiome
- Endothelial injury: HIV proteins and chronic inflammation drive microvascular dysfunction, creating fertile ground for both type 1 and type 2 MI [71].
- Therapy-related effects: While integrase inhibitors are generally safer, certain regimens, especially recent abacavir exposure, have been linked to abrupt rises in MI risk [71].
6. Clinical Implication
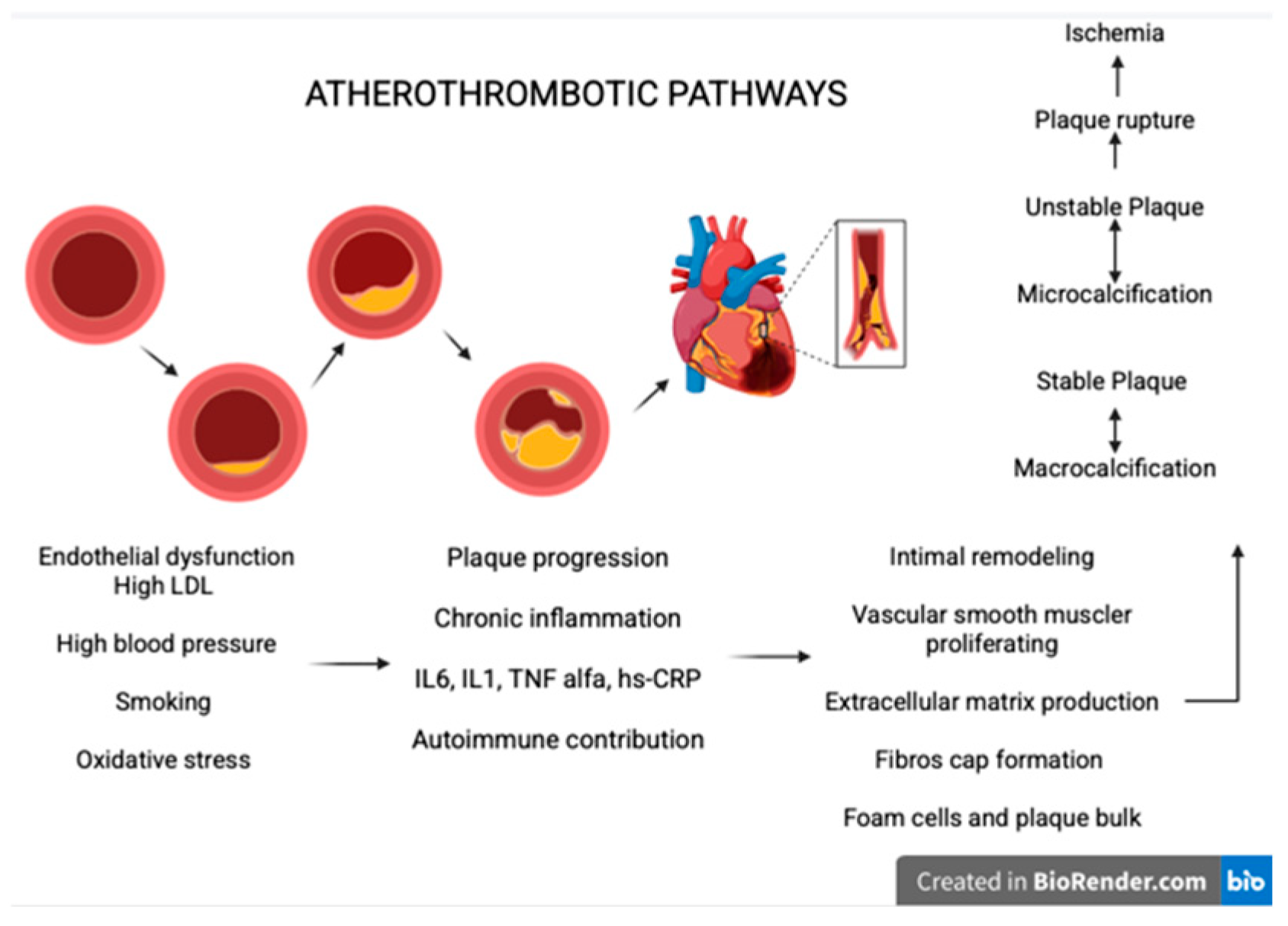
7. Atherothrombotic Pathways
- Endothelial dysfunctions and the response to the injury [84]: The main factors that lead to this process include high LDL cholesterol, elevated systolic blood pressure, smoking, and oxidative stress. These factors increase endothelial permeability, allowing LDL particles to penetrate the intima, where they undergo oxidation and trigger inflammation. At this stage, macrophage apoptosis contributes to further inflammation and endothelial dysfunction [85].
- Intimal remodulating: The vascular smooth muscle cells respond to inflammation by proliferating and producing extracellular matrix leading to fibrous cap formation and by transforming into foam cells contributing to plaque bulk and potentially necrotic formation [88].
- Calcification and plaque stability: Unstable plaques are typically characterized by microcalcifications, whereas stable plaques tend to show macrocalcifications. This distinction explains why unstable plaques rupture more frequently, promoting ischemic events [89].
8. Non-Atherosclerotic Mechanism
- SCAD primarily affects young women, often without traditional risk factors (23–36% in women suffering from AMI). In SCAD, the most common mechanism involves an intimal tear that allows blood to enter the media, or rupture of the vasa vasorum leading to intramural hematoma. These two events compress the true lumen of the vessel, producing luminal obstruction. SCAD is associated with fibromuscular dysplasia (pregnancy-related vascular changes, connective tissue disorders, and emotional or physical stressors). The main treatment strategy is conservative management (aspirin + beta-blockers), and the PCI strategy is reserved only for high-risk patients [92,93,94].
- Vasospasm is a very frequent cause of MINOCA in young people, affecting 20% of them. The main mechanism involves automatic overstimulation, endothelial dysfunction, oxidative stress, and hyperreactivity of smooth muscle contractility. The main treatment strategy is represented by calcium channel blockers and nitroglycerin, avoid beta-blockers, and lifestyle intervention [95].
