Feminine Intimate Hygiene: A Review of Healthy and Unhealthy Habits in Women
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Search Strategy
2.3. Data Extraction and Analysis
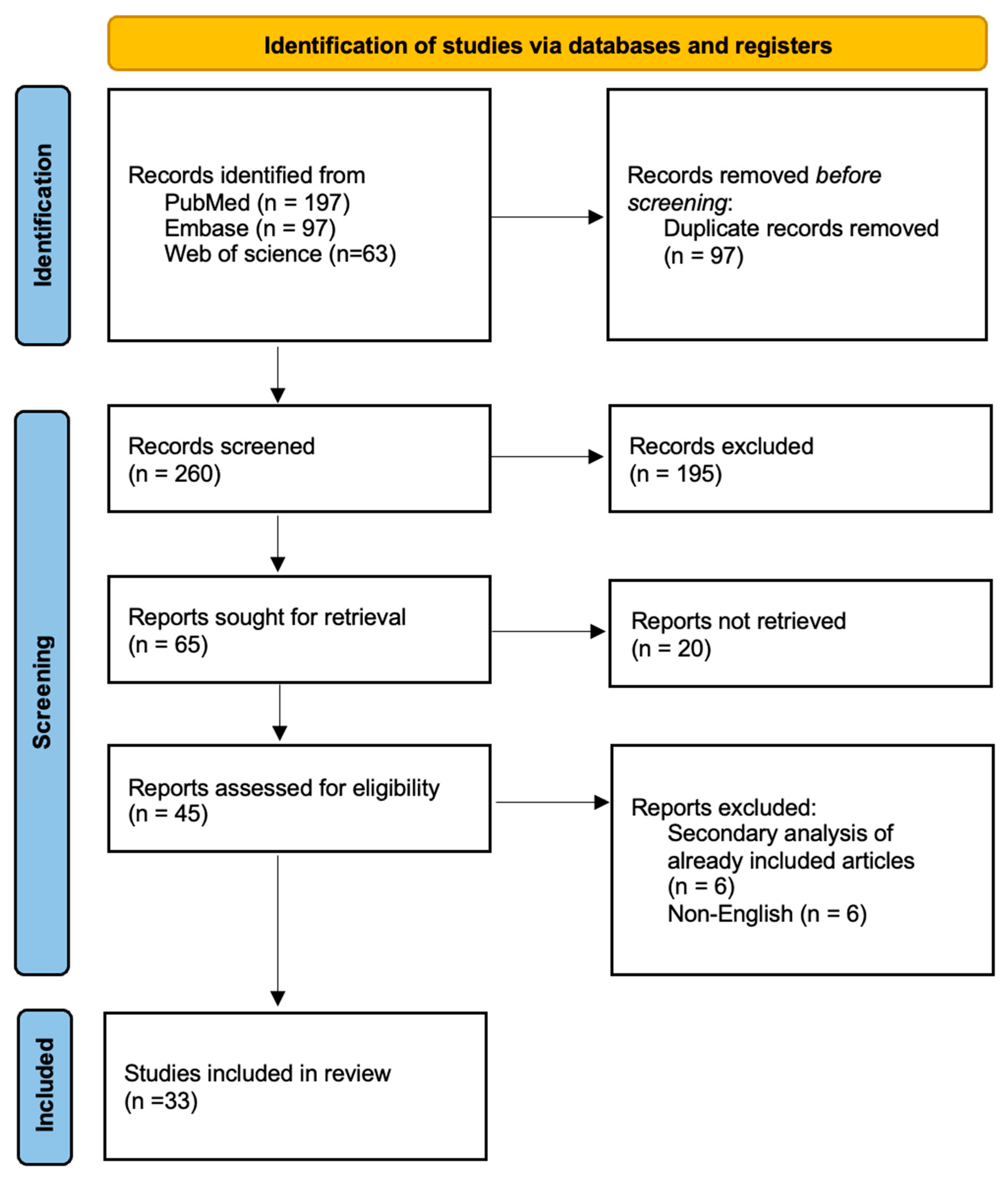
3. Results
3.1. Study Selection and Study Characteristics
3.2. Washing Habits
3.3. Underwear
3.4. Pubic Hair Grooming
3.5. Bias Analysis
4. Discussion
4.1. Washing Habits
4.2. Underwear
4.3. Intimate Area Hair Removal Methods
4.4. Guidelines on Feminine Hygiene
4.5. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, Y.; Bruning, E.; Rubino, J.; Eder, S.E. Role of female intimate hygiene in vulvovaginal health: Global hygiene practices and product usage. Women’s Health 2017, 13, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Wakashin, K. Sanitary napkin contact dermatitis of the vulva: Location-dependent differences in skin surface conditions may play a role in negative patch test results. J. Dermatol. 2007, 34, 834–837. [Google Scholar] [CrossRef] [PubMed]
- Britz, M.B.; Maibach, H.I. Human cutaneous vulvar reactivity to irritants. Contact Dermat. 1979, 5, 375–377. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.J.; Wong, M.; Davis, C.C.; Kanti, A.; Zhou, X.; Forney, L.J. Preliminary characterisation of the normal microbiota of the human vulva using cultivation-independent methods. J. Med. Microbiol. 2007, 56, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Elsner, P.; Oriba, H.A.; Maibach, H.I. Physiology of the skin of the vulva: New aspects. Hautarzt 1989, 40, 411–417. [Google Scholar] [PubMed]
- Komesu, Y.M.; Dinwiddie, D.L.; Richter, H.E.; Lukacz, E.S.; Sung, V.W.; Siddiqui, N.Y.; Zyczynski, H.M.; Ridgeway, B.; Rogers, R.G.; Arya, L.A.; et al. Defining the relationship between vaginal and urinary microbiomes. AJOG 2020, 222, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Farage, M.A.; Bramante, M. Genital hygiene: Culture, practices, and health impact. In The Vulva; CRC Press: Boca Raton, FL, USA, 2017; Volume 2, pp. 297–312. [Google Scholar]
- Elsner, P.; Maibach, H.I. The effect of prolonged drying on transepidermal water loss, capacitance and pH of human vulvar and forearm skin. Acta Derm.-Venereol. 1990, 70, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.B.; Ravel, J. The vaginal microbiota, host defence and reproductive physiology. J. Physiol. 2017, 595, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Belec, L. Defenses of the female genital tract against infection. J. Gynécol. Obstét. Biol. Reprod. 2002, 31, 4S45–4S59. [Google Scholar]
- Spear, G.T.; French, A.L.; Gilbert, D.; Zariffard, M.R.; Mirmonsef, P.; Sullivan, T.H.; Spear, W.W.; Landay, A.; Micci, S.; Lee, B.-H.; et al. Human α-amylase present in lower-genital-tract mucosal fluid processes glycogen to support vaginal colonization by Lactobacillus. J. Infect. Dis. 2014, 210, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Mastromarino, P.; Di Pietro, M.; Schiavoni, G.; Nardis, C.; Gentile, M.; Sessa, R. Effects of vaginal lactobacilli in Chlamydia trachomatis infection. Int. J. Med. Microbiol. 2014, 304, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Tomás, M.S.J.; Duhart, C.I.S.; De Gregorio, P.R.; Pingitore, E.V.; Nader-Macías, M.E. Urogenital pathogen inhibition and compatibility between vaginal Lactobacillus strains to be considered as probiotic candidates. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 159, 399–406. [Google Scholar] [CrossRef] [PubMed]
