Does the Interaction Between Alcohol Use and Depression Exacerbate Hyperglycemia Risk? A Cross-Sectional Study Beyond Additive Effects
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Data Collection and Measurements
2.3. Assessment of Depression and Alcohol Consumption
2.4. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics
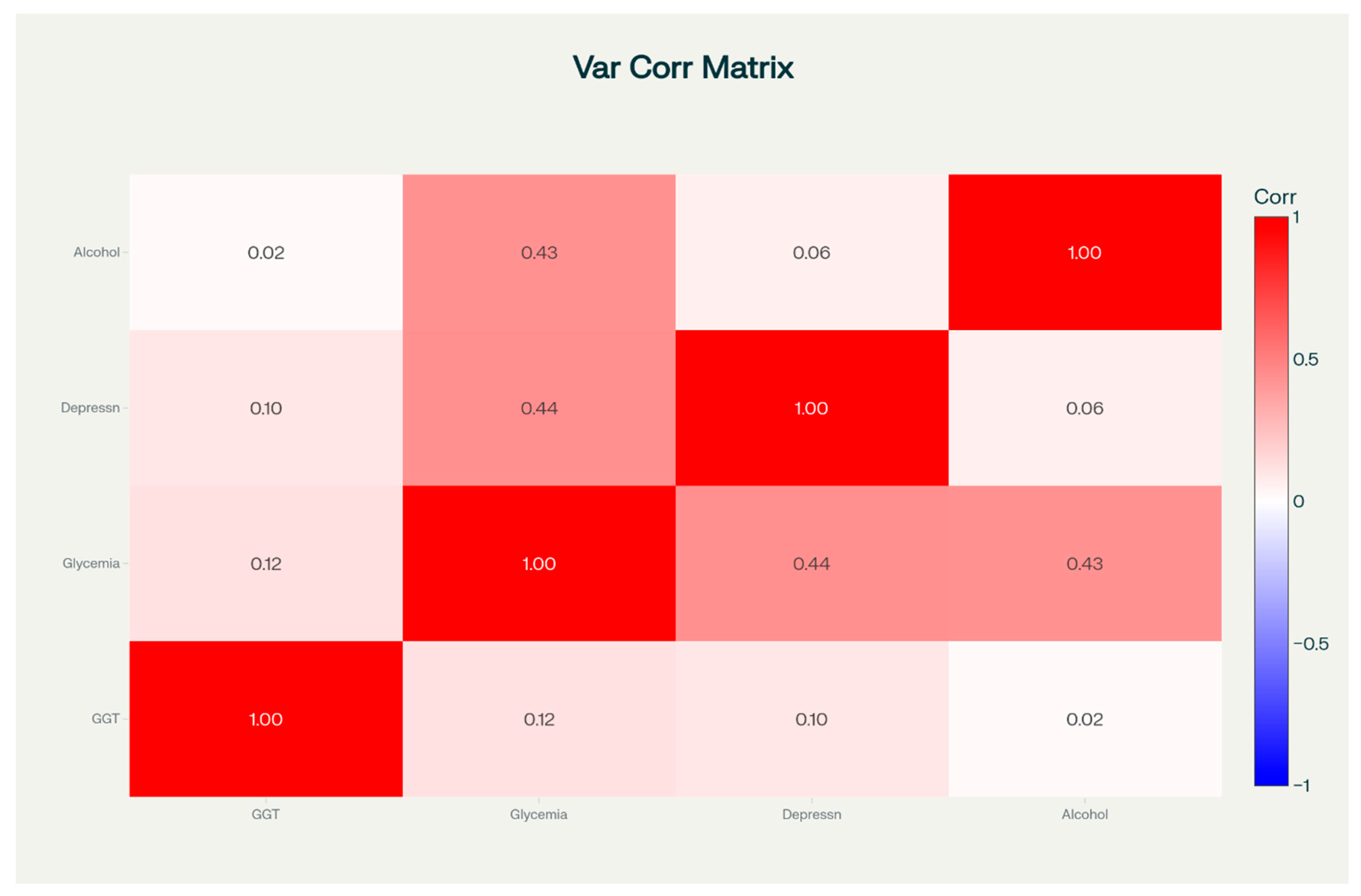
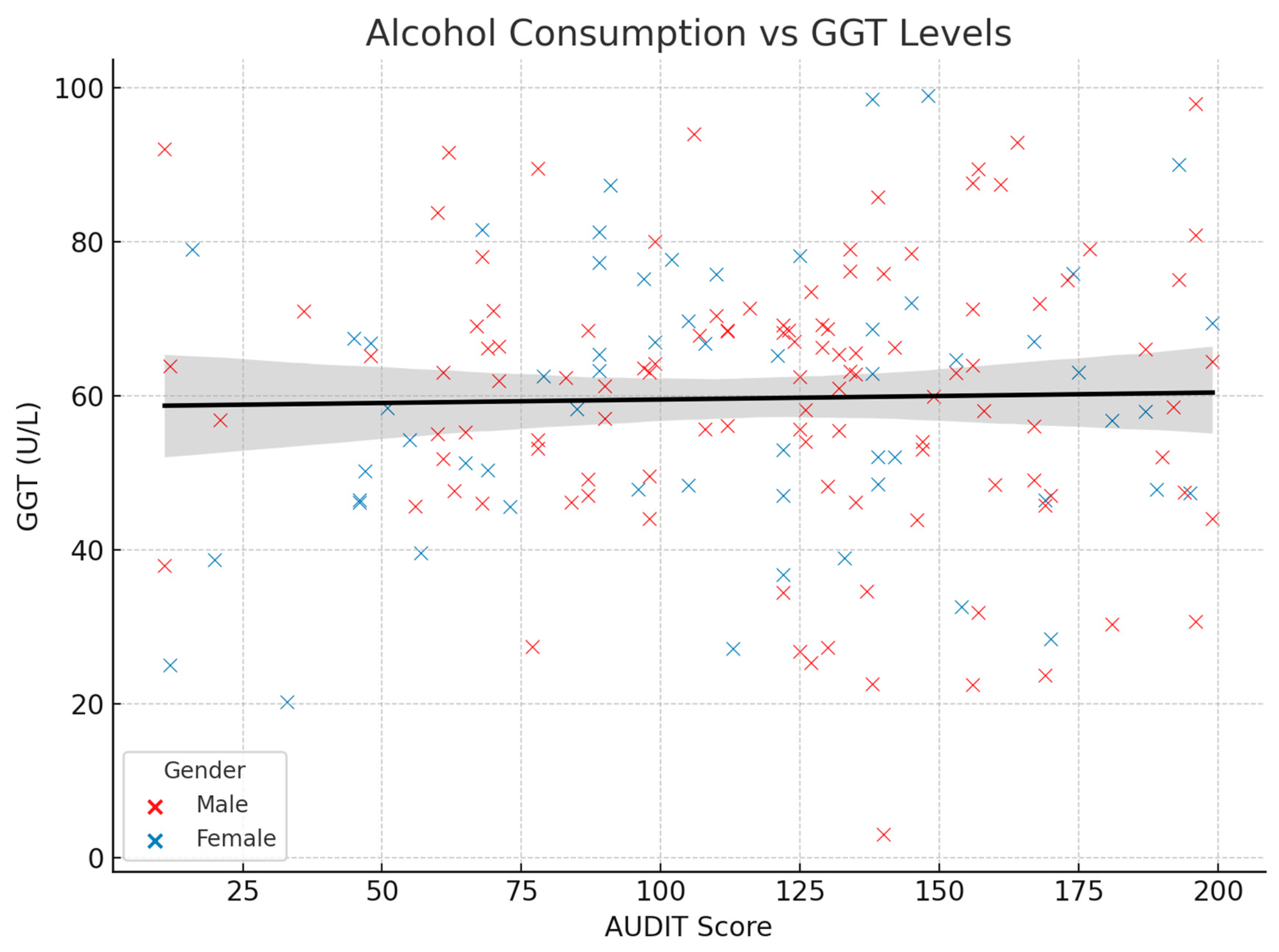
3.2. Correlations Between Variables of Interest
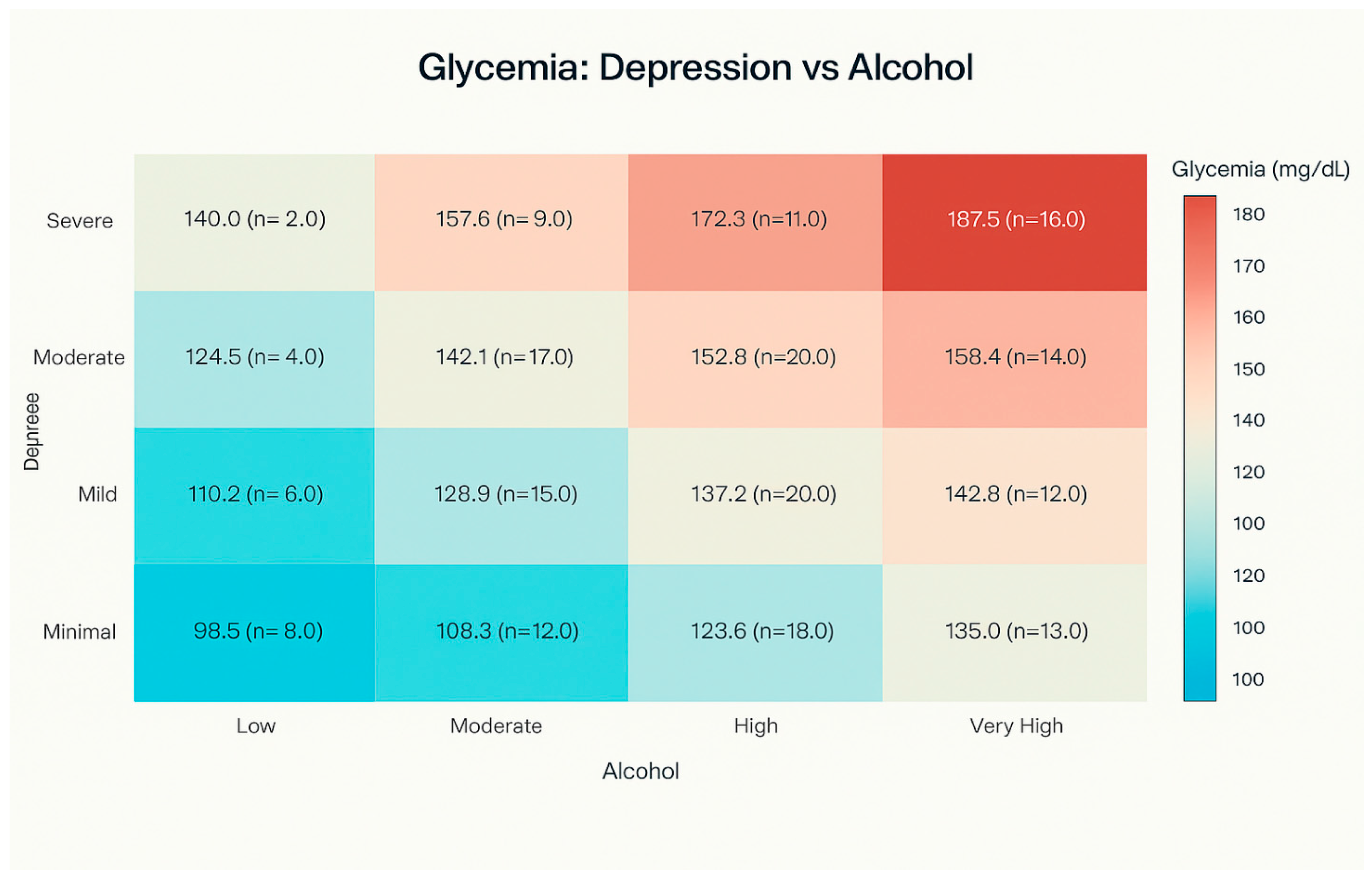
3.3. Glycemia Levels by Depression Category and Alcohol Consumption
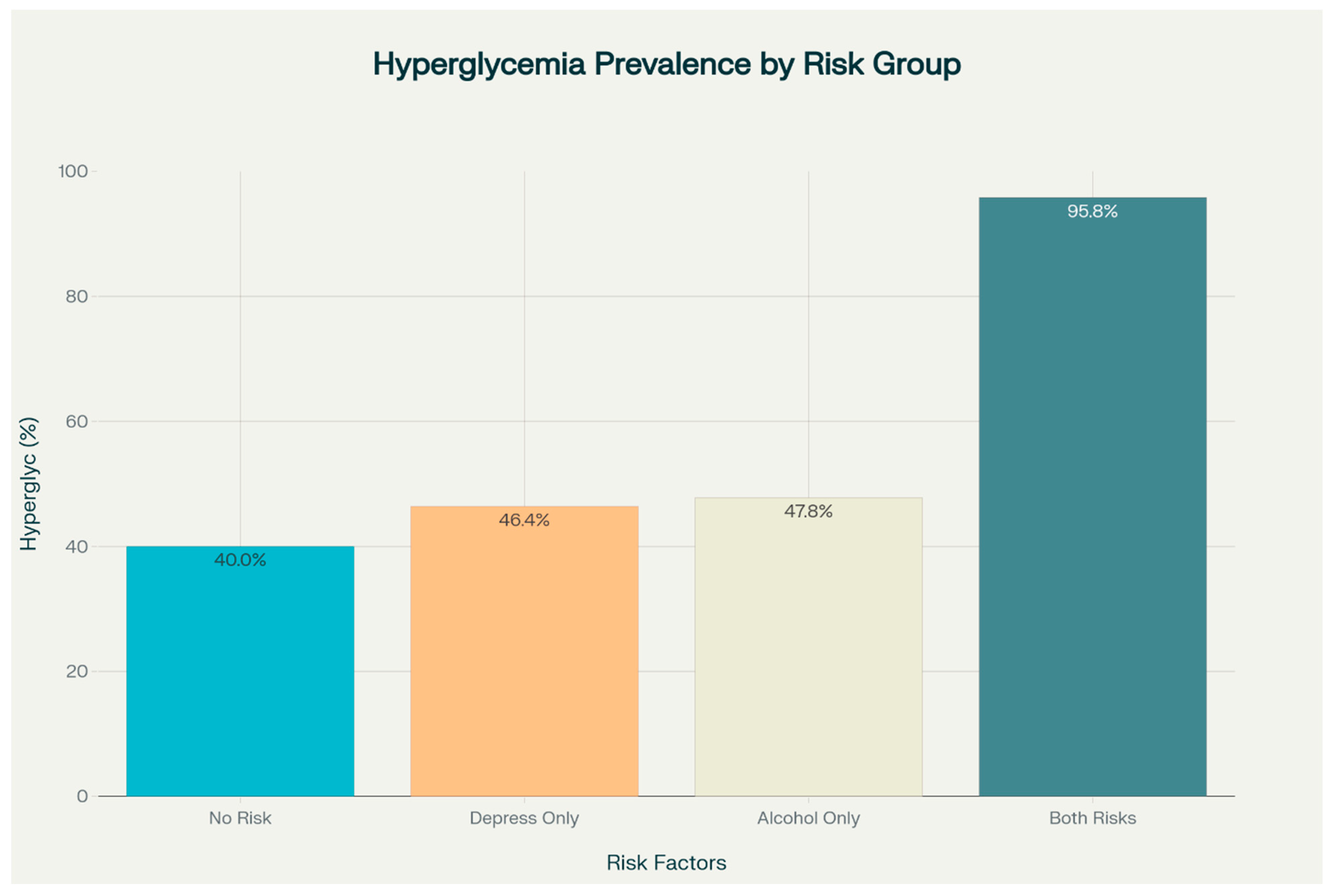
3.4. Prevalence of Hyperglycemia by Risk Factors
3.5. Regression Analysis and Risk Estimation
3.6. Relationship Between Risk Factors and Mean Glycemia Levels
3.7. Mediation Analysis
4. Discussion
4.1. The Association Between Alcohol Consumption, Depression, and Hyperglycemia in the Context of the Scientific Literature
4.2. Pathophysiological Mechanisms of the Alcohol–Depression–Diabetes Interaction
4.3. Dysfunction of HPA Axis
4.4. Systemic Inflammation and Neuroinflammation
4.5. Neurotransmitter Systems
4.6. Therapeutic Implications and Integrated Management
4.7. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| T2DM | Type 2 Diabetes Mellitus |
| GGT | gamma-Glutamyl Transferase |
| BDI | Beck Depression Inventory |
| AUDIT | Alcohol Use Disorder Identification Test |
| HPA | Hypothalamic–Pituitary–Adrenal |
| 5-HTT | Serotonin Transporter Gene |
| BMI | Body Mass Index |
References
- Derese, A.; Sirgu, S.; Gebreegzhiabhere, Y.; Hanlon, C. Mechanisms and Pathways Linking Depression and Type 2 Diabetes Outcomes: A Scoping Review. medRXiv 2025. [Google Scholar] [CrossRef]
- Virtanen, M.; Ferrie, J.E.; Tabak, A.G.; Akbaraly, T.N.; Vahtera, J.; Singh-Manoux, A.; Kivimäki, M. Psychological Distress and Incidence of Type 2 Diabetes in High-Risk and Low-Risk Populations: The Whitehall II Cohort Study. Diabetes Care 2014, 37, 2091–2097. [Google Scholar] [CrossRef] [PubMed]