- Microvascular dysfunction can result from distal thrombus embolization during PCI, ischemia–reperfusion injury (leading to endothelial swelling, pericyte contraction, glycocalyx shedding, and capillary obstruction), or from microvascular inflammation and oxidative stress, which promote chronic dysfunction and remodeling. Diagnostic approaches include invasive measurements such as the index of microvascular resistance, hyperemic microvascular resistance, and resistance reserve ratio, as well as non-invasive tools like PET imaging to detect obstruction and quantify flow reserve. Management strategies include beta-blockers, calcium channel blockers, ACE inhibitors/ARBs, statins, SGLT-2 inhibitors, and colchicine [4,95,96].
- Myocardial Infarction in Pregnancy and Postpartum.
- SCAD accounts for up to 40% of pregnancy-related acute coronary syndromes, particularly in the late third trimester and early postpartum period. Hormonal changes, increased hemodynamic stress, and peripartum vascular remodeling are believed to weaken the arterial wall and predispose to dissection. Management is typically conservative, as most cases heal spontaneously; percutaneous or surgical revascularization is reserved for ongoing ischemia, hemodynamic instability, or left main involvement.
- Takotsubo syndrome is less frequent but has been reported in pregnancy and postpartum, often triggered by emotional or physical stress. It mimics acute coronary syndrome but is characterized by transient left ventricular dysfunction, usually with a favorable recovery. However, its occurrence during pregnancy can complicate maternal and fetal outcomes.
9. Actionable Clinical Recommendations for Preventing Myocardial Infarction in Young Adults (<55 Years)
9.1. Dyslipidaemia and Lipoprotein(a)
9.2. Smoking and Substance Use
9.3. Hypertension and Metabolic Risk
9.4. Obesity and Lifestyle Interventions
9.5. Psychosocial Factors
10. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LDL-C | Low density lipoprotein cholesterol |
| HDL-C | High density lipoprotein cholesterol |
| HR | Hazard Ration |
| CI | Confidence Interval |
| NSAID | Non-steroidal anti-inflammatory drug |
| ASCVD | Atherosclerotic cardiovascular disease |
| ACS | Acute coronary syndrome |
| TIMI | Thrombolysis in myocardical infarction flow |
| PCI | Percutaneous coronary intervention |
| ECG | Electrocardiogram |
| STEMI | ST-Elevation myocardical infarction |
| NSTEMI | Non-ST- Elevation myocardical infarction |
| ACE | Angiotensin-Converting Enzyme |
| ARBS | Angiotensin II receptor blockers |
| AHA | American heart association |
| ACC | American college of cardiology |
| GLP-1 | Gluconat-Like peptide-1 |
| MACE | Major adverse cardiovascular events |
| MI | Myocardical infarction |
| CAD | Coronary artery disease |
| MINOCA | Myocardical infarction with non-obtrustive coronary arteries |
| CHD | Coronary heart disease |
| AMI | Acut myocardical infarction |
| BMI | Body mass index |
| SID | Systemic inflammation |
| RA | Rheumatoid arthritis |
| CV | Cardiovascular |
| SLE | Systemic lupus eerythematosus |
| AS | Ankylosing spondylitis |
| hsCRP | High-sensitivity C-reactive protein |
| MPO | Myeloperoxidase |
| Lp-PLA2 | Lipoprotein-associated phospholipase A2 |
| TMAO | Trimethylamine-N-oxide |
| LpA | Lipoprotein A |
| SCAD | Spontaneous coronary artery dissection |
References
- Prakash, S.; Thomas, J.M.; Anantharaman, R. Clinical and Angiographic Profiles of Myocardial Infarction in a Young South Indian Population. Cureus 2024, 16, e63949. [Google Scholar] [CrossRef]
- Li, H.; Zheng, J.; Qian, F.; Zou, X.; Zou, S.; Wu, Z.; Guo, X.; Su, P. Demographic and regional trends of acute myocardial infarction-related mortality among young adults in the US, 1999–2020. npj Cardiovasc. Health 2025, 2, 9. [Google Scholar] [CrossRef]
- Schultz, W.M.; Kelli, H.M.; Lisko, J.C.; Varghese, T.; Shen, J.; Sandesara, P.; Quyymi, A.; Taylor, H.; Gulati, M.; Harold, J.; et al. Socioeconomic Status and Cardiovascular Outcomes: Challenges and Interventions. Circulation 2018, 137, 2166–2178. [Google Scholar] [CrossRef]
- Zaheen, M.; Pender, P.; Dang, Q.M.; Sinha, E.; Chong, J.J.H.; Chow, C.K.; Zaman, S. Myocardial Infarction in the Young: Aetiology, Emerging Risk Factors, and the Role of Novel Biomarkers. J. Cardiovasc. Dev. Dis. 2025, 12, 148. [Google Scholar] [CrossRef]
- Dimitrova, I.N. Acute Myocardial Infarction in Young Individuals: Demographic and Risk Factor Profile, Clinical Features, Angiographic Findings and In-Hospital Outcome. Cureus 2023, 15, e45803. [Google Scholar] [CrossRef]
- Parlati, A.L.M.; Nardi, E.; Sucato, V.; Madaudo, C.; Leo, G.; Rajah, T.; Marzano, F.; Prastaro, M.; Gargiulo, P.; Paolillo, S.; et al. ANOCA, INOCA, MINOCA: The New Frontier of Coronary Syndromes. J. Cardiovasc. Dev. Dis. 2025, 12, 64. [Google Scholar] [CrossRef]
