Coronary Angioplasty with Drug-Coated Balloons: Pharmacological Foundations, Clinical Efficacy, and Future Directions
Abstract
1. Introduction
2. Advantages of Drug-Coated Balloons Compared to Stents
3. Pharmacological Premises
- (a)
- Drug Selection and Mechanism of Action
- (b)
- Drug Delivery Technologies and Excipient Systems
4. Clinical Applications and Indications of DCBs
- (a)
- In-Stent Restenosis (ISR)
- (b)
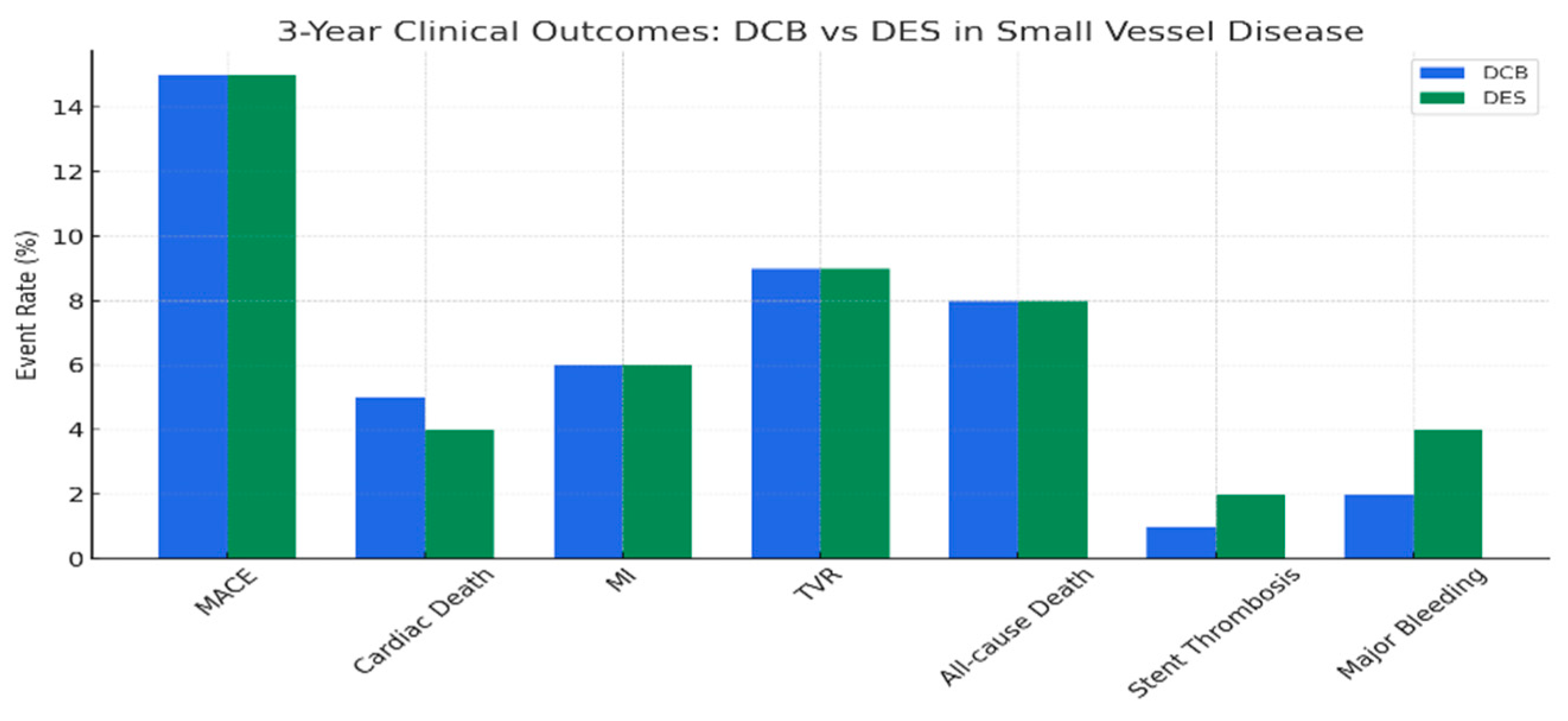
- Small Vessel Disease (SVD)
- (c)
- De Novo Lesions
- (d)
- Bifurcation Lesions
- (e)
- High-Bleeding-Risk (HBR) Patients
- (f)
- Potential roles of DCBs
4.1. Unstable Plaques (ACS, Ruptured Lesions)
4.2. Diffuse Coronary Disease
5. Comparative Efficacy: Paclitaxel vs. Sirolimus
- (a)
- Randomized Trials and Meta-Analyses
- (b)
- Safety and Healing
6. Technical Considerations
6.1. Predilatation
6.2. Semi-Compliant and Scoring Balloons
6.3. Imaging Guidance (IVUS/OCT)
6.4. Role of Coronary Physiology in DCB Angioplasty
6.5. FFR in DCB Angioplasty
6.6. iFR in DCB Angioplasty
6.7. Post-PCI Role
7. Discussion
8. Future Perspectives
- Dual-Drug DCBs: Combining paclitaxel and sirolimus could leverage synergistic effects, potentially reducing restenosis rates further [12].
- Ultra-Thin Balloons: These enhance deliverability in complex anatomies, minimizing vessel trauma [27].
- Novel Coatings: Biodegradable matrices and nanoparticle-based systems may improve drug elution and reduce inflammation [12].
- AI Integration: AI-driven algorithms for lesion characterization, balloon sizing, and patient selection could optimize outcomes [61].
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Grüntzig, A.R.; Senning, A.; Siegenthaler, W.E. Nonoperative dilatation of coronary artery stenosis: Percutaneous transluminal coronary angioplasty. N. Engl. J. Med. 1978, 301, 61–68. [Google Scholar] [CrossRef]
- Jeremias, A.; Davies, J.E.; Maehara, A.; Matsumura, M.; Schneider, J.; Tang, K.; Talwar, S.; Marques, K.; Shammas, N.W.; Gruberg, L.; et al. Blinded Physiological Assessment of Residual Ischemia After Successful Angiographic Percutaneous Coronary Intervention: The DEFINE PCI Study. JACC Cardiovasc. Interv. 2019, 12, 1991–2001. [Google Scholar] [CrossRef] [PubMed]
- Serruys, P.W.; Luijten, H.E.; Beatt, K.J.; Geuskens, R.; de Feyter, P.J.; van den Brand, M.; Reiber, J.H.; ten Katen, H.J.; van Es, G.A.; Hugenholtz, P.G. Incidence of restenosis after successful coronary angioplasty: A time-related phenomenon. A quantitative angiographic study in 342 consecutive patients at 1, 2, 3, and 4 months. Circulation 1988, 77, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Gallinoro, E.; Almendarez, M.; Alvarez-Velasco, R.; Barbato, E.; Avanzas, P. Bioresorbable stents: Is the game over? Int. J. Cardiol. 