- Beulens, J.W.J.; van der Schouw, Y.T.; Moons, K.G.M.; Boshuizen, H.C.; van der, A.D.L.; Groenwold, R.H.H. Estimating the mediating effect of different biomarkers on the relation of alcohol consumption with the risk of type 2 diabetes. Ann. Epidemiol. 2013, 23, 193–197. [Google Scholar] [CrossRef] [PubMed]
- van Beek, J.H.; de Moor, M.H.; Geels, L.M.; Sinke, M.R.; de Geus, E.J.; Lubke, G.H.; Kluft, C.; Neuteboom, J.; Vink, J.M.; Willemsen, G.; et al. The association of alcohol intake with gamma-glutamyl transferase (GGT) levels: Evidence for correlated genetic effects. Drug Alcohol. Depend. 2014, 134, 99–105. [Google Scholar] [CrossRef]
- Peterson, K. Biomarkers for Alcohol Use and Abuse. Alcohol. Res. Health 2004, 28, 30–37. [Google Scholar]
- Heo, J.H.; Kang, J.G.; Han, K.; Lee, K.J. Association between Gamma-Glutamyl Transferase Levels and Pancreatobiliary Cancer Risk in Patients with Diabetes: Evidence from the National Health Insurance Cooperation Health Checkup 2009 to 2012. Gut Liver 2025, 19, 286–296. [Google Scholar] [CrossRef]
- Bijnens, E.M.; Derom, C.; Thiery, E.; Martens, D.S.; Loos, R.J.F.; Weyers, S.; Nawrot, T.S. Serum gamma-glutamyl transferase, a marker of alcohol intake, is associated with telomere length and cardiometabolic risk in young adulthood. Sci. Rep. 2021, 11, 12407. [Google Scholar] [CrossRef]
- Baliunas, D.O.; Taylor, B.J.; Irving, H.; Roerecke, M.; Patra, J.; Mohapatra, S.; Rehm, J. Alcohol as a Risk Factor for Type 2 Diabetes: A systematic review and meta-analysis. Diabetes Care 2009, 32, 2123–2132. [Google Scholar] [CrossRef]
- Zheng, C.; Yin, J.; Wu, L.; Hu, Z.; Zhang, Y.; Cao, L.; Qu, Y. Association between depression and diabetes among American adults using NHANES data from 2005 to 2020. Sci. Rep. 2024, 14, 27735. [Google Scholar] [CrossRef]
- Magzoub, M.; Ali, E.; Elzubair, Q.A.; Alfeel, A.; Yousif, I.; Gopinath, K. Evaluation of Gamma-Glutamine Transferase (γ-GT) as a Marker of Alcohol Abuse. Biomed. Pharmacol. J. 2024, 17, 2709–2715. [Google Scholar] [CrossRef]
