Dental Disease as a Clinical Marker for Coronary Artery Disease Severity: A Narrative Review of Current Evidence and Mechanisms
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Information Sources
2.2. Study Selection and Screening
2.3. Eligibility Criteria (Inclusion/Exclusion)
- Population: adults (≥18 years) assessed for coronary artery disease by means of angiography, CT coronary angiography, or clinically adjudicated CAD.
- Exposure/index test: tooth loss (number of missing teeth, edentulism, partial edentulism) or validated measures of oral health that report tooth count. Studies that used periodontitis as an exposure but also reported tooth loss were eligible.
- Comparator: patients with fewer/no missing teeth or analyses treating tooth loss as continuous or categorical exposure.
- Outcomes: measures of CAD severity (angiographic scores such as SYNTAX, Gensini, number of vessels involved, and extent of stenosis), major adverse cardiovascular events (MACE) stratified by CAD severity, or other clinically meaningful CAD severity indices.
- Study design: observational cohort (prospective or retrospective), case–control, cross-sectional studies, and meta-analyses. Clinical reports and mechanistic studies were included for contextual discussion but excluded from pooled quantitative analysis.
- Timeframe: published between 2000 and 2025, English language (or language with accessible translation).
- Case reports, small case series (<10 participants), editorials, narrative reviews (except for background), in vitro or animal studies (except in mechanistic discussion).
- Studies that only assessed periodontal indices without reporting tooth loss (unless tooth loss data were available upon request).
- Studies in pediatric populations.
- Studies lacking quantitative outcomes related to CAD severity or not reporting extractable data.
3. Results
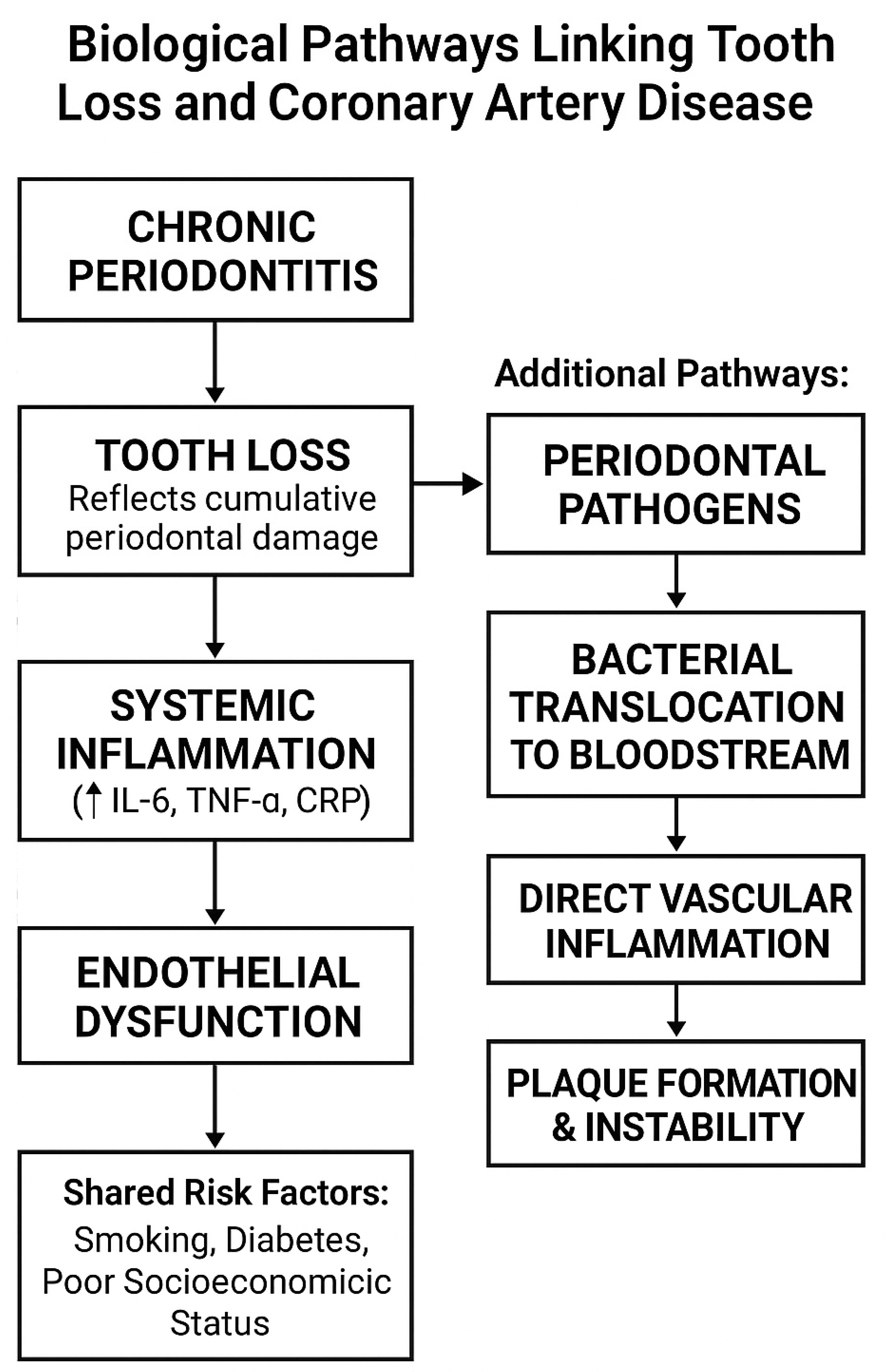
3.1. Oral Health and Systemic Inflammation
3.2. Mechanisms Linking Periodontal Disease to CAD
3.3. Evidence Linking Tooth Loss Disease to CAD
- Buhlin et al. (2003): Patients undergoing coronary angiography had more severe periodontal disease with deeper periodontal pockets and reduced numbers of teeth compared to controls. The results supported an association between CAD and oral pathology. A limitation of the study could be its size [42].
- Elter et al. (2003): In this cross-sectional study, individuals who were edentulous for more than nine teeth were at higher risk of multivessel CAD and stroke/transient ischemic attacks [44].