- O’Hanlon, D.E.; Come, R.A.; Moench, T.R. Vaginal pH measured in vivo: Lactobacilli determine pH and lactic acid concentration. BMC Microbiol. 2019, 19, 13. [Google Scholar] [CrossRef] [PubMed]
- Martino, J.L.; Vermund, S.H. Vaginal douching: Evidence for risks or benefits to women’s health. Epidemiol. Rev. 2002, 24, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Park, M.G.; Cho, S.; Oh, M.M. Menopausal Changes in the Microbiome—A Review Focused on the Genitourinary Microbiome. Diagnostics 2023, 13, 1193. [Google Scholar] [CrossRef] [PubMed]
- Hummelen, R.; Macklaim, J.M.; Bisanz, J.E.; Hammond, J.A.; McMillan, A.; Vongsa, R.; Koenig, D.; Gloor, G.B.; Reid, G. Vaginal microbiome and epithelial gene array in post-menopausal women with moderate to severe dryness. PLoS ONE 2011, 6, e26602. [Google Scholar] [CrossRef] [PubMed]
- Caliskan, D.; Subasi, N.; Sarisen, O. Vaginal douching and associated factors among married women attending a family planning clinic or a gynecology clinic. Eur. J. Obstet. Gynecol. Reprod. Biol. 2006, 127, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Hilber, A.M.; Hull, T.H.; Preston-Whyte, E.; Bagnol, B.; Smit, J.; Wacharasin, C.; Widyantoro, N.; WHO GSVP Study Group. A cross cultural study of vaginal practices and sexuality: Implications for sexual health. Soc. Sci. Med. 2010, 70, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Thomas, A.G.; Leybovich, E. Vaginal douching and adverse health effects: A meta-analysis. Am. J. Public Health 1997, 87, 1207–1211. [Google Scholar] [CrossRef] [PubMed]
- Meyer, L.; Brouselle, C.; Soulat, C.; Gros, I. Vaginal Douching and Ectopic Pregnancy. J. Am. Med. Assoc. 1991, 265, 2670–2671. [Google Scholar] [CrossRef]
- Baird, D.D.; Weinberg, C.R.; Voigt, L.F.; Daling, J.R. Vaginal Douching and Reduced Fertility. Am. J. Public Health 1996, 86, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Klann, A.M.; Rosenberg, J.; Wang, T.; Parker, S.E.; Harlow, B.L. Exploring Hygienic Behaviors and Vulvodynia. J. Low. Genit. Tract Dis. 2019, 23, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, C.; Giraldo, P.C.; Sanches, J.M.; Reis, V.; Beghini, J.; Laguna, C.; Amaral, R.L. Daily genital cares of female gynecologists: A descriptive study. Rev. Assoc. Med. Bras. 2019, 65, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Crann, S.E.; Cunningham, S.; Albert, A.; Money, D.M.; O’Doherty, K.C. Vaginal health and hygiene practices and product use in Canada: A national cross-sectional survey. BMC Women’s Health 2018, 18, 52. [Google Scholar] [CrossRef] [PubMed]
- Leo, V.D.; Benvenuti, C. Pharmacological, Microbiological and Clinical Activity of Feminine IntimateCleansers Based on Plant Extracts Active Principles (Saugella Line). J. Women’s Health Care 2015, 4, 244. [Google Scholar]
- Ness, R.B.; Soper, D.E.; Holley, R.L.; Peipert, J.; Randall, H.; Sweet, R.L.; Sondheimer, S.J.; Hendrix, S.L.; Hillier, S.L.; Amortegui, A.; et al. Douching and endometritis: Results from the PID evaluation and clinical health (PEACH) study. Sex. Transm. Dis. 2001, 28, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Ness, R.B.; Hillier, S.L.; Richter, H.E.; Soper, D.E.; Stamm, C.; McGregor, J.; Bass, D.C.; Sweet, R.L.; Rice, P. Douching in relation to bacterial vaginosis, lactobacilli, and facultative bacteria in the vagina. Obstet. Gynecol. 2002, 100, 765–772. [Google Scholar] [PubMed]
