Exercise Interventions in Breast Cancer: Molecular Mechanisms, Physical Benefits, and Practical Recommendations
Abstract
1. Introducing the Framework Between Exercise and Breast Cancer
2. Exercise Reduces BC Risk and Recurrence and Enhances Survival
3. Molecular Mechanisms Underlying Exercise in BC Patients
3.1. Sex Hormones and Exercise
3.1.1. Estrogen and SHBG
3.1.2. Progesterone, Androgens and Cortisol
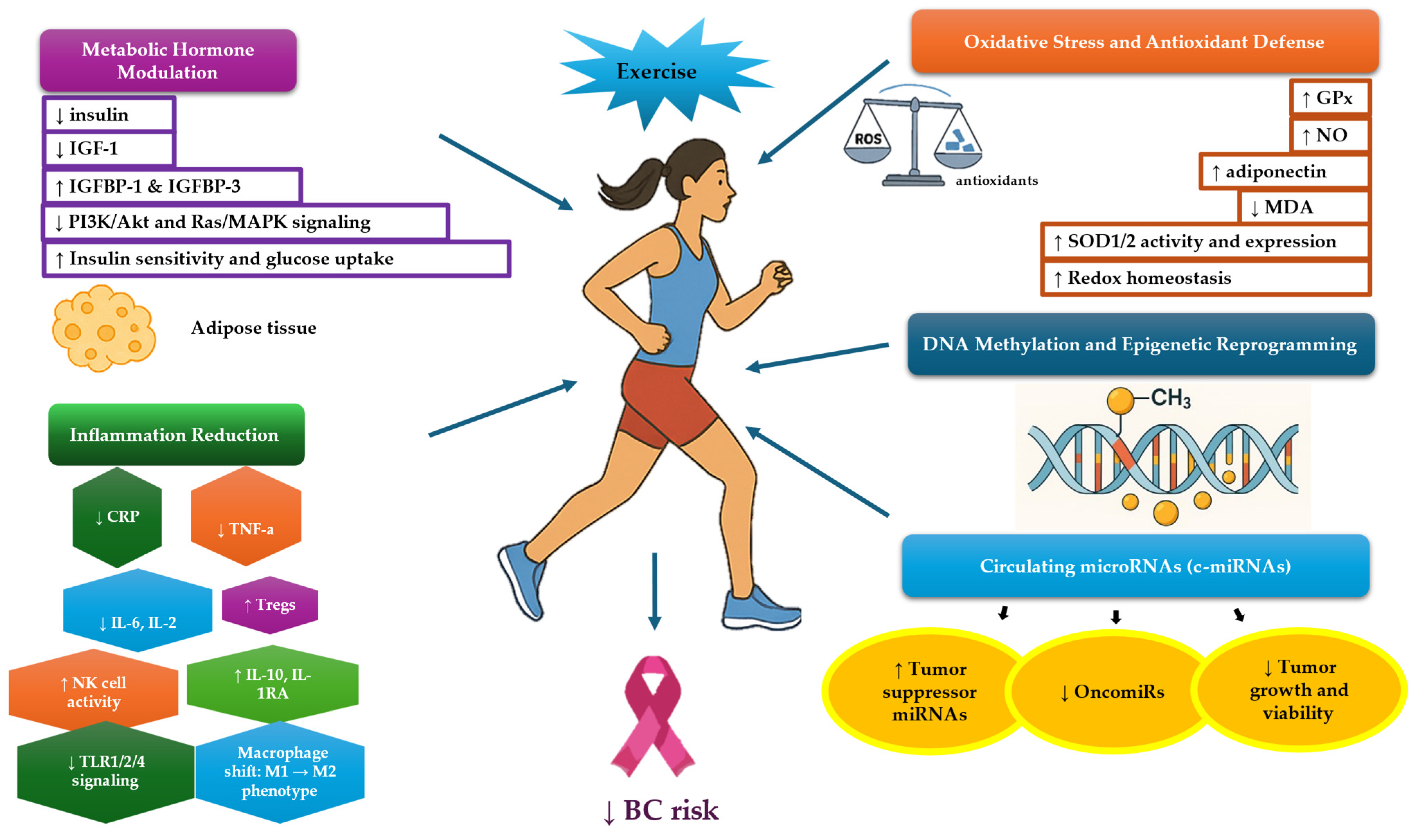
3.2. Metabolic Hormones and Exercise
3.3. Inflammation and Exercise
3.4. Oxidative Stress and Exercise
3.5. Exercise-Induced Circulating microRNAs
3.6. BC-Related DNA Methylation and Exercise
3.7. Apoptosis/Angiogenesis and Exercise
3.8. Exercise-Induced Exosomes
4. Exercise-Induced Effects in BC Patients: What Do We Know So Far?
4.1. Studies in BC Patients With or Without Related Lymphedema and Survivors
4.2. Studies in BRCA1/2 Carriers
5. Treatment or Dietary Strategies with Possible Synergetic Effects in BC
5.1. The Synergetic Effects of Treatment and Exercise Training
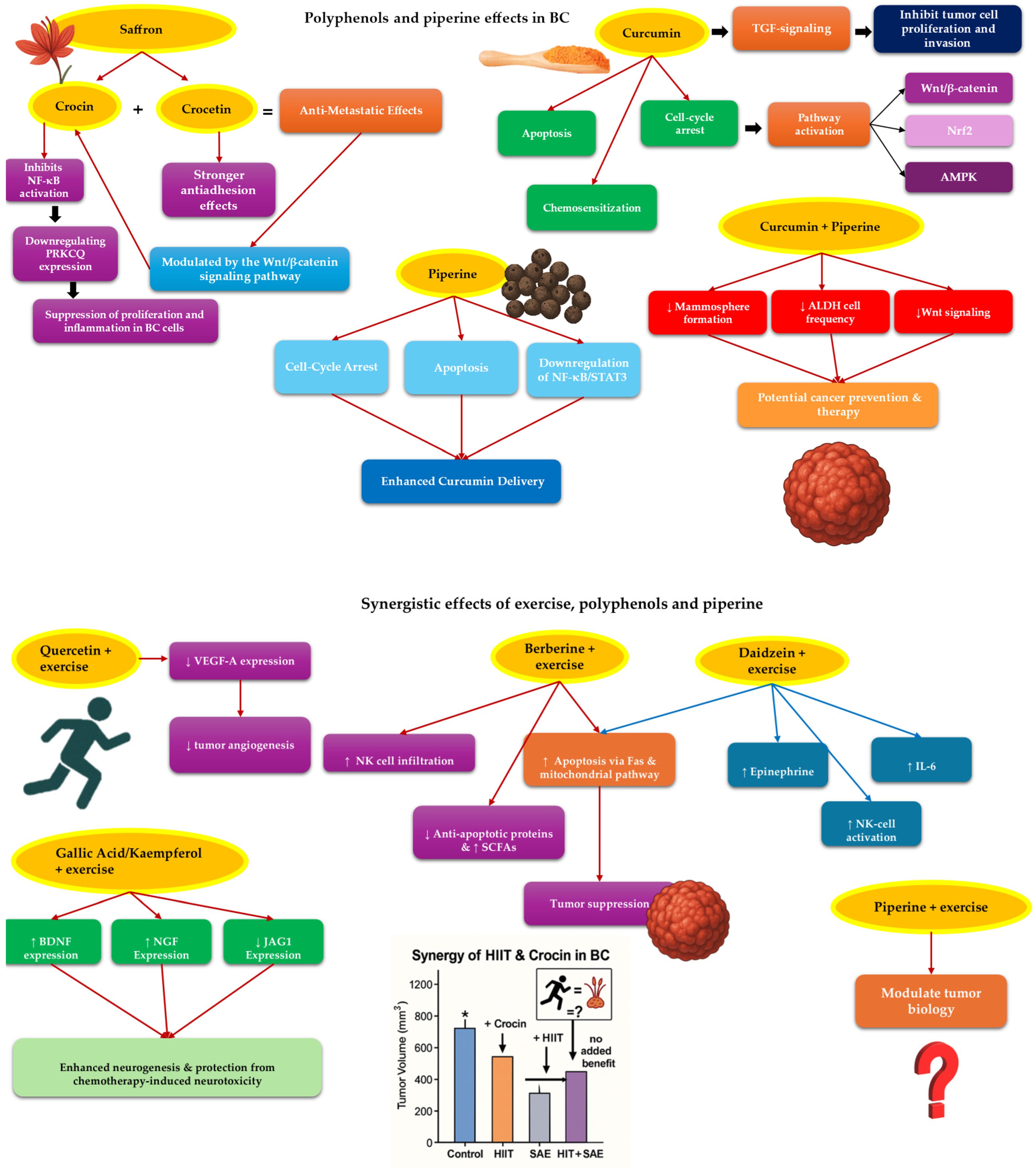
5.2. The Synergetic Effects of Polyphenol and Exercise Training in BC Patients [176]
5.2.1. Saffron and Exercise Training
5.2.2. Curcumin and Exercise Training
5.2.3. Other Substances and Exercise Training
5.2.4. What Is More to Be Explored?
6. Exercise Recommendations for BC Patients
Key-Recommendations
- Individualized Exercise Prescription: Exercise programs should be tailored to each patient’s specific cancer type, stage, treatment regimen, and overall health status. This personalization ensures safety and maximizes therapeutic benefits.
- Multimodal Approach: A combination of aerobic and resistance training is advocated. Moderate- to high-intensity exercises are generally appropriate, but the exact regimen should be adjusted based on individual capabilities and treatment phases.
- Safety and Feasibility: Prior to initiating an exercise program, a thorough assessment should be conducted to identify any contraindications or limitations. This ensures that the prescribed exercises are both safe and feasible for the patient.
- Integration Across the Cancer Care Continuum: Exercise should be incorporated at all stages of cancer care—pre-treatment, during treatment, and post-treatment, to mitigate side effects, enhance physical function, and improve quality of life.
- Addressing Barriers: Recognizing and addressing potential barriers to exercise, such as fatigue, psychological distress, or logistical challenges, is crucial. Providing behavioral support and education can enhance adherence and outcomes.
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Health Observatory; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- European Comission. Knowledge service. In Breast Cancer Burden and Screening Inequalities in Europe; European Comission: Brussels, Belgium, 2025. [Google Scholar]
- European Comission. Health Promotion Knowledge Gateway. In Age-Standardised Incidence of Breast Cancer Per 100 000 Women in the EU in 2020; European Comission: Brussels, Belgium, 2023. [Google Scholar]
- Akram, M.; Iqbal, M.; Daniyal, M.; Khan, A.U. Awareness and current knowledge of breast cancer. Biol. Res. 2017, 50, 33. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, K.H.; Courneya, K.S.; Matthews, C.; Demark-Wahnefried, W.; Galvão, D.A.; Pinto, B.M.; Irwin, M.L.; Wolin, K.Y.; Segal, R.J.; Lucia, A.; et al. American College of Sports Medicine Roundtable on Exercise Guidelines for Cancer Survivors. Med. Sci. Sports Exerc. 2010, 42, 1409–1426. [Google Scholar] [CrossRef] [PubMed]
- Neil-Sztramko, S.E.; Kirkham, A.A.; Hung, S.H.; Niksirat, N.; Nishikawa, K.; Campbell, K.L. Aerobic capacity and upper limb strength are reduced in women diagnosed with breast cancer: A systematic review. J. Physiother. 2014, 60, 189–200. [Google Scholar] [CrossRef]
- Yu, A.F.; Jones, L.W. Breast cancer treatment-associated cardiovascular toxicity and effects of exercise countermeasures. Cardio-Oncology 2016, 2, 1. [Google Scholar] [CrossRef]
