Abstract
Background and objectives: Trismus is a frequent and debilitating complication in people with head and neck cancer (HNC) which leads to significant functional limitations and reduced quality of life. Rehabilitation interventions are commonly recommended to manage or prevent trismus. However, in many randomized controlled trials (RCTs), the theoretical justification for these interventions is poorly articulated, and the underlying biological or physiological mechanisms are not described in detail, limiting our understanding of why certain treatments may (or may not) work. This review aimed to identify and analyze how RCTs report the rationale for rehabilitation interventions and the explanations used to manage this population. Materials and Methods: A scoping review was conducted in accordance with the PRISMA-ScR guidelines. Five databases (PubMed, PEDro, Web of Science, Scopus, and EMBASE) were searched up to May 2025 for RCTs evaluating rehabilitation interventions for the management or prevention of treatment-induced trismus in patients with HNC. Data were extracted and synthesized narratively, focusing on the type of intervention, the rationale for its use, and the proposed mechanisms of action. Results: Of 2215 records identified, 24 RCTs met the inclusion criteria. Thirteen studies focused on preventive interventions—primarily exercise therapy—while the remainder addressed established trismus using exercise, manual therapy, electrotherapy, or combined treatment modalities. The rationales provided for intervention selection were heterogeneous and often lacked depth, with most studies justifying interventions based on their potential to improve mouth opening or reduce fibrosis but rarely grounding these claims in detailed pathophysiological models. Only half of the studies provided any mechanistic explanation for the intervention’s effects, and these were typically generic or speculative. Conclusions: RCTs investigating rehabilitation interventions for treatment-induced trismus in patients with HNC frequently lack comprehensive rationales and mechanistic explanations for their interventions. This gap limits the ability to refine and optimize treatment approaches, as the underlying processes driving clinical improvements remain poorly understood. Future research should be guided by theoretical models and include objective outcomes to better elucidate the mechanisms of action of interventions to inform clinical practice.
1. Introduction
Trismus, defined as mouth opening of 35 mm or less [1], is a well-known complication in people with head and neck cancer (HNC) [2,3]. While the prevalence varies enormously due to patient and physician under-reporting, it has been estimated to occur in 5–38% of patients with HNC [1,4]. It can appear early during treatment or develop progressively in the post-treatment phase, and its presence can lead to problems with speech, oral hygiene, and dental treatment, among other issues, ultimately impacting mandibular functioning and quality of life [5,6].
The etiology of trismus is multifactorial and often reflects a combination of anatomical, treatment-related, and functional mechanisms. Tumor invasion may directly infiltrate or compress the muscles of mastication, the temporomandibular joint, or the surrounding connective tissue, leading to a mechanical obstruction or even a limitation in the range of motion of the jaw due to pain [7]. In addition, in malignancies related to the oral cavity, oropharynx, nasopharynx, or infratemporal fossa, surgical resections of tumor-bearing tissues may provoke postoperative fibrosis, scarring, or motor nerve impairment [8]. However, the most frequent cause of trismus is radiation-induced fibrosis. High-dose radiotherapy, especially when applied over regions involving the masticatory apparatus, can trigger progressive fibrotic changes in muscles and soft tissues. These fibrotic changes may lead to chronic inflammation, microvascular damage, and excessive deposition of collagen, reducing tissue elasticity properties and ultimately affecting range of motion [9]. Due to increasing awareness of the functional burden of trismus, rehabilitation treatments often aim to increase maximal interincisal opening (MIO) for these patients. [10]. Nonetheless, rehabilitation approaches remain heterogenous, with limited evidence to support the effectiveness of rehabilitation interventions for trismus apart from exercise [11]. In rehabilitation, the justification/rationale for an intervention, and the proposed explanation of the mechanisms of effect are essential to ensure the translation of evidence into practice [12]. This would ensure that the understanding and development of complex interventions are aligned with the complexity of the health disorder being addressed [13]. However, in the domain of treatment-induced trismus in people with HNC, the extent to which authors report their rationale for treatment selection and the mechanisms underlying the effects of the intervention within randomized controlled trials (RCT) remains unclear.
Therefore, the aim of this scoping review is to identify and analyze how RCTs report the rationale for the chosen rehabilitation interventions used to manage or prevent treatment-induced trismus in individuals with HNC and the explanation of the mechanisms of treatment effects.