- Ylöstalo et al. (2006) established a dose-dependent relationship between missing teeth count and elevated CRP levels, suggesting a systemic inflammatory link [39].
- Gul et al. (2012): A cross-sectional study that reviewed the coronary angiograms of patients and reported that patients with fewer than 10 teeth had considerably higher Gensini scores, compared to those with normal dentition. The limitations of the study are the lack of certain clinical data: door-to-balloon time (important in STEMI cases) or the exact onset of infarction, which can impact the accuracy of the Gensini score [45].
- Liljestrand et al. (2015) followed over 1500 Finnish adults for more than a decade.
- This prospective cohort study demonstrated that edentulous individuals had increased incidence of myocardial infarction, coronary artery calcification, and all-cause mortality, independent of traditional cardiovascular risk factors. Its longitudinal design strengthens the temporal association between tooth loss and cardiovascular outcomes. The most important limitation is the lack of reasons or diagnosis for tooth extractions [15].
- Elevated coronary artery calcium scores were associated with tooth loss by Donders et al. (2020). The study is limited by its size [40].
- Gao et al. (2021) showed an association between coronary heart disease, number of teeth, and periodontitis. The limitations of the study include the fact that the number of teeth does not totally represent oral inflammation; also, the grouping of individuals based on the number of teeth was performed differently in the various articles, and this study ignored the differences in individual teeth [5].
- Shen M et al. (2023) showed that the number of missing teeth is associated with the degree of coronary atherosclerosis, especially in young patients and short-duration diabetic patients. Limitations of the study are the size of the study and the use of Coronary Artery Calcium score as the primary outcome measure; a causal relationship could not be identified due to a lack of information on the time sequence of events [43].
3.4. Clinical Implications
- It may also be a sign of low socioeconomic status, often with worse outcomes and fewer opportunities to access services [54].
- Psychological impacts of tooth loss (e.g., social withdrawal and depression) are aggravated by cardiometabolic risk profiles [55].
3.5. Prevention Approaches
3.6. Core Insights for Cardiologists and General Practitioners
- Tooth loss is more than a dental concern—it can be a visible, easily assessed marker of systemic inflammation and an independent indicator of coronary artery disease (CAD) severity.