- Parker, P.A.; Youssef, A.; Walker, S.; Basen-Engquist, K.; Cohen, L.; Gritz, E.R.; Wei, Q.X.; Robb, G.L. Short-Term and Long-Term Psychosocial Adjustment and Quality of Life in Women Undergoing Different Surgical Procedures for Breast Cancer. Ann. Surg. Oncol. 2007, 14, 3078–3089. [Google Scholar] [CrossRef]
- Lucas, A.R.; Levine, B.J.; Avis, N.E. Posttreatment trajectories of physical activity in breast cancer survivors. Cancer 2017, 123, 2773–2780. [Google Scholar] [CrossRef]
- Huy, C.; Schmidt, M.E.; Vrieling, A.; Chang-Claude, J.; Steindorf, K. Physical activity in a German breast cancer patient cohort: One-year trends and characteristics associated with change in activity level. Eur. J. Cancer 2012, 48, 297–304. [Google Scholar] [CrossRef]
- Spei, M.E.; Samoli, E.; Bravi, F.; La Vecchia, C.; Bamia, C.; Benetou, V. Physical activity in breast cancer survivors: A systematic review and meta-analysis on overall and breast cancer survival. Breast 2019, 44, 144–152. [Google Scholar] [CrossRef]
- Singh, B.; Spence, R.R.; Steele, M.L.; Sandler, C.X.; Peake, J.M.; Hayes, S.C. A Systematic Review and Meta-Analysis of the Safety, Feasibility, and Effect of Exercise in Women With Stage II+ Breast Cancer. Arch. Phys. Med. Rehabil. 2018, 99, 2621–2636. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, M.G. Effects of Exercise Interventions on Breast Cancer Patients During Adjuvant Therapy. Cancer Nurs. 2020, 43, 115–125. [Google Scholar] [CrossRef]
- Gebruers, N.; Camberlin, M.; Theunissen, F.; Tjalma, W.; Verbelen, H.; Van Soom, T.; van Breda, E. The effect of training interventions on physical performance, quality of life, and fatigue in patients receiving breast cancer treatment: A systematic review. Support. Care Cancer 2019, 27, 109–122. [Google Scholar] [CrossRef]
- Mctiernan, A.; Friedenreich, C.M.; Katzmarzyk, P.T.; Powell, K.E.; Macko, R.; Buchner, D.; Pescatello, L.S.; Bloodgood, B.; Tennant, B.; Vaux-Bjerke, A.; et al. Physical Activity in Cancer Prevention and Survival: A Systematic Review. Med. Sci. Sports Exerc. 2019, 51, 1252–1261. [Google Scholar] [CrossRef]
- Lynch, B.M.; Neilson, H.K.; Friedenreich, C.M. Physical Activity and Breast Cancer Prevention. Phys. Act. Cancer 2010, 186, 13–42. [Google Scholar]
- Moore, S.C.; Lee, I.M.; Weiderpass, E.; Campbell, P.T.; Sampson, J.N.; Kitahara, C.M.; Keadle, S.K.; Arem, H.; Berrington de Gonzalez, A.; Hartge, P.; et al. Association of Leisure-Time Physical Activity with Risk of 26 Types of Cancer in 1.44 Million Adults. JAMA Intern. Med. 2016, 176, 816. [Google Scholar] [CrossRef]
- McNeely, M.L. Effects of exercise on breast cancer patients and survivors: A systematic review and meta-analysis. Can. Med. Assoc. J. 2006, 175, 34–41. [Google Scholar] [CrossRef]
- Dimeo, F.C. Effects of exercise on cancer-related fatigue. Cancer 2001, 92 (Suppl. 6), 1689–1693. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.M.; Zabor, E.C.; Schwitzer, E.; Koelwyn, G.J.; Adams, S.C.; Nilsen, T.S.; Moskowitz, C.S.; Matsoukas, K.; Iyengar, N.M.; Dang, C.T.; et al. Efficacy of Exercise Therapy on Cardiorespiratory Fitness in Patients with Cancer: A Systematic Review and Meta-Analysis. J. Clin. Oncol. 2018, 36, 2297–2305. [Google Scholar] [CrossRef]
- Lee, J. A Meta-analysis of the Association Between Physical Activity and Breast Cancer Mortality. Cancer Nurs. 2019, 42, 271–285. [Google Scholar] [CrossRef]
- Holmes, M.D. Physical Activity and Survival After Breast Cancer Diagnosis. JAMA 2005, 293, 2479. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, D.; Kang, S. Physical activity and risk of breast cancer: A meta-analysis of prospective studies. Breast Cancer Res. Treat. 2013, 137, 869–882. [Google Scholar] [CrossRef] [PubMed]
- de Boer, M.C.; Wörner, E.A.; Verlaan, D.; van Leeuwen, P.A.M. The Mechanisms and Effects of Physical Activity on Breast Cancer. Clin. Breast Cancer 2017, 17, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhou, W. Roles and molecular mechanisms of physical exercise in cancer prevention and treatment. J. Sport. Health Sci. 2021, 10, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Hawley, J.A.; Hargreaves, M.; Joyner, M.J.; Zierath, J.R. Integrative Biology of Exercise. Cell 2014, 159, 738–749. [Google Scholar] [CrossRef]