2022, 361, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Sigwart, U.; Puel, J.; Mirkovitch, V.; Joffre, F.; Kappenberger, L. Intravascular stents to prevent occlusion and restenosis after transluminal angioplasty. N. Engl. J. Med. 1987, 316, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Fischman, D.L.; Leon, M.B.; Baim, D.S.; Schatz, R.A.; Savage, M.P.; Penn, I.; Detre, K.; Veltri, L.; Ricci, D.; Nobuyoshi, M.; et al. A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. Stent Restenosis Study Investigators. N. Engl. J. Med. 1994, 331, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Morice, M.C.; Serruys, P.W.; Sousa, J.E.; Fajadet, J.; Ban Hayashi, E.; Perin, M.; Colombo, A.; Schuler, G.; Barragan, P.; Guagliumi, G.; et al. Randomized Study with the Sirolimus-Coated Bx Velocity Balloon-Expandable Stent in the Treatment of Patients with de Novo Native Coronary Artery Lesions. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N. Engl. J. Med. 2002, 346, 1773–1780. [Google Scholar] [CrossRef] [PubMed]
- McFadden, E.P.; Stabile, E.; Regar, E.; Cheneau, E.; Ong, A.T.; Kinnaird, T.; Suddath, W.O.; Weissman, N.J.; Torguson, R.; Kent, K.M.; et al. Late thrombosis in drug-eluting coronary stents after discontinuation of antiplatelet therapy. Lancet 2004, 364, 1519–1521. [Google Scholar] [CrossRef] [PubMed]
- Scheller, B.; Speck, U.; Abramjuk, C.; Bernhardt, U.; Böhm, M.; Nickenig, G. Paclitaxel balloon coating, a novel method for prevention and therapy of restenosis. Circulation 2004, 110, 810–814. [Google Scholar] [CrossRef] [PubMed]
- Byrne, R.A.; Neumann, F.J.; Mehilli, J.; Pinieck, S.; Wolff, B.; Tiroch, K.; Schulz, S.; Fusaro, M.; Ott, I.; Ibrahim, T.; et al. Paclitaxel-eluting balloons, paclitaxel-eluting stents, and balloon angioplasty in patients with restenosis after implantation of a drug-eluting stent (ISAR-DESIRE 3): A randomised, open-label trial. Lancet 2013, 381, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Kleber, F.X.; Schulz, A.; Waliszewski, M.; Hauschild, T.; Böhm, M.; Dietz, U.; Cremers, B.; Scheller, B.; Clever, Y.P. Local paclitaxel induces late lumen enlargement in coronary arteries after balloon angioplasty. Clin. Res. Cardiol. 2015, 104, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Buccheri, D.; Lombardo, R.; Cortese, B. Drug-coated balloons for coronary artery disease: Current concepts and controversies. Future Cardiol. 2019, 15, 437–454. [Google Scholar] [CrossRef] [PubMed]
- Jeger, R.V.; Farah, A.; Ohlow, M.A.; Mangner, N.; Möbius-Winkler, S.; Leibundgut, G.; Weilenmann, D.; Wöhrle, J.; Richter, S.; Schreiber, M.; et al. Drug-coated balloons for small coronary artery disease (BASKET-SMALL 2): An open-label randomised non-inferiority trial. Lancet 2018, 392, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Cortese, B.; Di Palma, G.; Guimaraes, M.G.; Piraino, D.; Orrego, P.S.; Buccheri, D.; Rivero, F.; Perotto, A.; Zambelli, G.; Alfonso, F. Drug-Coated Balloon Versus Drug-Eluting Stent for Small Coronary Vessel Disease: PICCOLETO II Randomized Clinical Trial. JACC Cardiovasc. Interv. 2020, 13, 2840–2849. [Google Scholar] [CrossRef] [PubMed]