- Shared risk factors (age, smoking, diabetes, obesity, and low socioeconomic status) complicate causality, but the biological plausibility is supported by mechanistic evidence involving Th1/Th17 activation, endothelial dysfunction, and pro-thrombotic states.
- Risk stratification: Patients with significant tooth loss, especially when unexplained by trauma or localized pathology, should be considered for enhanced cardiovascular evaluation.
- Multidisciplinary care: Collaboration between dental and medical professionals can improve early detection, risk modification, and patient outcomes.
- Prevention pays: Maintaining periodontal health through regular dental care may reduce systemic inflammatory burden and potentially slow atherosclerotic disease progression.
4. Conclusion, Confounding Variables, Limitations, and Future Research
- Most are cross-sectional or retrospective cohort studies, vulnerable to reverse causation and selection bias.
- Key confounders (e.g., smoking, diabetes, and access to dental care) are inconsistently controlled.
- The outcome measures vary widely—from CRP levels to coronary calcium scores to clinical endpoints—making synthesis challenging.
- Prospective studies incorporating oral microbiome sequencing and inflammatory biomarkers could strengthen biological plausibility.
- Randomized trials of periodontal interventions with cardiovascular outcomes are needed to explore causality.
- Socioeconomic and dietary factors must be integrated into multivariate models to assess the independent effects of oral health.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Malakar, A.K.; Choudhury, D.; Halder, B.; Paul, P.; Uddin, A.; Chakraborty, S. A Review on Coronary Artery Disease, Its Risk Factors, and Therapeutics. J. Cell. Physiol. 2019, 234, 16812–16823. [Google Scholar] [CrossRef]
- Lee, H.; Kim, H.-L.; Jin, K.N.; Oh, S.; Han, Y.-S.; Jung, D.-U.; Sim, H.-Y.; Kim, H.-S.; Lim, W.-H.; Seo, J.-B.; et al. Association between Dental Health and Obstructive Coronary Artery Disease: An Observational Study. BMC Cardiovasc. Disord. 2019, 19, 98. [Google Scholar] [CrossRef]
- Czerniuk, M.R.; Surma, S.; Romańczyk, M.; Nowak, J.M.; Wojtowicz, A.; Filipiak, K.J. Unexpected Relationships: Periodontal Diseases: Atherosclerosis–Plaque Destabilization? From the Teeth to a Coronary Event. Biology 2022, 11, 272. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Tian, J.; Li, Y.; Liu, T.; Li, R.; Yang, L.; Xing, Z. Periodontitis and Number of Teeth in the Risk of Coronary Heart Disease: An Updated Meta-Analysis. Med. Sci. Monit. 2021, 27, e930112. [Google Scholar] [CrossRef]
- Salminen, A.; Kopra, E.; Lahdentausta, L.; Liljestrand, J.; Paju, S. Association between oral infections and cardiovascular diseases. Tidende 2020, 131, 122–127. [Google Scholar] [CrossRef]
- Nazir, M.; Al-Ansari, A.; Al-Khalifa, K.; Alhareky, M.; Gaffar, B.; Almas, K. Global Prevalence of Periodontal Disease and Lack of Its Surveillance. Sci. World J. 2020, 2020, 2146160. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Marco Del Castillo, A.; Jepsen, S.; Gonzalez-Juanatey, J.R.; D’Aiuto, F.; Bouchard, P.; Chapple, I.; Dietrich, T.; Gotsman, I.; Graziani, F.; et al. Periodontitis and Cardiovascular Diseases: Consensus Report. J. Clin. Periodontol. 2020, 47, 268–288. [Google Scholar] [CrossRef]