- Gabriel, B.M.; Zierath, J.R. The Limits of Exercise Physiology: From Performance to Health. Cell Metab. 2017, 25, 1000–1011. [Google Scholar] [CrossRef]
- Neufer, P.D.; Bamman, M.M.; Muoio, D.M.; Bouchard, C.; Cooper, D.M.; Goodpaster, B.H.; Booth, F.W.; Kohrt, W.M.; Gerszten, R.E.; Mattson, M.P.; et al. Understanding the Cellular and Molecular Mechanisms of Physical Activity-Induced Health Benefits. Cell Metab. 2015, 22, 4–11. [Google Scholar] [CrossRef]
- Sasso, J.P.; Eves, N.D.; Christensen, J.F.; Koelwyn, G.J.; Scott, J.; Jones, L.W. A framework for prescription in exercise-oncology research. J. Cachexia Sarcopenia Muscle 2015, 6, 115–124. [Google Scholar] [CrossRef]
- Ballard-Barbash, R.; Friedenreich, C.M.; Courneya, K.S.; Siddiqi, S.M.; McTiernan, A.; Alfano, C.M. Physical Activity, Biomarkers, and Disease Outcomes in Cancer Survivors: A Systematic Review. JNCI J. Natl. Cancer Inst. 2012, 104, 815–840. [Google Scholar] [CrossRef]
- Pizot, C.; Boniol, M.; Mullie, P.; Koechlin, A.; Boniol, M.; Boyle, P.; Autier, P. Physical activity, hormone replacement therapy and breast cancer risk: A meta-analysis of prospective studies. Eur. J. Cancer 2016, 52, 138–154. [Google Scholar] [CrossRef]
- Emaus, A.; Veierød, M.B.; Furberg, A.S.; Espetvedt, S.; Friedenreich, C.; Ellison, P.; Jasienska, G.; Andersen, L.B.; Thune, I. Physical Activity, Heart Rate, Metabolic Profile, and Estradiol in Premenopausal Women. Med. Sci. Sports Exerc. 2008, 40, 1022–1030. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gonçalves, A.K.; Florêncio, G.L.D.; de Atayde Silva, M.J.M.; Cobucci, R.N.; Giraldo, P.C.; Cote, N.M. Effects of Physical Activity on Breast Cancer Prevention: A Systematic Review. J. Phys. Act. Health 2014, 11, 445–454. [Google Scholar] [CrossRef]
- Howard, R.A.; Leitzmann, M.F.; Linet, M.S.; Freedman, D.M. Physical activity and breast cancer risk among pre- and postmenopausal women in the U.S. Radiologic Technologists cohort. Cancer Causes Control 2009, 20, 323–333. [Google Scholar] [CrossRef]
- Friedenreich, C.M.; Neilson, H.K.; Wang, Q.; Stanczyk, F.Z.; Yasui, Y.; Duha, A.; MacLaughlin, S.; Kallal, C.; Forbes, C.C.; Courneya, K.S. Effects of exercise dose on endogenous estrogens in postmenopausal women: A randomized trial. Endocr. Relat. Cancer 2015, 22, 863–876. [Google Scholar] [CrossRef] [PubMed]
- McTiernan, A.; Wu, L.; Chen, C.; Chlebowski, R.; Mossavar-Rahmani, Y.; Modugno, F.; Perri, M.G.; Stanczyk, F.Z.; Van Horn, L.; Wang, C.; et al. Relation of BMI and Physical Activity to Sex Hormones in Postmenopausal Women. Obesity 2006, 14, 1662–1677. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, W.; Dai, Z.; Wang, M.; Tian, T.; Liu, X.; Kang, H.; Guan, H.; Zhang, S.; Dai, Z. Association between body mass index and breast cancer risk: Evidence based on a dose–response meta-analysis. Cancer Manag. Res. 2018, 10, 143–151. [Google Scholar]
- Guo, Z.; Wang, J.; Tian, X.; Fang, Z.; Gao, Y.; Ping, Z.; Liu, L. Body mass index increases the recurrence risk of breast cancer: A dose–response meta-analysis from 21 prospective cohort studies. Public Health 2022, 210, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, L.; Zhou, Q.; Imam, M.U.; Cai, J.; Wang, Y.; Qi, M.; Sun, P.; Ping, Z.; Fu, X. Body mass index had different effects on premenopausal and postmenopausal breast cancer risks: A dose-response meta-analysis with 3,318,796 subjects from 31 cohort studies. BMC Public Health 2017, 17, 936. [Google Scholar] [CrossRef]
- Diaz Font, A.; Thompson, R.; Wiseman, M.; Mitrou, G.; Brown, S.; Allen, K. The 2017 WCRF/AICR CUP Report on diet, nutrition, physical activity and breast cancer: Recent findings and future priorities. Eur. J. Cancer 2018, 92, S22–S23. [Google Scholar] [CrossRef]
- Díaz-Balboa, E.; Peña-Gil, C.; Rodríguez-Romero, B.; Cuesta-Vargas, A.I.; Lado-Baleato, O.; Martínez-Monzonís, A.; Pedreira-Pérez, M.; Palacios-Ozores, P.; López-López, R.; González-Juanatey, J.R.; et al. Exercise-based cardio-oncology rehabilitation for cardiotoxicity prevention during breast cancer chemotherapy: The ONCORE randomized controlled trial. Prog. Cardiovasc. Dis. 2024, 85, 74–81. [Google Scholar] [CrossRef]