- Niemelä, O. Biomarker-Based Approaches for Assessing Alcohol Use Disorders. Int. J. Env. Res. Public Health 2016, 13, 166. [Google Scholar] [CrossRef]
- Subba, R.; Sandhir, R.; Singh, S.P.; Mallick, B.N.; Mondal, A.C. Pathophysiology linking depression and type 2 diabetes: Psychotherapy, physical exercise, and fecal microbiome transplantation as damage control. Eur. J. Neurosci. 2021, 53, 2870–2900. [Google Scholar] [CrossRef] [PubMed]
- Bellos, S.; Skapinakis, P.; Rai, D.; Zitko, P.; Araya, R.; Lewis, G.; Lionis, C.; Mavreas, V. Longitudinal association between different levels of alcohol consumption and a new onset of depression and generalized anxiety disorder: Results from an international study in primary care. Psychiatry Res. 2016, 243, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Shayani, G.; Raka, J.; Sonali, J.; Ravindra, R.; Kumar, M.A. Alcohol Biomarkers and their Relevance in Detection of Alcohol Consumption in Clinical Settings. Int. Arch. Subst. Abus. Rehabil. 2019, 1. [Google Scholar] [CrossRef]
- Monteiro, M.G.; Masur, J. Alcohol: How Useful Is Comb. Gamma Glutamyl Transferase Differ. Biochem. Markers? Drug Alcohol Depend. Drug Alcohol. Depend. 1985, 16, 31–37. [Google Scholar] [CrossRef]
- Munukutla, S.; Pan, G.; Deshpande, M.; Thandavarayan, R.A.; Krishnamurthy, P.; Palaniyandi, S.S. Alcohol Toxic. Diabetes Its Complicat: A Double Trouble? Alcohol. Clin. Exp. Res. 2016, 40, 686–697. [Google Scholar] [CrossRef]
- Gan, Y.; Tian, F.; Fan, X.; Wang, H.; Zhou, J.; Yang, N.; Qi, H. A study of the relationship between social support, depression, alexithymia and glycemic control in patients with type 2 diabetes mellitus: A structural equation modeling approach. Front. Endocrinol. 2024, 15, 1390564. [Google Scholar] [CrossRef]
- Mitincu-Caramfil, S.D.; Moroianu, L.-A.; Bradeanu, A.V.; Isailă, O.-M.; Curis, C.; Drima, E. The Correlation Between Emotionality Changes and Alcohol Consumption in Young Persons: A Pilot Study. Healthcare 2025, 13, 987. [Google Scholar] [CrossRef]
