The Effectiveness of Semaglutide on a Composite Endpoint of Glycemic Control and Weight Reduction and Its Effect on Lipid Profile Among Obese Type 2 Diabetes Patients
Abstract
1. Introduction
2. Methods
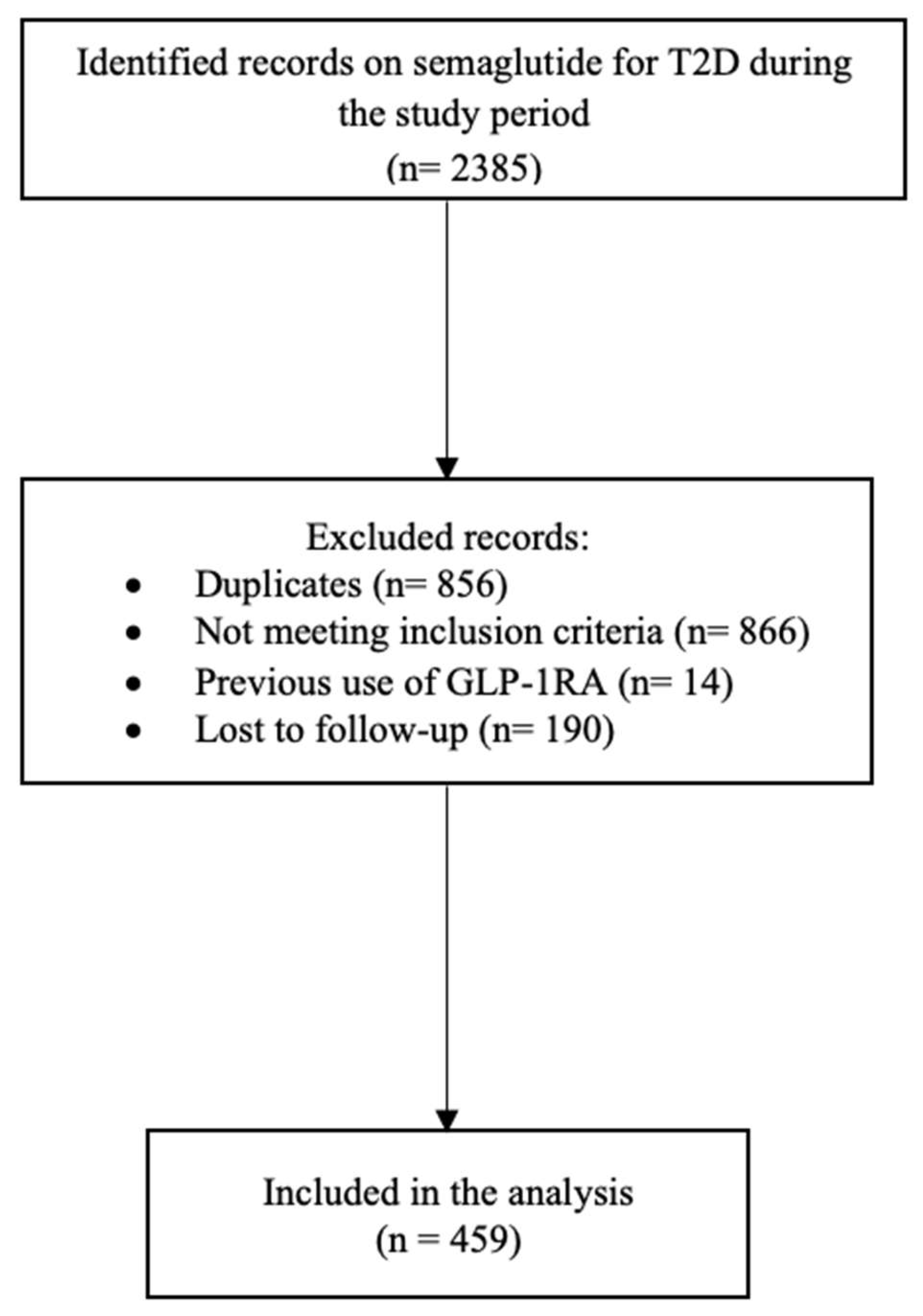
2.1. Study Design, Setting, and Population
2.2. Sample Size Calculation
2.3. Data Collection
2.4. Study Endpoints
2.5. Ethical Considerations
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Composite Endpoint
3.3. Subgroup Analysis
3.4. Efficacy Outcomes and Metabolic Profile Changes
3.5. Factors Associated with Achieving the Composite Endpoint
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body mass index |
| A1C | Hemoglobin A1C |
| CI | Confidence interval |
| CVD | Cardiovascular disease |
| IHD | Ischemic heart disease |
| eGFR | Estimated glomerular filtration rate |
| FBG | Fasting blood glucose |
| GLP1-RA | Glucagon-like peptide-1 receptor agonist |
| HDL | High-density lipoprotein |
| LDL | Low-density lipoprotein |
| SGLT2-I | Sodium-glucose cotransporter-2 inhibitors |
| T2D | Type 2 diabetes mellitus |
| TG | Triglycerides |
| DPP4-I | Dipeptidyl peptidase-4 |
References
- Aras, M.; Tchang, B.G.; Pape, J. Obesity and Diabetes. Nurs. Clin. N. Am. 2021, 56, 527–541. [Google Scholar] [CrossRef] [PubMed]
- Boden, G. Obesity, insulin resistance and free fatty acids. Curr. Opin. Endocrinol. Diabetes Obes. 2011, 18, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Katsiki, N.; Anagnostis, P.; Kotsa, K.; Goulis, D.G.; Mikhailidis, D.P. Obesity, Metabolic Syndrome and the Risk of Microvascular Complications in Patients with Diabetes mellitus. Curr. Pharm. Des. 2019, 25, 2051–2059. [Google Scholar] [CrossRef] [PubMed]
- Althumiri, N.A.; Basyouni, M.H.; AlMousa, N.; AlJuwaysim, M.F.; Almubark, R.A.; BinDhim, N.F.; Alkhamaali, Z.; Alqahtani, S.A. Obesity in Saudi Arabia in 2020: Prevalence, Distribution, and Its Current Association with Various Health Conditions. Healthcare 2021, 9, 311. [Google Scholar] [CrossRef] [PubMed]
- Chong, B.; Jayabaskaran, J.; Kong, G.; Chan, Y.H.; Chin, Y.H.; Goh, R.; Kannan, S.; Ng, C.H.; Loong, S.; Kueh, M.T.W. Trends and predictions of malnutrition and obesity in 204 countries and territories: An analysis of the Global Burden of Disease Study 2019. eClinicalMedicine 2023, 57, 101850. [Google Scholar] [CrossRef] [PubMed]
- Ammori, B.J.; Skarulis, M.C.; Soran, H.; Syed, A.A.; Eledrisi, M.; Malik, R.A. Medical and surgical management of obesity and diabetes: What’s new? Diabet. Med. 2020, 37, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J.; Aroda, V.R.; Collins, B.S.; Gabbay, R.A.; Green, J.; Maruthur, N.M.; Rosas, S.E.; Del Prato, S.; Mathieu, C.; Mingrone, G.; et al. Management of Hyperglycemia in Type 2 Diabetes, 2022. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2022, 45, 2753–2786. [Google Scholar] [CrossRef] [PubMed]