- Dieli-Conwright, C.M.; Courneya, K.S.; Demark-Wahnefried, W.; Sami, N.; Lee, K.; Buchanan, T.A.; Spicer, D.V.; Tripathy, D.; Bernstein, L.; Mortimer, J.E. Effects of Aerobic and Resistance Exercise on Metabolic Syndrome, Sarcopenic Obesity, and Circulating Biomarkers in Overweight or Obese Survivors of Breast Cancer: A Randomized Controlled Trial. J. Clin. Oncol. 2018, 36, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, E.M.; Al-Homaidh, A. Physical activity and survival after breast cancer diagnosis: Meta-analysis of published studies. Med. Oncol. 2011, 28, 753–765. [Google Scholar] [CrossRef]
- Schmidt, M.E.; Chang-Claude, J.; Vrieling, A.; Seibold, P.; Heinz, J.; Obi, N.; Flesch-Janys, D.; Steindorf, K. Association of pre-diagnosis physical activity with recurrence and mortality among women with breast cancer. Int. J. Cancer 2013, 133, 1431–1440. [Google Scholar] [CrossRef] [PubMed]
- Irwin, M.L.; Smith, A.W.; McTiernan, A.; Ballard-Barbash, R.; Cronin, K.; Gilliland, F.D.; Baumgartner, R.N.; Baumgartner, K.B.; Bernstein, L. Influence of Pre- and Postdiagnosis Physical Activity on Mortality in Breast Cancer Survivors: The Health, Eating, Activity, and Lifestyle Study. J. Clin. Oncol. 2008, 26, 3958–3964. [Google Scholar] [CrossRef]
- Holick, C.N.; Newcomb, P.A.; Trentham-Dietz, A.; Titus-Ernstoff, L.; Bersch, A.J.; Stampfer, M.J.; Baron, J.A.; Egan, K.M.; Willett, W.C. Physical Activity and Survival after Diagnosis of Invasive Breast Cancer. Cancer Epidemiol. Biomark. Prev. 2008, 17, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Graf, C.; Wessely, N. Physical Activity in the Prevention and Therapy of Breast Cancer. Breast Care 2010, 5, 389–394. [Google Scholar] [CrossRef]
- Pollan, M.; Torres, A.; Ramon YCajal, T.; Llort, G.; Castello, A.; Fisas, D.; Yague, C.; Lopez, C.; Leon, M.T.; Pollan, M. Effects of lifestyle and diet as modifiers of risk of breast cancer in BRCA1 and BRCA2 carriers. J. Clin. Oncol. 2017, 35, 1505. [Google Scholar] [CrossRef]
- Kehm, R.D.; Genkinger, J.M.; MacInnis, R.J.; John, E.M.; Phillips, K.A.; Dite, G.S.; Milne, R.L.; Zeinomar, N.; Liao, Y.; Knight, J.A.; et al. Recreational Physical Activity Is Associated with Reduced Breast Cancer Risk in Adult Women at High Risk for Breast Cancer: A Cohort Study of Women Selected for Familial and Genetic Risk. Cancer Res. 2020, 80, 116–125. [Google Scholar] [CrossRef]
- Lammert, J.; Lubinski, J.; Gronwald, J.; Huzarski, T.; Armel, S.; Eisen, A.; Meschino, W.S.; Lynch, H.T.; Snyder, C.; Eng, C.; et al. Physical activity during adolescence and young adulthood and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers. Breast Cancer Res. Treat. 2018, 169, 561–571. [Google Scholar] [CrossRef]
- Grill, S.; Yahiaoui-Doktor, M.; Dukatz, R.; Lammert, J.; Ullrich, M.; Engel, C.; Pfeifer, K.; Basrai, M.; Siniatchkin, M.; Schmidt, T.; et al. Smoking and physical inactivity increase cancer prevalence in BRCA-1 and BRCA-2 mutation carriers: Results from a retrospective observational analysis. Arch. Gynecol. Obstet. 2017, 296, 1135–1144. [Google Scholar] [CrossRef]
- Pijpe, A.; Manders, P.; Brohet, R.M.; Collée, J.M.; Verhoef, S.; Vasen, H.F.A. Physical activity and the risk of breast cancer in BRCA1/2 mutation carriers. Breast Cancer Res. Treat. 2010, 120, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, B.E.; Haskell, W.L.; Whitt, M.C.; Irwin, M.L.; Swartz, A.M.; Strath, S.J.; O’Brien, W.L.; Bassett, D.R., Jr.; Schmitz, K.H.; Emplaincourt, P.O.; et al. Compendium of physical activities: An update of activity codes and MET intensities. Med. Sci. Sports Exerc. 2000, 32 (Suppl. 9), S498–S504. [Google Scholar] [CrossRef] [PubMed]
- Friedenreich, C.M.; Gregory, J.; Kopciuk, K.A.; Mackey, J.R.; Courneya, K.S. Prospective cohort study of lifetime physical activity and breast cancer survival. Int. J. Cancer 2009, 124, 1954–1962. [Google Scholar] [CrossRef]