- World Health Organization; International Diabetes Federation. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycaemia: Report of a WHO/IDF Consultation; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Beck, A.T.; Steer, R.A.; Brown, G.K. Manual for the Beck Depression Inventory-II; Psychological Corporation: San Antonio, TX, USA, 1996. [Google Scholar]
- Beck, A.T. Depression: Causes and Treatment; 13. Print; University of Pennsylvania Press: Philadelphia, PA, USA, 1991. [Google Scholar]
- Upton, J. Beck Depression Inventory (BDI). In Encyclopedia of Behavioral Medicine; Springer: New York, NY, USA, 2013; pp. 178–179. [Google Scholar] [CrossRef]
- Saunders, J.B.; Aasland, O.G.; Babor, T.F.; De La Fuente, J.R.; Grant, M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption-II. Addiction 1993, 88, 791–804. [Google Scholar] [CrossRef]
- Babor, T.; Higgins-Biddle, J.C.; Saunders, J.B.; Monteiro, M.G. AUDIT: The Alcohol Use Disorders Identification Test: Guidelines for Use in Primary Health Care, 2nd ed.; World Health Organization: Geneva, Switzerland, 2001. [Google Scholar]
- Droahnă, A.R.; Moroianu, L.-A.; Pietroșel, V.-A.; Bica, C.I.; Salmen, T.; Curis, C.; Merlo, E.M.; Stoica, R.A.; Moroianu, M. Anxio-depressive disorders in a pandemic context: A comparative analysis: Year 2019 versus 2020. J. Mind Med. Sci. 2023, 10, 156–162. [Google Scholar] [CrossRef]
- Capasso, A.; Jones, A.M.; Ali, S.H.; Foreman, J.; Tozan, Y.; DiClemente, R.J. Increased alcohol use during the COVID-19 pandemic: The effect of mental health and age in a cross-sectional sample of social media users in the U.S. Prev. Med. 2021, 145, 106422. [Google Scholar] [CrossRef] [PubMed]
- Acuff, S.F.; Strickland, J.C.; Tucker, J.A.; Murphy, J.G. Changes in alcohol use during COVID-19 and associations with contextual and individual difference variables: A systematic review and meta-analysis. Psychol. Addict. Behav. 2022, 36, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Wang, S.; Smith, E.; Goyal, D.; Zhu, K.; Zhang, Z.