- D’Agostino, R.B.S.; Pencina, M.J.; Massaro, J.M.; Coady, S. Cardiovascular Disease Risk Assessment: Insights from Framingham. Glob. Heart 2013, 8, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Krishnaswami, S.; Prasad, N.K.; Jacob Jose, V. A study of lipid levels in Indian patients with coronary arterial disease. Int. J. Cardiol. 1989, 24, 337–345. [Google Scholar] [CrossRef]
- Dreyer, R.P.; Wang, Y.; Strait, K.M.; Lorenze, N.P.; D’Onofrio, G.; Bueno, H.; Lichtman, J.; Spertus, J.; Krumholz, H. Gender differences in the trajectory of recovery in health status among young patients with acute myocardial infarction: Results from the variation in recovery: Role of gender on outcomes of young AMI patients (VIRGO) study. Circulation 2015, 131, 1971–1980. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekhar, J.; Gill, A.; Mehran, R. Acute myocardial infarction in young women: Current perspectives. Int. J. Women’s Health 2018, 10, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, P.; Wickramasinghe, K.; Wilkins, E.; Townsend, N. Trends in the epidemiology of cardiovascular disease in the UK. Heart 2016, 102, 1945–1952. [Google Scholar] [CrossRef]
- Lecoeur, E.; Domengé, O.; Fayol, A.; Jannot, A.-S.; Hulot, J.-S. Epidemiology of heart failure in young adults: A French nationwide cohort study. Eur. Heart J. 2023, 44, 383–392. [Google Scholar] [CrossRef]
- Allen, A.M.; Oncken, C.; Hatsukami, D. Women and Smoking: The Effect of Gender on the Epidemiology, Health Effects, and Cessation of Smoking. Curr. Addict. Rep. 2014, 1, 53–60. [Google Scholar] [CrossRef]
- Gleerup, H.B.; Dahm, C.C.; Thim, T.; Jensen, S.E.; Jensen, L.O.; Kristensen, S.D.; Botker, H.E.; Maeng, M. Smoking is the dominating modifiable risk factor in younger patients with STEMI. Eur. Heart J. Acute Cardiovasc. Care 2020, 9, 70–75. [Google Scholar] [CrossRef]
- Wu, W.Y.; Berman, A.N.; Biery, D.W.; Blankstein, R. Recent trends in acute myocardial infarction among the young. Curr. Opin. Cardiol. 2020, 35, 524–530. [Google Scholar] [CrossRef]
- Fotedar, S.; Garg, A.; Arora, A.; Chawla, S. Study of lipid profile in young patients (age 40 years or below) with acute coronary syndrome. J. Fam. Med. Prim. Care 2022, 11, 3034–3039. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-B.; Kim, D.H.; Lee, H.; Hwang, I.-C.; Yoon, Y.E.; Park, H.E.; Kim, Y.J.; Cho, G.-Y.; Kim, H.-K. Mildly Abnormal Lipid Levels, but Not High Lipid Variability, Are Associated With Increased Risk of Myocardial Infarction and Stroke in “Statin-Naive” Young Population A Nationwide Cohort Study. Circ. Res. 2020, 126, 824–835. [Google Scholar] [CrossRef]
- Arnesen, E.K.; Retterstøl, K. Secular trends in serum lipid profiles in young adults in Norway, 2001–2019. Atheroscler. Plus 2022, 48, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Han, K.; Yoo, S.J.; Kim, M.K. Low-Density Lipoprotein Cholesterol Level, Statin Use and Myocardial Infarction Risk in Young Adults. J. Lipid Atheroscler. 2022, 11, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Johnson, H.M.; Thorpe, C.T.; Bartels, C.M.; Schumacher, J.R.; Palta, M.; Pandhi, N.; Sheehy, A.N.; Smith, M.A. Undiagnosed hypertension among young adults with regular primary care use. J. Hypertens. 2014, 32, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Antza, C.; Gallo, A.; Boutari, C.; Ershova, A.; Gurses, K.M.; Lewek, J.; Mirmalsudov, M.; Silbernageli, G.; Sandstedt, J. Prevention of cardiovascular disease in young adults: Focus on gender differences. A collaborative review from the EAS Young Fellows. Atherosclerosis 2023, 384, 117272. [Google Scholar] [CrossRef] [PubMed]
- Rachma, P.; AzamMahalul Ainun, N.; Ika, F.; KanthaweePhitsanuruk Abdul, S. Prevalence and Risk Factors of Hypertension among Young Adults: An Indonesian Basic Health Survey. Open Public Health J. 2025, 18, e18749445361291. [Google Scholar] [CrossRef]
- Moledina, S.M.; Shoaib, A.; Sun, L.Y.; Myint, P.K.; Kotronias, R.A.; Shah, B.N.; Gale, C.P.; Quan, H.; Bagur, R.; Mamas, A. Impact of the admitting ward on care quality and outcomes in non-ST-segment elevation myocardial infarction: Insights from a national registry. Eur. Heart J. Qual. Care Clin. Outcomes 2022, 8, 681–691. [Google Scholar] [CrossRef]
- Biery, D.W.; Berman, A.N.; Singh, A.; Divakaran, S.; DeFilippis, E.M.; Collins, B.L.; Divakaran, S.; DeFilippis, E.M.; Collins, B.L.; Gupta, A.; et al. Association of Smoking Cessation and Survival Among Young Adults With Myocardial Infarction in the Partners YOUNG-MI Registry. JAMA Netw. Open 2020, 3, e209649. [Google Scholar] [CrossRef] [PubMed]
- Stătescu, C.; Anghel, L.; Benchea, L.-C.; Tudurachi, B.-S.; Leonte, A.; Zăvoi, A.; Zota, I.M.; Prisacariu, C.; Radu, R.; Serban, I.L.; et al. A Systematic Review on the Risk Modulators of Myocardial Infarction in the “Young”—Implications of Lipoprotein (a). Int. J. Mol. Sci. 2023, 24, 5927. [Google Scholar] [CrossRef]