- King, S.; Chow, C.K.; Eberhard, J. Oral Health and Cardiometabolic Disease: Understanding the Relationship. Intern. Med. J. 2022, 52, 198–205. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Van Dyke, T.E.; on behalf of working group 1 of the joint EFP/AAP workshop. Periodontitis and Atherosclerotic Cardiovascular Disease: Consensus Report of the Joint EFP/AAPWorkshop on Periodontitis and Systemic Diseases. J. Periodontol. 2013, 84, S24–S29. [Google Scholar] [CrossRef] [PubMed]
- Degasperi, G.R.; Etchegaray, A.; Marcelino, L.; Sicard, A.; Villalpando, K.; Pinheiro, S.L. Periodontal Disease: General Aspects from Biofilm to the Immune Response Driven by Periodontal Pathogens. AiM 2018, 8, 1–17. [Google Scholar] [CrossRef]
- Carrizales-Sepúlveda, E.F.; Ordaz-Farías, A.; Vera-Pineda, R.; Flores-Ramírez, R. Periodontal Disease, Systemic Inflammation and the Risk of Cardiovascular Disease. Heart Lung Circ. 2018, 27, 1327–1334. [Google Scholar] [CrossRef]
- Aminoshariae, A.; Nosrat, A.; Jakovljevic, A.; Jaćimović, J.; Narasimhan, S.; Nagendrababu, V. Tooth Loss Is a Risk Factor for Cardiovascular Disease Mortality: A Systematic Review with Meta-Analyses. J. Endod. 2024, 50, 1370–1380. [Google Scholar] [CrossRef]
- Joshy, G.; Arora, M.; Korda, R.J.; Chalmers, J.; Banks, E. Is Poor Oral Health a Risk Marker for Incident Cardiovascular Disease Hospitalisation and All-Cause Mortality? Findings from 172 630 Participants from the Prospective 45 and Up Study. BMJ Open 2016, 6, e012386. [Google Scholar] [CrossRef] [PubMed]
- Liljestrand, J.M.; Havulinna, A.S.; Paju, S.; Männistö, S.; Salomaa, V.; Pussinen, P.J. Missing Teeth Predict Incident Cardiovascular Events, Diabetes, and Death. J. Dent. Res. 2015, 94, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Dhungana, G.; Srisai, D.; Sampath, C.; Soliman, J.; Kelly, R.M.; Saleh, H.Y.; Sedik, A.; Raynes, E.; Ferguson, A.; Alluri, L.S.C.; et al. Unveiling the Molecular Crosstalk Between Periodontal and Cardiovascular Diseases: A Systematic Review. Dent. J. 2025, 13, 98. [Google Scholar] [CrossRef]
- Farooq, V.; Head, S.J.; Kappetein, A.P.; Serruys, P.W. Widening Clinical Applications of the SYNTAX Score. Heart 2014, 100, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Aksu, F.; Ahmed, S. Gensini Score’s Severity and Its Relationship with Risk Factors for Coronary Artery Disease Among Patients Who Underwent Angiography in Somalia’s Largest PCI Centre. IJGM 2024, 17, 187–192. [Google Scholar] [CrossRef]
- Dietrich, T.; Sharma, P.; Walter, C.; Weston, P.; Beck, J. The Epidemiological Evidence behind the Association between Periodontitis and Incident Atherosclerotic Cardiovascular Disease. J. Clin. Periodontol. 2013, 40, S70–S84. [Google Scholar] [CrossRef]
- Gheorghita, D.; Eördegh, G.; Nagy, F.; Antal, M. Periodontal disease as a risk factor for atherosclerotic cardiovascular disease. Med. Wkly. 2019, 160, 419–425. [Google Scholar] [CrossRef]
- Herrera, D.; Molina, A.; Buhlin, K.; Klinge, B. Periodontal Diseases and Association with Atherosclerotic Disease. Periodontology 2000 2020, 83, 66–89. [Google Scholar] [CrossRef]
- Schenkein, H.A.; Papapanou, P.N.; Genco, R.; Sanz, M. Mechanisms Underlying the Association between Periodontitis and Atherosclerotic Disease. Periodontology 2000 2020, 83, 90–106. [Google Scholar] [CrossRef] [PubMed]
- Gianos, E.; Jackson, E.A.; Tejpal, A.; Aspry, K.; O’Keefe, J.; Aggarwal, M.; Jain, A.; Itchhaporia, D.; Williams, K.; Batts, T.; et al. Oral Health and Atherosclerotic Cardiovascular Disease: A Review. Am. J. Prev. Cardiol. 2021, 7, 100179. [Google Scholar] [CrossRef]
