Redefining the Diagnostic Approach to Adrenal Insufficiency: Re-Assessment of Baseline and Cortisol Increment Cut-Offs with the 1 µg Synacthen Test
Abstract
1. Introduction
2. Materials and Methods
2.1. Collected Data
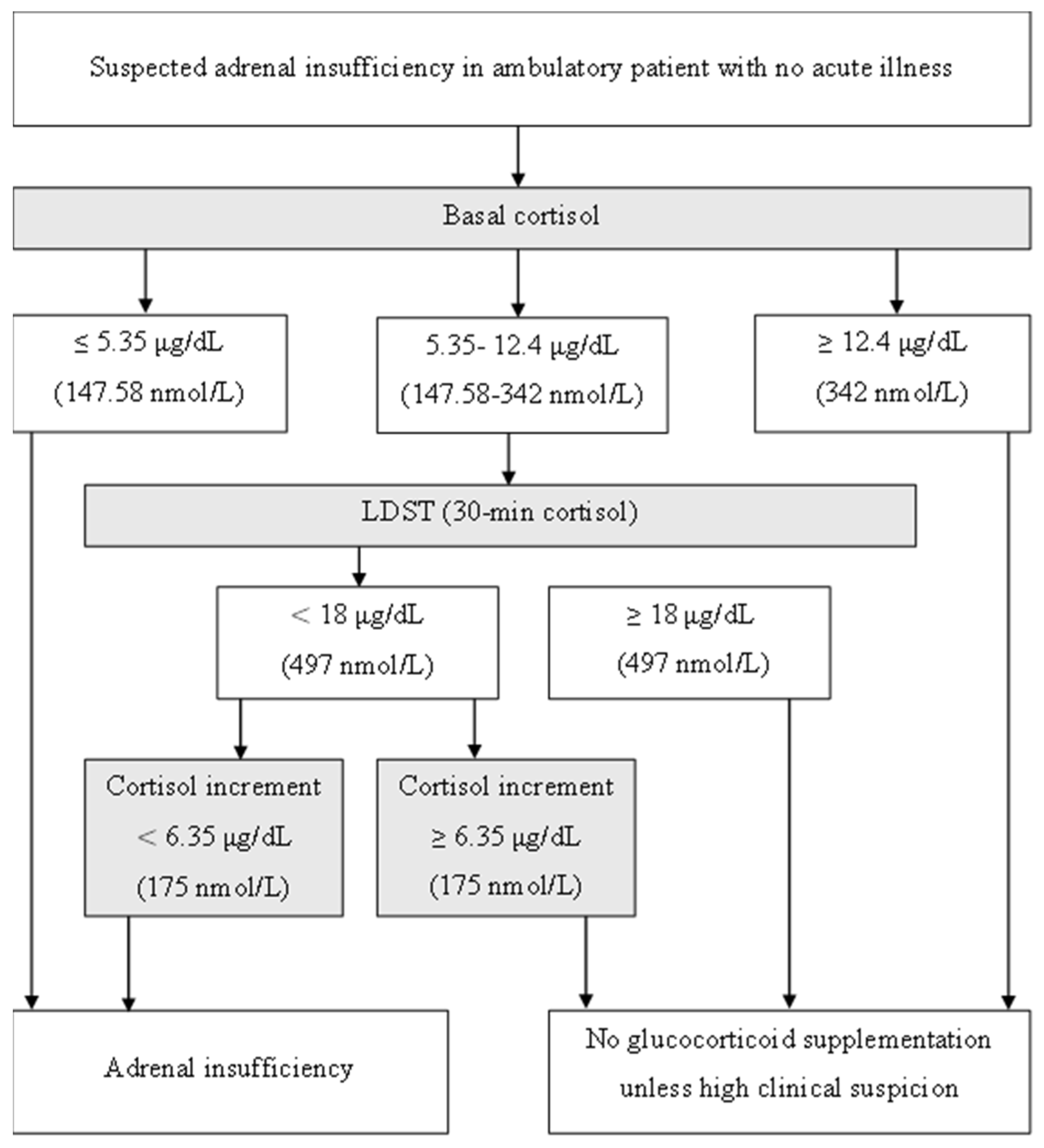
2.2. Test Protocol and Interpretation
2.3. Statistical Analyses
3. Results
3.1. Patients
3.2. Analysis of the Corticotrope Axis During LDST
3.3. Baseline Cortisol and LDST Outcome Analysis
3.4. 30 and 60 Min Cortisol Measurements and LDST
3.5. Cortisol Increment and Predictive Performance for AI Diagnosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hahner, S.; Ross, R.J.; Arlt, W.; Bancos, I.; Burger-Stritt, S.; Torpy, D.J. Adrenal insufficiency. Nat. Rev. Dis. Primers 2024, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Arlt, W.; Allolio, B. Adrenal insufficiency. Lancet 2003, 361, 1881–1893. [Google Scholar] [CrossRef] [PubMed]
- Regal, M.; Páramo, C.; Sierra, S.M.; Garcia-Mayor, R.V. Prevalence and incidence of hypopituitarism in an adult Caucasian population in northwestern Spain. Clin. Endocrinol. 2001, 55, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Løvås, K.; Husebye, E.S. High prevalence and increasing incidence of Addison’s disease in western Norway. Clin. Endocrinol. 2002, 56, 787–791. [Google Scholar] [CrossRef] [PubMed]
- Laureti, S.; Vecchi, L.; Santeusanio, F.; Falorni, A. Is the prevalence of Addison’s disease underestimated? J. Clin. Endocrinol. Metab. 1999, 84, 1762. [Google Scholar] [CrossRef] [PubMed]
- Ach, T.; Yosra, H.; Jihen, M.; Ben Abdelkarim, A.; Maha, K.; Molka, C. Cortisol cut-points for the glucagon stimulation test in the evaluation of hypothalamic pituitary adrenal axis. Endocr. J. 2018, 65, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Khare, S.; Anjum, F. Adrenocorticotropic Hormone (Cosyntropin) Stimulation Test. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: http://www.ncbi.nlm.nih.gov/books/NBK555940/ (accessed on 10 August 2024).