2. Materials and Methods
We conducted a scoping review of RCTs evaluating the management of treatment-induced trismus in people with HNC. The protocol for this review was registered in Open Science Framework (http://osf.io). Based on the framework outlined by Arksey and O’Malley [14] and later developed by Levac et al. [15], we used the PRISMA extension for Scoping Reviews (PRISMA-ScR) tool [16] to increase the quality of this study. Our review was performed based on five steps: (a) identifying the research questions, (b) searching for relevant studies, (c) selection of studies, (d) charting the data, and (e) collating, summarizing, and reporting the results.
2.1. Identifying the Research Question
This review aimed to address the following question: “How is the rationale for rehabilitation interventions described in RCTs for the management or prevention of treatment-induced trismus in individuals with HNC and what explanations are provided to describe the mechanisms of effect of the treatment investigated?”. Based on this question, three sub-questions were of interest:
What types of rehabilitation interventions have been evaluated in RCTs for the management or prevention of treatment-induced trismus in people with HNC?
What rationale is provided for selecting these interventions? For this purpose, the introduction section of each article was assessed.
What explanations or mechanisms of action are provided to support the expected therapeutic effects of these interventions? For this purpose, the discussion of each article was evaluated.
The participant, concept, and context (PCC) [16] framework of this scoping review can be found in Appendix A.
2.2. Identifying Relevant Studies
Eligibility Criteria
This review included RCTs involving participants with treatment-induced trismus secondary to HNC. Eligible studies had to be published in peer-reviewed journals, in English or Spanish, with publication dates up to 10 May 2025. We considered trials assessing rehabilitation interventions, including but not limited to exercise, manual therapy, electrotherapy, and other physical agents or conservative modalities. Exclusion criteria included publications in languages other than English or Spanish and studies involving surgical or pharmacological interventions.
2.3. Information Sources
We searched the following electronic databases: PubMed, PEDro, Web of Science, Scopus, and EMBASE. In addition, hand-searching of reference lists from relevant articles and contact with field experts were performed to identify further eligible studies.
2.3.1. Search Strategy
A comprehensive search strategy was developed using a combination of Medical Subject Headings (MeSH) and free-text terms related to “Trismus,” “Rehabilitation,” and “Physical Therapy.” An example search strategy for MEDLINE is presented in Appendix B. This search strategy was designed in collaboration with a librarian and finalized through author consensus. The search was conducted by a single reviewer (EA), and the results were stored in Mendeley.
2.3.2. Selecting the Studies
Following duplicate removal, two reviewers (EA and CB) independently assessed titles and abstracts according to predefined eligibility criteria and retrieved the full texts of studies meeting the eligibility criteria. Inclusion required agreement between both reviewers; disagreements were resolved through discussion or adjudicated by a third reviewer (DF) when needed. The same process was applied to the full-text screening stage by the same reviewers.
2.4. Charting the Data
2.4.1. Data Extraction
A data extraction spreadsheet was developed, and the data were extracted by EA and checked by AB. Disagreement was resolved by a third reviewer (DF) when needed.
2.4.2. Data Charting
Data extracted included authors, intervention, rationale for the application of the intervention in relation to trismus, presence or absence of positive effects of the treatment compared to a control group, and explanation of the mechanisms of effect of the treatment.
2.4.3. Collating, Summarizing, and Reporting the Results
In line with the scoping review methodology, the objective of this synthesis was to map the existing literature and examine how intervention rationales and mechanisms of treatment effect are reported in RCTs addressing treatment-induced trismus. Thus, a narrative synthesis was performed, structured according to the extracted data and sub-questions. Findings were grouped thematically and presented with descriptive summaries. The full research team was involved in finalizing the synthesis and the interpretation of the results.
3. Results
A total of 2215 records were identified, of which 18 were identified through hand searching and the rest through electronic database searches. After the removal of duplicates, 1498 unique records remained. Titles and abstracts were screened for eligibility, leading to the selection of 104 articles for full-text review. Of these, 25 studies met the inclusion criteria and were included in the final synthesis. The study selection process is illustrated in the PRISMA Flow Diagram (Figure 1).
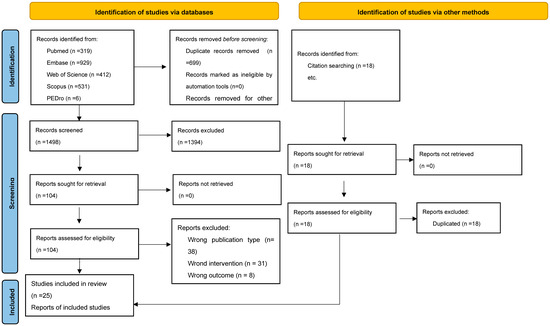
Figure 1.