- Apovian, C.M.; Okemah, J.; O’Neil, P.M. Body Weight Considerations in the Management of Type 2 Diabetes. Adv. Ther. 2019, 36, 44–58. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.; Isaacs, D.; Clements, J.N. Pharmacokinetics and Clinical Implications of Semaglutide: A New Glucagon-Like Peptide (GLP)-1 Receptor Agonist. Clin. Pharmacokinet. 2018, 57, 1529–1538. [Google Scholar] [CrossRef] [PubMed]
- Sorli, C.; Harashima, S.I.; Tsoukas, G.M.; Unger, J.; Karsbøl, J.D.; Hansen, T.; Bain, S.C. Efficacy and safety of once-weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): A double-blind, randomised, placebo-controlled, parallel-group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol. 2017, 5, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, M.; Narukawa, M. Assessment of Cardiovascular Risk With Glucagon-Like Peptide 1 Receptor Agonists in Patients With Type 2 Diabetes Using an Alternative Measure to the Hazard Ratio. Ann. Pharmacother. 2018, 52, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Smits, M.M.; Van Raalte, D.H. Safety of Semaglutide. Front. Endocrinol. 2021, 12, 645563. [Google Scholar] [CrossRef] [PubMed]
- Al Hayek, A.A.; Al Dawish, M.A. Evaluation of Patient-Reported Satisfaction and Clinical Efficacy of Once-Weekly Semaglutide in Patients with Type 2 Diabetes: An Ambispective Study. Adv. Ther. 2022, 39, 1582–1595. [Google Scholar] [CrossRef] [PubMed]
- Einarson, T.R.; Garg, M.; Kaur, V.; Hemels, M.E.H. Composite endpoints in trials of type-2 diabetes. Diabetes Obes. Metab. 2014, 16, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Yale, J.F.; Bodholdt, U.; Catarig, A.M.; Catrina, S.; Clark, A.; Ekberg, N.R.; Erhan, U.; Holmes, P.; Knudsen, S.T.; Liutkus, J.; et al. Real-world use of once-weekly semaglutide in patients with type 2 diabetes: Pooled analysis of data from four SURE studies by baseline characteristic subgroups. BMJ Open Diabetes Res Care. 2022, 10, e002619. [Google Scholar] [CrossRef] [PubMed]
- Dungan, K.M.; Bardtrum, L.; Christiansen, E.; Eliasson, J.; Mellbin, L.; Woo, V.C.; Vilsbøll, T. Greater Combined Reductions of HbA1c ≥ 1.0% and Body Weight Loss ≥ 5.0% or ≥10.0% with Orally Administered Semaglutide Versus Comparators. Diabetes Ther. 2023, 14, 1415–1425. [Google Scholar] [CrossRef] [PubMed]