- Ainsworth, B.E.; Haskell, W.L.; Leon, A.S.; Jacobs, D.R., Jr.; Montoye, H.J.; Sallis, J.F.; Paffenbarger, R.S., Jr. Compendium of Physical Activities: Classification of energy costs of human physical activities. Med. Sci. Sports Exerc. 1993, 25, 71–80. [Google Scholar] [CrossRef]
- Nkondjock, A.; Robidoux, A.; Paredes, Y.; Narod, S.A.; Ghadirian, P. Diet, lifestyle and BRCA-related breast cancer risk among French-Canadians. Breast Cancer Res Treat. 2006, 98, 285–294. [Google Scholar] [CrossRef]
- King, M.C.; Marks, J.H.; Mandell, J.B. Breast and Ovarian Cancer Risks Due to Inherited Mutations in BRCA1 and BRCA2. Science 2003, 302, 643–646. [Google Scholar] [CrossRef]
- The Endogenous Hormones and Breast Cancer Collaborative Group. Endogenous Sex Hormones and Breast Cancer in Postmenopausal Women: Reanalysis of Nine Prospective Studies. CancerSpectrum Knowl. Environ. 2002, 94, 606–616. [Google Scholar]
- Folkerd, E.; Dowsett, M. Sex hormones and breast cancer risk and prognosis. Breast 2013, 22, S38–S43. [Google Scholar] [CrossRef]
- Yager, J.D.; Davidson, N.E. Estrogen Carcinogenesis in Breast Cancer. N. Engl. J. Med. 2006, 354, 270–282. [Google Scholar] [CrossRef]
- Królik, M.; Milnerowicz, H. The effect of using estrogens in the light of scientific research. Adv. Clin. Exp. Med. 2012, 21, 535–543. [Google Scholar]
- Fortunati, N.; Catalano, M. Sex Hormone-binding Globulin (SHBG) and Estradiol Cross-talk in Breast Cancer Cells. Horm. Metab. Res. 2006, 38, 236–240. [Google Scholar] [CrossRef]
- Friedenreich, C.M. Physical Activity and Breast Cancer Risk: The Effect of Menopausal Status. Exerc. Sport Sci. Rev. 2004, 32, 180–184. [Google Scholar] [CrossRef]
- Tin Tin, S.; Reeves, G.K.; Key, T.J. Endogenous hormones and risk of invasive breast cancer in pre- and post-menopausal women: Findings from the UK Biobank. Br. J. Cancer 2021, 125, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Drummond, A.E.; Swain, C.T.V.; Brown, K.A.; Dixon-Suen, S.C.; Boing, L.; van Roekel, E.H.; Moore, M.M.; Gaunt, T.R.; Milne, R.L.; English, D.R.; et al. Linking Physical Activity to Breast Cancer via Sex Steroid Hormones, Part 2: The Effect of Sex Steroid Hormones on Breast Cancer Risk. Cancer Epidemiol. Biomark. Prev. 2022, 31, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.-F.; Dowsett, M.; Folkerd, E.; Bingham, S.; Wareham, N.; Luben, R.; Welch, A.; Khaw, K.-T. Usual physical activity and endogenous sex hormones in postmenopausal women: The European prospective investigation into cancer-norfolk population study. Cancer Epidemiol. Biomark. Prev. 2007, 16, 900–905. [Google Scholar] [CrossRef]
- De Cree, C. Sex Steroid Metabolism and Menstrual Irregularities in the Exercising Female. Sports Med. 1998, 25, 369–406. [Google Scholar] [CrossRef] [PubMed]
- Verkasalo, P.K.; Thomas, H.V.; Appleby, P.N.; Davey, G.K.; Key, T.J. Circulating levels of sex hormones and their relation to risk factors for breast cancer: A cross-sectional study in 1092 pre- and postmenopausal women (United Kingdom). Cancer Causes Control 2001, 12, 47–59. [Google Scholar] [CrossRef]
- Kaaks, R.; Lukanova, A.; Rinaldi, S.; Biessy, C.; Söderberg, S.; Olsson, T.; Stenman, U.-H.; Riboli, E.; Hallmans, G.; Stattin, P.; et al. Interrelationships between plasma testosterone, SHBG, IGF-I, insulin and leptin in prostate cancer cases and controls. Eur. J. Cancer Prev. 2003, 12, 309–315. [Google Scholar] [CrossRef]
- Friedenreich, C.M.; Neilson, H.K.; Lynch, B.M. State of the epidemiological evidence on physical activity and cancer prevention. Eur. J. Cancer 2010, 46, 2593–2604. [Google Scholar] [CrossRef]
- Conn, V.S.; Koopman, R.J.; Ruppar, T.M.; Phillips, L.J.; Mehr, D.R.; Hafdahl, A.R. Insulin Sensitivity Following Exercise Interventions. J. Prim. Care Community Health 2014, 5, 211–222. [Google Scholar] [CrossRef]
- Hargreaves, M. Skeletal Muscle Glucose Metabolism during Exercise: Implications for Health and Performance. J. Sci. Med. Sport. 1998, 1, 195–202. [Google Scholar] [CrossRef]