-F.; Smith, B.; Niu, Z.; Lei, L.; Freudenheim, J.L.; et al. Alcohol Consumption and Mental Health during the COVID-19 Pandemic. Alcohol. Alcohol. 2023, 58, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Curis, C.; Ardeleanu, V.; Moroianu, L.A.; Manole, C.; Stoica, R.A.; Gherghiceanu, F.; Stoian, A.P. Obesity in Children: Systematic Review over a 6-Year Period, Including the COVID-19 Pandemic. J. Mind Med. Sci. 2024, 11, 310–320. [Google Scholar] [CrossRef]
- Elgendy, R.; Deschênes, S.S.; Burns, R.J.; Levy, M.; Schmitz, N. Alcohol consumption, depressive symptoms, and the incidence of diabetes-related complications. J. Diabetes 2019, 11, 14–22. [Google Scholar] [CrossRef]
- Fanelli, G.; Raschi, E.; Hafez, G.; Matura, S.; Schiweck, C.; Poluzzi, E.; Lunghi, C. The interface of depression and diabetes: Treatment considerations. Transl. Psychiatry 2025, 15, 22. [Google Scholar] [CrossRef]
- Afif. Diabetes, Depression, and Alcohol: Understanding the Risks. Tap Health. 2025. Available online: https://tap.health/diabetes-depression-alcohol-risks/ (accessed on 21 June 2025).
- Liu, Y.; Huang, S.-Y.; Liu, D.-L.; Zeng, X.-X.; Pan, X.-R.; Peng, J. Bidirectional relationship between diabetes mellitus and depression: Mechanisms and epidemiology. World J. Psychiatry 2024, 14, 1429–1436. [Google Scholar] [CrossRef]
- Joseph, J.J.; Golden, S.H. Cortisol dysregulation: The bidirectional link between stress, depression, and type 2 diabetes mellitus. Ann. N. Y. Acad. Sci. 2017, 1391, 20–34. [Google Scholar] [CrossRef]
- Jang, H.B.; Go, M.J.; Park, S.I.; Lee, H.-J.; Cho, S.B. Chronic heavy alcohol consumption influences the association between genetic variants of GCK or INSR and the development of diabetes in men: A 12-year follow-up study. Sci. Rep. 2019, 9, 20029. [Google Scholar] [CrossRef]
- Kim, S.-J.; Kim, D.-J. Alcoholism and Diabetes Mellitus. Diabetes Metab. J. 2012, 36, 108–115. [Google Scholar] [CrossRef]
