Value of Early Kinetics of Procalcitonin with Point-of-Care Test to Predict Postoperative Abscess Following Non-Complicated Acute Appendicitis: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
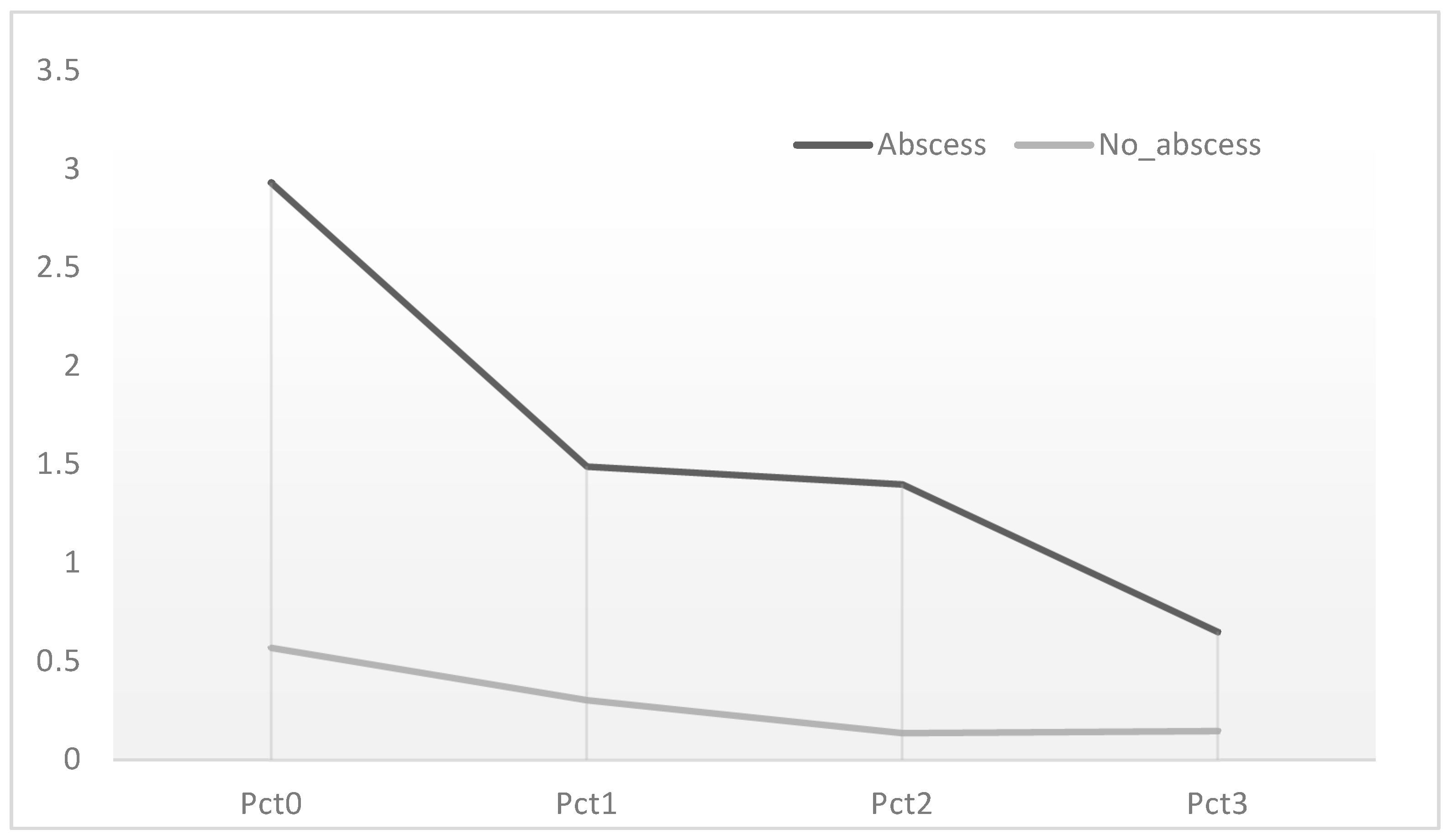
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CRP | C-reactive protein |
| PCT | Procalcitonin |
| POCT | Point-of-care testing |
| LDH | Lactate dehydrogenase |
| PLT | Platelets |
| WBC | White blood cells |
References
- Bianchi, V.; Giambusso, M.; De Iacob, A.; Chiarello, M.M.; Brisinda, G. Artificial intelligence in the diagnosis and treatment of acute appendicitis: A narrative review. Updates Surg. 2024, 76, 783–792. [Google Scholar] [CrossRef]
- Brucchi, F.; Bracchetti, G.; Fugazzola, P.; Vigano, J.; Filisetti, C.; Ansaloni, L.; Dal Mas, F.; Cobianchi, L.; Danelli, P. A meta-analysis and trial sequential analysis comparing nonoperative versus operative management for uncomplicated appendicitis: A focus on randomized controlled trials. World J. Emerg. Surg. 2024, 19, 2. [Google Scholar] [CrossRef]
- Guaitoli, E.; Gallo, G.; Cardone, E.; Conti, L.; Famularo, S.; Formisano, G.; Galli, F.; Giuliani, G.; Martino, A.; Pasculli, A.; et al. Consensus Statement of the Italian Polispecialistic Society of Young Surgeons (SPIGC): Diagnosis and Treatment of Acute Appendicitis. J. Investig. Surg. 2021, 34, 1089–1103. [Google Scholar] [CrossRef]
- Ball, C.G.; Murphy, P.; Verhoeff, K.; Albusadi, O.; Patterson, M.; Widder, S.; Hameed, S.M.; Parry, N.; Vogt, K.; Kortbeek, J.B.; et al. A 30-day prospective audit of all inpatient complications following acute care surgery: How well do we really perform? Can. J. Surg. 2020, 63, E150–E154. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, S.; Kato, H.; Asano, Y.; Horiguchi, A.; Yamamoto, M.; Miura, F.; Okamoto, K.; Kimura, Y.; Sakaguchi, T.; Yoshida, M. Emergency appendectomy versus elective appendectomy following conservative treatment for acute appendicitis: A multicenter retrospective clinical study by the Japanese Society for Abdominal Emergency Medicine. Surg. Today 2022, 52, 1607–1619. [Google Scholar] [CrossRef]
- D’Souza, N. Appendicitis. BMJ Clin. Evid. 2011, 2011, 0408. [Google Scholar] [PubMed]
- Poon, S.H.T.; Lee, J.W.Y.; Ng, K.M.; Chiu, G.W.Y.; Wong, B.Y.K.; Foo, C.C.; Law, W.L. The current management of acute uncomplicated appendicitis: Should there be a change in paradigm? A systematic review of the literatures and analysis of treatment performance. World J. Emerg. Surg. 2017, 12, 46. [Google Scholar] [CrossRef] [PubMed]