- Holzman, C.; Leventhal, J.M.; Qui, H.; Jones, N.M.; Wang, J.; BV Study Group. Factors linked to bacterial vaginosis in nonpregnant women. Am. J. Public Health 2001, 91, 1664–1669. [Google Scholar] [CrossRef] [PubMed]
- Sunay, D.; Kaya, E.; Ergün, Y. Vaginal douching behavior of women and relationship among vaginal douching and vaginal discharge and demographic factors. J. Turk. Soc. Obstet. Gynecol. 2011, 8, 264–267. [Google Scholar] [CrossRef]
- Heng, L.S.; Yatsuya, H.; Morita, S.; Sakamoto, J. Vaginal douching in Cambodian women: Its prevalence and association with vaginal candidiasis. J. Epidemiol. 2010, 20, 70–76. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Klebanoff, M.A.; Nansel, T.R.; Brotman, R.M.; Zhang, J.; Yu, K.F.; Schwebke, J.R.; Andrews, W.W. Personal hygienic behaviors and bacterial vaginosis. Sex. Transm. Dis. 2010, 37, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, O.M.; Shlatout, A.; Abbas, A.M.; Youssef, A.A. Internal vaginal douching increases the rate of vaginal infection in intrauterine contraceptive device users: A cross sectional study. Fertil. Steril. 2021, 116, e407. [Google Scholar] [CrossRef]
- McClelland, R.S.; Lavreys, L.; Hassan, W.M.; Mandaliya, K.; Ndinya-Achola, J.O.; Baeten, J.M. Vaginal washing and increased risk of HIV-1 acquisition among African women: A 10-year prospective study. AIDS 2006, 20, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Güzel, A.I.; Kuyumcuoğlu, U.; Celik, Y. Vaginal douching practice and related symptoms in a rural area of Turkey. Arch. Gynecol. Obstet. 2011, 284, 1153–1156. [Google Scholar] [CrossRef] [PubMed]
- McKee, M.D.; Baquero, M.; Fletcher, J. Vaginal hygiene practices and perceptions among women in the urban Northeast. Women Health 2009, 49, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Foch, B.J.; McDaniel, N.D.; Chacko, M.R. Racial differences in vaginal douching knowledge, attitude, and practices among sexually active adolescents. J. Pediatr. Adolesc. Gynecol. 2001, 14, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Yıldırım, R.; Vural, G.; Koçoğlu, E. Effect of vaginal douching on vaginal flora and genital infection. J. Turk. Ger. Gynecol. Assoc. 2020, 21, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Murina, F.; Caimi, C.; Felice, R.; Francesco, S.; Cetin, I. Characterization of female intimate hygiene practices and vulvar health: A randomized double-blind controlled trial. J. Cosmet. Dermatol. 2020, 19, 2721–2726. [Google Scholar] [CrossRef] [PubMed]
- Bahamondes, M.V.; Portugal, P.M.; Brolazo, E.M.; Simões, J.A.; Bahamondes, L. Use of a lactic acid plus lactoserum intimate liquid soap for external hygiene in the prevention of bacterial vaginosis recurrence after metronidazole oral treatment. Rev. Assoc. Med. Bras. 2011, 57, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Bahram, A.; Hamid, B.; Zohre, T. Prevalence of bacterial vaginosis and impact of genital hygiene practices in non-pregnant women in Zanjan, Iran. Oman Med. J. 2009, 24, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Murina, F.; Di Francesco, S.; Ratti, M. Is it relevant a correct intimate cleansing in the treatment of vulvar dermatosis? Open J. Obstet. Gynecol. 2014, 4, 4. [Google Scholar] [CrossRef]