- Lindtner, C.; Scherer, T.; Zielinski, E.; Filatova, N.; Fasshauer, M.; Tonks, N.K.; Puchowicz, M.; Buettner, C. Binge Drinking Induces Whole-Body Insulin Resistance by Impairing Hypothalamic Insulin Action. Sci. Transl. Med. 2013, 5, ra14–ra170. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wang, G.-J.; Kojori, E.S.; Fowler, J.S.; Benveniste, H.; Tomasi, D. Alcohol Decreases Baseline Brain Glucose Metabolism More in Heavy Drinkers Than Controls but Has No Effect on Stimulation-Induced Metabolic Increases. J. Neurosci. 2015, 35, 3248–3255. [Google Scholar] [CrossRef] [PubMed]
- Curis, C.; Moroianu, M.; Moroianu, L.-A.; Pintilei, D.R.; Ardeleanu, V.; Bobirca, A.; Mahler, B.; Stoian, A.P. Intuitive Nutrition Co-factor of Emotional Equilibrium for Maintaining Nutritional Balance: A Prospective Study. Mediterr. J. Clin. Psychol. 2023, 11. [Google Scholar] [CrossRef]
- Rehm, J.; Gmel, G.E., Sr.; Gmel, G.; Hasan, O.S.M.; Imtiaz, S.; Popova, S.; Probst, C.; Roerecke, M.; Room, R.; Samokhvalov, A.V.; et al. The relationship between different dimensions of alcohol use and the burden of disease-an update. Addiction 2017, 112, 968–1001. [Google Scholar] [CrossRef] [PubMed]
- Pan, A.; Lucas, M.; Sun, Q.; van Dam, R.M.; Franco, O.H.; Willett, W.C.; Manson, J.E.; Rexrode, K.M.; Ascherio, A.; Hu, F.B. Increased Mortality Risk in Women with Depression and Diabetes Mellitus. Arch. Gen. Psychiatry 2011, 68, 42–50. [Google Scholar] [CrossRef]
- Atlantis, E.; Baker, M. Obesity effects on depression: Systematic review of epidemiological studies. Int. J. Obes. 2008, 32, 881–891. [Google Scholar] [CrossRef]
- Fox, H.C.; Milivojevic, V.; MacDougall, A.; LaVallee, H.; Simpson, C.; Angarita, G.A.; Sinha, R. Stress-related suppression of peripheral cytokines predicts future relapse in alcohol-dependent individuals with and without subclinical depression. Addict. Biol. 2020, 25, e12832. [Google Scholar] [CrossRef]
- Kelley, K.W.; Dantzer, R. Alcoholism and inflammation: Neuroimmunology of behavioral and mood disorders. Brain Behav. Immun. 2011, 25, S13–S20. [Google Scholar] [CrossRef]