- Coccolini, F.; Licitra, G.; De’Angelis, N.; Martinez Perez, A.; Cremonini, C.; Musetti, S.; Strambi, S.; Zampieri, F.; Cengeli, I.; Tartaglia, D.; et al. Complication analysis in acute appendicitis, results from an international multicenter study. Eur. J. Trauma Emerg. Surg. 2024, 50, 305–314. [Google Scholar] [CrossRef]
- Afzal, B.; Cirocchi, R.; Dawani, A.; Desiderio, J.; Di Cintio, A.; Di Nardo, D.; Farinacci, F.; Fung, J.; Gemini, A.; Guerci, L.; et al. Is it possible to predict the severity of acute appendicitis? Reliability of predictive models based on easily available blood variables. World J. Emerg. Surg. 2023, 18, 10. [Google Scholar] [CrossRef]
- Lindberg, M.; Hole, A.; Johnsen, H.; Asberg, A.; Rydning, A.; Myrvold, H.E.; Bjerve, K.S. Reference intervals for procalcitonin and C-reactive protein after major abdominal surgery. Scand. J. Clin. Lab. Investig. 2002, 62, 189–194. [Google Scholar] [CrossRef]
- AlRawahi, A.N.; AlHinai, F.A.; Doig, C.J.; Ball, C.G.; Dixon, E.; Xiao, Z.; Kirkpatrick, A.W. The prognostic value of serum procalcitonin measurements in critically injured patients: A systematic review. Crit. Care 2019, 23, 390. [Google Scholar] [CrossRef]
- Godinez-Vidal, A.R.; Alcantara-Gordillo, R.; Aguirre-Rojano, V.I.; Lopez-Romero, S.C.; Gonzalez-Calatayud, M.; Gonzalez-Perez, L.G.; Pulido-Cejudo, A.; Gracida-Mancilla, N.I. Evaluation of C-reactive protein, procalcitonin and the PCR/PCT index as indicators of mortality in abdominal sepsis. Cirugía Y Cir. 2020, 88, 150–153. [Google Scholar] [CrossRef]
- Novotny, T.; Staffa, R.; Tomandl, J.; Krivka, T.; Kruzliak, P.; Tomandlova, M.; Slaby, O.; Sponiar, J.; Caprnda, M.; Gaspar, L.; et al. Procalcitonin kinetics following abdominal aortic surgery and its value for postoperative intestinal ischaemia detection. Vascular 2023, 31, 1061–1068. [Google Scholar] [CrossRef]
- Albuali, W.H. The impact of procalcitonin in assessing outcomes in pediatrics severe trauma cases: A three-year experience from a tertiary hospital. Biomedicine 2023, 13, 39–45. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Borruel Nacenta, S.; Ibanez Sanz, L.; Sanz Lucas, R.; Depetris, M.A.; Martinez Chamorro, E. Update on acute appendicitis: Typical and untypical findings. Radiologia 2023, 65 (Suppl. 1), S81–S91. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Mustafa, M.S.; Shafique, M.A.; Haseeb, A.; Rangwala, H.S.; Kumar, H.; Rangwala, B.S.; Raja, A.; Raja, S.; Ali, S.M.S. Comparison of polymeric clip and endoloop in laparoscopic appendectomy: A systematic review and meta-analysis. Surgery 2024, 176, 1329–1336. [Google Scholar] [CrossRef] [PubMed]