- Di Pietro, M.; Filardo, S.; Falasca, F.; Turriziani, O.; Sessa, R. Infectious Agents in Atherosclerotic Cardiovascular Diseases through Oxidative Stress. IJMS 2017, 18, 2459. [Google Scholar] [CrossRef]
- Haraszthy, V.I.; Zambon, J.J.; Trevisan, M.; Zeid, M.; Genco, R.J. Identification of Periodontal Pathogens in Atheromatous Plaques. J. Periodontol. 2000, 71, 1554–1560. [Google Scholar] [CrossRef]
- Joshi, C.; Bapat, R.; Anderson, W.; Dawson, D.; Hijazi, K.; Cherukara, G. Detection of Periodontal Microorganisms in Coronary Atheromatous Plaque Specimens of Myocardial Infarction Patients: A Systematic Review and Meta-Analysis. Trends Cardiovasc. Med. 2021, 31, 69–82. [Google Scholar] [CrossRef]
- Kędzia, A.; Ciecierski, M.; Kufel, A.; Wierzbowska, M.; Kwapisz, E. Isolation of anaerobic bacteria from atherosclerotic plaques from carotid arteries. Acta Angiol. 2012, 18, 59–67. [Google Scholar]
- Kim, H.-J.; Cha, G.S.; Kim, H.-J.; Kwon, E.-Y.; Lee, J.-Y.; Choi, J.; Joo, J.-Y. Porphyromonas gingivalis Accelerates Atherosclerosis through Oxidation of High-Density Lipoprotein. J. Periodontal Implant. Sci. 2018, 48, 60. [Google Scholar] [CrossRef] [PubMed]
- Joo, J.; Cha, G.S.; Chung, J.; Lee, J.; Kim, S.; Choi, J. Peptide 19 of Porphyromonas gingivalis Heat Shock Protein Is a Potent Inducer of Low-Density Lipoprotein Oxidation. J. Periodontol. 2017, 88, e58–e64. [Google Scholar] [CrossRef]
- Rao, A.; D’Souza, C.; Subramanyam, K.; Rai, P.; Thomas, B.; Gopalakrishnan, M.; Karunasagar, I.; Kumar, B.K. Molecular Analysis Shows the Presence of Periodontal Bacterial DNA in Atherosclerotic Plaques from Patients with Coronary Artery Disease. Indian. Heart J. 2021, 73, 218–220. [Google Scholar] [CrossRef]
- Farrugia, C.; Stafford, G.P.; Murdoch, C. Porphyromonas gingivalis Outer Membrane Vesicles Increase Vascular Permeability. J. Dent. Res. 2020, 99, 1494–1501. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, C.; Stafford, G.P.; Potempa, J.; Wilkinson, R.N.; Chen, Y.; Murdoch, C.; Widziolek, M. Mechanisms of Vascular Damage by Systemic Dissemination of the Oral Pathogen Porphyromonas gingivalis. FEBS J. 2021, 288, 1479–1495. [Google Scholar] [CrossRef]
- Bugueno, I.M.; Zobairi El-Ghazouani, F.; Batool, F.; El Itawi, H.; Anglès-Cano, E.; Benkirane-Jessel, N.; Toti, F.; Huck, O. Porphyromonas Gingivalis Triggers the Shedding of Inflammatory Endothelial Microvesicles That Act as Autocrine Effectors of Endothelial Dysfunction. Sci. Rep. 2020, 10, 1778. [Google Scholar] [CrossRef]
- Zhang, B.; Elmabsout, A.; Khalaf, H.; Basic, V.T.; Jayaprakash, K.; Kruse, R.; Bengtsson, T.; Sirsjö, A. The Periodontal Pathogen Porphyromonas Gingivalis Changes the Gene Expression in Vascular Smooth Muscle Cells Involving the TGFbeta/Notch Signalling Pathway and Increased Cell Proliferation. BMC Genom. 2013, 14, 770. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-J.; Kim, Y.; Kim, M.-K.; Park, H.-R.; Kim, H.-J.; Bae, S.-K.; Bae, M.-K. Infection of Porphyromonas Gingivalis Increases Phosphate-Induced Calcification of Vascular Smooth Muscle Cells. Cells 2020, 9, 2694. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Lu, R.; Meng, H.; Wang, X.; Hou, J. Platelet Activation and Platelet–Leukocyte Interaction in Generalized Aggressive Periodontitis. J. Leukoc. Biol. 2016, 100, 1155–1166. [Google Scholar] [CrossRef]