- Schmitz, K.H.; Williams, N.I.; Kontos, D.; Domchek, S.; Morales, K.H.; Hwang, W.-T.; Grant, L.L.; DiGiovanni, L.; Salvatore, D.; Fenderson, D.; et al. Dose–response effects of aerobic exercise on estrogen among women at high risk for breast cancer: A randomized controlled trial. Breast Cancer Res. Treat. 2015, 154, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Kong, Z.; Sun, S.; Liu, M.; Shi, Q. Short-Term High-Intensity Interval Training on Body Composition and Blood Glucose in Overweight and Obese Young Women. J. Diabetes Res. 2016, 2016, 4073618. [Google Scholar] [CrossRef] [PubMed]
- Kong, Z.; Fan, X.; Sun, S.; Song, L.; Shi, Q.; Nie, J. Comparison of High-Intensity Interval Training and Moderate-to-Vigorous Continuous Training for Cardiometabolic Health and Exercise Enjoyment in Obese Young Women: A Randomized Controlled Trial. PLoS ONE 2016, 11, e0158589. [Google Scholar] [CrossRef] [PubMed]
- Swain, C.T.; Drummond, A.E.; Boing, L.; Milne, R.L.; English, D.R.; Brown, K.A.; van Roekel, E.H.; Dixon-Suen, S.C.; Lynch, M.J.; Moore, M.M.; et al. Linking Physical Activity to Breast Cancer via Sex Hormones, Part 1: The Effect of Physical Activity on Sex Steroid Hormones. Cancer Epidemiol. Biomark. Prev. 2022, 31, 16–27. [Google Scholar] [CrossRef]
- Choudhury, F.; Bernstein, L.; Hodis, H.N.; Stanczyk, F.Z.; Mack, W.J. Physical activity and sex hormone levels in estradiol- and placebo-treated postmenopausal women. Menopause 2011, 18, 1079–1086. [Google Scholar] [CrossRef]
- Copeland, J.L.; Consitt, L.A.; Tremblay, M.S. Hormonal Responses to Endurance and Resistance Exercise in Females Aged 19-69 Years. J. Gerontol. A Biol. Sci. Med. Sci. 2002, 57, B158–B165. [Google Scholar] [CrossRef]
- Consitt, L.A.; Copeland, J.L.; Tremblay, M.S. Hormone Responses to Resistance vs. Endurance Exercise in Premenopausal Females. Can. J. Appl. Physiol. 2001, 26, 574–587. [Google Scholar] [CrossRef]
- Vajda, M.; Kovárová, J.; Okuliarová, M.; Cvečka, J.; Schickhofer, P.; Böhmerová, Ľ.; Marián, V.; Tomáš, K.; Dušan, H.; Zeman, M.; et al. Acute hormonal and neuromuscular response to different resistance loading in young pre- and middle-aged postmenopausal women. Gazz. Medica Ital. Arch. Sci. Mediche 2018, 177, 443–451. [Google Scholar] [CrossRef]
- Hong, B.S.; Lee, K.P. A systematic review of the biological mechanisms linking physical activity and breast cancer. Phys. Act. Nutr. 2020, 24, 25–31. [Google Scholar] [CrossRef]
- Wolf, I.; Sadetzki, S.; Catane, R.; Karasik, A.; Kaufman, B. Diabetes mellitus and breast cancer. Lancet Oncol. 2005, 6, 103–111. [Google Scholar] [CrossRef]
- Gunter, M.J.; Hoover, D.R.; Yu, H.; Wassertheil-Smoller, S.; Rohan, T.E.; Manson, J.E.; Li, J.; Ho, G.Y.; Xue, X.; Anderson, G.L.; et al. Insulin, Insulin-Like Growth Factor-I, and Risk of Breast Cancer in Postmenopausal Women. JNCI J. Natl. Cancer Inst. 2009, 101, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Irwin, M.L.; Varma, K.; Alvarez-Reeves, M.; Cadmus, L.; Wiley, A.; Chung, G.G.; Dipietro, L.; Mayne, S.T.; Yu, H. Randomized Controlled Trial of Aerobic Exercise on Insulin and Insulin-like Growth Factors in Breast Cancer Survivors: The Yale Exercise and Survivorship Study. Cancer Epidemiol. Biomark. Prev. 2009, 18, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Kaaks, R.; Lukanova, A. Energy balance and cancer: The role of insulin and insulin-like growth factor-I. Proc. Nutr. Soc. 2001, 60, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Lann, D.; LeRoith, D. The Role of Endocrine Insulin-Like Growth Factor-I and Insulin in Breast Cancer. J. Mammary Gland. Biol. Neoplasia 2008, 13, 371–379. [Google Scholar] [CrossRef]
- Frank, L.L.; Sorensen, B.E.; Yasui, Y.; Tworoger, S.S.; Schwartz, R.S.; Ulrich, C.M.; Irwin, M.L.; Rudolph, R.E.; Rajan, K.B.; Stanczyk, F.; et al. Effects of Exercise on Metabolic Risk Variables in Overweight Postmenopausal Women: A Randomized Clinical Trial. Obes. Res. 2005, 13, 615–625. [Google Scholar] [CrossRef]
- Thomas, R.; Kenfield, S.A.; Yanagisawa, Y.; Newton, R.U. Why exercise has a crucial role in cancer prevention, risk reduction and improved outcomes. Br. Med. Bull. 2021, 139, 100–119. [Google Scholar] [CrossRef]