- Podda, M.; Gerardi, C.; Cillara, N.; Fearnhead, N.; Gomes, C.A.; Birindelli, A.; Mulliri, A.; Davies, R.J.; Di Saverio, S. Antibiotic Treatment and Appendectomy for Uncomplicated Acute Appendicitis in Adults and Children: A Systematic Review and Meta-analysis. Ann. Surg. 2019, 270, 1028–1040. [Google Scholar] [CrossRef]
- Sallinen, V.; Akl, E.A.; You, J.J.; Agarwal, A.; Shoucair, S.; Vandvik, P.O.; Agoritsas, T.; Heels-Ansdell, D.; Guyatt, G.H.; Tikkinen, K.A. Meta-analysis of antibiotics versus appendicectomy for non-perforated acute appendicitis. Br. J. Surg. 2016, 103, 656–667. [Google Scholar] [CrossRef] [PubMed]
- Huston, J.M.; Kao, L.S.; Chang, P.K.; Sanders, J.M.; Buckman, S.; Adams, C.A.; Cocanour, C.S.; Parli, S.E.; Grabowski, J.; Diaz, J.; et al. Antibiotics vs. Appendectomy for Acute Uncomplicated Appendicitis in Adults: Review of the Evidence and Future Directions. Surg. Infect. 2017, 18, 527–535. [Google Scholar] [CrossRef]
- Di Saverio, S.; Podda, M.; De Simone, B.; Ceresoli, M.; Augustin, G.; Gori, A.; Boermeester, M.; Sartelli, M.; Coccolini, F.; Tarasconi, A.; et al. Diagnosis and treatment of acute appendicitis: 2020 update of the WSES Jerusalem guidelines. World J. Emerg. Surg. 2020, 15, 27. [Google Scholar] [CrossRef] [PubMed]
- Sauerland, S.; Lefering, R.; Neugebauer, E.A. Laparoscopic versus open surgery for suspected appendicitis. Cochrane Database Syst. Rev. 2004, 10, CD001546. [Google Scholar] [CrossRef]
- van Rossem, C.C.; van Geloven, A.A.; Schreinemacher, M.H.; Bemelman, W.A.; snapshot appendicitis collaborative study group. Endoloops or endostapler use in laparoscopic appendectomy for acute uncomplicated and complicated appendicitis: No difference in infectious complications. Surg. Endosc. 2017, 31, 178–184. [Google Scholar] [CrossRef]
- Swank, H.A.; van Rossem, C.C.; van Geloven, A.A.; in’t Hof, K.H.; Kazemier, G.; Meijerink, W.J.; Lange, J.F.; Bemelman, W.A. Endostapler or endoloops for securing the appendiceal stump in laparoscopic appendectomy: A retrospective cohort study. Surg. Endosc. 2014, 28, 576–583. [Google Scholar] [CrossRef]
- Ceresoli, M.; Tamini, N.; Gianotti, L.; Braga, M.; Nespoli, L. Are endoscopic loop ties safe even in complicated acute appendicitis? A systematic review and meta-analysis. Int. J. Surg. 2019, 68, 40–47. [Google Scholar] [CrossRef]
- Knight, S.R.; Ibrahim, A.; Makaram, N.; Patil, P.; Wilson, M.S.J. The use of polymeric clips in securing the appendiceal stump during laparoscopic appendicectomy: A systematic review. Eur. J. Trauma Emerg. Surg. 2019, 45, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhao, L.; Cheng, Y.; Cheng, N.; Deng, Y. Abdominal drainage to prevent intra-peritoneal abscess after open appendectomy for complicated appendicitis. Cochrane Database Syst. Rev. 2018, 5, CD010168. [Google Scholar] [CrossRef]