- Scheller, B.; Mangner, N.; Jeger, R.V.; Afan, S.; Mahfoud, F.; Woitek, F.J.; Fahrni, G.; Schwenke, C.; Schnorr, B.; Kleber, F. A randomised trial of sirolimus- versus paclitaxel-coated balloons for de novo coronary lesions. EuroIntervention 2024, 20, e1322–e1329. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hu, W.; Jiang, J. Hypersensitivity and in-stent restenosis in coronary stent materials. Front. Bioeng. Biotechnol. 2022, 10, 1003322. [Google Scholar] [CrossRef]
- Todd, M.; Liu, L.B.; Saul, J.M.; Yazdani, S.K. Pre-clinical investigation of liquid sirolimus for local drug delivery. Front. Cardiovasc. Med. 2023, 10, 1184816. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Unverdorben, M.; Vallbracht, C.; Cremers, B.; Heuer, H.; Hengstenberg, C.; Maikowski, C.; Werner, G.S.; Antoni, D.; Kleber, F.X.; Bocksch, W.; et al. Paclitaxel-coated balloon catheter versus paclitaxel-coated stent for the treatment of coronary in-stent restenosis: The three-year results of the PEPCAD II ISR study. EuroIntervention 2015, 11, 926–934. [Google Scholar] [CrossRef] [PubMed]
- Rissanen, T.T.; Uskela, S.; Eränen, J.; Mäntylä, P.; Olli, A.; Romppanen, H.; Siljander, A.; Pietilä, M.; Minkkinen, M.J.; Tervo, J.; et al. Drug-coated balloon for treatment of de-novo coronary artery lesions in patients with high bleeding risk (DEBUT): A single-blind, randomised, non-inferiority trial. Lancet 2019, 394, 230–239, Erratum in Lancet 2019, 394, 218. https://doi.org/10.1016/S0140-6736(19)31464-3. [Google Scholar] [CrossRef] [PubMed]
- Gurgoglione, F.L.; De Gregorio, M.; Benatti, G.; Donelli, D.; Vignali, L.; Solinas, E.; Tadonio, I.; Denegri, A.; Covani, M.; Dallaglio, G.; et al. Paclitaxel-Coated Versus Sirolimus-Coated Eluting Balloons for Percutaneous Coronary Interventions: Pharmacodynamic Properties, Clinical Evidence, and Future Perspectives. Future Pharmacol. 2024, 4, 775–787. [Google Scholar] [CrossRef]
- Teichgräber, U.; Ingwersen, M.; Platzer, S.; Lehmann, T.; Zeller, T.; Aschenbach, R.; Scheinert, D. Head-to-head comparison of sirolimus- versus paclitaxel-coated balloon angioplasty in the femoropopliteal artery: Study protocol for the randomized controlled SIRONA trial. Trials 2021, 22, 665. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Long, B.H.; Fairchild, C.R. Paclitaxel inhibits progression of mitotic cells to G1 phase by interference with spindle formation without affecting other microtubule functions during anaphase and telephase. Cancer Res. 1994, 54, 4355–4361. [Google Scholar]