- Mościcka, P.; Chróst, N.; Terlikowski, R.; Przylipiak, M.; Wolosik, K.; Przylipiak, A. Hygienic and cosmetic care habits in Polish women during COVID-19 pandemic. J. Cosmet. Dermatol. 2020, 19, 1840–1845. [Google Scholar] [CrossRef] [PubMed]
- Schafer, P.; Bewick-Sonntag, C.; Capri, M.G.; Berardesca, E. Physiological changes in skin barrier function in relation to occlusion level, exposure time and climatic conditions. Ski. Pharmacol. Appl. Ski. Physiol. 2002, 15, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Runeman, B.; Rybo, G.; Larkö, O.; Faergemann, J. The vulva skin microclimate: Influence of panty liners on temperature, humidity and pH. Acta Derm. Venereol. 2003, 83, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Runeman, B.; Rybo, G.; Forsgren-Brusk, U.; Larkö, O.; Larsson, P.; Faergemann, J. The vulvar skin microenvironment: Impact of tight-fitting underwear on microclimate, pH and microflora. Acta Derm. Venereol. 2005, 85, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Rouzi, A.A.; Berg, R.C.; Turkistani, J.; Alamoudi, R.; Alsinani, N.; Alkafy, S.; Alwazzan, A. Practices and complications of pubic hair removal among Saudi women. BMC Women’s Health 2018, 18, 172. [Google Scholar] [CrossRef] [PubMed]
- DeMaria, A.L.; Flores, M.; Hirth, J.M.; Berenson, A.B. Complications related to pubic hair removal. Am. J. Obstet. Gynecol. 2014, 210, 528.e1–528.e5. [Google Scholar] [CrossRef] [PubMed]
- Butler, S.M.; Smith, N.K.; Collazo, E.; Caltabiano, L.; Herbenick, D. Pubic hair preferences, reasons for removal, and associated genital symptoms: Comparisons between men and women. J. Sex. Med. 2015, 12, 48–58. [Google Scholar] [CrossRef] [PubMed]
- DeMaria, A.L.; Sundstrom, B.; McInnis, S.M.; Rogers, E. Perceptions and correlates of pubic hair removal and grooming among college-aged women: A mixed methods approach. Sex. Health 2016, 13, 248–256. [Google Scholar] [CrossRef] [PubMed]
- DeMaria, A.L.; Berenson, A.B. Prevalence and correlates of pubic hair grooming among low-income Hispanic, Black, and White women. Body Image 2013, 10, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Trager, J.D. Pubic hair removal—Pearls and pitfalls. J. Pediatr. Adolesc. Gynecol. 2006, 19, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Dendle, C.; Mulvey, S.; Pyrlis, F.; Grayson, M.L.; Johnson, P.D. Severe complications of a “Brazilian” bikini wax. Clin. Infect. Dis. 2007, 45, e29–e31. [Google Scholar] [CrossRef] [PubMed]
- Herbenick, D.; Schick, V.; Reece, M.; Sanders, S.; Fortenberry, J.D. Pubic hair removal among women in the United States: Prevalence, methods, and characteristics. J. Sex. Med. 2010, 7, 3322–3330. [Google Scholar] [CrossRef] [PubMed]
- Herbenick, D.; Hensel, D.; Smith, N.K.; Schick, V.; Reece, M.; Sanders, S.A.; Fortenberry, J.D. Pubic hair removal and sexual behavior: Findings from a prospective daily diary study of sexually active women in the United States. J. Sex. Med. 2013, 10, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Gunasekaran, G.; Prakash, C.; Periyasamy, S. Preparation, characterisation of bamboo charcoal particles and the effect of their application on thermo-physiological comfort properties of woven fabrics. J. Text. Inst. 2019, 111, 318–325. [Google Scholar] [CrossRef]
- Royal College of General Practitioners. The Management of Vulval Skin Disorders. 2011. Available online: https://mrcog.womanhospital.cn/ueditor/php/upload/file/20190821/1566377001.pdf (accessed on 27 December 2023).