- Hamrahian, A.H.; Fleseriu, M.; AACE Adrenal Scientific Committee. Evaluation and Management of Adrenal Insufficiency in Critically Ill Patients: Disease State Review. Endocr. Pract. 2017, 23, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Wassif, W.S. Adrenal insufficiency. J. Clin. Pathol. 2022, 75, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Doi, S.A.R.; Lasheen, I.; Al-Humood, K.; Al-Shoumer, K.A.S. Relationship between cortisol increment and basal cortisol: Implications for the low-dose short adrenocorticotropic hormone stimulation test. Clin. Chem. 2006, 52, 746–749. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yo, W.S.; Toh, L.M.; Brown, S.J.; Howe, W.D.; Henley, D.E.; Lim, E.M. How good is a morning cortisol in predicting an adequate response to intramuscular synacthen stimulation? Clin. Endocrinol. 2014, 81, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, S.D.; Gifford, R.M.; Boyle, L.D.; Crane, M.S.; Strachan, M.W.J.; Gibb, F.W. Validated criteria for the interpretation of a single measurement of serum cortisol in the investigation of suspected adrenal insufficiency. Clin. Endocrinol. 2019, 91, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Hägg, E.; Asplund, K.; Lithner, F. Value of basal plasma cortisol assays in the assessment of pituitary-adrenal insufficiency. Clin. Endocrinol. 1987, 26, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Aji, A.; Colyer, S.; Burn, S.; Dimitri, P.; Wright, N.; Krone, N. The relationship of baseline, incremental and peak cortisol following a Short Synacthen Test—Single-centre analysis of three years. In Endocrine Abstracts; Bioscientifica: Bristol, UK, 2018; Available online: https://www.endocrine-abstracts.org/ea/0058/ea0058oc2.2 (accessed on 14 August 2024).