- Scheller, B.; Hehrlein, C.; Bocksch, W.; Rutsch, W.; Haghi, D.; Dietz, U.; Böhm, M.; Speck, U. Treatment of coronary in-stent restenosis with a paclitaxel-coated balloon catheter. N. Engl. J. Med. 2006, 355, 2113–2124. [Google Scholar] [CrossRef] [PubMed]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165, Erratum in Eur. Heart J. 2019, 40, 3096. https://doi.org/10.1093/eurheartj/ehz507. [Google Scholar] [CrossRef] [PubMed]
- Axel, D.I.; Kunert, W.; Göggelmann, C.; Oberhoff, M.; Herdeg, C.; Küttner, A.; Wild, D.H.; Brehm, B.R.; Riessen, R.; Köveker, G.; et al. Paclitaxel inhibits arterial smooth muscle cell proliferation and migration in vitro and in vivo using local drug delivery. Circulation 1997, 96, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Marx, S.O.; Marks, A.R. Bench to bedside: The development of rapamycin and its application to stent restenosis. Circulation 2001, 104, 852–855. [Google Scholar] [CrossRef] [PubMed]
- Sciahbasi, A.; Salvi, N.; Heang, T.M.; Perez, I.S.; Geraci, S.; Vaccaro, G.; Benincasa, S.; Nuruddin, A.A.; Ocaranza, R.; Giannini, F.; et al. Long term clinical outcome of sirolimus drug coated balloons in large coronary vessels. Catheter. Cardiovasc. Interv. 2024, 103, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Siontis, G.C.; Stefanini, G.G.; Mavridis, D.; Siontis, K.C.; Alfonso, F.; Pérez-Vizcayno, M.J.; Byrne, R.A.; Kastrati, A.; Meier, B.; Salanti, G.; et al. Percutaneous coronary interventional strategies for treatment of in-stent restenosis: A network meta-analysis. Lancet 2015, 386, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Baan, J., Jr.; Claessen, B.E.; Dijk, K.B.; Vendrik, J.; van der Schaaf, R.J.; Meuwissen, M.; van Royen, N.; Gosselink, A.T.M.; van Wely, M.H.; Dirkali, A.; et al. A Randomized Comparison of Paclitaxel-Eluting Balloon Versus Everolimus-Eluting Stent for the Treatment of Any In-Stent Restenosis: The DARE Trial. JACC Cardiovasc. Interv. 2018, 11, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Alfonso, F.; Pérez-Vizcayno, M.J.; Cárdenas, A.; García Del Blanco, B.; Seidelberger, B.; Iñiguez, A.; Gómez-Recio, M.; Masotti, M.; Velázquez, M.T.; Sanchís, J.; et al. A randomized comparison of drug-eluting balloon versus everolimus-eluting stent in patients with bare-metal stent-in-stent restenosis: The RIBS V Clinical Trial (Restenosis Intra-stent of Bare Metal Stents: Paclitaxel-eluting balloon vs. everolimus-eluting stent). J. Am. Coll. Cardiol. 2014, 63, 1378–1386. [Google Scholar] [CrossRef] [PubMed]