- Arab, H.; Almadani, L.; Tahlak, M.; Chawla, M.; Ashouri, M.; Tehranian, A.; Ghasemi, A.; Taheripanah; Gulyaf, M.; Khalil, A.; et al. The Middle East and Central Asia guidelines on female genital hygiene. BMJ Middle East 2011, 19, 99–106. [Google Scholar]
| Article (Author, Year) | Study Design | Country | Participant Number | Population | Topic | Results | Quality |
|---|---|---|---|---|---|---|---|
| Bahamondes et al. (2011) [41] | Prospective cohort study | Brazil | 123 | Women with bacterial diagnoses | Lactic soap post-metronidazole to prevent BV recurrence | The use of a lactic acid plus lactoserum liquid soap for external intimate hygiene may be an effective option for preventing BV recurrence after oral metronidazole treatment. Significant improvements in quality of life were noted, indicating potential benefits beyond infection prevention. | O |
| Bahram et al. (2009) [42] | Descriptive-analytic cross-sectional study | Iran | 500 | Non-pregnant married women | BV and hygiene | BV prevalence among non-pregnant Iranian women (16.2%) was substantial. Statistical analysis showed a significant correlation between BV menstrual status and individual vaginal hygiene (p < 0.01 and p < 0.001, respectively). In contrast, no significant correlation was observed between BV and coital hygiene. Genital and menstrual hygiene practices appeared to influence BV risk significantly. Education programs on hygienic behaviours may help reduce BV prevalence in similar contexts. | O |
| Butler et al. (2015) [50] | Cross-sectional survey. | USA | 1110 participants (671 women and 439 men)—data about women participants were included in the study. | Healthy reproductive-age women and men | Pubic hair preferences/symptoms | Pubic hair grooming and removal are prevalent among college-age individuals, with women more likely to engage in grooming and prefer a hair-free appearance. Genital itching is a common side effect associated with pubic hair grooming. The motivations for grooming vary, with women associating grooming with cleanliness, comfort, and sex appeal, while men may be influenced by partner preferences. Women’s total pubic hair removal was associated with younger age (p < 0.05); heterosexual orientation (p < 0.01); race/ethnicity (self-identified Asian/Asian American women and women in non-specified “other” race/ethnicity categories were significantly less likely to report complete hair removal as compared with white women; p < 0.01); and having either a monogamous (p < 0.01) or nonmonogamous sexual partner(s) (p < 0.01), as compared to having no sexual partner. | O |
| Crann et al. (2018) [26] | Cross-sectional online survey | Canada | 1435 | Healthy reproductive-age women | Vaginal health and hygiene practices | Widespread use of vaginal/genital hygiene products in Canada, with high self-reported rates of adverse symptoms (anti-itch creams, moisturisers/lubricants, gel sanitisers, feminine wipes, baby wipes, feminine washes/gels, and douches). Observed strong associations between product use and genital health complaints—though causal direction is uncertain (i.e., product use may follow symptoms rather than cause them). | O |
| DeMaria & Berenson (2013) [52] | Cross-sectional survey | USA | 1677 | Low-income women | Grooming among low-income women | Pubic hair grooming is pervasive among low-income women across racial backgrounds. Grooming behaviour varied significantly with race, age, income, body weight, and sexual history. The majority of women who had ever groomed indicated having used a razor and shaving cream for pubic hair grooming (77.2%; n = 1108), followed by trimming with scissors (23.1%; n = 392) and hair removal cream (18.7%; n = 268). | O |
| DeMaria et al. (2014) [49] | Cross-sectional survey | USA | 333 | Healthy reproductive-age women | Complications from pubic hair removal | Pubic hair removal is prevalent among women, with a significant proportion experiencing complications, particularly when using razors. Overweight/obese women and White women are more likely to report complications. There is a need for increased awareness and education on safe pubic hair removal practices. | O |
| DeMaria et al. (2016) [51] | Mixed-methods study combining quantitative and qualitative approaches | USA | 663 | Healthy reproductive-age women | Pubic hair grooming perceptions, mixed methods | Pubic hair removal is influenced by personal reasons and external factors, such as family, friends, and media. Initiation age, genital self-image, and sexual behaviours are significantly related to pubic hair removal. There is a need for further education regarding safe pubic hair removal methods, especially for those who initiate pubic hair removal and sexual behaviours concurrently. The most prevalent complications ever experienced due to pubic hair removal included razor burn (70.0%; n = 464) and ingrown hairs (69.8%; n = 463), with some participants reporting severe itching (31.1%; n = 206) and cuts (32.1%; n = 213). Those who identified as hair-free were significantly more likely to suffer from razor burn (p = 0.02), ingrown hairs (p = 0.02), and severe itching (p < 0.01). | O |