- Park, J.; Titman, A.; Lancaster, G.; Selvarajah, B.; Collingwood, C.; Powell, D. Baseline and Peak Cortisol Response to the Low-Dose Short Synacthen Test Relates to Indication for Testing, Age, and Sex. J. Endocr. Soc. 2022, 6, bvac043. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, R.; Carter, J.; Kumar, A.; Capatana, F.; Khan, I.; Adlan, M. Pre-test Cortisol Levels in Predicting Short Synacthen Test Outcome: A Retrospective Analysis. Clin. Med. Insights Endocrinol. Diabetes 2022, 15, 117955142210933. [Google Scholar] [CrossRef] [PubMed]
- Ach, M.T.; Zaouali, M.; Hasni, Y.; Abdelkarim, A.B.; Maaroufi, A.; Maha, K. Relationship between cortisol increment and basal cortisol: Implications for the insulin tolerance test in assessing corticotrop insufficiency. In Endocrine Abstracts; Bioscientifica: Bristol, UK, 2018; Available online: https://www.endocrine-abstracts.org/ea/0056/ea0056p791 (accessed on 14 August 2024).
- Perton, F.T.; Mijnhout, G.S.; Kollen, B.J.; Rondeel, J.M.M.; Franken, A.A.M.; Groeneveld, P.H.P. Validation of the 1 µg short synacthen test: An assessment of morning cortisol cut-off values and other predictors. Neth. J. Med. 2017, 75, 14–20. [Google Scholar] [PubMed]
- Oguz, Y.; Oktenli, C.; Ozata, M.; Ozgurtas, T.; Sanisoglu, Y.; Yenicesu, M. The midnight-to-morning urinary cortisol increment method is not reliable for the assessment of hypothalamic-pituitary-adrenal insufficiency in patients with end-stage kidney disease. J. Endocrinol. Investig. 2003, 26, 609–615. [Google Scholar] [CrossRef] [PubMed]
- El-Farhan, N.; Pickett, A.; Ducroq, D.; Bailey, C.; Mitchem, K.; Morgan, N. Method-specific serum cortisol responses to the adrenocorticotrophin test: Comparison of gas chromatography-mass spectrometry and five automated immunoassays. Clin. Endocrinol. 2013, 78, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Javorsky, B.R.; Raff, H.; Carroll, T.B.; Algeciras-Schimnich, A.; Singh, R.J.; Colón-Franco, J.M. New Cutoffs for the Biochemical Diagnosis of Adrenal Insufficiency after ACTH Stimulation using Specific Cortisol Assays. J. Endocr. Soc. 2021, 5, bvab022. [Google Scholar] [CrossRef] [PubMed]