- Alenzi, S.; Alzahrani, A.; Aljaloud, A.; Alanazi, K.; Alarfaj, S.J. The effectiveness of 0.5 mg and 1 mg of semaglutide in patients with type two diabetes and predictors of response: A retrospective cohort study. Front. Endocrinol. 2024, 15, 1395651. [Google Scholar] [CrossRef] [PubMed]
- Rodbard, H.W.; Bellary, S.; Hramiak, I.; Seino, Y.; Silver, R.; Damgaard, L.H.; Nayak, G.; Zacho, J.; Aroda, V.R. Greater Combined Reductions in HbA1c ≥1.0% and Weight ≥5.0% with Semaglutide versus Comparators In Type 2 Diabetes. Endocr. Pr. 2019, 25, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Calle, E.E.; Thun, M.J.; Petrelli, J.M.; Rodriguez, C.; Heath, C.W. Body-mass index and mortality in a prospective cohort of U.S. adults. N. Engl. J. Med. 1999, 341, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Wing, R.R.; Lang, W.; Wadden, T.A.; Safford, M.; Knowler, W.C.; Bertoni, A.G.; Hill, J.O.; Brancati, F.L.; Peters, A.; Wagenknecht, L.; et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care 2011, 34, 1481–1486. [Google Scholar] [CrossRef] [PubMed]
- Ryan, D.H.; Yockey, S.R. Weight Loss and Improvement in Comorbidity: Differences at 5%, 10%, 15%, and Over. Curr. Obes. Rep. 2017, 6, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Magkos, F.; Fraterrigo, G.; Yoshino, J.; Luecking, C.; Kirbach, K.; Kelly, S.C.; de Las Fuentes, L.; He, S.; Okunade, A.L.; Patterson, B.W.; et al. Effects of Moderate and Subsequent Progressive Weight Loss on Metabolic Function and Adipose Tissue Biology in Humans with Obesity. Cell Metab. 2016, 23, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Lingvay, I.; Sumithran, P.; Cohen, R.V.; le Roux, C.W. Obesity management as a primary treatment goal for type 2 diabetes: Time to reframe the conversation. Lancet 2022, 399, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Lean, M.E.; Leslie, W.S.; Barnes, A.C.; Brosnahan, N.; Thom, G.; McCombie, L.; Peters, C.; Zhyzhneuskaya, S.; Al-Mrabeh, A.; Hollingsworth, K.G.; et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): An open-label, cluster-randomised trial. Lancet 2018, 391, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Unick, J.L.; Beavers, D.; Jakicic, J.M.; Kitabchi, A.E.; Knowler, W.C.; Wadden, T.A.; Wing, R.R. Effectiveness of lifestyle interventions for individuals with severe obesity and type 2 diabetes: Results from the Look AHEAD trial. Diabetes Care 2011, 34, 2152–2157. [Google Scholar] [CrossRef] [PubMed]