- Allemann, P.; Probst, H.; Demartines, N.; Schafer, M. Prevention of infectious complications after laparoscopic appendectomy for complicated acute appendicitis—The role of routine abdominal drainage. Langenbecks Arch. Surg. 2011, 396, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Schlottmann, F.; Reino, R.; Sadava, E.E.; Campos Arbulu, A.; Rotholtz, N.A. Could an abdominal drainage be avoided in complicated acute appendicitis? Lessons learned after 1300 laparoscopic appendectomies. Int. J. Surg. 2016, 36, 40–43. [Google Scholar] [CrossRef]
- Siotos, C.; Stergios, K.; Prasath, V.; Seal, S.M.; Duncan, M.D.; Sakran, J.V.; Habibi, M. Irrigation Versus Suction in Laparoscopic Appendectomy for Complicated Appendicitis: A Meta-analysis. J. Surg. Res. 2019, 235, 237–243. [Google Scholar] [CrossRef]
- Hernandez, M.C.; Finnesgard, E.J.; Aho, J.M.; Jenkins, D.H.; Zielinski, M.D. Association of postoperative organ space infection after intraoperative irrigation in appendicitis. J. Trauma Acute Care Surg. 2018, 84, 628–635. [Google Scholar] [CrossRef]
- Antoniou, S.A.; Mavridis, D.; Hajibandeh, S.; Antoniou, G.A.; Gorter, R.; Tenhagen, M.; Koutras, C.; Pointner, R.; Chalkiadakis, G.E.; Granderath, F.-A.; et al. Optimal stump management in laparoscopic appendectomy: A network meta-analysis by the Minimally Invasive Surgery Synthesis of Interventions and Outcomes Network. Surgery 2017, 162, 994–1005. [Google Scholar] [CrossRef] [PubMed]
- Mitsuma, S.F.; Mansour, M.K.; Dekker, J.P.; Kim, J.; Rahman, M.Z.; Tweed-Kent, A.; Schuetz, P. Promising new assays and technologies for the diagnosis and management of infectious diseases. Clin. Infect. Dis. 2013, 56, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Uzzan, B.; Cohen, R.; Nicolas, P.; Cucherat, M.; Perret, G.Y. Procalcitonin as a diagnostic test for sepsis in critically ill adults and after surgery or trauma: A systematic review and meta-analysis. Crit. Care Med. 2006, 34, 1996–2003. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Agnoletti, V.; Ansaloni, L.; Coccolini, F.; Bravi, F.; Sartelli, M.; Vallicelli, C.; Catena, F. Management of Intra-Abdominal Infections: The Role of Procalcitonin. Antibiotics 2023, 12, 1406. [Google Scholar] [CrossRef]
- Schuetz, P.; Birkhahn, R.; Sherwin, R.; Jones, A.E.; Singer, A.; Kline, J.A.; Runyon, M.S.; Self, W.H.; Courtney, D.M.; Nowak, R.M.; et al. Serial Procalcitonin Predicts Mortality in Severe Sepsis Patients: Results From the Multicenter Procalcitonin MOnitoring SEpsis (MOSES) Study. Crit. Care Med. 2017, 45, 781–789. [Google Scholar] [CrossRef]