- Raverot, V.; Richet, C.; Morel, Y.; Raverot, G.; Borson-Chazot, F. Establishment of revised diagnostic cut-offs for adrenal laboratory investigation using the new Roche Diagnostics Elecsys® Cortisol II assay. Ann. Endocrinol. 2016, 77, 620–622. [Google Scholar] [CrossRef] [PubMed]
- Dickstein, G.; Shechner, C.; Nicholson, W.E.; Rosner, I.; Shen-Orr, Z.; Adawi, F. Adrenocorticotropin Stimulation Test: Effects of Basal Cortisol Level, Time of Day, and Suggested New Sensitive Low Dose Test. J. Clin. Endocrinol. Metab. 1991, 72, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Tordjman, K.; Jaffe, A.; Grazas, N.; Apter, C.; Stern, N. The role of the low dose (1 microgram) adrenocorticotropin test in the evaluation of patients with pituitary diseases. J. Clin. Endocrinol. Metab. 1995, 80, 1301–1305. [Google Scholar] [PubMed]
- Weintrob, N.; Sprecher, E.; Josefsberg, Z.; Weininger, C.; Aurbach-Klipper, Y.; Lazard, D. Standard and low-dose short adrenocorticotropin test compared with insulin-induced hypoglycemia for assessment of the hypothalamic-pituitary-adrenal axis in children with idiopathic multiple pituitary hormone deficiencies. J. Clin. Endocrinol. Metab. 1998, 83, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.H.; Tiu, S.C.; Shek, C.C.; Choi, K.L.; Chan, F.K.W.; Kong, P.S. Use of the low-dose corticotropin stimulation test for the diagnosis of secondary adrenocortical insufficiency. Hong Kong Med. J. 2002, 8, 427–434. [Google Scholar] [PubMed]