- Pussinen, P.J.; Alfthan, G.; Rissanen, H.; Reunanen, A.; Asikainen, S.; Knekt, P. Antibodies to Periodontal Pathogens and Stroke Risk. Stroke 2004, 35, 2020–2023. [Google Scholar] [CrossRef]
- Buhlin, K. Risk Factors for Cardiovascular Disease in Patients with Periodontitis. Eur. Heart J. 2003, 24, 2099–2107. [Google Scholar] [CrossRef]
- Ylöstalo, P.; Suominen-Taipale, L.; Reunanen, A.; Knuuttila, M. Association between Body Weight and Periodontal Infection. J. Clin. Periodontol. 2008, 35, 297–304. [Google Scholar] [CrossRef]
- Donders, H.C.M.; IJzerman, L.M.; Soffner, M.; Van ‘T Hof, A.W.J.; Loos, B.G.; De Lange, J. Elevated Coronary Artery Calcium Scores Are Associated with Tooth Loss. PLoS ONE 2020, 15, e0243232. [Google Scholar] [CrossRef]
- Pham, T.D.; Zou, L.; Patel, M.; Holmes, S.B.; Coulthard, P. Impact of Tooth Loss and Patient Characteristics on Coronary Artery Calcium Score Classification and Prediction. Sci. Rep. 2024, 14, 28315. [Google Scholar] [CrossRef]
- Buhlin, K.; Mäntylä, P.; Paju, S.; Peltola, J.S.; Nieminen, M.S.; Sinisalo, J.; Pussinen, P.J. Periodontitis Is Associated with Angiographically Verified Coronary Artery Disease. J. Clin. Periodontol. 2011, 38, 1007–1014. [Google Scholar] [CrossRef]
- Shen, M.; Li, Z.; Li, H.; Yan, X.; Feng, B.; Xu, L. Association of Periodontitis and Tooth Loss with Extent of Coronary Atherosclerosis in Patients with Type 2 Diabetes Mellitus. Front. Endocrinol. 2023, 14, 1243992. [Google Scholar] [CrossRef]
- Elter, J.R.; Offenbacher, S.; Toole, J.F.; Beck, J.D. Relationship of Periodontal Disease and Edentulism to Stroke/TIA. J. Dent. Res. 2003, 82, 998–1001. [Google Scholar] [CrossRef]
- Gul, M.; Gurses, D.; Yalcin, F.; Buyukkaya, E.; Turan, C.; Inan, O. The Relationship between Tooth Loss and Coronary Artery Disease. Acta Cardiol. 2012, 67, 209–215. [Google Scholar]
- Romandini, M.; Baima, G.; Antonoglou, G.; Bueno, J.; Figuero, E.; Sanz, M. Periodontitis, Edentulism, and Risk of Mortality: A Systematic Review with Meta-Analyses. J. Dent. Res. 2021, 100, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.-T.; Leng, W.-D.; Lam, Y.-Y.; Yan, B.P.; Wei, X.-M.; Weng, H.; Kwong, J.S.W. Periodontal Disease and Carotid Atherosclerosis: A Meta-Analysis of 17,330 Participants. Int. J. Cardiol. 2016, 203, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Geng, X.; Sun, J.; Zhang, S.; Yu, W.; Zhang, X.; Liu, H. The Risk of Periodontitis for Peripheral Vascular Disease: A Systematic Review and Meta-Analysis. Rev. Cardiovasc. Med. 2019, 20, 81. [Google Scholar] [CrossRef] [PubMed]
- Bahekar, A.A.; Singh, S.; Saha, S.; Molnar, J.; Arora, R. The Prevalence and Incidence of Coronary Heart Disease Is Significantly Increased in Periodontitis: A Meta-Analysis. Am. Heart J. 2007, 154, 830–837. [Google Scholar] [CrossRef]
- Bengtsson, V.W.; Persson, G.R.; Berglund, J.S.; Renvert, S. Periodontitis Related to Cardiovascular Events and Mortality: A Long-Time Longitudinal Study. Clin. Oral Investig. 2021, 25, 4085–4095. [Google Scholar] [CrossRef]
- Holmlund, A.; Holm, G.; Lind, L. Number of Teeth as a Predictor of Cardiovascular Mortality in a Cohort of 7,674 Subjects Followed for 12 Years. J. Periodontol. 2010, 81, 870–876. [Google Scholar] [CrossRef]