PRISMA Flow Diagram.
A summary of the included studies is presented in Table 1.

Table 1.
Summary of the included studies.
3.1. Interventions
Of the 25 studies included in this scoping review, 12 focused on interventions aimed at preventing the development of treatment-induced trismus in patients undergoing therapy for HNC, with all of these prevention studies using exercise therapy as the intervention. The remaining 13 studies addressed the management of established trismus, including 2 that evaluated manual therapy alone, 2 that assessed electrotherapy alone, 5 that investigated exercise therapy as a standalone approach, and 4 that examined combined interventions such as exercise, manual therapy, and electrotherapy. In summary, 17 studies examined the effects of exercise (whether it be for prevention or management), 2 evaluated manual therapy, 2 investigated electrotherapy, and 4 assessed combination therapies.
3.2. Rationale
Across the 25 RCTs included in this review, we identified different rationales for the proposed interventions; thus, there was heterogeneity in both content and depth. Most studies justified the use of preventive or therapeutic active interventions based on their theoretical potential to maintain or improve the MIO, reduce fibrosis, and preserve oropharyngeal muscle function. As such, some authors cited the risk of muscle deterioration or loss of function following chemoradiotherapy [17,19], while others emphasized the potential of exercise to mitigate inflammation, endothelial injury, and radiation-induced fibrosis [20].
The rationale for manual therapy interventions was usually justified according to their capacity to relieve fascial restrictions and myofascial impairments [21]; nonetheless, some studies explicitly linked these effects to trismus-related anatomical dysfunction [41]. Studies combining multiple modalities (e.g., exercise therapy, electrotherapy, manual therapy) tended to provide more descriptive rationales but did not always clarify how each component contributed to the expected therapeutic benefit [22,25].
3.3. Explanation of Treatment Effects
A central finding of this review is the limited and inconsistent reporting of explanatory mechanisms that could explain the observed effects of the interventions: in 11 of 25 studies (44%), no pathophysiological explanation was provided for how the intervention might exert its therapeutic effects. In the remaining studies, explanations were often partial, generic, or speculative, with few directly linking the intervention to underlying mechanisms contributing to trismus (e.g., fibrosis, tissue remodeling, neuromuscular activation).
Nonetheless, some studies offered more integrated models. For example, it was suggested that exercise can produce an inflammatory modulation and connective tissue remodeling [32]. Moreover, vascular and thermal effects were also suggested as a potential explanation for the effects of low-level laser therapy [23]. Finally, a biomechanical connection between cervical manipulation and mandibular kinematics was also proposed as a potential explanation [40]. However, these are speculations on the potential mechanisms.
4. Discussion
This scoping review aimed to summarize and synthesize the existing literature describing the rationale for and explanation of treatment effects given for rehabilitation interventions evaluated in RCTs involving HNC patients with treatment-induced trismus. While many studies have assessed the effects of interventions for trismus, and some systematic reviews have been published on this topic [42,43], this is the first study to investigate why interventions are proposed and what the reasoning behind the explanation of their findings is. Our review reveals high heterogeneity in how RCTs justify and explain rehabilitation interventions for these patients. While most studies reported clinical outcomes, specifically those involving exercise therapy, these benefits were often presented without sufficient theoretical or mechanistic context.
Overall, these findings reflect positive trends for rehabilitative interventions—especially those combining exercise and manual therapy—but also highlight significant variability across trials. Most of the studies were based on active interventions, demonstrating a trend to involve the patient in their treatment. However, despite the overall recognition of the need for early or preventive interventions, only a few studies grounded their rationale in pathophysiological models specific to trismus or to the sequelae of cancer treatment. Among them, the most frequent explanation was a reduction in treatment-induced fibrosis after the intervention [20,21,31,33,40]. Other explanations were a reduction in inflammation [32,35] or an increase in vascularization [24,34,38,40]. However, 11 studies of 25 did not include a pathophysiological explanation for their findings. This lack of explanation may limit the replicability, refinement, and clinical translation of the interventions evaluated.