| Dendle et al. (2007) [54] | Case report | Australia | 1 | A 20-year-old woman | hair removing | Case underscores the need for caution and hygiene awareness—particularly for medically vulnerable individuals. Physicians should warn immunosuppressed patients of the risks of extensive body hair removal (in particular, removal of pubic hair) and suggest that they attend hygienic and reputable establishments. Advice on shaving techniques, ensuring that the wax is not too hot, and testing of products on nongenital areas can be offered. Patients should attend hygienic beauty salons, where therapists regularly wash their hands and wear gloves. Patient support groups, such as the Diabetes Council, should also be aware of and warn patients of the risks. | N |
| Foch et al. (2001) [38] | Cross-sectional, descriptive study | USA | 169 | Caucasian and African-American adolescent females (≤19 years) | Douching in adolescents | Sixty-nine % of participants reported vaginal douching, primarily for hygienic reasons (68%). Those who reported douching were more likely to have a history of sexual intercourse (p < 0.01) and a history of one or more sexually transmitted diseases (p < 0.05). The age at first douching correlated positively with the age at first sexual intercourse (r = 0.34, p < 0.001). African-Americans did not douche to a greater degree than Caucasians. However, racial differences were noted in knowledge of and attitude toward vaginal douching. | O |
| Güzel et al. (2011) [36] | Cross-sectional, observational study | Turkey | 393 | Reproductive-age women | Douching in rural Turkey | The major symptoms of the subjects were itching and vaginal discharge. Of the 393 women, 317 (80.66%) performed vaginal douching, and all of them had recurrent or treatment-resistant mixed-agent vulvovaginitis. The majority of the women douched for ritual cleansing or washing before prayer (n = 278; 91.6%). The majority of the cases (n = 354; 90.1%) were of lower socioeconomic and educational status. The odds ratios and 95% confidence interval (CI) of the risk variables—vaginal douching frequency, cervical motion tenderness, dyspareunia, and vaginal itching—were 9.39 (2.07–42.48), 7.31 (2.08–25.64), 6.52 (2.26–18.78), and 1.46 (1.22–1.74). | O |
| Heng L.S. et al. (2010) [32] | Cross-sectional, observational study | Cambodia | 451 | Reproductive-age women with normal and abnormal vaginal discharge and different douching habits | Douching and candidiasis | Vaginal douching is highly prevalent among Cambodian women—76.7% (n = 346). Douching was significantly more prevalent in urban than in rural women (85.7%, n = 198 vs. 67.3%, n = 148; p < 0.001). The practice is significantly associated with increased odds of vaginal candidiasis (dysuria with trichomoniasis (p = 0.061) and itching with BV (p = 0.063)). | O |
| Herbenick et al. (2010) [55] | Cross-sectional, internet-based survey | USA | 2451 | Sexually active women in US | US prevalence of pubic hair removal | Women reported a range of hair removal practices, from trimming to complete removal. Significantly associated with younger age, being partnered (not single or married), having received cunnilingus in past 4 weeks, recently examining one’s genitals, higher scores on genital self-image (FGSIS), and overall sexual function (FSFI; except orgasm subscale). | O |
| Herbenick et al. (2013) [56] | Prospective event-level daily diary study | USA | 2453 | Sexually active women in US | Pubic hair and sexual behaviour | A total of 15.2% of daily entries involved pubic hair removal, almost entirely shaving (~99%). Predictors of grooming: Events of grooming were significantly more likely on days when women reported younger age, interest in sex, vaginal fingering or finger-clitoral stimulation, casual partner contact, use of vaginal hygiene products, and application of creams to the genitals. Grooming was modestly linked to longer episodes of vaginal intercourse. | O |
| Holzman C. et al. (2001) [30] | Cross-sectional, multi-site epidemiologic study | USA | 496 | Symptomatic and asymptomatic women who douche | Risk factors associated with douching | Frequent douching correlated significantly with bacterial vaginosis even after adjusting for sexual risk factors. After adjustment for ethnicity and education, symptomatic bacterial vaginosis was more strongly associated with vaginal douching (OR = 5.8, 95% CI = 2.2, 16.6) than was asymptomatic bacterial vaginosis (OR = 2.6, 95% CI = 1.3, 5.2). | P |
| Klann et al. (2019) [24] | Cross-sectional study | USA (Boston University/Minnesota) | 434 | Women with vulvodynia | Vulvodynia and hygiene behaviours | Women with vulvodynia reported more frequent use of specialised vulvar cleansers and avoidance of soaps compared to controls. Wearing tight-fitting jeans or pants 4 times per week or more was crudely associated with vulvodynia. | O |
| Klebanoff et al. (2010) [33] | Prospective cohort study | USA | 3620 | Healthy reproductive-age women | Hygiene and BV | Vaginal douching is significantly associated with BV, suggesting a potential causal relationship. Other feminine hygiene behaviours assessed in this study were not strongly associated with BV, indicating that they may not be significant risk factors. | P |
| Leo & Benvenuti (2015) [27] | Combined in vitro laboratory assays and non-randomised clinical evaluation | Italy | 2631 | Reproductive age, pre-adolescents, adolescents, pre-menopausal and menopausal women | Plant-based cleansers | The experimental data showed the antibacterial, antimycotic, anti-inflammatory, and antioxidant activity of the natural principles contained in the four extracts. The clinical studies evidenced the significant reduction of genital signs and symptoms (itching, burning, erythema, oedema, vaginal dryness, dyspareunia) and the improvement of sexual female disorder. The cleanser selection should be tailored to the age and physiopathological condition of the woman. | O |