- Giacoppo, D.; Alfonso, F.; Xu, B.; Claessen, B.E.P.M.; Adriaenssens, T.; Jensen, C.; Pérez-Vizcayno, M.J.; Kang, D.Y.; Degenhardt, R.; Pleva, L.; et al. Paclitaxel-coated balloon angioplasty vs. drug-eluting stenting for the treatment of coronary in-stent restenosis: A comprehensive, collaborative, individual patient data meta-analysis of 10 randomized clinical trials (DAEDALUS study). Eur. Heart J. 2020, 41, 3715–3728, Erratum in Eur. Heart J. 2020, 41, 3728. https://doi.org/10.1093/eurheartj/ehz861Erratum in Eur. Heart J. 2025, 46, 2485. https://doi.org/10.1093/eurheartj/ehaf217. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yeh, R.W.; Shlofmitz, R.; Moses, J.; Bachinsky, W.; Dohad, S.; Rudick, S.; Stoler, R.; Jefferson, B.K.; Nicholson, W.; Altman, J.; et al. Paclitaxel-Coated Balloon vs Uncoated Balloon for Coronary In-Stent Restenosis: The AGENT IDE Randomized Clinical Trial. JAMA. 2024, 331, 1015–1024. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, K.; Fu, G.; Tong, Q.; Liu, B.; Han, X.; Zhang, J.; Ma, G.; Yang, Q.; Li, H.; Zhou, Y.; et al. Biolimus-Coated Balloon in Small-Vessel Coronary Artery Disease: The BIO-RISE CHINA Study. JACC Cardiovasc. Interv. 2022, 15, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Qiao, S.; Su, X.; Chen, Y.; Jin, Z.; Chen, H.; Xu, B.; Kong, X.; Pang, W.; Liu, Y.; et al. Drug-Coated Balloon Versus Drug-Eluting Stent for Small-Vessel Disease: The RESTORE SVD China Randomized Trial. JACC Cardiovasc. Interv. 2018, 11, 2381–2392. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, W.; Nuruddin, A.; Ali, M.; Kader, A.; Houng, T.; Liew, B.; Ali, R.; Zuhdi, A.; Ismail, M.; Yusof, A.; et al. Treatment of Coronary De Novo Lesions by a Sirolimus- or Paclitaxel-Coated Balloon. JACC Cardiovasc. Interv. 2022, 15, 770–779. [Google Scholar] [CrossRef] [PubMed]
- Vos, N.S.; Fagel, N.D.; Amoroso, G.; Herrman, J.R.; Patterson, M.S.; Piers, L.H.; van der Schaaf, R.J.; Slagboom, T.; Vink, M.A. Paclitaxel-Coated Balloon Angioplasty Versus Drug-Eluting Stent in Acute Myocardial Infarction: The REVELATION Randomized Trial. JACC Cardiovasc. Interv. 2019, 12, 1691–1699. [Google Scholar] [CrossRef] [PubMed]
- Rathore, S.; Tehrani, S.; Prvulovic, D.; Araya, M.; Lefèvre, T.; Banning, A.P.; Burzotta, F.; Rigatelli, G.; Gutierrez-Chico, J.L.; Bonaventura, K.; et al. Drug coated balloons and their role in bifurcation coronary angioplasty: Appraisal of the current evidence and future directions. Expert. Rev. Med. Devices. 2020, 17, 1021–1033. [Google Scholar] [CrossRef] [PubMed]
- Aihara, K.; Aihara, K.; Torii, S.; Ito, M.; Koseki, K.; Shiozaki, M.; Sato, Y.; Nakamura, N.; Yoshikawa, A.; Ikari, Y.; et al. Comparison of vessel healing in rabbit iliac arteries treated with sirolimus- and paclitaxel-coated balloons. JACC Cardiovasc. Interv. 2024, 17, 401–410. [Google Scholar] [CrossRef]
- Scheller, B.; Ohlow, M.A.; Ewen, S.; Kische, S.; Rudolph, T.K.; Clever, Y.P.; Wagner, A.; Richter, S.; El-Garhy, M.; Böhm, M.; et al. Bare metal or drug-eluting stent versus drug-coated balloon in non-ST-elevation myocardial infarction: The randomised PEPCAD NSTEMI trial. EuroIntervention. 2020, 15, 1527–1533. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.W.; Christiansen, E.H.; Ali, Z.A.; Andreasen, L.N.; Maehara, A.; Ahmad, Y.; Landmesser, U.; Holm, N.R. Intravascular imaging-guided coronary drug-eluting stent implantation: An updated network meta-analysis. Lancet 2024, 403, 824–837. [Google Scholar] [CrossRef] [PubMed]