In contrast to the observations in this scoping review, some evidence has suggested the rationale for and/or explanation of the mechanisms involved in the effectiveness of rehabilitation treatments in temporomandibular disorders. For example, it has been suggested that manual therapy can improve mouth opening as a result of the restoration of joint glide, reduction in capsular rigidity, and analgesic effects mediated by the central nervous system and peripheral mechanisms [44]. Moreover, exercise therapy of the craniomandibular muscles may improve mouth opening by increasing blood perfusion, reducing muscle fatigue, and promoting neuromuscular plasticity [45]. In the field of treatment-induced trismus, there has been enormous progress in rehabilitation research, with the proposal and recognition of different treatments for the management of treatment-induced trismus in people with HNC. However, we cannot currently provide an evidence-based explanation for how or why even exercise, the most studied intervention, produced changes. Understanding of how and why rehabilitation is offered to patients is an essential starting point towards more responsive rehabilitation services [46].
In health research, connecting the design of interventions and the selection of outcomes with the understanding of the mechanisms of effect on an intervention is essential [47]. Without a clear understanding of the physiological mechanisms exerted by a treatment, we would be unable to identify which components of a treatment are truly beneficial, which are redundant, or how we could optimize future RCT protocols [48]. It has been recognized that the effectiveness of a rehabilitative intervention will be influenced by many uncontrollable and interrelated factors [49]. It is notable that, with regard to manual therapy, biomechanic and/or pathological explanations were more frequently suggested, indicating a likely improvement in myofascial structures [21] or an improvement via the close biomechanical relationship between the temporomandibular joint and cervical spine [41]. However, this is not in line with previously published models of manual therapy, which suggested that the effects of this kind of treatment should not only be considered in anatomical and/or biomechanical terms but also from a neurophysiological point of view [50]. Thus, it would be interesting to investigate the mechanisms underlying the effects of manual therapy for trismus, especially considering that different types of manual therapy interventions can be applied and could achieve their effect via different mechanisms [50]. In that sense, the UK Medical Research Council (MRC) framework for complex interventions highlights the need to ground intervention development in both a theoretical and empirical understanding, including a clear explanation of the causal mechanisms linking an intervention component to outcomes [51]. Thus, rehabilitation interventions should be grounded in a mechanistic model that explains how the treatment components are expected to produce change, considering factors such as neuromuscular plasticity, facial dynamics, or tissue remodeling.
4.1. Study Limitations
One of the main limitations of this scoping review is that we only included RCTs; other studies, such as prospective studies, may include other rationales/and or explanations that could help us to understand the mechanisms proposed for the different treatments more deeply. Moreover, the heterogeneity in protocols and the lack of standardization in reporting made it difficult to compare different studies and to reach firm conclusions. In addition, in line with the purpose and methodology of a scoping review, we did not perform a formal risk-of-bias or quality assessment of the included RCTs, as our goal was to map the available evidence rather than critically evaluate it. Additionally, we restricted the inclusion criteria to studies published in English or Spanish, which may have introduced a language-based selection bias. It is possible that relevant studies published in other languages were missed, potentially limiting the comprehensiveness of our findings. Finally, the rationale and/or explanations were limited, with no studies performing a real assessment of the mechanisms produced by their intervention, which would be necessary to validate proposed mechanisms. This represents a significant gap in the literature and limits the replication and advancement of mechanistically informed rehabilitation approaches.
4.2. Implications for Future Research
Despite many studies in this field, very few studies employed treatment theory or mechanistic frameworks to articulate how and why a specific intervention was expected to improve trismus outcomes. This observation indicates a need for a stronger theoretical underpinning in future trials. Understanding these mechanisms could facilitate the reproducibility of studies and enhance study design. In that sense, comprehension of the mechanisms of effect of different interventions may guide future trials to combine different therapies to maximize treatment benefit. Moreover, future studies should consider the use of assessment techniques that allow for objective measures of the changes that occur at a pathoanatomical and/or physiological level to improve our understanding of the mechanisms underlying post-treatment changes. For example, future studies could consider evaluating masticatory muscles with ultrasound or electromyography, before and after the intervention.
5. Conclusions
This scoping review summarizes the literature describing the rationale for a chosen treatment and the explanation of treatment effects of interventions for the management and prevention of trismus in people with HNC. The most common rationale for and explanation of therapeutic effects provided was the reduction in treatment-induced fibrosis. Nonetheless, there is a general lack of knowledge about the proposed mechanisms underlying the therapeutic effects of the studied interventions. Standardization of protocols and outcome measurements, as well as the inclusion of diverse methodological designs that complement the evidence generated by RCTs, is needed.