- Yu, Y.-H.; Cheung, W.S.; Steffensen, B.; Miller, D.R. Number of Teeth Is Associated with All-Cause and Disease-Specific Mortality. BMC Oral Health 2021, 21, 568. [Google Scholar] [CrossRef]
- Glick, M.; Greenberg, B.L. The Potential Role of Dentists in Identifying Patients' Risk of Experiencing Coronary Heart Disease Events. J. Am. Dent. Assoc. 2005, 136, 1541–1546. [Google Scholar] [CrossRef] [PubMed]
- Denny, C.; Natarajan, S.; Jophy, J.; Kp, N.; Lewis, A.J.; Yellapurkar, S. Assessing Socioeconomic Status through Dental and Associated Tissue Characteristics: A Cross-Sectional Study for Human Identification. F1000Research 2025, 14, 220. [Google Scholar] [CrossRef] [PubMed]
- Davis, D.M.; Fiske, J.; Scott, B.; Radford, D.R. The Emotional Effects of Tooth Loss: A Preliminary Quantitative Study. Br. Dent. J. 2000, 188, 503–506. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Park, S.-Y.; Kim, S.-H.; Kang, S.-H.; Yoon, C.-H.; Lee, H.-J.; Yun, P.-Y.; Youn, T.-J.; Chae, I.-H. Improved Oral Hygiene Care Attenuates the Cardiovascular Risk of Oral Health Disease: A Population-Based Study from Korea. Eur. Heart J. 2019, 40, 1138–1145. [Google Scholar] [CrossRef]
| Author | Year | Study Type | N/Population | Method | Outcome |
|---|---|---|---|---|---|
| Buhlin et al. [42] | 2003 | Observational | 96 patients | Coronary Angiography | Fewer teeth, severe periodontal disease |
| Elter et al. [44] | 2003 | Cross-sectional | 10,906 patients | Multivessel CAD + Stroke/TIA | Increased risk of multivessel CAD |
| Ylöstalo et al. [39] | 2006 | Observational | 8690 patients | CRP | Dose–response relationship between missing teeth and inflammation |
| Gul et al. [45] | 2012 | Cross-sectional | 321 patients | Gensini score | Significantly higher Gensini score |
| Liljestrand et al. [15] | 2015 | Longitudinal cohort | 1500 adults | Cardiovascular Events | All cardiovascular markers increase with edentulism |
| Donders et al. [40] | 2020 | Observational | 212 adults | Cardiovascular Events | Elevated scores in those with missing teeth |
| Gao et al. [5] | 2021 | Meta-analysis | Over 200,000 participants | Periodontitis and the number of teeth alongside CAD | Increased risk of CAD with missing teeth |
| Shen M et al. [43] | 2023 | Observational | 272 patients | Coronary artery calcium computed tomography scan | More missing teeth → more severe CAD |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cinezan, C.; Rus, C.B.; Ilias, I.T.; Cinezan, A. Dental Disease as a Clinical Marker for Coronary Artery Disease Severity: A Narrative Review of Current Evidence and Mechanisms. Medicina 2025, 61, 1714. https://doi.org/10.3390/medicina61091714
Cinezan C, Rus CB, Ilias IT, Cinezan A. Dental Disease as a Clinical Marker for Coronary Artery Disease Severity: A Narrative Review of Current Evidence and Mechanisms. Medicina. 2025; 61(9):1714. https://doi.org/10.3390/medicina61091714
Chicago/Turabian StyleCinezan, Corina, Camelia Bianca Rus, Ioana Tiberia Ilias, and Alexandra Cinezan. 2025. "Dental Disease as a Clinical Marker for Coronary Artery Disease Severity: A Narrative Review of Current Evidence and Mechanisms" Medicina 61, no. 9: 1714. https://doi.org/10.3390/medicina61091714
APA StyleCinezan, C., Rus, C. B., Ilias, I. T., & Cinezan, A. (2025). Dental Disease as a Clinical Marker for Coronary Artery Disease Severity: A Narrative Review of Current Evidence and Mechanisms. Medicina, 61(9), 1714. https://doi.org/10.3390/medicina61091714