- Cullins, A. Does Alcohol Cause Inflammation? Drinking and the Risk of Chronic Illness. Ria Health. 2024. Available online: https://riahealth.com/blog/alcohol-and-inflammation/ (accessed on 21 June 2025).
- Wang, H.J.; Zakhari, S.; Jung, M.K. Alcohol, inflammation, and gut-liver-brain interactions in tissue damage and disease development. World J. Gastroenterol. 2010, 16, 1304–1313. [Google Scholar] [CrossRef]
- Neupane, S.P. Neuroimmune Interface in the Comorbidity between Alcohol Use Disorder and Major Depression. Front. Immunol. 2016, 7, 655. [Google Scholar] [CrossRef]
- Leclercq, S.; Le Roy, T.; Furgiuele, S.; Coste, V.; Bindels, L.B.; Leyrolle, Q.; Neyrinck, A.M.; Quoilin, C.; Amadieu, C.; Petit, G.; et al. Gut Microbiota-Induced Changes in β-Hydroxybutyrate Metabolism Are Linked to Altered Sociability and Depression in Alcohol Use Disorder. Cell Rep. 2020, 33, 108238. [Google Scholar] [CrossRef] [PubMed]
- de Bartolomeis, A.; De Simone, G.; De Prisco, M.; Barone, A.; Napoli, R.; Beguinot, F.; Billeci, M.; Fornaro, M. Insulin effects on core neurotransmitter pathways involved in schizophrenia neurobiology: A meta-analysis of preclinical studies. Implications for the treatment. Mol. Psychiatry 2023, 28, 2811–2825. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Singla, R.; Maheshwari, O.; Fontaine, C.J.; Gil-Mohapel, J. Alcohol Use Disorder: Neurobiology and Therapeutics. Biomedicines 2022, 10, 1192. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, V.; Gupta, D.; Kanade, P.; Radhakrishnan, M. Diabetes-associated depression: The serotonergic system as a novel multifunctional target. Indian J. Pharmacol. 2015, 47, 4–10. [Google Scholar] [CrossRef]
- The Effects of Alcohol & Drug Addiction on Serotonin & Dopamine. ARK Behavioral Health. Available online: https://www.arkbh.com/addiction-serotonin-and-dopamine/ (accessed on 21 June 2025).
- Okey, B. What Are the Effects of Alcohol on GABA? 2023. Available online: https://www.ardurecoverycenter.com/alcohol-effects-on-gaba-neurotransmitters/ (accessed on 21 June 2025).