- Dikaiou, P.; Björck, L.; Adiels, M.; Lundberg, C.E.; Mandalenakis, Z.; Manhem, K.; Rosengren, A. Obesity, overweight and risk for cardiovascular disease and mortality in young women. Eur. J. Prev. Cardiol. 2021, 28, 1351–1359. [Google Scholar] [CrossRef]
- Chen, J.; Huang, W.; Liang, N. Blood glucose fluctuation and in-hospital mortality among patients with acute myocardial infarction: eICU collaborative research database. PLoS ONE 2024, 19, e0300323. [Google Scholar] [CrossRef]
- Patel, R.S.; Manocha, P.; Patel, J.; Patel, R.; Tankersley, W.E. Cannabis Use Is an Independent Predictor for Acute Myocardial Infarction Related Hospitalization in Younger Population. J. Adolesc. Health 2020, 66, 79–85. [Google Scholar] [CrossRef]
- Kim, S.T.; Park, T. Acute and Chronic Effects of Cocaine on Cardiovascular Health. Int. J. Mol. Sci. 2019, 20, 584. [Google Scholar] [CrossRef]
- Billman, G.E. Cocaine: A review of its toxic actions on cardiac function. Crit. Rev. Toxicol. 1995, 25, 113–132. [Google Scholar] [CrossRef]
- Benzaquen, B.S.; Cohen, V.; Eisenberg, M.J. Effects of cocaine on the coronary arteries. Am. Heart J. 2001, 142, 402–410. [Google Scholar] [CrossRef]
- DeFilippis, E.M.; Singh, A.; Divakaran, S.; Gupta, A.; Collins, B.L.; Biery, D.W.; Qamar, A.; Fatima, A.; Ramsis, M.; Pipilas, D.; et al. Cocaine and Marijuana Use Among Young Adults With Myocardial Infarction. J. Am. Coll. Cardiol. 2018, 71, 2540–2551. [Google Scholar] [CrossRef]
- Gresnigt, F.M.J.; Hulshof, M.; Franssen, E.J.F.; Vanhommerig, J.W.; de Lange, D.W.; Riezebos, R.K. Recreational drug use among young, hospitalized patients with acute coronary syndrome: A retrospective study. Toxicol. Rep. 2022, 9, 1993–1999. [Google Scholar] [CrossRef]
- Weber, B.; Biery, D.W.; Singh, A.; Divakaran, S.; Berman, A.N.; Wu, W.Y.; Brown, J.C.; Hainer, J.; Nasir, K.; Liao, K.P.; et al. Association of inflammatory disease and long-term outcomes among young adults with myocardial infarction: The Mass General Brigham YOUNG-MI Registry. Eur. J. Prev. Cardiol. 2022, 29, 352–359. [Google Scholar] [CrossRef]
- Houge, I.S.; Hoff, M.; Thomas, R.; Videm, V. Mortality is increased in patients with rheumatoid arthritis or diabetes compared to the general population—The Nord-Trøndelag Health Study. Sci. Rep. 2020, 10, 3593. [Google Scholar] [CrossRef]
- Yan, J.; Yang, S.; Han, L.; Ba, X.; Shen, P.; Lin, W.; Li, J.; Zhang, J.; Huang, Y.; Chen, Z.; et al. Dyslipidemia in rheumatoid arthritis: The possible mechanisms. Front. Immunol. 2023, 14, 1254753. [Google Scholar] [CrossRef] [PubMed]
- Meune, C.; Touzé, E.; Trinquart, L.; Allanore, Y. Abstract 1639: Trends in Cardiovascular Mortality in Patients With Rheumatoid Arthritis Over 50 years: A Systematic Review and Meta-analysis of Cohort Studies. Circulation 2009, 120, S536. [Google Scholar] [CrossRef]
- Chen, H.W.; Liu, J.; Yang, D.M.; Xie, Y.; Peterson, E.D.; Navar, A.M.; Li, X.; Zhang, Y.; Wang, J.; Zhou, L.; et al. Incidence and Prevalence of Atherosclerotic Cardiovascular Disease in Cutaneous Lupus Erythematosus. JAMA Dermatol. 2025, 161, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-Y.; Lai, Y.-F.; Chien, W.-C.; Chen, Y.-H.; Chung, C.-H.; Chen, J.-T.; Lee, Y.-C.; Huang, C.-W.; Hsu, C.-Y.; Chang, W.-Y.; et al. Impact of Endophthalmitis on the Risk of Acute Myocardial Infarction in Ankylosing Spondylitis Patients: A Population-Based Retrospective Cohort Study. J. Clin. Med. 2023, 12, 1211. [Google Scholar] [CrossRef]
- Kwon, O.C.; Lee, H.S.; Yang, J.; Park, M.-C. Cardiovascular risk according to biological agent exposure in patients with ankylosing spondylitis: A nationwide population-based study. Clin. Rheumatol. 2025, 44, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Garshick, M.S.; Ward, N.L.; Krueger, J.G.; Berger, J.S. Cardiovascular Risk in Patients With Psoriasis. JACC 2021, 77, 1670–1680. [Google Scholar] [CrossRef] [PubMed]
- Piaserico, S.; Papadavid, E.; Cecere, A.; Orlando, G.; Theodoropoulos, K.; Katsimbri, P.; Mazzotta, A.; Gkalpakiotis, S.; Bobyr, I.; Gisondi, P.; et al. Coronary Microvascular Dysfunction in Asymptomatic Patients with Severe Psoriasis. J. Investig. Dermatol. 2023, 143, 1929–1936.e2. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-L.; Fan, Y.-H.; Fan, K.-S.; Juan, C.-K.; Chen, Y.-J.; Wu, C.-Y. Cardiovascular disease risk in patients with psoriasis receiving biologics targeting TNF-α, IL-12/23, IL-17, and IL-23: A population-based retrospective cohort study. PLoS Med. 2025, 22, e1004591. [Google Scholar] [CrossRef]
- Dzhus, M.; Mostbauer, H. Coronary artery lesions in Takayasu arteritis. Reumatologia 2023, 61, 460–472. [Google Scholar] [CrossRef]