- Rasmuson, S.; Olsson, T.; Hagg, E. A low dose ACTH test to assess the function of the hypothalamic-pituitary-adrenal axis. Clin. Endocrinol. 1996, 44, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Cartaya, J.; Misra, M. The low-dose ACTH stimulation test: Is 30 minutes long enough? Endocr. Pract. 2015, 21, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Chitale, A.; Musonda, P.; McGregor, A.M.; Dhatariya, K.K. Determining the utility of the 60 min cortisol measurement in the short synacthen test. Clin. Endocrinol. 2013, 79, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Carr, P.; Moore, K.; Rajput, Z.; Ward, L.; Wassif, W.S. Do we need 30 min cortisol measurement in the short synacthen test: A retrospective study. Postgrad. Med. J. 2020, 96, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Abufarhaneh, M. Comparing the utility of 30- and 60-minute cortisol levels after the standard short synacthen test to determine adrenal insufficiency A retrospective cross-sectional study. Medicine 2020, 99, e22621. [Google Scholar]
- Mansoor, S.; Islam, N.; Siddiqui, I.; Jabbar, A. Sixty-minute post-Synacthen serum cortisol level: A reliable and cost-effective screening test for excluding adrenal insufficiency compared to the conventional short Synacthen test. Singap. Med. J. 2007, 48, 519–523. [Google Scholar]
- Zueger, T.; Jordi, M.; Laimer, M.; Stettler, C. Utility of 30 and 60 minute cortisol samples after high-dose synthetic ACTH-1-24 injection in the diagnosis of adrenal insufficiency. Swiss Med. Wkly. 2014, 144, w13987. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Struja, T.; Briner, L.; Meier, A.; Kutz, A.; Mundwiler, E.; Huber, A. Diagnostic accuracy of basal cortisol level to predict adrenal insufficiency in cosyntropin testing: Results from an observational cohort study with 804 patients. Endocr. Pract. 2017, 23, 949–961. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.M.; Seckl, J.R.; Corrie, J.; Edwards, C.R.W.; Padfield, P.L. A rational approach for assessing the hypothalamo-pituitary-adrenal axis. Lancet 1988, 331, 1208–1210. [Google Scholar] [CrossRef] [PubMed]