- Hankinson, S.E.; Willett, W.C.; Colditz, G.A.; Hunter, D.J.; Michaud, D.S.; Deroo, B.; Rosner, B.; Speizer, F.E.; Pollak, M. Circulating concentrations of insulin-like growth factor I and risk of breast cancer. Lancet 1998, 351, 1393–1396. [Google Scholar] [CrossRef]
- Fairey, A.S.; Courneya, K.S.; Field, C.J.; Bell, G.J.; Jones, L.W.; Mackey, J.R. Effects of exercise training on fasting insulin, insulin resistance, insulin-like growth factors, and insulin-like growth factor binding proteins in postmenopausal breast cancer survivors: A randomized controlled trial. Cancer Epidemiol. Biomark. Prev. 2003, 12, 721–727. [Google Scholar]
- Xie, L.; Wang, W. Weight control and cancer preventive mechanisms: Role of insulin growth factor-1-mediated signaling pathways. Exp. Biol. Med. 2013, 238, 127–132. [Google Scholar] [CrossRef]
- Schmitz, K.H.; Ahmed, R.L.; Yee, D. Effects of a 9-month strength training intervention on insulin, insulin-like growth factor (IGF)-I, IGF-binding protein (IGFBP)-1, and IGFBP-3 in 30–50-year-old women. Cancer Epidemiol. Biomark. Prev. 2002, 11, 1597–1604. [Google Scholar]
- Ross, R.; Janssen, I.; Dawson, J.; Kungl, A.; Kuk, J.L.; Wong, S.L.; Nguyen-Duy, T.B.; Lee, S.; Kilpatrick, K.; Hudson, R. Exercise-Induced Reduction in Obesity and Insulin Resistance in Women: A Randomized Controlled Trial. Obes. Res. 2004, 12, 789–798. [Google Scholar] [CrossRef]
- Boulé, N.G.; Haddad, E.; Kenny, G.P.; Wells, G.A.; Sigal, R.J. Effects of Exercise on Glycemic Control and Body Mass in Type 2 Diabetes Mellitus. JAMA 2001, 286, 1218. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, M.; Bishop, N.C.; Stensel, D.J.; Lindley, M.R.; Mastana, S.S.; Nimmo, M.A. The anti-inflammatory effects of exercise: Mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 2011, 11, 607–615. [Google Scholar] [CrossRef]
- Kawanishi, N.; Mizokami, T.; Yano, H.; Suzuki, K. Exercise Attenuates M1 Macrophages and CD8+ T Cells in the Adipose Tissue of Obese Mice. Med. Sci. Sports Exerc. 2013, 45, 1684–1693. [Google Scholar] [CrossRef]
- Karimi, N.; Roshan, V.D. Change in Adiponectin and Oxidative Stress after Modifiable Lifestyle Interventions in Breast Cancer Cases. Asian Pac. J. Cancer Prev. 2013, 14, 2845–2850. [Google Scholar] [CrossRef]
- Delrieu, L.; Touillaud, M.; Pérol, O.; Morelle, M.; Martin, A.; Friedenreich, C.M.; Mury, P.; Dufresne, A.; Bachelot, T.; Heudel, P.E.; et al. Impact of Physical Activity on Oxidative Stress Markers in Patients with Metastatic Breast Cancer. Oxid. Med. Cell. Longev. 2021, 2021, 6694594. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.C.; Sturgeon, K.; Sarwer, D.B.; Troxel, A.B.; DeMichele, A.M.; Denlinger, C.S.; Schmitz, K.H. The effects of exercise and diet on oxidative stress and telomere length in breast cancer survivors. Breast Cancer Res. Treat. 2023, 199, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Werner, C.; Fürster, T.; Widmann, T.; Pöss, J.; Roggia, C.; Hanhoun, M.; Scharhag, J.; Büchner, N.; Meyer, T.; Kindermann, W.; et al. Physical Exercise Prevents Cellular Senescence in Circulating Leukocytes and in the Vessel Wall. Circulation 2009, 120, 2438–2447. [Google Scholar] [CrossRef]
- Sánchez-González, J.L.; Sánchez-Rodríguez, J.L.; Varela-Rodríguez, S.; González-Sarmiento, R.; Rivera-Picón, C.; Juárez-Vela, R.; Tejada-Garrido, C.I.; Martín-Vallejo, J.; Navarro-López, V. Effects of Physical Exercise on Telomere Length in Healthy Adults: Systematic Review, Meta-Analysis, and Meta-Regression. JMIR Public Health Surveill. 2024, 10, e46019. [Google Scholar] [CrossRef]
- Ornish, D.; Lin, J.; Daubenmier, J.; Weidner, G.; Epel, E.; Kemp, C.; Magbanua, M.J.; Marlin, R.; Yglecias, L.; Carroll, P.R.; et al. Increased telomerase activity and comprehensive lifestyle changes: A pilot study. Lancet Oncol. 2008, 9, 1048–1057. [Google Scholar] [CrossRef] [PubMed]
- Santa-Maria, C.A.; Coughlin, J.W.; Sharma, D.; Armanios, M.; Blackford, A.L.; Schreyer, C.; Dalcin, A.; Carpenter, A.; Jerome, G.J.; Armstrong, D.K.; et al. The Effects of a Remote-based Weight Loss Program on Adipocytokines, Metabolic Markers, and Telomere Length in Breast Cancer Survivors: The POWER-Remote Trial. Clin. Cancer Res. 2020, 26, 3024–3034. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Gao, Y.T.; Shu, X.O.; Yang, G.; Milne, G.; Cai, Q.; Li, H.; Xiang, Y.; Chow, W.H.; Zheng, W. Oxidative Stress, Obesity, and Breast Cancer Risk: Results From the Shanghai Women’s Health Study. J. Clin. Oncol. 2009, 27, 2482–2488. [Google Scholar] [CrossRef]