Author Contributions
All authors contributed to study design, data interpretation, and manuscript preparation. C.B.-U. and D.F. were consulted in case of doubt in the selection procedure. C.R.-B. and D.T.-L. developed the main manuscript. A.B.-V. and E.A.-L. performed the literature search. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A

Table A1.
The participant, context, and concept framework used in this study for defining the eligibility of the studies for the primary research question.
Table A1.
The participant, context, and concept framework used in this study for defining the eligibility of the studies for the primary research question.
| P: Population | People with treatment-induced trismus associated with head and neck cancer |
| C: Concept | Characteristics of different rehabilitation modalities for the management of treatment-induced trismus in people with head and neck cancer |
| C: Context | Studies including observational or interventional trials are of interest for this review if they include the implementation of rehabilitation strategies for the management of trismus |
Appendix B

Table A2.
Search strategy on Pubmed.
Table A2.
Search strategy on Pubmed.
| Search Strategy | |
|---|---|
| Terms | |
| #1 | “Physical Therapy Modalities” “[Mesh]” |
| #2 | “Rehabilitation” “[Mesh]” |
| #3 | “Education” “[Mesh]” |
| #4 | “Exercise” “[Mesh]” |
| #5 | “Exercise Therapy” [Mesh] |
| #6 | “Electrotherapy” “[Mesh]” |
| #7 | “Manual Therapy” “[Mesh]” |
| #8 | “jaw exercises” |
| #9 | “physiotherapy” |
| #10 | “physical therapy” |
| #11 | “Trismus” “[Mesh]” |
| #12 | “trismus” |
| #13 | “mouth opening” |
| #14 | “jaw opening” |
| #15 | “Neck” “[Mesh]” |
| #16 | “Head” “[Mesh]” |
| #17 | “Neoplasms” “[Mesh]” |
| #18 | “Head and Neck Neoplasm” “[Mesh]” |
| #19 | “head and neck cancer” |
| #20 | #1 OR #2 OR #3 OR #4 OR #5 OR#6 OR #7 OR #8 OR #9 OR #10 |
| #21 | #11 OR #12 OR #13 OR #14 |
| #22 | #15 OR #16 OR #17 OR #18 OR #19 |
| #23 | #20 AND #21 AND #22 |
References
- Dijkstra, P.U.; Huisman, P.M.; Roodenburg, J.L. Criteria for trismus in head and neck oncology. Int. J. Oral Maxillofac. Surg. 2006, 35, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.; van As-Brooks, C.J.; Fagerberg-Mohlin, B.; Finizia, C. Trismus in head and neck cancer patients in Sweden: Incidence and risk factors. Med. Sci. Monit. 2010, 16, CR278–CR282. [Google Scholar] [PubMed]
- van der Molen, L.; van Rossum, M.A.; Ackerstaff, A.H.; Smeele, L.E.; Rasch, C.R.; Hilgers, F.J. Pretreatment organ function in patients with advanced head and neck cancer: Clinical outcome measures and patients’ views. BMC Ear Nose Throat Disord. 2009, 9, 10. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stubblefield, M.D. Radiation fibrosis syndrome: Neuromuscular and musculoskeletal complications in cancer survivors. PM R 2011, 3, 1041–1054. [Google Scholar] [CrossRef] [PubMed]
- Melchers, L.J.; Van Weert, E.; Beurskens, C.H.; Reintsema, H.; Slagter, A.P.; Roodenburg, J.L.; Dijkstra, P.U. Exercise adherence in patients with trismus due to head and neck oncology: A qualitative study into the use of the Therabite. Int. J. Oral Maxillofac. Surg. 2009, 38, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Scott, B.; D’Souza, J.; Perinparajah, N.; Lowe, D.; Rogers, S.N. Longitudinal evaluation of restricted mouth opening (trismus) in patients following primary surgery for oral and oropharyngeal squamous cell carcinoma. Br. J. Oral Maxillofac. Surg. 2011, 49, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Ichimura, K.; Tanaka, T. Trismus in patients with malignant tumours in the head and neck. J. Laryngol. Otol. 1993, 107, 1017–1020. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.E.; LaMonte, S.J.; Erb, N.L.; Beckman, K.L.; Sadeghi, N.; Hutcheson, K.A.; Stubblefield, M.D.; Abbott, D.M.; Fisher, P.S.; Stein, K.D.; et al. American Cancer Society Head and Neck Cancer Survivorship Care Guideline. CA Cancer J. Clin. 2016, 66, 203–239, Erratum in: CA Cancer J. Clin. 2016, 66, 351. https://doi.org/10.3322/caac.21353. [Google Scholar] [CrossRef] [PubMed]