- Krystal, J.H.; Staley, J.; Mason, G.; Petrakis, I.L.; Kaufman, J.; Harris, R.A.; Gelernter, J.; Lappalainen, J. γ-Aminobutyric Acid Type A Receptors and Alcoholism: Intoxication, Dependence, Vulnerability, and Treatment. Arch. Gen. Psychiatry 2006, 63, 957–968. [Google Scholar] [CrossRef]
- Mitincu-Caramfil, S.D.; Drima, E.; Pascu, L.S.; Moroianu, L.-A.; Gherghiceanu, F.; Popoviciu, M.-S.; Stoian, A.P. The Relationship Between Alcohol Consumption and Depression: An Analysis of Secondary Affections and Therapeutic Interventions. J. Mind Med. Sci. 2025, 12, 24. [Google Scholar] [CrossRef]
- Moroianu, L.-A.; Curis, C.; Moroianu, M.; Stefanescu, C.; Ciubara, A. Optimism as a Resource and Form of Manifesting Positive Affectiveness in the Elderly and Young Adults Pre-Pandemic vs Pandemic—Comparative Study. BRAIN Broad Res. Artif. Intell. Neurosci. 2024, 15, 240–257. [Google Scholar] [CrossRef]



| Variable | Total (n = 172) | Men (n = 113) | Women (n = 59) | p-Value |
|---|---|---|---|---|
| Age (years) | 43.5 ± 12.3 | 44.1 ± 11.7 | 42.3 ± 13.4 | 0.412 |
| BMI (kg/m2) | 27.8 ± 4.2 | 27.5 ± 4.5 | 28.4 ± 3.7 | 0.264 |
| Glycemia (mg/dL) | 144.7 ± 26.2 | 146.2 ± 27.1 | 142.0 ± 24.5 | 0.305 |
| Hyperglycemia prevalence | 58.7% | 61.9% | 52.5% | 0.305 |
| GGT (U/L) | 59.7 ± 17.6 | 61.2 ± 18.3 | 56.8 ± 16.0 | 0.172 |
| Severe depression (BDI > 25) | 45.9% | 39.8% | 52.5% | 0.152 |
| High alcohol use (AUDIT > 20) | 54.1% | 59.3% | 45.8% | 0.126 |
| Model | Comparison | OR (95% CI) | p-Value |
|---|---|---|---|
| 1 | High Alcohol vs. Low | 1.38 (0.61–3.09) | 0.441 |
| 2 | Severe Depression vs. None | 1.30 (0.51–3.31) | 0.582 |
| 3 | Interaction (High Alcohol × Severe Depression) | 19.30 (3.22–115.81) | 0.001 |
| 4 | Both Risk Factors vs. Neither | 28.85 (4.81–173.02) | <0.001 |
| High Alcohol | High Depression | Mean | Std | Count |
|---|---|---|---|---|
| 0 | 0 | 133.9 | 25.9 | 50 |
| 0 | 1 | 134.5 | 24.1 | 28 |
| 1 | 0 | 143.3 | 25.5 | 46 |
| 1 | 1 | 163.3 | 17.4 | 48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitincu-Caramfil, S.-D.; Stoian, A.P.; Moroianu, L.-A.; Plesea-Condratovici, C.; Bradeanu, A.V.; Drima, E. Does the Interaction Between Alcohol Use and Depression Exacerbate Hyperglycemia Risk? A Cross-Sectional Study Beyond Additive Effects. Medicina 2025, 61, 1380. https://doi.org/10.3390/medicina61081380
Mitincu-Caramfil S-D, Stoian AP, Moroianu L-A, Plesea-Condratovici C, Bradeanu AV, Drima E. Does the Interaction Between Alcohol Use and Depression Exacerbate Hyperglycemia Risk? A Cross-Sectional Study Beyond Additive Effects. Medicina. 2025; 61(8):1380. https://doi.org/10.3390/medicina61081380
Chicago/Turabian StyleMitincu-Caramfil, Simona-Dana, Anca Pantea Stoian, Lavinia-Alexandra Moroianu, Catalin Plesea-Condratovici, Andrei Vlad Bradeanu, and Eduard Drima. 2025. "Does the Interaction Between Alcohol Use and Depression Exacerbate Hyperglycemia Risk? A Cross-Sectional Study Beyond Additive Effects" Medicina 61, no. 8: 1380. https://doi.org/10.3390/medicina61081380
APA StyleMitincu-Caramfil, S.-D., Stoian, A. P., Moroianu, L.-A., Plesea-Condratovici, C., Bradeanu, A. V., & Drima, E. (2025). Does the Interaction Between Alcohol Use and Depression Exacerbate Hyperglycemia Risk? A Cross-Sectional Study Beyond Additive Effects. Medicina, 61(8), 1380. https://doi.org/10.3390/medicina61081380