- Lind, M.; Imberg, H.; Coleman, R.L.; Nerman, O.; Holman, R.R. Historical HbA1c Values May Explain the Type 2 Diabetes Legacy Effect: UKPDS 88. Diabetes Care 2021, 44, 2231–2237. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Hisland, L.; Grenet, G.; Gueyffier, F.; Cornu, C.; Jaafari, N.; Boussageon, R. Reappraisal of the efficacy of intensive glycaemic control on microvascular complications in patients with type 2 diabetes: A meta-analysis of randomised control-trials. Therapies 2022, 77, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, L.; Azad, N.; Bahn, G.D.; Ge, L.; Reaven, P.D.; Hayward, R.A.; Reda, D.J.; Emanuele, N.V.; VADT Study Group. Long-term follow-up of intensive glycaemic control on renal outcomes in the Veterans Affairs Diabetes Trial (VADT). Diabetologia 2018, 61, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Zaazouee, M.S.; Hamdallah, A.; Helmy, S.K.; Hasabo, E.A.; Sayed, A.K.; Gbreel, M.I.; Elmegeed, A.A.; Aladwan, H.; Elshanbary, A.A.; Abdel-Aziz, W.; et al. Semaglutide for the treatment of type 2 Diabetes Mellitus: A systematic review and network meta-analysis of safety and efficacy outcomes. Diabetes Metab. Syndr. 2022, 16, 102511. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.H.; Li, H.; Cui, M.; Zhang, Z.L.; Gu, Z.C.; Liu, X.Y. Efficacy and Safety of Once-Weekly Semaglutide for the Treatment of Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Pharmacol. 2018, 9, 576. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, A.; Yokokawa, H.; Nagamine, T.; Fukuda, H.; Hisaoka, T.; Naito, T. Efficacy and safety of semaglutide in glycemic control, body weight management, lipid profiles and other biomarkers among obese type 2 diabetes patients initiated or switched to semaglutide from other GLP-1 receptor agonists. J. Diabetes Metab. Disord. 2021, 20, 2121–2128. [Google Scholar] [CrossRef]
- Aroda, V.R.; Ahmann, A.; Cariou, B.; Chow, F.; Davies, M.J.; Jódar, E.; Mehta, R.; Woo, V.; Lingvay, I. Comparative efficacy, safety, and cardiovascular outcomes with once-weekly subcutaneous semaglutide in the treatment of type 2 diabetes: Insights from the SUSTAIN 1-7 trials. Diabetes Metab. 2019, 45, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Yoshida, M.; Funazaki, S.; Morimoto, J.; Tonezawa, S.; Takahashi, A.; Nagashima, S.; Masahiko, K.; Kiyoshi, O.; Hara, K. Retrospective Analysis of the Effectiveness of Oral Semaglutide in Type 2 Diabetes Mellitus and Its Effect on Cardiometabolic Parameters in Japanese Clinical Settings. J. Cardiovasc. Dev. Dis. 2023, 10, 176. [Google Scholar] [CrossRef]