| McClelland et al. (2006) [35] | Prospective cohort study | Kenya | 1270 | Kenyan female sex workers enrolled between ~1992 and 2002, HIV-negative at baseline | Vaginal washing and HIV risk | Compared with women who did not perform vaginal washing, there was an increased risk for acquiring HIV-1 among women who used water (adjusted HR, 2.64; 95% CI, 1.00–6.97) or soap (adjusted HR, 3.84; 95% CI, 1.51–9.77) to clean inside the vagina. Women who performed vaginal washing with soap or other substances were at higher risk for HIV-1 compared with those who used water alone (adjusted HR, 1.47; 95% CI, 1.02–2.13). | P |
| McKee et al. (2009) [37] | Cross-sectional survey | USA | 335 | Reproductive-age women | Urban women’s hygiene practices | Prevalence of vaginal douching: Approximately 30.7% of women reported never having douched, while 51.1% of those who had ever douched indicated they no longer do. Ethnic Differences: Hispanic women were more likely to report never douching and to use imported products compared to Black women. Beliefs about douching: Women who currently douche held more positive beliefs about its benefits and safety—douching products are safe, douching promotes vaginal health, and that men find women who douche more attractive. | O |
| Mościcka et al. (2020) [44] | Cross-sectional questionnaire survey | Poland | 140 | Healthy reproductive-age women | Polish women’s hygiene during COVID | Many women reported a notable increase in showering or bathing immediately after returning home, as part of hygiene routines to reduce perceived infection risk. Slight decline in hair washing frequency, possibly due to reduced public appearances | O |
| Murina et al. (2014) [43] | Randomised controlled trial | Italy | 32 | Female patients diagnosed with vulvar dermatosis—either lichen sclerosus (LS) or lichen simplex chronicus (LSC) | Cleansing in vulvar dermatosis | No significant difference between cleansers in rubbing, applicability, or pleasantness ratings. Cleaner A (plant-based formula (almond, malva, jojoba oil, hyaluronic acid) BID + daily lanolin cream) users required significantly fewer lanolin applications (mean: 5.28 vs. 8.25; p < 0.001), indicating better symptomatic control compared to cleaner B (amino-acid surfactant with oat extract, maltodextrins, caprylic glycol, plus the same lanolin regimen). The aloe/jojoba/hyaluronic-based cleanser A might enhance skin soothing and hydration, reducing reliance on emollient rescue therapy. | O |
| Murina et al. (2020) [40] | Randomised, double-blind, controlled trial | Italy | 40 | Healthy reproductive-age women | Intimate hygiene and vulvar health | Both cleansers tested showed high performance for safety and tolerability on vulvar skin, but a plant-extract intimate cleanser (Saugella Hydraserum) may better support vulvar skin health compared to conventional lactic-acid washes—preserving hydration, maintaining optimal pH, and reducing sebum changes. | P |
| Ness R.B. et al. (2001) [28] | Cross-sectional analysis within a multicentre study | USA | 654 | Women with PID symptoms and douching habits | Douching and endometritis | Douching prior to/during PID treatment was associated with higher rates of endometritis and pelvic pain. Vaginal flora and douching (%): normal (28.2%), intermediate (36.3%), and bacterial vaginosis (50.8%) (p < 0.001). | P |
| Ness R.B. et al. (2002) [29] | Cross-sectional, multicentre study | USA | 1200 | Women with different douching habits and vaginal microfloral changes. | Douching and BV/lactobacilli | Douchers had an increased prevalence of bacterial vaginosis and reduced lactobacilli counts. Douching at least once per month was associated with an increased frequency of bacterial vaginosis. Those who douched recently (within 7 days) were at highest risk [odds ratio (OR) 2.1, 95% confidence interval (CI) 1.3, 3.1]. Douching for symptoms (OR 1.7, 95% CI 1.1, 2.6) and for hygiene (OR 1.3, 95% CI 1.0, 1.9), both related to bacterial vaginosis risk. | P |
| Rouzi et al. (2018) [48] | Cross-sectional survey | Saudi Arabia | 400 | Healthy reproductive-age women | Pubic hair removal complications in Saudi women | Pubic hair removal is a common practice among Saudi women, often initiated in early adolescence. While most complications are minor, a significant proportion of women experience issues requiring medical attention. | O |
| Ruiz et al. (2019) [25] | Descriptive observational | Brazil | 220 | Gynaecologists | Daily genital care in gynaecologists | Less than half adhered to ideal genital hygiene practices (use of water and soap), and nearly half reported vaginal discharge, indicating room for improvement. | O |
| Runeman et al. (2003) [46] | Experimental study with repeated measures | Sweden | 12 | Healthy reproductive-age women | Panty liners and vulvar microclimate | The use of conventional panty liners with non-breathable back sheets significantly alters the vulvar skin microclimate by increasing temperature and humidity and altering pH. Panty liners with breathable back sheets help maintain the vulvar skin microclimate closer to the natural state. | P |