- 45. Shiono, Y.; Takahata, M.; Ino, Y.; Tanimoto, T.; Kakimoto, N.; Suenaga, T.; Nakashima, K.; Harada, M.; Yamaguchi, T.; Okada, Y.; et al. Pathological Alterations of Coronary Arteries Late After Kawasaki Disease: An Optical Coherence Tomography Study. JACC Adv. 2024, 3, 100937. [Google Scholar] [CrossRef]
- Borgas, Y.; Mohammad, M.A.; Gisslander, K.; Rathmann, J.; Erlinge, D.; Jayne, D.; Eriksson, P.; Smith, J.G.; Lund, L.H.; Lindholm, C.R.; et al. Myocardial infarction in ANCA-associated vasculitis: A population-based cohort study. RMD Open 2025, 11, e005055. [Google Scholar] [CrossRef]
- Walter, D.J.; Bigham, G.E.; Lahti, S.; Haider, S.W. Shifting perspectives in coronary involvement of polyarteritis nodosa: Case of 3-vessel occlusion treated with 4-vessel CABG and review of literature. BMC Cardiovasc. Disord. 2024, 24, 190. [Google Scholar] [CrossRef]
- Liao, K.P.; Playford, M.P.; Frits, M.; Coblyn, J.S.; Iannaccone, C.; Weinblatt, M.E.; Shadick, N.A.; Costenbader, K.H.; Karlson, E.W.; Solomon, D.H.; et al. The association between reduction in inflammation and changes in lipoprotein levels and HDL cholesterol efflux capacity in rheumatoid arthritis. J. Am. Heart Assoc. 2015, 4, e001588. [Google Scholar] [CrossRef]
- Țieranu, E.N.; Cureraru, S.I.; Târtea, G.C.; Vlăduțu, V.-C.; Cojocaru, P.A.; Piorescu, M.T.L.; Dincă, D.; Popescu, R.; Militaru, C.; Donoiu, I.; et al. Acute Myocardial Infarction and Diffuse Coronary Artery Disease in a Patient with Multiple Sclerosis: A Case Report and Literature Review. J. Clin. Med. 2025, 14, 4304. [Google Scholar] [CrossRef] [PubMed]
- Alfaddagh, A.; Martin, S.S.; Leucker, T.M.; Michos, E.D.; Blaha, M.J.; Lowenstein, C.J.; Jones, S.R.; Toth, P.P.; Karalis, D.G.; Martin, J.M.J.; et al. Inflammation and cardiovascular disease: From mechanisms to therapeutics. Am. J. Prev. Cardiol. 2020, 4, 100130. [Google Scholar] [CrossRef]
- Cosău, D.E.; Costache Enache, I.I.; Costache, A.D.; Tudorancea, I.; Ancuța, C.; Șerban, D.N.; Ciobanu, A.O.; Drăgănescu, M.; Bălănescu, A.; Stanciu, S.; et al. From Joints to the Heart: An Integrated Perspective on Systemic Inflammation. Life 2025, 15, 629. [Google Scholar] [CrossRef]
- Leib, A.D.; Foris, L.A.; Nguyen, T.; Khaddour, K. Dressler Syndrome; StatPearls: Treasure Island, FL, USA, 2025. [Google Scholar]
- Bogsrud, M.P.; Øyri, L.K.L.; Halvorsen, S.; Atar, D.; Leren, T.P.; Holven, K.B. Prevalence of genetically verified familial hypercholesterolemia among young (<45 years) Norwegian patients hospitalized with acute myocardial infarction. J. Clin. Lipidol. 2020, 14, 339–345. [Google Scholar] [CrossRef]
- Elmaghraby, K.M.; Abdel-Galeel, A.; Osman, A.H.; Hasan-Ali, H.; Abdelmegid, M.A.-K.F. Clinical and angiographic characteristics of patients with familial hypercholesterolemia presenting with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Sci. Rep. 2024, 14, 27098. [Google Scholar] [CrossRef]
- Chávez-González, E.; Rodríguez-Jiménez, A.E.; Ferrer-Rodríguez, C.J.; Donoiu, I. Ventricular arrhythmias are associated with increased QT interval and QRS dispersion in patients with ST-elevation myocardial infarction. Rev. Port. Cardiol. 2022, 41, 395–404. [Google Scholar] [CrossRef]
- Clair, V.; Zirille, F.M.; Gill, E. Rethinking cardiovascular risk: The emerging role of lipoprotein(a) screening. Am. J. Prev. Cardiol. 2025, 21, 100945. [Google Scholar] [CrossRef]
- 57. Doherty, S.; Hernandez, S.; Rikhi, R.; Mirzai, S.; De Los Reyes, C.; McIntosh, S.; Block, R.C.; Shapiro, M.D.; Christof, M.; Wong, N.D.; et al. Lipoprotein(a) as a Causal Risk Factor for Cardiovascular Disease. Curr. Cardiovasc. Risk Rep. 2025, 19, 8. [Google Scholar] [CrossRef]
- Buciu, I.C.; Țieranu, E.N.; Pîrcălabu, A.Ș.; Istratoaie, O.; Zlatian, O.M.; Cioboată, R.; Donoiu, I.; Militaru, C.; Militaru, S.; Găman, A.; et al. Exploring the Relationship Between Lipoprotein (a) Level and Myocardial Infarction Risk: An Observational Study. Medicina 2024, 60, 1878. [Google Scholar] [CrossRef]
- Buciu, I.C.; Țieranu, E.N.; Pîrcălabu, A.Ș.; Zlatian, O.M.; Donoiu, I.; Militaru, C.; Militaru, S.; Militaru, C.; Istratoaie, O.; Cioboată, R.; et al. The Relationship between Lipoprotein A and the Prevalence of Multivessel Coronary Artery Disease in Young Patients with Acute Myocardial Infarction: An Observational Study. Biomedicines 2024, 12, 2159. [Google Scholar] [CrossRef] [PubMed]
- Buciu, I.C.; Țieranu, E.N.; Pîrcălabu, A.Ș.; Zlatian, O.M.; Donoiu, I.; Cioboată, R.; Militaru, C.; Militaru, S.; Găman, A.; Istratoaie, O.; et al. Lipoprotein (a) in the context of atherosclerosis: Pathological implications and therapeutic perspectives in myocardial infarction. A narrative review. Rom. J. Morphol. Embryol. 2024, 65, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Bertolín-Boronat, C.; Marcos-Garcés, V.; Merenciano-González, H.; Martínez Mas, M.L.; Climent Alberola, J.I.; Perez, N. Familial Hypercholesterolemia Screening in a Cardiac Rehabilitation Program After Myocardial Infarction. Cardiogenetics 2025, 15, 6. [Google Scholar] [CrossRef]