- Ielasi, A.; Buono, A.; Pellicano, M.; Tedeschi, D.; Loffi, M.; Donahue, M.; Regazzoli, D.; De Angelis, G.; Danzi, G.; Reimers, B.; et al. A HYbrid APproach Evaluating a DRug-Coated Balloon in Combination With a New-Generation Drug-Eluting Stent in the Treatment of De Novo Diffuse Coronary Artery Disease: The HYPER Pilot Study. Cardiovasc. Revasc. Med. 2021, 28, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Lansky, A.; Grubman, D.; Scheller, B. Paclitaxel-coated balloons: A safe alternative to drug-eluting stents for coronary in-stent restenosis. Eur. Heart J. 2020, 41, 3729–3731. [Google Scholar] [CrossRef] [PubMed]
- Cortese, B.; Testa, L.; Heang, T.M.; Ielasi, A.; Bossi, I.; Latini, R.A.; Lee, C.Y.; Perez, I.S.; Milazzo, D.; Caiazzo, G.; et al. Sirolimus-Coated Balloon in an All-Comer Population of Coronary Artery Disease Patients: The EASTBOURNE Prospective Registry. JACC Cardiovasc. Interv. 2023, 16, 1794–1803. [Google Scholar] [CrossRef] [PubMed]
- Jeger, R.V.; Farah, A.; Ohlow, M.A.; Mangner, N.; Möbius-Winkler, S.; Weilenmann, D.; Wöhrle, J.; Stachel, G.; Markovic, S.; Leibundgut, G.; et al. Long-term efficacy and safety of drug-coated balloons versus drug-eluting stents for small coronary artery disease (BASKET-SMALL 2): 3-year follow-up of a randomised, non-inferiority trial. Lancet 2020, 396, 1504–1510, Erratum in Lancet 2020, 396, 1490. https://doi.org/10.1016/S0140-6736(20)32283-2. [Google Scholar] [CrossRef] [PubMed]
- Bhogal, S.; Hill, A.; Merdler, I.; Wermers, J.; Ben-Dor, Y.; Waksman, R. Drug-coated balloons for coronary artery disease: An updated review with future perspectives. Cardiovasc. Revascularization Med. 2024, 69, 79–89. [Google Scholar] [CrossRef]
- SPACIOUS Investigators. Sirolimus-coated versus paclitaxel-coated balloons in coronary bifurcation lesions: The SPACIOUS trial. Catheter. Cardiovasc. Interv. 2025, 105, 89–97. [Google Scholar] [CrossRef]
- Liu, H.; Li, Y.; Fu, G.; An, J.; Chen, S.; Zhong, Z.; Liu, B.; Qiu, C.; Ma, L.; Cong, H.; et al. Sirolimus- vs Paclitaxel-Coated Balloon for the Treatment of Coronary In-Stent Restenosis: The SIBLINT-ISR Randomized Trial. JACC Cardiovasc. Interv. 2025, 18, 963–971. [Google Scholar] [CrossRef]
- Cortese, B.; Caiazzo, G.; Di Palma, G.; De Rosa, S. Comparison Between Sirolimus- and Paclitaxel-Coated Balloon for Revascularization of Coronary Arteries: The SIRPAC (SIRolimus-PAClitaxel) Study. Cardiovasc. Revascularization Med. 2021, 28, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Choke, E.; Peh, E.; Tang, T.; Cheng, S.; Tay, J.; Aw, D.; Vijaykumar, K. MagicTouch PTA Sirolimus-Coated Balloon for Femoropopliteal and Below-the-Knee Disease: 3-Year Outcomes of the XTOSI Trial. Ann. Vasc. Surg. 2024, 106, 8–15. [Google Scholar] [CrossRef]