- Annane, D.; Pastores, S.M.; Rochwerg, B.; Arlt, W.; Balk, R.A.; Beishuizen, A. Guidelines for the Diagnosis and Management of Critical Illness-Related Corticosteroid Insufficiency (CIRCI) in Critically Ill Patients (Part I): Society of Critical Care Medicine (SCCM) and European Society of Intensive Care Medicine (ESICM) 2017. Crit. Care Med. 2017, 45, 2078–2088. [Google Scholar] [CrossRef] [PubMed]
- Kadiyala, R.; Kamath, C.; Baglioni, P.; Geen, J.; Okosieme, O.E. Can a random serum cortisol reduce the need for short synacthen tests in acute medical admissions? Ann. Clin. Biochem. 2010, 47, 378–380. [Google Scholar] [CrossRef] [PubMed]
- Lassoued, N.; Zantour, B.; Alaya, W.; Sfar, M.H. The use of baseline cortisol level in predicting the outcome of 1 μg Synacthen tests in an outpatient endocrinology unit. Heliyon 2022, 8, e09559. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, I.L.; Lahner, H.; Mann, K.; Petersenn, S. Diagnosis of Adrenal Insufficiency: Evaluation of the Corticotropin-Releasing Hormone Test and Basal Serum Cortisol in Comparison to the Insulin Tolerance Test in Patients with Hypothalamic-Pituitary-Adrenal Disease. J. Clin. Endocrinol. Metab. 2003, 88, 4193–4198. [Google Scholar] [CrossRef] [PubMed]

| Variable | G1 (n = 65) | G2 (n = 98) | p-Value |
|---|---|---|---|
| Average Age (years ± SD) | 43.26 ± 15.95 | 42.82 ± 17.18 | 0.358 |
| Gender | |||
| Males | 23 (35.4%) | 37 (37.8%) | 0.642 |
| Females | 42 (64.6%) | 61 (62.2%) | 0.759 |
| Personal History | |||
| High blood pressure | 12 (18.5%) | 16 (16.3) | 0.314 |
| Dysthyroidism | 9 (13.8) | 9 (9.2) | 0.352 |
| Cardiovascular disease | 2 (3.1%) | 4 (4.1%) | 0.214 |
| Pituitary axis defect | 4 (6.2) | 2 (2) | 0.172 |
| Type 1 diabetes | 4 (6.2) | 8 (8.2) | 0.631 |
| Consanguinity | 0 | 1 (1) | 0.414 |
| Celiac disease | 0 | 2 (2) | 0.247 |
| Vitiligo | 0 | 0 | - |
| BMI (kg/m2 ± SD) | 26.34 (±4.41) | 25.63 (±4.96) | 0.241 |
| Abnormal BMI n (%) | 34 (52.3) | 49 (50) | 0.773 |
| Underweight | 0 | 3 (3.1) | 0.276 |