- Faresjö, Å.; Karlsson, J.-E.; Segerberg, H.; Lebena, A.; Faresjö, T. Cardiovascular and psychosocial risks among patients below age 50 with acute myocardial infarction. BMC Cardiovasc. Disord. 2023, 23, 121. [Google Scholar] [CrossRef]
- Chong, R.J.; Hao, Y.; Tan, E.W.Q.; Mok, G.J.L.; Sia, C.-H.; Ho, J.S.Y.; Chan, M.Y.Y.; Ho, A.F.W.; Koh, Z.X.; Ong, M.E.H.; et al. Prevalence of Depression, Anxiety and Post-Traumatic Stress Disorder (PTSD) After Acute Myocardial Infarction: A Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 1786. [Google Scholar] [CrossRef]
- Rahman, M.M.; Islam, F.; Or-Rashid, M.H.; Mamun, A.A.; Rahaman, M.S.; Islam, M.M.; Mubarak, M.S.; Rahman, M.H.; Hossain, M.A.; Mondal, B.; et al. The Gut Microbiota (Microbiome) in Cardiovascular Disease and Its Therapeutic Regulation. Front. Cell. Infect. Microbiol. 2022, 12, 903570. [Google Scholar] [CrossRef]
- Calborean, V.; Gheorman, V.; Istratoaie, O.; Mustafa, R.E.; Cojocaru, P.A.; Alexandru, D.O.; Militaru, C.; Militaru, S.; Țieranu, E.N.; Cioboată, R.; et al. QT interval analysis in patients with chronic liver disease. Rev. Chim. 2018, 69, 1134–1138. [Google Scholar] [CrossRef]
- Verhaar, B.J.H.; Prodan, A.; Nieuwdorp, M.; Muller, M. Gut Microbiota in Hypertension and Atherosclerosis: A Review. Nutrients 2020, 12, 2982. [Google Scholar] [CrossRef]
- Sessa, R.; Pietro MDi Filardo, S.; Turriziani, O. Infectious burden and atherosclerosis: A clinical issue. World J. Clin. Cases 2014, 2, 240–249. [Google Scholar] [CrossRef]
- Kaplan, H.; Thompson, R.C.; Trumble, B.C.; Wann, L.S.; Allam, A.H.; Beheim, B.; Frohlich, B.; Sutherland, M.L.; Sutherland, J.D.; Stieglitz, J.; et al. Coronary atherosclerosis in indigenous South American Tsimane: A cross-sectional cohort study. Lancet 2017, 389, 1730–1739. [Google Scholar] [CrossRef]
- Lei, S.; Chen, T.; Zhou, J.; Zhu, L.; Zhang, Z.; Xie, X.; Wang, Y.; Liu, Y.; Huang, Z.; Zhao, J.; et al. Distinct blood and oral microbiome profiles reveal altered microbial composition and functional pathways in myocardial infarction patients. Front. Cell. Infect. Microbiol. 2025, 15, 1506382. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.S.V.; Stelzle, D.; Lee, K.K.; Beck, E.J.; Alam, S.; Clifford, S.; Longenecker, C.T.; Strachan, F.; Bagchi, S.; Whiteley, W.; et al. Global Burden of Atherosclerotic Cardiovascular Disease in People Living With HIV: Systematic Review and Meta-Analysis. Circulation 2018, 138, 1100–1112. [Google Scholar] [CrossRef] [PubMed]
- Longenecker, C.T.; Sullivan, C.; Baker, J.V. Immune activation and cardiovascular disease in chronic HIV infection. Curr. Opin. HIV AIDS 2016, 11, 216–225. [Google Scholar] [CrossRef]
- Kwok, C.S.; Bennett, S.; Holroyd, E.; Satchithananda, D.; Borovac, J.A.; Will, M.; Potts, J.; Mallen, C.; Chew-Graham, C.A.; Gale, C.P.; et al. Characteristics and outcomes of patients with acute coronary syndrome who present with atypical symptoms: A systematic review, pooled analysis and meta-analysis. Coron. Artery Dis. 2025, 36, 240–251. [Google Scholar] [CrossRef]
- Sood, A.; Singh, A.; Gadkari, C. Myocardial Infarction in Young Individuals: A Review Article. Cureus 2023, 15, e37102. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.A.; Karim, H.M.R.; Panda, C.K.; Ahmed, G.; Nayak, S. Atypical Presentations of Myocardial Infarction: A Systematic Review of Case Reports. Cureus 2023, 15, e35492. [Google Scholar] [CrossRef] [PubMed]
- Peerwani, G.; Hanif, B.; Rahim, K.A.; Kashif, M.; Virani, S.S.; Sheikh, S. Presentation, management, and early outcomes of young acute coronary syndrome patients- analysis of 23,560 South Asian patients from 2012 to 2021. BMC Cardiovasc. Disord. 2024, 24, 378. [Google Scholar] [CrossRef]
- Inoue, A.; Mizobe, M.; Takahashi, J.; Funakoshi, H. Factors for delays in door-to-balloon time ≤ 90 min in an electrocardiogram triage system among patients with ST-segment elevation myocardial infarction: A retrospective study. Int. J. Emerg. Med. 2023, 16, 77. [Google Scholar] [CrossRef] [PubMed]
- Brush, J.E., Jr.; Chaudhry, S.I.; Dreyer, R.P.; D’Onofrio, G.; Greene, E.J.; Hajduk, A.M.; Spatz, E.S.; Krumholz, H.M.; Murugiah, K.; Lu, D.Y.; et al. Sex Differences in Symptom Complexity and Door-to-Balloon Time in Patients With ST-Elevation Myocardial Infarction. Am. J. Cardiol. 2023, 197, 101–107. [Google Scholar] [CrossRef]
- Fischer, A.J.; Feld, J.; Lange, S.A.; Günster, C.; Dröge, P.; Engelbertz, C.; Jung, J.; Maier, L.S.; Werdan, K.; Störk, S.; et al. Impact of Guideline-Directed Drug Therapy after ST-Elevation Myocardial Infarction on Outcome in Young Patients—Age and Sex-Specific Factors. J. Clin. Med. 2024, 13, 3788. [Google Scholar] [CrossRef]