- Gregoriano, C.; Heilmann, E.; Molitor, A.; Schuetz, P. Role of procalcitonin use in the management of sepsis. J. Thorac. Dis. 2020, 12, S5–S15. [Google Scholar] [CrossRef]
- Linscheid, P.; Seboek, D.; Schaer, D.J.; Zulewski, H.; Keller, U.; Muller, B. Expression and secretion of procalcitonin and calcitonin gene-related peptide by adherent monocytes and by macrophage-activated adipocytes. Crit. Care Med. 2004, 32, 1715–1721. [Google Scholar] [CrossRef]
- Llewelyn, M.J.; Berger, M.; Gregory, M.; Ramaiah, R.; Taylor, A.L.; Curdt, I.; Lajaunias, F.; Graf, R.; Blincko, S.J.; Drage, S.; et al. Sepsis biomarkers in unselected patients on admission to intensive or high-dependency care. Crit. Care 2013, 17, R60. [Google Scholar] [CrossRef]
- Mokart, D.; Merlin, M.; Sannini, A.; Brun, J.P.; Delpero, J.R.; Houvenaeghel, G.; Moutardier, V.; Blache, J.L. Procalcitonin, interleukin 6 and systemic inflammatory response syndrome (SIRS): Early markers of postoperative sepsis after major surgery. Br. J. Anaesth. 2005, 94, 767–773. [Google Scholar] [CrossRef]
- Sager, R.; Kutz, A.; Mueller, B.; Schuetz, P. Procalcitonin-guided diagnosis and antibiotic stewardship revisited. BMC Med. 2017, 15, 15. [Google Scholar] [CrossRef] [PubMed]
- Jerome, E.; McPhail, M.J.; Menon, K. Diagnostic accuracy of procalcitonin and interleukin-6 for postoperative infection in major gastrointestinal surgery: A systematic review and meta-analysis. Ann. R. Coll. Surg. Engl. 2022, 104, 561–570. [Google Scholar] [CrossRef]
- Giaccaglia, V.; Salvi, P.F.; Antonelli, M.S.; Nigri, G.; Pirozzi, F.; Casagranda, B.; Giacca, M.; Corcione, F.; de Manzini, N.; Balducci, G.; et al. Procalcitonin Reveals Early Dehiscence in Colorectal Surgery: The PREDICS Study. Ann. Surg. 2016, 263, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Hoeboer, S.H.; Groeneveld, A.B.; Engels, N.; van Genderen, M.; Wijnhoven, B.P.; van Bommel, J. Rising C-reactive protein and procalcitonin levels precede early complications after esophagectomy. J. Gastrointest. Surg. 2015, 19, 613–624. [Google Scholar] [CrossRef]
- Hata, T.; Mizuma, M.; Motoi, F.; Hayashi, H.; Ishida, M.; Ohtsuka, H.; Nakagawa, K.; Morikawa, T.; Kamei, T.; Unno, M. Serum procalcitonin as an early diagnostic marker of severe postoperative complications after elective pancreaticoduodenectomy. J. Hepato Biliary Pancreat. Sci. 2020, 27, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Hu, D.; Cao, Y.; Liu, Z.; Ding, C.; Tian, H.; Gong, J.; Zhu, W.; Li, N.; Li, J. Procalcitonin in Crohn’s disease with fever episodes, a variable to differentiate intra-abdominal abscess from disease flares. Int. J. Surg. 2016, 36, 34–39. [Google Scholar] [CrossRef]