- Di Loreto, C.; Minarelli, V.; Nasini, G.; Norgiolini, R.; Del Sindaco, P. Effectiveness in Real World of Once Weekly Semaglutide in People with Type 2 Diabetes: Glucagon-Like Peptide Receptor Agonist Naïve or Switchers from Other Glucagon-Like Peptide Receptor Agonists: Results from a Retrospective Observational Study in Umbria. Diabetes Ther. 2022, 13, 551–567. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Belmonte, L.M.; Sanz-Cánovas, J.; García de Lucas, M.D.; Ricci, M.; Avilés-Bueno, B.; Cobos-Palacios, L.; Pérez-Velasco, M.A.; López-Sampalo, A.; Bernal-López, M.R.; Jansen-Chaparro, S.; et al. Efficacy and Safety of Semaglutide for the Management of Obese Patients With Type 2 Diabetes and Chronic Heart Failure in Real-World Clinical Practice. Front. Endocrinol. 2022, 13, 851035. [Google Scholar] [CrossRef] [PubMed]
- Masaki, T.; Ozeki, Y.; Yoshida, Y.; Okamoto, M.; Miyamoto, S.; Gotoh, K.; Shibata, H. Glucagon-Like Peptide-1 Receptor Agonist Semaglutide Improves Eating Behavior and Glycemic Control in Japanese Obese Type 2 Diabetic Patients. Metabolites 2022, 12, 147. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, R.B. Hyperlipidemia and cardiovascular risk factors in patients with type 2 diabetes. Am. J. Manag. Care 2000, 6 (Suppl. 13), S682–S691, discussion S692–S696. [Google Scholar] [PubMed]
- Lee, S.H.; Park, S.Y.; Choi, C.S. Insulin Resistance: From Mechanisms to Therapeutic Strategies. Diabetes Metab. J. 2022, 46, 15–37. [Google Scholar] [CrossRef] [PubMed]
- Russell-Jones, D.; Khan, R. Insulin-associated weight gain in diabetes -causes, effects and coping strategies. Diabetes Obes. Metab. 2007, 9, 799–812. [Google Scholar] [CrossRef] [PubMed]
- Anekwe, C.V.; Ahn, Y.J.; Bajaj, S.S.; Stanford, F.C. Pharmacotherapy causing weight gain and metabolic alteration in those with obesity and obesity-related conditions: A review. Ann. N. Y. Acad. Sci. 2024, 1533, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, V.A.; Capehorn, M.S.; Garg, S.K.; Gimeno, E.J.; Hansen, O.H.; Holst, A.G.; Nayak, G.; Seufert, J. Reductions in Insulin Resistance are Mediated Primarily via Weight Loss in Subjects with Type 2 Diabetes on Semaglutide. J. Clin. Endocrinol. Metab. 2019, 104, 4078–4086. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, D.; Vermund, S.H. Advantages and Limitations of the Body Mass Index (BMI) to Assess Adult Obesity. Int. J. Environ. Res. Public Health 2024, 21, 757. [Google Scholar] [CrossRef] [PubMed]

| Composite Endpoint | Total | Not Achieved | Achieved | p Value |
|---|---|---|---|---|
| Number of patients, n (%) | 459 | 266 (58%) | 193 (42%) | |
| Age (years), mean ± SD | 52.7 ± 8.5 | 53.3 ± 8 | 51.8 ± 9.2 | 0.053 a |
| Diabetes duration (years) | 14.2 ± 7.8 | 14.6 ± 7.6 | 13.6 ± 8.1 | 0.201 a |
| Sex/Gender | ||||
| Male | 213 (46.4%) | 126 (47.4%) | 87 (45.1%) | 0.637 b |
| Female | 246 (53.6%) | 140 (52.6%) | 106 (54.9%) | |