- Yang, J.; Biery, D.W.; Singh, A.; Divakaran, S.; DeFilippis, E.M.; Wu, W.Y.; Berman, A.N.; Qamar, A.; Mutharasan, R.K.; Klein, J.; et al. Risk Factors and Outcomes of Very Young Adults Who Experience Myocardial Infarction: The Partners YOUNG-MI Registry. Am. J. Med. 2020, 133, 605–612.e1. [Google Scholar] [CrossRef]
- Roston, T.M.; Aghanya, V.; Savu, A.; Fordyce, C.B.; Lawler, P.R.; Jentzer, J.; Fudim, M.; Austin, P.C.; Ko, D.T.; Goodman, S.G.; et al. Premature Acute Myocardial Infarction Treated With Invasive Revascularization: Comparing STEMI With NSTEMI in a Population-Based Study of Young Patients. Can. J. Cardiol. 2024, 40, 2079–2088. [Google Scholar] [CrossRef]
- uan-Salvadores, P.; De La Torre Fonseca, L.M.; Calderon-Cruz, B.; Veiga, C.; Pintos-Rodríguez, S.; Fernandez Barbeira, S.; Fernández, A.L.; Amigo-Vázquez, I.; Paredes-Galán, E.; Garcia, J.; et al. Ischaemia-reperfusion time differences in ST-elevation myocardial infarction in very young patients: A cohort study. Open Heart 2025, 12, e002957. [Google Scholar] [CrossRef]
- Kelly, C.; Lan, N.S.R.; Phan, J.; Hng, C.; Matthews, A.; Rankin, J.M.; Yudi, M.B.; Clark, D.; Walters, D.L.; Chan, W.; et al. Characteristics and Outcomes of Young Patients With ST-Elevation Myocardial Infarction Without Standard Modifiable Risk Factors. Am. J. Cardiol. 2023, 202, 81–89. [Google Scholar] [CrossRef]
- Porapakkham, P.; Porapakkham, P.; Srimahachota, S.; Limpijankit, T.; Kiatchoosakun, S.; Chandavimol, M.; Chee-watanakornkul, S.; Tangcharoen, T.; Krittayaphong, R.; Tansuphaswadikul, S.; et al. The contemporary management and coronary angioplasty outcomes in young patients with ST-Elevation myocardial infarction (STEMI) age <40 years old: The insight from nationwide Thai PCI registry. BMC Cardiovasc. Disord. 2024, 24, 548. [Google Scholar] [CrossRef]
- Rotaru-Zavaleanu, A.D.; Neacşu, A.I.; Cojocaru, A.; Osiac, E.; Gheonea, D.I. Heterogeneity in the Number of Astrocytes in the Central Nervous System after Peritonitis. Curr. Health Sci. J. 2021, 47, 164–169. [Google Scholar] [CrossRef]
- Kerr, P.; Tam, R.; Plane, F. Endothelium. In Mechanisms of Vascular Disease: A Reference Book for Vascular Specialists [Internet]; Fitridge, R., Thompson, M., Eds.; University of Adelaide Press: Adelaide, Australia, 2011. Available online: https://www.ncbi.nlm.nih.gov/books/NBK534266/ (accessed on 3 September 2025).
- Sterpetti, A.V. Inflammatory Cytokines and Atherosclerotic Plaque Progression. Therapeutic Implications. Curr. Atheroscler. Rep. 2020, 22, 75. [Google Scholar] [CrossRef]
- Sircana, M.C.; Erre, G.L.; Castagna, F.; Manetti, R. Crosstalk between Inflammation and Atherosclerosis in Rheumatoid Arthritis and Systemic Lupus Erythematosus: Is There a Common Basis? Life 2024, 14, 716. [Google Scholar] [CrossRef] [PubMed]
- Tasouli-Drakou, V.; Ogurek, I.; Shaikh, T.; Ringor, M.; DiCaro, M.V.; Lei, K. Atherosclerosis: A Comprehensive Review of Molecular Factors and Mechanisms. Int. J. Mol. Sci. 2025, 26, 1364. [Google Scholar] [CrossRef]
- Ajoolabady, A.; Praticò, D.; Lin, L.; Mantzoros, C.S.; Bahijri, S.; Tuomilehto, J.; Al-Rasheed, N.; Al-Rasheed, N.; Zhao, L.; Xu, Y.; et al. Inflammation in atherosclerosis: Pathophysiology and mechanisms. Cell Death Dis. 2024, 15, 817. [Google Scholar] [CrossRef]
- Țieranu, E.N.; Donoiu, I.; Istrătoaie, O.; Găman, A.E.; Țieranu, M.L.; Țieranu, C.G.; Osiac, E.; Gheonea, D.I.; Cojocaru, A.; Neacșu, A.I.; et al. Rare case of single coronary artery in a patient with liver cirrhosis. Rom. J. Morphol. Embryol. 2017, 58, 1505–1508. [Google Scholar]
- Ipek, G.; Nural, A.; Cebeci, A.C.; Yucedag, F.F.; Bolca, O. Long-term outcomes in very young patients with myocardial infarction with non-obstructive coronary arteries. Coron. Artery Dis. 2024, 35, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, M.; Chen, X.; Zhang, X.; Zhang, R.; Zuo, P.; Wang, L.; Huang, X.; Liu, Y.; Wang, Y.; et al. Spontaneous left main coronary artery dissection occurred in a young male: A case report and review of literature. BMC Cardiovasc. Disord. 2022, 22, 256. [Google Scholar] [CrossRef] [PubMed]
- Krittanawong, C.; Qadeer, Y.K.; Ang, S.P.; Wang, Z.; Alam, M.; Sharma, S.; Virani, S.S.; Jneid, H.; Tang, W.W.; Kapadia, S.R.; et al. Incidence and in-hospital mortality among women with acute myocardial infarction with or without SCAD. Curr. Probl. Cardiol. 2025, 50, 102921. [Google Scholar] [CrossRef]
- Meng, P.-N.; Xu, C.; You, W.; Wu, Z.-M.; Xie, D.-J.; Zhang, H.; Chen, Y.; Zhou, Y.; Wang, H.; Li, J.; et al. Spontaneous Coronary Artery Dissection as a Cause of Acute Myocardial Infarction in Young Female Population: A Single-center Study. Chin. Med. J. 2017, 130, 1534–1539. [Google Scholar] [CrossRef]