| Runeman et al. (2005) [47] | Crossover experimental study with repeated measures | Sweden | 32 | Healthy reproductive-age women | Underwear tightness effect | Wearing tight-fitting string panties with liners had negligible effect on vulvar skin microclimate, pH, or aerobic microflora compared to standard underwear. It should be emphasised that the two pantyliners used in this study were both equipped with breathable back sheets. The assumption that tighter underwear would increase contamination from anorectal bacteria was not supported. | P |
| Schafer et al. (2002) [45] | Randomised controlled trial | Germany and Sweden | 10 | Healthy reproductive-age women | Skin barrier and occlusion | Vapour-permeable hygiene articles better maintain a stable skin barrier (lower overhydration and moisture retention) by reducing skin surface water loss and excessive stratum corneum hydration, which otherwise may render the skin more vulnerable to irritating agents or mechanical forces. Occlusion effects: Both pad type and environment significantly influence skin microclimate and moisture balance. | P |
| Shaaban et al. (2021) [34] | Cross-sectional observational study | Egypt | 604 | Reproductive-age women with IUCD | Douching in IUD users | Internal douching in IUCD users is strongly linked to higher rates of both historical (perform internal VD 260 (88.1%) vs. do not perform internal VD 151 (43.4%), p < 0.001) and current vaginal infections (perform internal VD 275 (91.05%) vs. do not perform internal VD 115 (38.1%), p < 0.001). BV is the most frequent infection, followed by Candida. The findings support public health advice discouraging intravaginal douching, especially for women with IUDs. | O |
| Sunay et al. (2011) [31] | Analytical cross-sectional observational study | Turkey | 350 | Reproductive-age women with normal and abnormal vaginal discharge and different douching habits | Douching and changes in vaginal discharge | Douching was significantly associated with demographic factors like marital status and income, and those who douched had nearly four times higher odds of abnormal vaginal discharge. | O |
| Trager (2006) [53] | Case report | USA | 1 | A 17-year-old woman | Review of hair removal issues: “pearls and pitfalls” | Hair removal may cause skin microtrauma and subsequent spread of infectious agents throughout the pubic area. | N |
| Yıldırım et al. (2020) [39] | Cross-sectional observational (descriptive). | Turkey | 190 | Women with different douching habits and vaginal microfloral changes. | Douching effect on vaginal microflora | While douching was not linked to current infection or measurable changes in flora, it was correlated with a greater likelihood of past genital infections. (p < 0.01). | O |
| Topics | Articles Which Included Certain Topics, n = 21 (n/%) |
|---|---|
| Douching | 15/71 |
| 7/33 |
| Personal hygiene | 7/33 |
| Healthy (get rid of infection, prevent infection) | 7/33 |
| To decrease vaginal symptoms (itching, burning) | 4/19 |
| 11/52 |
| BV | 7/33 |
| Candida albicans | 3/14 |
| STI (Neisseria gonorrhoea, Chlamydia trachomatis) | 2/10 |
| Human immunodeficiency virus | 1/5 |
| Bathing and showering | 5/24 |
| Wash genitalia at least once a day. | 4/19 |
| I prefer shower over bathing as a routine washing method. | 3/14 |
| Increased bathing frequency is associated with BV development. | 3/14 |
| Washing product usage | 7/33 |
| Water influence on genitalia skin; | 3/14 |
| Specific intimate hygiene products (salvia, chamomile, non-irritating cleanser based on natural surfactants, lactic acid, lactose rum, etc.) | 4/19 |
| Topics | Articles Which Included Certain Topics, n = 7 (n/%) |
|---|---|
| Underwear | 3/43 |
| Changing frequency | 1/14 |
| Association with BV and douching | 2/29 |
| Underwear material and healthy state | 2/29 |
| Clothes and underwear tightness | 2/29 |
| Association with BV | 1/14 |
| Association with vulvodynia | 1/14 |
| Panty liners | 5/71 |
| Changing frequency | 1/14 |
| Association with douching | 1/14 |
| Association with vaginal symptoms (pruritus, burning) | 1/14 |
| Influence of different types of panty liners on perineal skin | 3/43 |
| Topics | Articles Which Included Certain Topics, n = 11 (n/%) |
|---|---|
| Pubic hair removing | 8/73 |
| |
| Aesthetics of the intimate area | 5/46 |
| Hygienic purposes | 3/27 |
| Self-confidence | 2/18 |
| |
| Family | 3/27 |
| Friends | 2/18 |
| Social media | 1/9 |
| |
| Younger age | 5/46 |
| Sexual activity | 3/27 |
| Preferred pubic hair removing methods | 9/82 |
| Shaving | 7/64 |
| Waxing (hot/cold) | 2/18 |
| Complications | 5/46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lohova-Matisa, E.; Rezeberga, D.; Miskova, A. Feminine Intimate Hygiene: A Review of Healthy and Unhealthy Habits in Women. Medicina 2025, 61, 1302. https://doi.org/10.3390/medicina61071302
Lohova-Matisa E, Rezeberga D, Miskova A. Feminine Intimate Hygiene: A Review of Healthy and Unhealthy Habits in Women. Medicina. 2025; 61(7):1302. https://doi.org/10.3390/medicina61071302
Chicago/Turabian StyleLohova-Matisa, Elizabeta, Dace Rezeberga, and Anna Miskova. 2025. "Feminine Intimate Hygiene: A Review of Healthy and Unhealthy Habits in Women" Medicina 61, no. 7: 1302. https://doi.org/10.3390/medicina61071302
APA StyleLohova-Matisa, E., Rezeberga, D., & Miskova, A. (2025). Feminine Intimate Hygiene: A Review of Healthy and Unhealthy Habits in Women. Medicina, 61(7), 1302. https://doi.org/10.3390/medicina61071302