- Sroussi, H.Y.; Epstein, J.B.; Bensadoun, R.J.; Saunders, D.P.; Lalla, R.V.; Migliorati, C.A.; Heaivilin, N.; Zumsteg, Z.S. Common oral complications of head and neck cancer radiation therapy: Mucositis, infections, saliva change, fibrosis, sensory dysfunctions, dental caries, periodontal disease, and osteoradionecrosis. Cancer Med. 2017, 6, 2918–2931. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chee, S.; Byrnes, Y.M.; Chorath, K.T.; Rajasekaran, K.; Deng, J. Interventions for Trismus in Head and Neck Cancer Patients: A Systematic Review of Randomized Controlled Trials. Integr. Cancer Ther. 2021, 20, 15347354211006474. [Google Scholar] [CrossRef] [PubMed]
- Kamstra, J.I.; Roodenburg, J.L.; Beurskens, C.H.; Reintsema, H.; Dijkstra, P.U. TheraBite exercises to treat trismus secondary to head and neck cancer. Support. Care Cancer 2013, 21, 951–957. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Whyte, J. Contributions of treatment theory and enablement theory to rehabilitation research and practice. Arch. Phys. Med. Rehabil. 2014, 95, S17–S23. [Google Scholar] [CrossRef] [PubMed]
- Levack, W.M.; Weatherall, M.; Hay-Smith, J.C.; Dean, S.G.; McPherson, K.; Siegert, R.J. Goal setting and strategies to enhance goal pursuit in adult rehabilitation: Summary of a Cochrane systematic review and meta-analysis. Eur. J. Phys. Rehabil. Med. 2016, 52, 400–416. [Google Scholar] [PubMed]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Levac, D.; Colquhoun, H.; O’Brien, K.K. Scoping studies: Advancing the methodology. Implement. Sci. 2010, 5, 69. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Hajdú, S.F.; Wessel, I.; Dalton, S.O.; Eskildsen, S.J.; Johansen, C. Swallowing Exercise During Head and Neck Cancer Treatment: Results of a Randomized Trial. Dysphagia 2022, 37, 749–762. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Petersson, K.; Finizia, C.; Pauli, N.; Tuomi, L. Preventing radiation-induced dysphagia and trismus in head and neck cancer—A randomized controlled trial. Head Neck 2025, 47, 159–174. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Messing, B.P.; Ward, E.C.; Lazarus, C.L.; Kim, M.; Zhou, X.; Silinonte, J.; Gold, D.; Harrer, K.; Ulmer, K.; Merritt, S.; et al. Prophylactic Swallow Therapy for Patients with Head and Neck Cancer Undergoing Chemoradiotherapy: A Randomized Trial. Dysphagia 2017, 32, 487–500. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saghafi, E.; Kadhim, K.; Andås, C.A.; Cahlin, B.J.; Finizia, C.; Axelsson, T.; Kjeller, G.; Tuomi, L. Jaw exercise in head and neck cancer patients for prevention of temporomandibular disorders: A randomized controlled trial. J. Cancer Surviv. 2024. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Comino, L.; Martín-Martín, L.; Galiano-Castillo, N.; Castro-Martín, E.; Fernández-Gualda, M.Á.; Lozano-Lozano, M.; Fernández-Lao, C. The effects of myofascial induction therapy in survivors of head and neck cancer: A randomized, controlled clinical trial. Support. Care Cancer 2022, 31, 49. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dai, Y.M.; Virtanen, H.; Zhu, Y.; Wan, H. Effectiveness of mHealth intervention for trismus exercise in patients with head and neck cancer undergoing proton and heavy ion therapy: A randomized control trial. Support. Care Cancer 2024, 32, 470. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Zhao, D. Effects of resistance exercise on complications, cancer-related fatigue and quality of life in nasopharyngeal carcinoma patients undergoing chemoradiotherapy: A randomised controlled trial. Eur. J. Cancer Care 2021, 30, e13355. [Google Scholar] [CrossRef] [PubMed]