- Squires, I.; Khalil, S.; Patel, A.; Katukuri, N. MINOCA: Predictors and Future Management. Indian J. Cardiovasc. Dis. Women 2025, 10, 45–50. [Google Scholar] [CrossRef]
- Cojocaru, A.; Zavaleanu, A.D.; Călina, D.C.; Gheonea, D.I.; Osiac, E.; Boboc, I.K.S.; Militaru, C.; Militaru, S.; Buciu, I.C.; Țieranu, E.N.; et al. Different Age Related Neurological and Cardiac Effects of Verapamil on a Transgenic Mouse Model of Alzheimer’s Disease. Curr. Health Sci. J. 2021, 47, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Iliakis, P.; Pitsillidi, A.; Pyrpyris, N.; Fragkoulis, C.; Leontsinis, I.; Koutsopoulos, G.; Vassilopoulos, D.; Kordossis, T.; Pappas, C.; Pappas, C.; et al. Pregnancy-Associated Takotsubo Syndrome: A Narrative Review of the Literature. J. Clin. Med. 2025, 14, 2356. [Google Scholar] [CrossRef]
- Makhmudova, U.; Samadifar, B.; Maloku, A.; Haxhikadrija, P.; Geiling, J.A.; Römer, R.; Schneider, G.; Weismann, D.; Angermann, C.E.; Rizas, K.D.; et al. Intensive lipid-lowering therapy for early achievement of guideline-recommended LDL-cholesterol levels in patients with ST-elevation myocardial infarction (“Jena auf Ziel”). Clin. Res. Cardiol. 2023, 112, 1212–1219. [Google Scholar] [CrossRef] [PubMed]
- Kronenberg, F.; Mora, S.; Stroes, E.S.G. Consensus and guidelines on lipoprotein(a)—Seeing the forest through the trees. Curr. Opin. Lipidol. 2022, 33, 342–352. [Google Scholar] [CrossRef]
- Zhang, Y.; de Ferranti, S.D.; Moran, A.E. Genetic testing for familial hypercholesterolemia. Curr. Opin. Lipidol. 2024, 35, 93–100. [Google Scholar] [CrossRef]
- Ramphul, K.; Mejias, S.G.; Joynauth, J. Cocaine, Amphetamine, and Cannabis Use Increases the Risk of Acute Myocardial Infarction in Teenagers. Am. J. Cardiol. 2019, 123, 354. [Google Scholar] [CrossRef]
- Grundy, S.M.; Feingold, K.R. Guidelines for the Management of High Blood Cholesterol. In Endotext; MDText.com, Inc.: South Dartmouth, MA, USA, 2025. [Google Scholar]
- Beyond Glycemic Control: Sodium-Glucose Cotransporter-2 Inhibitor and Glucagon-Like Peptide-1 Receptor Agonist to Reduce the Burden of Peripheral Artery Disease—American College of Cardiology n.d. Available online: https://www.acc.org/Latest-in-Cardiology/Articles/2025/04/23/12/22/Beyond-Glycemic-Control (accessed on 2 September 2025).
- SELECT: Semaglutide Reduces Risk of MACE in Adults with Overweight or Obesity—American College of Cardiology n.d. Available online: https://www.acc.org/Latest-in-Cardiology/Articles/2023/08/10/14/29/SELECT-Semaglutide-Reduces-Risk-of-MACE-in-Adults-With-Overweight-or-Obesity (accessed on 2 September 2025).
- Kwapong, Y.A.; Boakye, E.; Khan, S.S.; Honigberg, M.C.; Martin, S.S.; Oyeka, C.P.; Khera, R.; Ndumele, C.E.; Gulati, M.; Ssentongo, P.; et al. Association of Depression and Poor Mental Health With Cardiovascular Disease and Suboptimal Cardiovascular Health Among Young Adults in the United States. J. Am. Heart Assoc. 2023, 12, e028332. [Google Scholar] [CrossRef] [PubMed]
| Traditional Risk Factors | Emerging Risk Factors |
|---|---|
| Dyslipidemia | Recreational drug use (cocaine, cannabis) |
| Hypertension | Systemic inflammation and autoimmune disease (SLE, RA, psoriasis, vasculitis) |
| Smoking | Hereditary conditions (hypercolesterolemia and LpA) |
| Obesity | Psyschosocial factors (stress, depression, burnout) |
| Diabetes Mellitus | Endothelial dysfunctions and microbiome |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cojocaru, P.A.; Țieranu, M.L.; Piorescu, M.T.L.; Buciu, I.C.; Belu, A.M.; Cureraru, S.I.; Țieranu, E.N.; Moise, G.C.; Istratoaie, O. Myocardical Infarction in Young Adults: Revisiting Risk Factors and Atherothrombotic Pathways. Medicina 2025, 61, 1615. https://doi.org/10.3390/medicina61091615
Cojocaru PA, Țieranu ML, Piorescu MTL, Buciu IC, Belu AM, Cureraru SI, Țieranu EN, Moise GC, Istratoaie O. Myocardical Infarction in Young Adults: Revisiting Risk Factors and Atherothrombotic Pathways. Medicina. 2025; 61(9):1615. https://doi.org/10.3390/medicina61091615
Chicago/Turabian StyleCojocaru, Petre Alexandru, Maria Loredana Țieranu, Mina Teodora Luminița Piorescu, Ionuț Cezar Buciu, Alexandru Mugurel Belu, Silvana Isabella Cureraru, Eugen Nicolae Țieranu, Gianina Cristiana Moise, and Octavian Istratoaie. 2025. "Myocardical Infarction in Young Adults: Revisiting Risk Factors and Atherothrombotic Pathways" Medicina 61, no. 9: 1615. https://doi.org/10.3390/medicina61091615
APA StyleCojocaru, P. A., Țieranu, M. L., Piorescu, M. T. L., Buciu, I. C., Belu, A. M., Cureraru, S. I., Țieranu, E. N., Moise, G. C., & Istratoaie, O. (2025). Myocardical Infarction in Young Adults: Revisiting Risk Factors and Atherothrombotic Pathways. Medicina, 61(9), 1615. https://doi.org/10.3390/medicina61091615