| Comorbidities | ||||
| Dyslipidemia | 417 (90.8%) | 241 (90.6%) | 176 (91.2%) | 0.871 b |
| Hypertension | 247 (53.8%) | 143 (53.8%) | 104 (53.9%) | 1 b |
| IHD | 49 (10.7%) | 29 (10.9%) | 20 (10.4%) | 0.880 b |
| Concomitant medications | ||||
| Sulfonylurea | 124 (27%) | 68 (25.6%) | 56 (29%) | 0.456 b |
| Biguanide | 450 (98%) | 261 (98.1%) | 189 (97.9%) | 1 c |
| Glinide | 2 (0.4%) | 1 (0.4%) | 1 (0.5%) | 1 c |
| Thiazolidinedione | 5 (1.1%) | 4 (1.5%) | 1 (0.5%) | 0.404 c |
| SGLT2-I | 216 (47.1%) | 120 (45.1%) | 96 (49.7%) | 0.344 b |
| DPP4-I | 14 (3.1%) | 9 (3.4%) | 5 (2.6%) | 0.786 c |
| Mixed regimen insulin | 240 (52.3%) | 160 (60.2%) | 80 (41.5%) | <0.001 b |
| Basal only | 21 (4.6%) | 9 (3.4%) | 12 (6.2%) | 0.177 c |
| Bolus only | 49 (10.7%) | 28 (10.5%) | 21 (10.9%) | 1 c |
| Dose at 6 months | ||||
| 0.25 mg | 1 (0.2%) | 0 (0%) | 1 (0.5%) | 0.351 c |
| 0.5 mg | 199 (43.4%) | 120 (45.1%) | 79 (40.9%) | |
| 1 mg | 259 (56.4%) | 146 (54.9%) | 113 (58.5%) | |
| Dose at 12 months | ||||
| 0.25 mg | 3 (0.7%) | 2 (0.8%) | 1 (0.5%) | 0.892 c |
| 0.5 mg | 106 (23.1%) | 63 (23.7%) | 43 (22.3%) | |
| 1 mg | 350 (76.3%) | 201 (75.6%) | 149 (77.2%) |
| Male | Female | p Value | |
|---|---|---|---|
| Number of patients, n (%) | 213 (46.4%) | 246 (53.6%) | |
| Age (years) | 51.7 ± 9.4 | 53.6 ± 7.6 | 0.021 a |
| Diabetes duration (years) | 13.6 ± 7.6 | 14.7 ± 8 | 0.136 a |
| Body weight (kg) baseline | 96.7 ± 16.5 | 91.4 ± 14.7 | <0.001 a |
| BMI baseline | 34.7 ± 5.3 | 37.8 ± 5.7 | <0.001 a |
| A1C (%) baseline | 9.8 ± 1.5 | 10.1 ± 1.5 | 0.041 a |
| FBG (mmol/L) baseline | 11.7 ± 3.9 | 12 ± 4.1 | 0.584 a |
| Total cholesterol (mmol/L) baseline | 4.43 ± 1.33 | 4.54 ± 1.05 | 0.331 a |
| HDL (mmol/L) baseline | 1.05 ± 0.23 | 1.27 ± 0.29 | 0.001 a |
| LDL (mmol/L) baseline | 2.96 ± 1.15 | 2.92 ± 0.96 | 0.713 a |
| TG (mmol/L) baseline | 2.11 ± 1.51 | 1.76 ± 0.82 | 0.003 a |
| Comorbidities | |||
| Dyslipidemia | 188 (88.3%) | 229 (93.1%) | 0.077 b |
| Hypertension | 119 (55.9%) | 128 (52%) | 0.453 b |
| IHD | 31 (14.6%) | 18 (7.3%) | 0.015 b |
| Diabetic retinopathy | 15 (7%) | 19 (7.7%) | 0.859 b |
| Diabetic nephropathy | 30 (14.1%) | 19 (7.7%) | 0.034 b |
| Concomitant medications | |||
| Sulfonylurea | 58 (27.2%) | 66 (26.8%) | 1 b |
| Biguanide | 208 (97.7%) | 242 (98.4%) | 0.739 c |
| Glinide | 1 (0.5%) | 1 (0.4%) | 1 c |
| Thiazolidinedione | 4 (1.9%) | 1 (0.4%) | 0.188 c |
| SGLT2 -I | 110 (51.6%) | 106 (43.1%) | 0.075 b |
| DPP4 inhibitor | 5 (2.3%) | 9 (3.7%) | 0.588 c |
| Insulin therapy | 104 (48.8%) | 136 (55.3%) | 0.190 b |