- Abbas, M.H.; Choudhry, M.N.; Hamza, N.; Ali, B.; Amin, A.A.; Ammori, B.J. Admission levels of serum amyloid a and procalcitonin are more predictive of the diagnosis of acute appendicitis compared with C-reactive protein. Surg. Laparosc. Endosc. Percutan Tech. 2014, 24, 488–494. [Google Scholar] [CrossRef]
- Chandel, V.; Batt, S.H.; Bhat, M.Y.; Kawoosa, N.U.; Yousuf, A.; Zargar, B.R. Procalcitonin as the biomarker of inflammation in diagnosis of appendicitis in pediatric patients and prevention of unnecessary appendectomies. Indian J. Surg. 2011, 73, 136–141. [Google Scholar] [CrossRef]
- Khan, A.N.; Sawan, A.; Likourezos, A.; Schnellinger, M.; Garcia, E. The usefulness of procalcitonin in the diagnosis of appendicitis in children: A pilot study. Emerg. Med. Int. 2012, 2012, 317504. [Google Scholar] [CrossRef]
- Kaya, B.; Sana, B.; Eris, C.; Karabulut, K.; Bat, O.; Kutanis, R. The diagnostic value of D-dimer, procalcitonin and CRP in acute appendicitis. Int. J. Med. Sci. 2012, 9, 909–915. [Google Scholar] [CrossRef]
- Wu, J.Y.; Chen, H.C.; Lee, S.H.; Chan, R.C.; Lee, C.C.; Chang, S.S. Diagnostic role of procalcitonin in patients with suspected appendicitis. World J. Surg. 2012, 36, 1744–1749. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.; Cheang, I.; Li, X. Procalcitonin as an excellent differential marker between uncomplicated and complicated acute appendicitis in adult patients. Eur. J. Trauma Emerg. Surg. 2020, 46, 853–858. [Google Scholar] [CrossRef]
- Kang, C.B.; Li, X.W.; Hou, S.Y.; Chi, X.Q.; Shan, H.F.; Zhang, Q.J.; Li, X.B.; Zhang, J.; Liu, T.J. Preoperatively predicting the pathological types of acute appendicitis using machine learning based on peripheral blood biomarkers and clinical features: A retrospective study. Ann. Transl. Med. 2021, 9, 835. [Google Scholar] [CrossRef]
- Song, J.; Lu, Y. Composite Inflammatory Indicators as Early Predictor of Intra-abdominal Infections after General Surgery. J. Inflamm. Res. 2021, 14, 7173–7179. [Google Scholar] [CrossRef]
- Lipinska-Gediga, M.; Mierzchala-Pasierb, M.; Durek, G. Procalcitonin kinetics—Prognostic and diagnostic significance in septic patients. Arch. Med. Sci. 2016, 12, 112–119. [Google Scholar] [CrossRef]
- Reinhart, K.; Carlet, J. Procalcitonin-a new marker of severe infection and sepsis. Intensiv. Care Med. 2000, 26 (Suppl 2), S145. [Google Scholar] [CrossRef]