- Elgohary, H.M.; Eladl, H.M.; Soliman, A.H.; Soliman, E.S. Effects of Ultrasound, Laser and Exercises on Temporomandibular Joint Pain and Trismus Following Head and Neck Cancer. Ann. Rehabil. Med. 2018, 42, 846–853. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, T.J.; Su, J.H.; Leung, K.W.; Liang, S.Y.; Wu, S.F.; Wang, H.M. Effects of a mouth-opening intervention with remote support on adherence, the maximum interincisal opening, and mandibular function of postoperative oral cancer patients: A randomized clinical trial. Eur. J. Oncol. Nurs. 2019, 40, 111–119, Erratum in Eur. J. Oncol. Nurs. 2019, 41, 195. https://doi.org/10.1016/j.ejon.2019.06.009. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Anderson, C.M.; Perkhounkova, Y.; Sleeuwenhoek, B.M.; Louison, R.R. Transcutaneous Electrical Nerve Stimulation Reduces Resting Pain in Head and Neck Cancer Patients: A Randomized and Placebo-Controlled Double-Blind Pilot Study. Cancer Nurs. 2019, 42, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Chen, X.; Cui, Y.; Zhang, M.; Bai, X. Effects of Baduanjin exercise in nasopharyngeal carcinoma patients after chemoradiotherapy: A randomized controlled trial. Support. Care Cancer 2022, 31, 79. [Google Scholar] [CrossRef] [PubMed]
- Høgdal, N.; Juhl, C.; Aadahl, M.; Gluud, C. Early preventive exercises versus usual care does not seem to reduce trismus in patients treated with radiotherapy for cancer in the oral cavity or oropharynx: A randomised clinical trial. Acta Oncol. 2015, 54, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Carnaby-Mann, G.; Crary, M.A.; Schmalfuss, I.; Amdur, R. “Pharyngocise”: Randomized controlled trial of preventative exercises to maintain muscle structure and swallowing function during head-and-neck chemoradiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Bragante, K.C.; Groisman, S.; Carboni, C.; Baiocchi, J.M.T.; da Motta, N.W.; Silva, M.F.; Pinto, R.C.; Plentz, R.D.M.; Wienandts, P.; Jotz, G.P. Efficacy of exercise therapy during radiotherapy to prevent reduction in mouth opening in patients with head and neck cancer: A randomized controlled trial. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2020, 129, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Sekar, M.; Kumaresan, A.; Srinivasan, V.; Surya, V. Effectiveness of Myofascial Release on Mouth Opening in Subjects with Post Operative Buccal Mucosa Carcinoma. Indian J. Physiother. Occup. Ther. 2024, 18, 346–351. [Google Scholar] [CrossRef]
- Loorents, V.; Rosell, J.; Karlsson, C.; Lidbäck, M.; Hultman, K.; Börjeson, S. Prophylactic training for the prevention of radiotherapy-induced trismus—A randomised study. Acta Oncol. 2014, 53, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Hao, G.; Yang, W.; Hou, L. The impact of different timing of mouth opening exercises on trismus in postoperative radiotherapy patients with oral cancer. J. Stomatol. Oral Maxillofac. Surg. 2025, 126, 102104. [Google Scholar] [CrossRef] [PubMed]
- Fong, S.S.; Ng, S.S.; Lee, H.W.; Pang, M.Y.; Luk, W.S.; Chung, J.W.; Wong, J.Y.; Masters, R.S. The effects of a 6-month Tai Chi Qigong training program on temporomandibular, cervical, and shoulder joint mobility and sleep problems in nasopharyngeal cancer survivors. Integr. Cancer Ther. 2015, 14, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Retèl, V.P.; van der Molen, L.; Steuten, L.M.; van den Brekel, M.W.; Hilgers, F.J. A cost-effectiveness analysis of using TheraBite in a preventive exercise program for patients with advanced head and neck cancer treated with concomitant chemo-radiotherapy. Eur. Arch. Oto-Rhino-Laryngol. 2016, 273, 709–718. [Google Scholar] [CrossRef] [PubMed]