| Sulfonylurea/insulin/thiazolidinedione | 170 (79.8%) | 221 (89.8%) | 0.004 b |
| Dose at 6 months | |||
| 0.25 mg | 1 (0.5%) | 0 (0%) | 0.026 c |
| 0.5 mg | 79 (37.1%) | 120 (48.8%) | |
| 1 mg | 133 (62.4%) | 126 (51.2%) | |
| Dose at 12 months | |||
| 0.25 mg | 1 (0.5%) | 2 (0.8%) | 0.866 c |
| 0.5 mg | 48 (22.5%) | 58 (23.6%) | |
| 1 mg | 164 (77%) | 186 (75.6%) |
| Composite Endpoint | Total | Not Achieved | Achieved | p Value |
|---|---|---|---|---|
| Number of patients, n (%) | 459 | 266 (58%) | 193 (42%) | |
| Composite at 6 months | 56 (12.2%) | 3 (1.1%) | 53 (27.5%) | <0.001 b |
| Body weight (kg) | ||||
| Baseline | 93.9 ± 15.8 | 95.5 ± 15.5 | 91.7 ± 15.9 | 0.011 a |
| 6 months | 90.6 ± 15.9 | 93.3 ± 15.5 | 86.9 ± 15.6 | <0.001 a |
| 12 months | 87.8 ± 15.9 | 91.7 ± 15.5 | 82.5 ± 14.9 | <0.001 a |
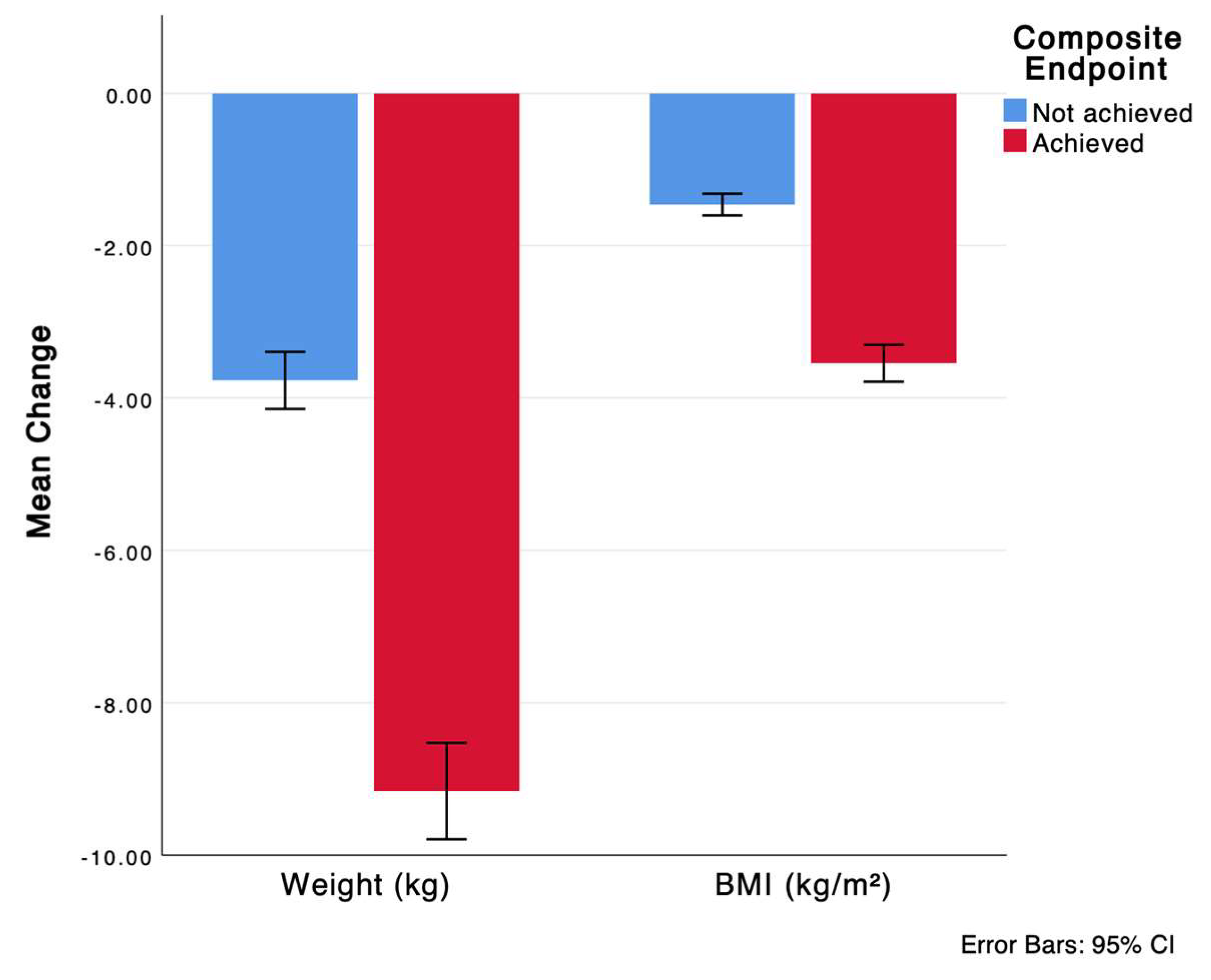
| Weight change at 12 months | −6 ± 4.6 | −3.7 ± 3.1 | −9.1 ± 4.4 | <0.001 |
| p value of the change from baseline to 12 months c | <0.001 | <0.001 | <0.001 | |
| BMI | ||||
| Baseline | 36.3 ± 5.7 | 36.7 ± 5.7 | 35.8 ± 5.7 | 0.111 a |
| 6 months | 35.1 ± 5.7 | 35.9 ± 5.7 | 34 ± 5.6 | 0.001 a |
| 12 months | 34 ± 5.8 | 35.2 ± 5.7 | 32.3 ± 5.5 | <0.001 a |
| BMI change at 12 months | −2.3 ± 1.8 | −1.5 ± 1.2 | −3.5 ± 1.7 | <0.001 |
| p value of the change from baseline to 12 months c | <0.001 | <0.001 | <0.001 | |
| A1C (%) | ||||
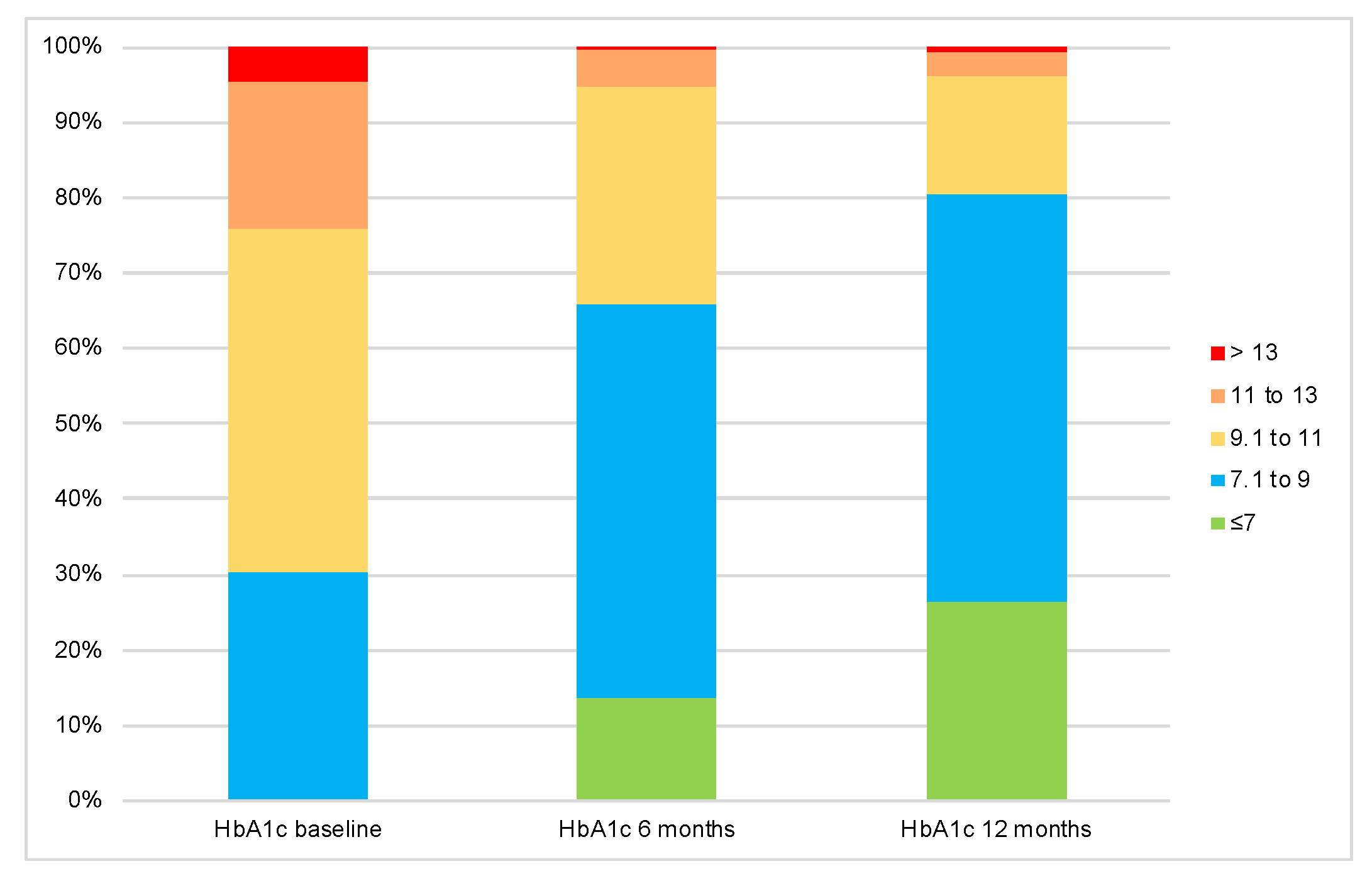
| Baseline | 10 ± 1.5 | 9.8 ± 1.5 | 10.2 ± 1.4 | 0.003 a |
| 6 months | 8.5 ± 1.5 | 8.8 ± 1.4 | 8.2 ± 1.4 | <0.001 a |
| 12 months | 7.9 ± 1.5 | 8.4 ± 1.5 | 7.2 ± 1.1 | <0.001 a |
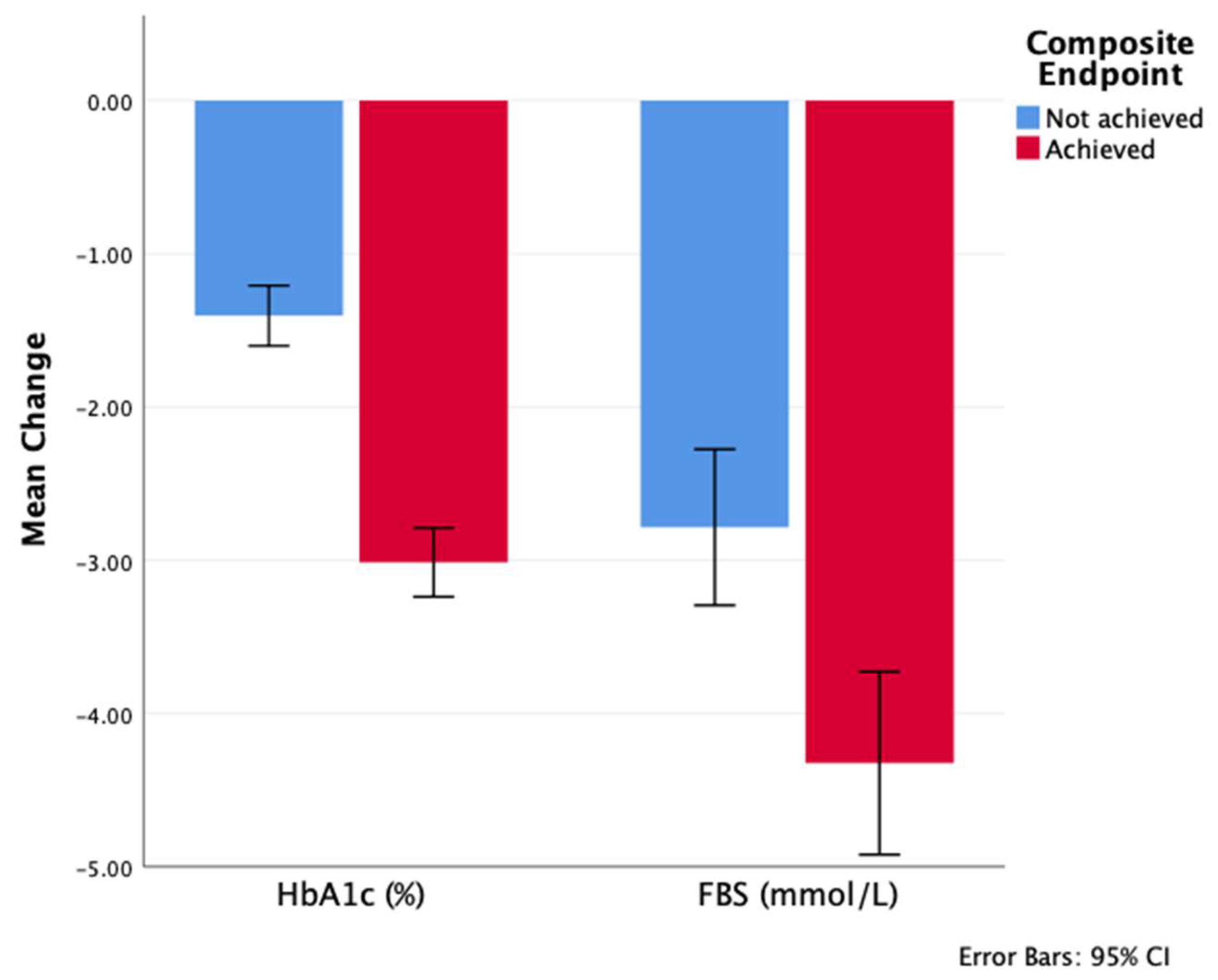
| A1c change at 12 months | −2.1 ± 1.8 | −1.4 ± 1.6 | −3 ± 1.6 | <0.001 |
| p value of the change from baseline to 12 months c | <0.001 | <0.001 | <0.001 | |
| FBG (mmol/L) | ||||
| Baseline | 11.9 ± 4 | 11.9 ± 4 | 11.7 ± 3.9 | 0.611 a |
| 6 months | 9.6 ± 3.6 | 10.1 ± 4 | 8.9 ± 3 | <0.001 a |
| 12 months | 8.4 ± 3.3 | 9.2 ± 3.6 | 7.4 ± 2.5 | <0.001 a |
| FBG change at 12 months | −3.4 ± 4.3 | −2.8 ± 4.2 | −4.3 ± 4.2 | <0.001 |
| p value of the change from baseline to 12 months c | <0.001 | <0.001 | <0.001 | |
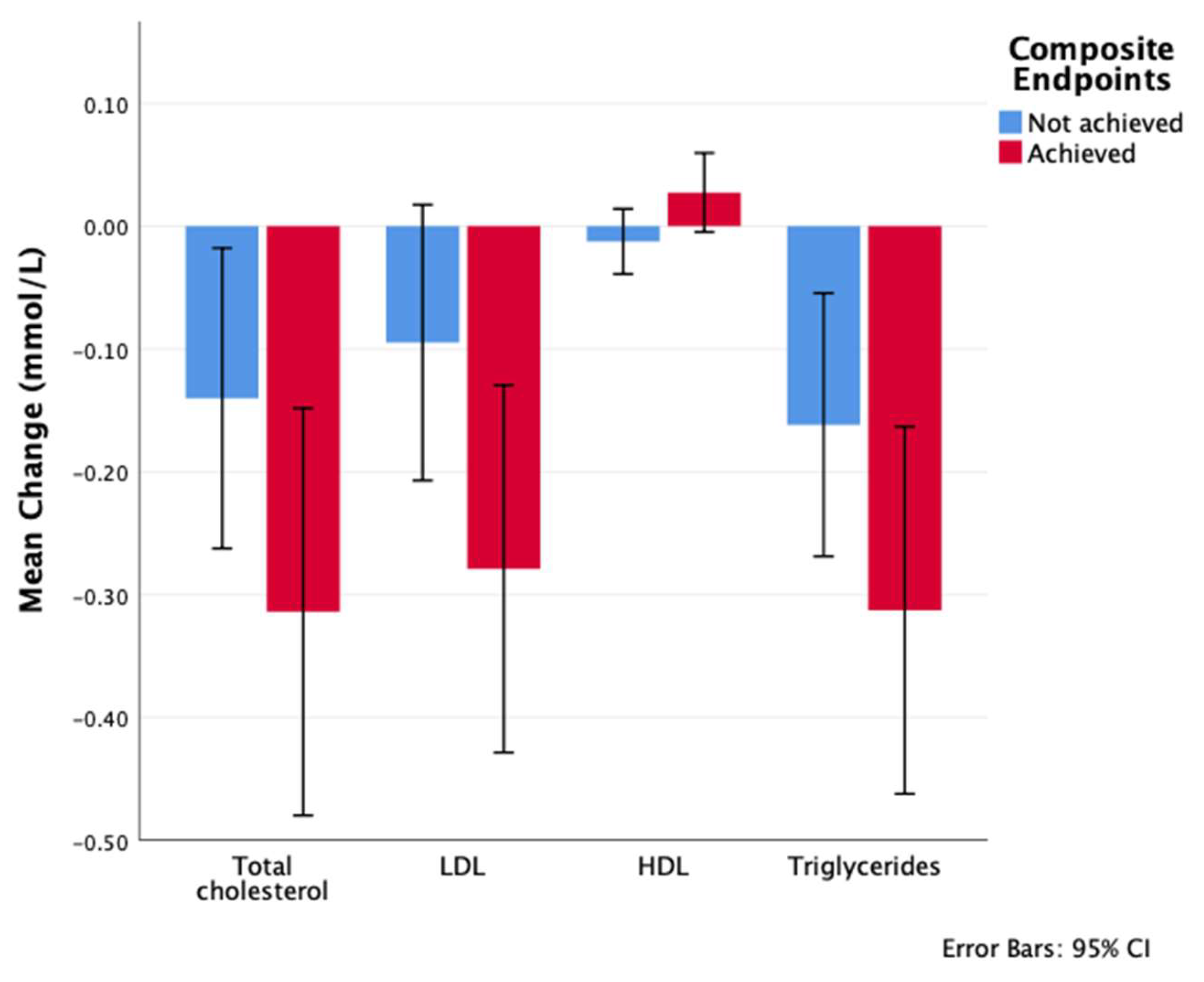
| Total cholesterol (mmol/L) | ||||
| Baseline | 4.49 ± 1.19 | 4.42 ± 1.15 | 4.59 ± 1.23 | 0.111 a |
| 6 months | 4.33 ± 1.08 | 4.29 ± 1.09 | 4.4 ± 1.08 | 0.295 a |
| 12 months | 4.27 ± 1.08 | 4.28 ± 1.11 | 4.27 ± 1.05 | 0.967 a |
| Cholesterol change at 12 months | −0.2 ± 1.1 | −0.1 ± 1 | −0.3 ± 1.2 | 0.090 |
| p value of the change from baseline to 12 months c | <0.001 | 0.025 | <0.001 | |
| HDL (mmol/L) | ||||