| Overweight | 20 (30.8) | 34 (34.7) | 0.602 |
| Obesity I | 9 (13.8) | 4 (4.1) | 0.036 |
| Obesity II | 4 (6.2) | 5 (5.1) | 0.516 |
| Obesity III | 0 | 3 (3.1) | 0.276 |
| Orthostatic hypotension n (%) | 4 (6.2) | 0 | 0.013 |
| Melanoderma n (%) | 10 (15.4) | 1 (1) | <0.001 |
| Laboratory test abnormalities n (%) | |||
| Hyponatremia | 13 (20) | 11 (11.2) | 0.250 |
| Hyperkalemia | 7 (10.8) | 4 (4.1) | 0.117 |
| Calcium disorders | 5 (7.7) | 4 (4.1) | 0.323 |
| Anemia | 17 (26.2) | 23 (23.5) | 0.697 |
| G1 (n = 65) | G2 (n = 98) | p-Value | |
|---|---|---|---|
| Functional signs | |||
| Asthenia | 39 (60%) | 55 (56.1%) | 0.372 |
| Anorexia | 6 (9.2%) | 11 (11.2%) | 0.448 |
| Fasting intolerance | 2 (3.1%) | 15 (15.3%) | 0.012 |
| Nausea and vomiting | 1 (1.5%) | 1 (1%) | 0.640 |
| Constipation | 1 (1.5%) | 1 (1%) | 0.640 |
| Diarrhea | 0 (0%) | 1 (1%) | 0.601 |
| Salt craving | 0 (0%) | 0 (0%) | - |
| Physical signs | |||
| Weight loss | 15 (23.1%) | 28 (28.6) | 0.276 |
| Melanoderma | 10 (15.4%) | 1 (1%) | <0.001 |
| Orthostatic hypotension | 4 (6.2%) | 0 (0%) | 0.013 |
| Laboratory findings | |||
| Hypoglycemia | 23 (35.4%) | 30 (30.6%) | 0.320 |
| Hyperkalemia | 0 (0%) | 1 (1%) | 0.601 |
| Hyponatremia | 0 (0%) | 0 (0%) | - |
| Clinical conditions | |||
| Long-term corticosteroid therapy | 25 (38.5%) | 26 (26.5%) | 0.076 |
| Corticosteroids withdrawal | 4 (6.2%) | 3 (3.1%) | 0.340 |
| Pituitary adenoma | 12 (18.5%) | 19 (19.4%) | 0.526 |
| Adrenal disease * | 12 (18.5%) | 5 (5.1%) | 0.006 |
| Post-pituitary radiotherapy | 6 (9.2%) | 11 (11.2%) | 0.448 |
| Post-pituitary surgery | 8 (12.3%) | 7 (7.1%) | 0.199 |
| T0 | T30 | T60 | ||
|---|---|---|---|---|
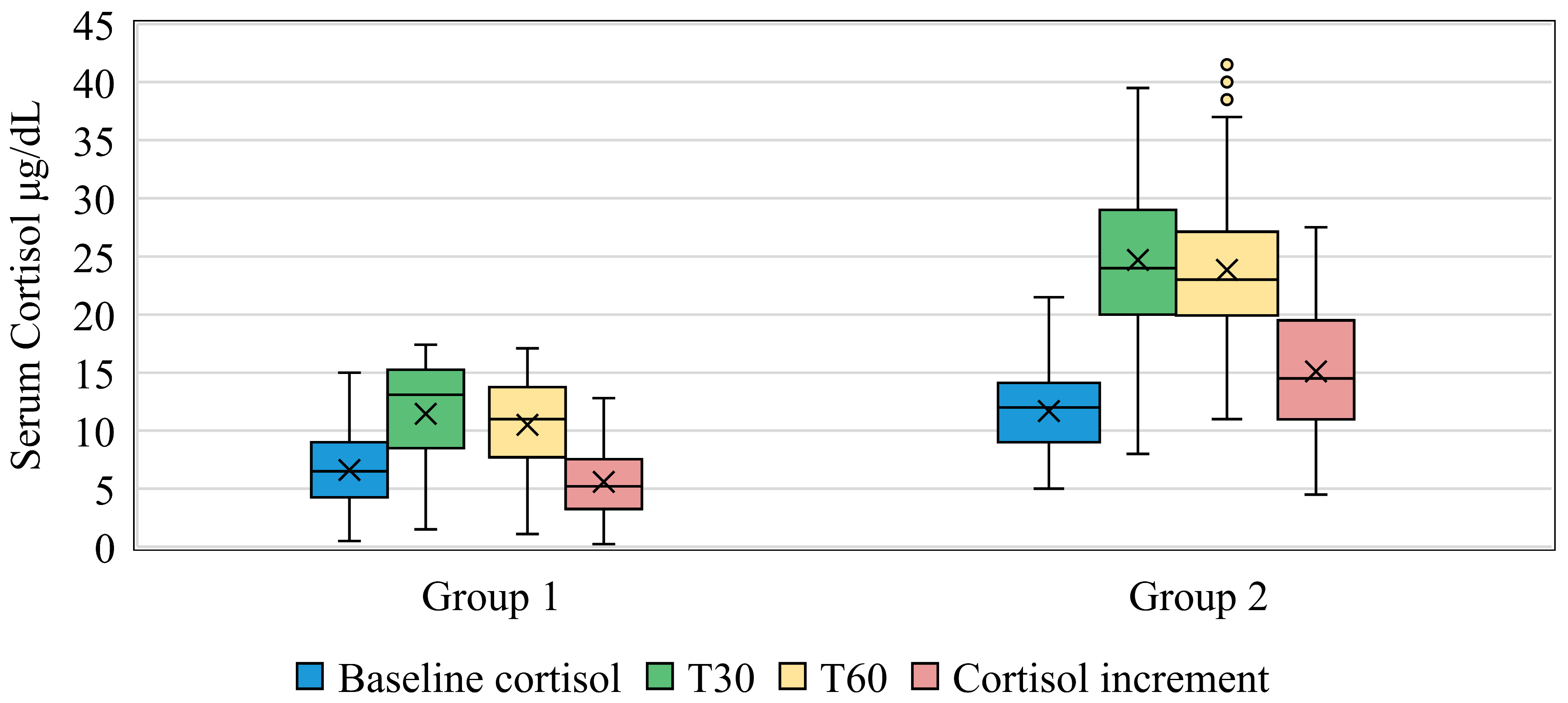
| Mean cortisol levels (µg/dL) | G1 | 5.26 ± 2.14 | 10.53 ± 4.18 | 10.03 ± 3.92 |
| G2 | 11.17 ± 2.73 | 24.68 ± 5.9 | 23.95 ± 6.01 | |
| p | <0.001 | <0.001 | <0.001 | |
| Frequency of cortisol peak n (%) | G1 | NA | 40 (61.5) | 25 (38.5) |