- Zheng, H.; Luo, Z.; Yi, Y.; Liu, K.; Huo, Z.; You, Y.; Li, H.; Tang, M. Assessment value of interleukin-6, procalcitonin, and C-reactive protein early kinetics for initial antibiotic efficacy in patients with febrile neutropenia: A prospective study. Cancer Med. 2024, 13, e7307. [Google Scholar] [CrossRef] [PubMed]
- Bahloul, M.; Bradii, S.; Turki, M.; Bouchaala, K.; Ben Hamida, C.; Chelly, H.; Ayedi, F.; Bouaziz, M. The value of sepsis biomarkers and their kinetics in the prognosis of septic shock due to bacterial infections. Anaesthesiol. Intensiv. Ther. 2021, 53, 312–318. [Google Scholar] [CrossRef]
- Waterfield, T.; Maney, J.A.; Hanna, M.; Fairley, D.; Shields, M.D. Point-of-care testing for procalcitonin in identifying bacterial infections in young infants: A diagnostic accuracy study. BMC Pediatr. 2018, 18, 387. [Google Scholar] [CrossRef] [PubMed]
- Waterfield, T.; Maney, J.A.; Lyttle, M.D.; McKenna, J.P.; Roland, D.; Corr, M.; Patenall, B.; Shields, M.D.; Woolfall, K.; Fairley, D.; et al. Diagnostic test accuracy of point-of-care procalcitonin to diagnose serious bacterial infections in children. BMC Pediatr. 2020, 20, 487. [Google Scholar] [CrossRef] [PubMed]
- Goyal, M.; Mascarenhas, D.; Rr, P.; Haribalakrishna, A. Diagnostic Accuracy of Point-of-Care Testing of C-Reactive Protein, Interleukin-6, and Procalcitonin in Neonates with Clinically Suspected Sepsis: A Prospective Observational Study. Med. Princ. Pract. 2024, 33, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Prkno, A.; Wacker, C.; Brunkhorst, F.M.; Schlattmann, P. Procalcitonin-guided therapy in intensive care unit patients with severe sepsis and septic shock--a systematic review and meta-analysis. Crit. Care 2013, 17, R291. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.L.; Yan, F.D.; Yu, J.Q.; Chen, Q.H.; Lin, H.; Zheng, R.Q. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment of sepsis patients. Zhonghua Yi Xue Za Zhi 2017, 97, 343–346. [Google Scholar] [CrossRef] [PubMed]
| Variables | N. of Patients | % |
|---|---|---|
| Age, year | 36.0 ± 17.9 | - |
| Male Sex | 17 | 51.5 |
| Blumberg’s sign | 13 | 39.4 |
| Pain migration | 5 | 15.1 |
| Symptom duration > 48 h | 9 | 27.3 |
| Comorbidities | 16 | 48.5 |
| Diabetes | 5 | 15.1 |
| Morbidity rate | 9 | 27.3 |
| Creatinine mg/dL | 0.7 ± 0.2 | - |
| Glycemia mg/dL | 109.0 ± 58.0 | - |
| Lactate Dehydrogenase H UI/L | 197.0 ± 100.0 | - |
| C-reactive protein mg/L | 49.9 ± 59.0 | - |
| Bilirubin mg/dL | 1.0 ± 0.7 | - |
| White blood cells count ×109/L | 12.4 ± 4.1 | - |
| Platelets ×109/L | 233.0 ± 68.0 | - |