- Tepe, G.; Laird, J.; Schneider, P.; Brodmann, M.; Krishnan, P.; Micari, A.; Metzger, C.; Scheinert, D.; Zeller, T.; Cohen, D.J.; et al. Drug-coated balloon versus standard percutaneous transluminal angioplasty for the treatment of superficial femoral and popliteal peripheral artery disease: 12-month results from the IN.PACT SFA randomized trial. Circulation 2015, 131, 495–502. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- SELUTION SLR Investigators. Sirolimus-eluting balloon in peripheral artery disease: Early results from, SELUTION SLR. J. Endovasc. Ther. 2023, 30, 178–186. [Google Scholar] [CrossRef]
- Secemsky, E.A.; Shen, C.; Schermerhorn, M.; Yeh, R.W. Longitudinal Assessment of Safety of Femoropopliteal Endovascular Treatment With Paclitaxel-Coated Devices Among Medicare Beneficiaries: The SAFE-PAD Study. JAMA Intern. Med. 2021, 181, 1071–1080. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Greco, A.; Sciahbasi, A.; Abizaid, A.; Mehran, R.; Rigattieri, S.; de la Torre Hernandez, J.M.; Alfonso, F.; Cortese, B. Sirolimus-coated balloon versus everolimus-eluting stent in de novo coronary artery disease: Rationale and design of the TRANSFORM II randomized clinical trial. Catheter. Cardiovasc. Interv. 2022, 100, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Katsanos, K.; Spiliopoulos, S.; Kitrou, P.; Krokidis, M.; Karnabatidis, D. Risk of Death Following Application of Paclitaxel-Coated Balloons and Stents in the Femoropopliteal Artery of the Leg: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2018, 7, e011245. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Otto, S.; Díaz, V.; Weilenmann, D.; Cuculi, F.; Nuruddin, A.; Leibundgut, G.; Alfonso, F.; Ahmad, W.; Pyxaras, S.; Rittger, H.; et al. Crystalline sirolimus-coated balloon (cSCB) angioplasty in an all-comers, patient population with stable and unstable coronary artery disease including chronic total occlusions: Rationale, methodology and design of the SCORE trial. BMC Cardiovasc. Disord. 2023, 23, 176. [Google Scholar] [CrossRef] [PubMed]
- Jeger, R.V.; Eccleshall, S.; Wan Ahmad, W.A.; Ge, J.; Poerner, T.C.; Shin, E.S.; Alfonso, F.; Latib, A.; Ong, P.J.; Rissanen, T.T.; et al. Drug-Coated Balloons for Coronary Artery Disease: Third Report of the International DCB Consensus Group. JACC Cardiovasc. Interv. 2020, 13, 1391–1402. [Google Scholar] [CrossRef] [PubMed]