| G2 | NA | 55 (56.1) | 43 (43.9) |
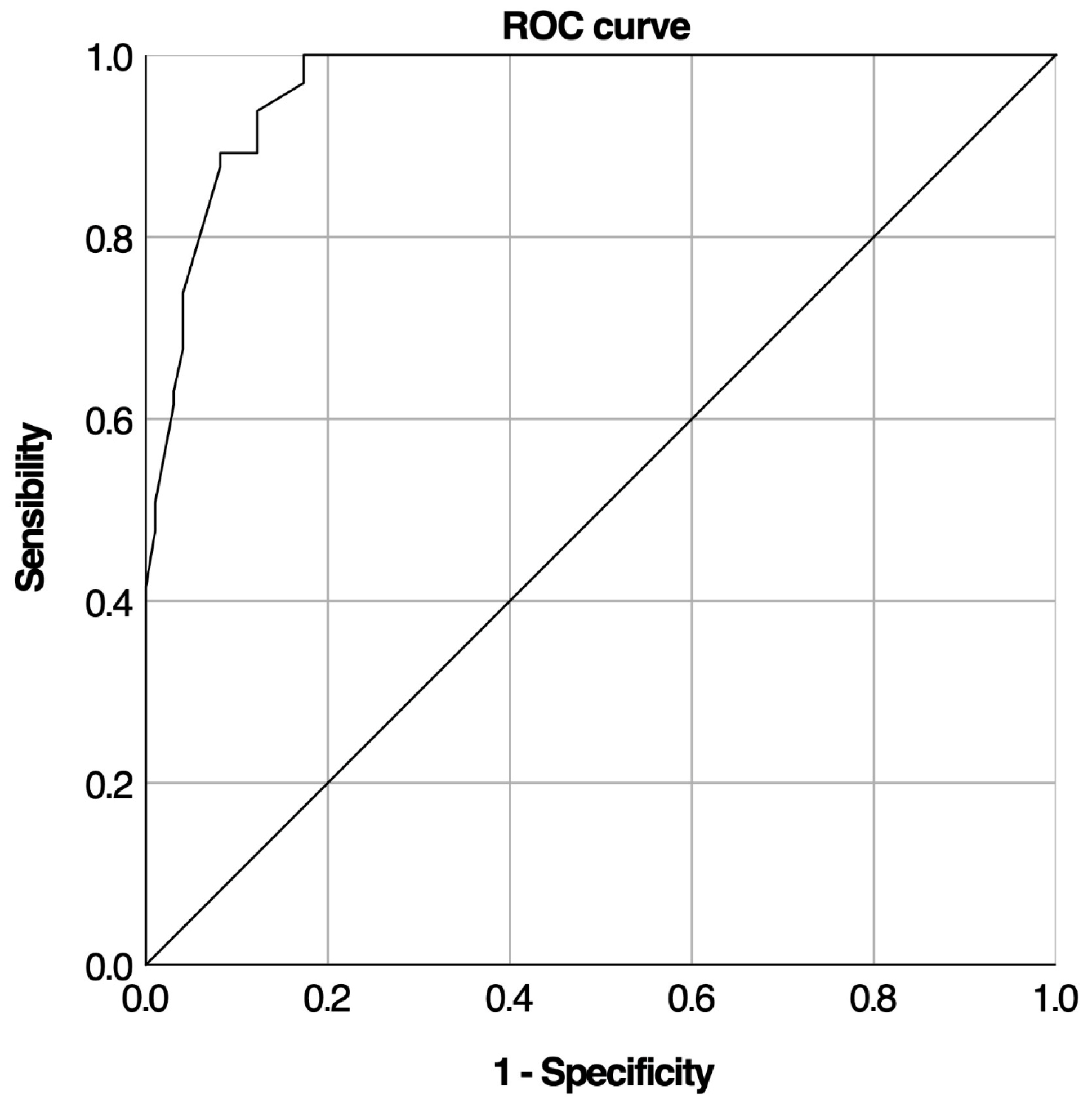
| Cut-Off | Spec% | Sens% | Sens-(1-Spec) | TP | TN | FP | FN |
|---|---|---|---|---|---|---|---|
| Baseline Cortisol | |||||||
| 5.35 | 100 | 41.5 | 0.415 | 27 | 98 | 0 | 38 |
| 12.4 | 45.9 | 100 | 0.459 | 65 | 45 | 53 | 0 |
| Cortisol Increment | |||||||
| 6.35 | 99 | 53.8 | 0.528 | 35 | 97 | 1 | 30 |
| 11.95 | 69.4 | 100 | 0.694 | 65 | 68 | 30 | 0 |
| 10.55 | 78.6 | 95.4 | 0.74 | 62 | 77 | 21 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ach, T.; Dhaffar, R.; Ammar, A.; Ghachem, A.; Halloul, I.; Saafi, W.; El Fekih, H.; Saad, G.; Hasni, Y.; Zaouali, M. Redefining the Diagnostic Approach to Adrenal Insufficiency: Re-Assessment of Baseline and Cortisol Increment Cut-Offs with the 1 µg Synacthen Test. Medicina 2025, 61, 1303. https://doi.org/10.3390/medicina61071303
Ach T, Dhaffar R, Ammar A, Ghachem A, Halloul I, Saafi W, El Fekih H, Saad G, Hasni Y, Zaouali M. Redefining the Diagnostic Approach to Adrenal Insufficiency: Re-Assessment of Baseline and Cortisol Increment Cut-Offs with the 1 µg Synacthen Test. Medicina. 2025; 61(7):1303. https://doi.org/10.3390/medicina61071303
Chicago/Turabian StyleAch, Taieb, Rim Dhaffar, Asma Ammar, Aycha Ghachem, Imen Halloul, Wiem Saafi, Hamza El Fekih, Ghada Saad, Yosra Hasni, and Monia Zaouali. 2025. "Redefining the Diagnostic Approach to Adrenal Insufficiency: Re-Assessment of Baseline and Cortisol Increment Cut-Offs with the 1 µg Synacthen Test" Medicina 61, no. 7: 1303. https://doi.org/10.3390/medicina61071303
APA StyleAch, T., Dhaffar, R., Ammar, A., Ghachem, A., Halloul, I., Saafi, W., El Fekih, H., Saad, G., Hasni, Y., & Zaouali, M. (2025). Redefining the Diagnostic Approach to Adrenal Insufficiency: Re-Assessment of Baseline and Cortisol Increment Cut-Offs with the 1 µg Synacthen Test. Medicina, 61(7), 1303. https://doi.org/10.3390/medicina61071303