| Time | PCT ng/mL |
|---|---|
| T0 | 2.03 ± 3.89 |
| T1 | 0.70 ± 1.51 |
| T2 | 0.90 ± 1.05 |
| T3 | 0.41 ± 0.37 |
| Variables 1 | Group 1 | Group 2 | p Value |
|---|---|---|---|
| N. of patients | 4 | 29 | - |
| Age, year | 46.0 ± 31.0 | 35.0 ± 15.7 | 0.02 |
| Male Sex | 3 | 13 | 0.3 |
| Blumberg’s sign | 2 | 11 | 0.6 |
| Pain migration | 2 | 3 | 0.5 |
| Comorbidities | 1 | 15 | 0.6 |
| Diabetes | 1 | 4 | 0.7 |
| Morbidity rate | 1 | 8 | 0.5 |
| Creatinine mg/dL | 0.9 ± 0.5 | 0.7 ± 0.2 | 0.2 |
| Glycemia mg/dL | 114.3 ± 22.4 | 108.2 ± 61.1 | 0.8 |
| LDH UI/L | 273.5 ± 170.4 | 190.4 ± 95.0 | 0.2 |
| CRP mg/L | 93.3 ± 94.0 | 44.6 ± 54.0 | 0.1 |
| Bilirubin mg/dL | 1.6 ± 0.8 | 0.9 ± 0.7 | 0.09 |
| WBC ×109/L | 13.4 ± 2.2 | 12.4 ± 4.3 | 0.6 |
| PLT ×109/L | 192.0 ± 126.0 | 237.5 ± 61.4 | 0.2 |
| Minimally invasive surgery | 1 | 0 | 0.6 |
| Variables | Group 1 | Group 2 |
|---|---|---|
| N. of patients | 4 | 29 |
| T0 vs. T1 | −49.15% | −47.37% |
| T1 vs. T2 | −6.04% | −53.33% |
| T2 vs. T3 | −53.57% | −7.14% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fransvea, P.; Fico, V.; Arcangeli, C.; Altieri, G.; Tropeano, G.; Di Grezia, M.; Pepe, G.; Misuriello, F.; Brisinda, G.; Sganga, G.; et al. Value of Early Kinetics of Procalcitonin with Point-of-Care Test to Predict Postoperative Abscess Following Non-Complicated Acute Appendicitis: A Pilot Study. Medicina 2025, 61, 1374. https://doi.org/10.3390/medicina61081374
Fransvea P, Fico V, Arcangeli C, Altieri G, Tropeano G, Di Grezia M, Pepe G, Misuriello F, Brisinda G, Sganga G, et al. Value of Early Kinetics of Procalcitonin with Point-of-Care Test to Predict Postoperative Abscess Following Non-Complicated Acute Appendicitis: A Pilot Study. Medicina. 2025; 61(8):1374. https://doi.org/10.3390/medicina61081374
Chicago/Turabian StyleFransvea, Pietro, Valeria Fico, Claudia Arcangeli, Gaia Altieri, Giuseppe Tropeano, Marta Di Grezia, Gilda Pepe, Filomena Misuriello, Giuseppe Brisinda, Gabriele Sganga, and et al. 2025. "Value of Early Kinetics of Procalcitonin with Point-of-Care Test to Predict Postoperative Abscess Following Non-Complicated Acute Appendicitis: A Pilot Study" Medicina 61, no. 8: 1374. https://doi.org/10.3390/medicina61081374
APA StyleFransvea, P., Fico, V., Arcangeli, C., Altieri, G., Tropeano, G., Di Grezia, M., Pepe, G., Misuriello, F., Brisinda, G., Sganga, G., & Alfieri, S. (2025). Value of Early Kinetics of Procalcitonin with Point-of-Care Test to Predict Postoperative Abscess Following Non-Complicated Acute Appendicitis: A Pilot Study. Medicina, 61(8), 1374. https://doi.org/10.3390/medicina61081374