- Waksman, R.; Pakala, R. Drug-eluting balloon: The comeback kid? Circ. Cardiovasc. Interv. 2009, 2, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.S.; Ann, S.H.; Balbir Singh, G.; Lim, K.H.; Kleber, F.X.; Koo, B.K. Fractional flow reserve-guided paclitaxel-coated balloon treatment for de novo coronary lesions. Catheter. Cardiovasc. Interv. 2016, 88, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Collet, C.; Johnson, N.P.; Mizukami, T.; Fearon, W.F.; Berry, C.; Sonck, J.; Collison, D.; Koo, B.K.; Meneveau, N.; Agarwal, S.K.; et al. Impact of Post-PCI FFR Stratified by Coronary Artery. JACC Cardiovasc. Interv. 2023, 16, 2396–2408. [Google Scholar] [CrossRef] [PubMed]
- Xaplanteris, P.; Fournier, S.; Pijls, N.H.J.; Fearon, W.F.; Barbato, E.; Tonino, P.A.L.; Engstrøm, T.; Kääb, S.; Dambrink, J.H.; Rioufol, G.; et al. Five-Year Outcomes with PCI Guided by Fractional Flow Reserve. N. Engl. J. Med. 2018, 379, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Medrano-Gracia, P.; Ormiston, J.; Webster, M.; Beier, S.; Ellis, C.; Wang, C.; Young, A.A.; Cowan, B.R. Construction of a coronary artery atlas from CT angiography. In Lecture Notes in Computer Science; Including Subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics; Springer: Berlin/Heidelberg, Germany, 2014; pp. 513–520. [Google Scholar]
- Lee, J.M.; Koo, B.K.; Shin, E.S.; Nam, C.W.; Doh, J.H.; Hwang, D.; Park, J.; Kim, K.J.; Zhang, J.; Hu, X.; et al. Clinical implications of three-vessel fractional flow reserve measurement in patients with coronary artery disease. Eur. Heart J. 2018, 39, 945–951. [Google Scholar] [CrossRef] [PubMed]





| Property | Paclitaxel | Sirolimus |
|---|---|---|
| Lipophilicity | High (logP 3.96) | Moderate (logP 2.5) |
| Mechanism | Microtubule stabilization | mTOR inhibition |
| Tissue Retention | Long (weeks) | Shorter (requires sustained delivery) |
| Excipient Examples | Shellac, urea, iopromide | BTHC, PLGA, phospholipids |
| Healing Profile | Delayed endothelialization | Faster endothelialization |
| Inflammatory Response | Moderate | Low |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chioncel, V.; Gherasie, F.; Iancu, A.; Avram, A.-G. Coronary Angioplasty with Drug-Coated Balloons: Pharmacological Foundations, Clinical Efficacy, and Future Directions. Medicina 2025, 61, 1470. https://doi.org/10.3390/medicina61081470
Chioncel V, Gherasie F, Iancu A, Avram A-G. Coronary Angioplasty with Drug-Coated Balloons: Pharmacological Foundations, Clinical Efficacy, and Future Directions. Medicina. 2025; 61(8):1470. https://doi.org/10.3390/medicina61081470
Chicago/Turabian StyleChioncel, Valentin, Flavius Gherasie, Alexandru Iancu, and Anamaria-Georgiana Avram. 2025. "Coronary Angioplasty with Drug-Coated Balloons: Pharmacological Foundations, Clinical Efficacy, and Future Directions" Medicina 61, no. 8: 1470. https://doi.org/10.3390/medicina61081470
APA StyleChioncel, V., Gherasie, F., Iancu, A., & Avram, A.-G. (2025). Coronary Angioplasty with Drug-Coated Balloons: Pharmacological Foundations, Clinical Efficacy, and Future Directions. Medicina, 61(8), 1470. https://doi.org/10.3390/medicina61081470