- van der Molen, L.; van Rossum, M.A.; Burkhead, L.M.; Smeele, L.E.; Rasch, C.R.; Hilgers, F.J. A randomized preventive rehabilitation trial in advanced head and neck cancer patients treated with chemoradiotherapy: Feasibility, compliance, and short-term effects. Dysphagia 2011, 26, 155–170. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, R.; Yeo, S.T.; Rogers, S.N.; Caress, A.L.; Molassiotis, A.; Ryder, D.; Sanghera, P.; Lunt, C.; Scott, B.; Keeley, P.; et al. Randomised feasibility study to compare the use of Therabite® with wooden spatulas to relieve and prevent trismus in patients with cancer of the head and neck. Br. J. Oral Maxillofac. Surg. 2018, 56, 283–291. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aboelez, M.A.; Ibrahim, A.M.; ElSawy, M.A.; Sayed El-Khamisy, N.E. Efficiency of different treatment modalities on radiation induced trismus for maxillofacial cases: A parallel randomized clinical trial. BMC Oral Health 2025, 25, 332. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sirapracha, J.; Sessirisombat, S. Comparative study on the maximum mouth opening between dynamic and static jaw exercise in irradiated head and neck cancer patients: A randomized control trial. J. Oral Maxillofac. Surg. Med. Pathol. 2018, 30, 307–312. [Google Scholar] [CrossRef]
- Tang, Y.; Shen, Q.; Wang, Y.; Lu, K.; Wang, Y.; Peng, Y. A randomized prospective study of rehabilitation therapy in the treatment of radiation-induced dysphagia and trismus. Strahlenther. Onkol. 2011, 187, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Castro-Martín, E.; Galiano-Castillo, N.; Fernández-Lao, C.; Ortiz-Comino, L.; Postigo-Martin, P.; Arroyo-Morales, M. Myofascial Induction Therapy Improves the Sequelae of Medical Treatment in Head and Neck Cancer Survivors: A Single-Blind, Placebo-Controlled, Randomized Cross-Over Study. J. Clin. Med. 2021, 10, 5003. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kamstra, J.I.; van Leeuwen, M.; Roodenburg, J.L.; Dijkstra, P.U. Exercise therapy for trismus secondary to head and neck cancer: A sys-tematic review. Head Neck 2017, 39, 160–169, Erratum in Head Neck 2017, 39, 2352–2362. https://doi.org/10.1002/hed.24859. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.H.; Chiang, C.C.; Huang, T.W. Exercise therapy for cancer treatment-induced trismus in patients with head and neck cancer: A systematic review and meta-analysis of randomized controlled trials. Radiother. Oncol. 2020, 151, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Valencia, A.; Ruiz-Muñoz, M.; Martin-Martin, J.; Cuesta-Vargas, A.; González-Sánchez, M. Effcacy of Manual Therapy in TemporomandibularJoint Disorders and Its Medium-and Long-TermEffects on Pain and Maximum Mouth Opening: A Systematic Review and Meta-Analysis. J. Clin. Med. 2020, 9, 3404. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nicolakis, P.; Erdogmus, B.; Kopf, A.; Djaber-Ansari, A.; Piehslinger, E.; Fialka-Moser, V. Exercise therapy for craniomandibular disorders. Arch. Phys. Med. Rehabil. 2000, 81, 1137–1142. [Google Scholar] [CrossRef] [PubMed]
- Seijas, V.; Maritz, R.; Fernandes, P.; Bernard, R.M.; Lugo, L.H.; Bickenbach, J.; Sabariego, C. Rehabilitation delivery models to foster healthy ageing—A scoping review. Front. Rehabil. Sci. 2024, 5, 1307536. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ostelo, R.W.; Deyo, R.A.; Stratford, P.; Waddell, G.; Croft, P.; Von Korff, M.; Bouter, L.M.; de Vet, H.C. Interpreting change scores for pain and functional status in low back pain: Towards international consensus regarding minimal important change. Spine 2008, 33, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Kazdin, A.E. Mediators and mechanisms of change in psychotherapy research. Annu. Rev. Clin. Psychol. 2007, 3, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Stockley, R.C.; Graham, I.S. The importance of embracing complexity in rehabilitation. J. Eval. Clin. Pract. 2023, 29, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Bialosky, J.E.; Bishop, M.D.; Price, D.D.; Robinson, M.E.; George, S.Z. The mechanisms of manual therapy in the treatment of musculoskeletal pain: A comprehensive model. Man. Ther. 2009, 14, 531–538. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Craig, P.; Dieppe, P.; Macintyre, S.; Michie, S.; Nazareth, I.; Petticrew, M. Medical Research Council Guidance. Developing and evaluating complex interventions: The new Medical Research Council guidance. BMJ 2008, 337, a1655. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).