| Baseline | 1.17 ± 0.29 | 1.16 ± 0.29 | 1.18 ± 0.28 | 0.558 a |
| 6 months | 1.16 ± 0.29 | 1.14 ± 0.3 | 1.19 ± 0.26 | 0.092 a |
| 12 months | 1.17 ± 0.28 | 1.15 ± 0.27 | 1.2 ± 0.29 | 0.032 a |
| HDL change at 12 months | 0 ± 0.7 | −0.1 ± 1 | 0 ± 0.2 | 0.421 |
| p value of the change from baseline to 12 months c | 0.803 | 0.360 | 0.093 | |
| LDL (mmol/L) | ||||
| Baseline | 2.94 ± 1.05 | 2.88 ± 1.03 | 3.02 ± 1.08 | 0.147 a |
| 6 months | 2.82 ± 0.97 | 2.79 ± 0.97 | 2.86 ± 0.98 | 0.450 a |
| 12 months | 2.76 ± 0.97 | 2.78 ± 0.99 | 2.73 ± 0.94 | 0.585 a |
| LDL change at 12 months | −0.2 ± 1 | −0.1 ± 0.9 | −0.3 ± 1.1 | 0.048 |
| p value of the change from baseline to 12 months c | <0.001 | 0.098 | <0.001 | |
| TG (mmol/L) | ||||
| Baseline | 1.92 ± 1.2 | 1.89 ± 1.01 | 1.98 ± 1.43 | 0.409 a |
| 6 months | 1.75 ± 0.79 | 1.76 ± 0.81 | 1.73 ± 0.77 | 0.608 a |
| 12 months | 1.7 ± 0.88 | 1.72 ± 0.86 | 1.67 ± 0.91 | 0.486 a |
| TG change at 12 months | −0.2 ± 1 | −0.2 ± 0.9 | −0.3 ± 1.1 | 0.097 a |
| p value of the change from baseline to 12 months c | <0.001 | 0.003 | <0.001 |
| aOR † | 95% CI | p Value | |
|---|---|---|---|
| Age (year) | 0.983 | 0.958 to 1.009 | 0.189 |
| Male | reference | ||
| Female | 1.273 | 0.846 to 1.915 | 0.247 |
| Duration of T2D | 1.006 | 0.978 to 1.035 | 0.686 |
| Baseline BMI | 0.953 | 0.915 to 0.992 | 0.02 |
| Baseline A1C | 1.213 | 1.062 to 1.385 | 0.004 |
| Insulin | 0.02 | 0.001 to 0.343 | 0.007 |
| Insulin * baseline BMI | 1.092 | 1.009 to 1.181 | 0.028 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alarfaj, S.J. The Effectiveness of Semaglutide on a Composite Endpoint of Glycemic Control and Weight Reduction and Its Effect on Lipid Profile Among Obese Type 2 Diabetes Patients. Medicina 2025, 61, 1393. https://doi.org/10.3390/medicina61081393
Alarfaj SJ. The Effectiveness of Semaglutide on a Composite Endpoint of Glycemic Control and Weight Reduction and Its Effect on Lipid Profile Among Obese Type 2 Diabetes Patients. Medicina. 2025; 61(8):1393. https://doi.org/10.3390/medicina61081393
Chicago/Turabian StyleAlarfaj, Sumaiah J. 2025. "The Effectiveness of Semaglutide on a Composite Endpoint of Glycemic Control and Weight Reduction and Its Effect on Lipid Profile Among Obese Type 2 Diabetes Patients" Medicina 61, no. 8: 1393. https://doi.org/10.3390/medicina61081393
APA StyleAlarfaj, S. J. (2025). The Effectiveness of Semaglutide on a Composite Endpoint of Glycemic Control and Weight Reduction and Its Effect on Lipid Profile Among Obese Type 2 Diabetes Patients. Medicina, 61(8), 1393. https://doi.org/10.3390/medicina61081393