- Figueira, A.; Cortinhas, A.; Soares, J.; Leitão, J.; Ferreira, R.; Duarte, J. Efficacy of Exercise on Breast Cancer Outcomes: A Systematic Review and Meta-analysis of Preclinical Data. Int. J. Sports Med. 2018, 39, 327–342. [Google Scholar] [CrossRef]
- Dethlefsen, C.; Lillelund, C.; Midtgaard, J.; Andersen, C.; Pedersen, B.K.; Christensen, J.F.; Hojman, P. Exercise regulates breast cancer cell viability: Systemic training adaptations versus acute exercise responses. Breast Cancer Res. Treat. 2016, 159, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Hojman, P.; Dethlefsen, C.; Brandt, C.; Hansen, J.; Pedersen, L.; Pedersen, B.K. Exercise-induced muscle-derived cytokines inhibit mammary cancer cell growth. Am. J. Physiol.-Endocrinol. Metab. 2011, 301, E504–E510. [Google Scholar] [CrossRef]
- Hojman, P.; Gehl, J.; Christensen, J.F.; Pedersen, B.K. Molecular Mechanisms Linking Exercise to Cancer Prevention and Treatment. Cell Metab. 2018, 27, 10–21. [Google Scholar] [CrossRef]
- Isanejad, A.; Alizadeh, A.M.; Amani Shalamzari, S.; Khodayari, H.; Khodayari, S.; Khori, V.; Khojastehnjad, N. MicroRNA-206, let-7a and microRNA-21 pathways involved in the anti-angiogenesis effects of the interval exercise training and hormone therapy in breast cancer. Life Sci. 2016, 151, 30–40. [Google Scholar] [CrossRef]
- Adams, B.D.; Arem, H.; Hubal, M.J.; Cartmel, B.; Li, F.; Harrigan, M.; Sanft, T.; Cheng, C.J.; Pusztai, L.; Irwin, M.L. Exercise and weight loss interventions and miRNA expression in women with breast cancer. Breast Cancer Res. Treat. 2018, 170, 55–67. [Google Scholar] [CrossRef]
- Hagstrom, A.; Denham, J. microRNAs in High and Low Responders to Resistance Training in Breast Cancer Survivors. Int. J. Sports Med. 2018, 39, 482–489. [Google Scholar] [CrossRef]
- Alizadeh, S.; Isanejad, A.; Sadighi, S.; Khalighfard, S.; Alizadeh, A.M. Effect of a high-intensity interval training on serum microRNA levels in women with breast cancer undergoing hormone therapy. A single-blind randomized trial. Ann. Phys. Rehabil. Med. 2019, 62, 329–335. [Google Scholar] [CrossRef]
- Pulliero, A.; You, M.; Chaluvally-Raghavan, P.; Marengo, B.; Domenicotti, C.; Banelli, B.; Degan, P.; Molfetta, L.; Gianiorio, F.; Izzotti, A. Anticancer effect of physical activity is mediated by modulation of extracellular microRNA in blood. Oncotarget 2020, 11, 2106–2119. [Google Scholar] [CrossRef] [PubMed]
- Telles, G.D.; Conceição, M.S.; Vechin, F.C.; Libardi, C.A.; Mori, M.A.d.S.; Derchain, S.; Ugrinowitsch, C. Exercise-Induced Circulating microRNAs: Potential Key Factors in the Control of Breast Cancer. Front. Physiol. 2022, 13, 800094. [Google Scholar] [CrossRef]
- Bryan, A.D.; Magnan, R.E.; Hooper, A.E.C.; Harlaar, N.; Hutchison, K.E. Physical Activity and Differential Methylation of Breast Cancer Genes Assayed from Saliva: A Preliminary Investigation. Ann. Behav. Med. 2013, 45, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Gillman, A.S.; Helmuth, T.; Koljack, C.E.; Hutchison, K.E.; Kohrt, W.M.; Bryan, A.D. The Effects of Exercise Duration and Intensity on Breast Cancer-Related DNA Methylation: A Randomized Controlled Trial. Cancers 2021, 13, 4128. [Google Scholar] [CrossRef] [PubMed]
- Moulton, C.; Murri, A.; Benotti, G.; Fantini, C.; Duranti, G.; Ceci, R.; Grazioli, E.; Cerulli, C.; Sgrò, P.; Rossi, C.; et al. The impact of physical activity on promoter-specific methylation of genes involved in the redox-status and disease progression: A longitudinal study on post-surgery female breast cancer patients undergoing medical treatment. Redox Biol. 2024, 70, 103033. [Google Scholar] [CrossRef]
- Gorski, P.P.; Raastad, T.; Ullrich, M.; Turner, D.C.; Hallén, J.; Savari, S.I.; Nilsen, T.S.; Sharples, A.P. Aerobic exercise training resets the human skeletal muscle methylome 10 years after breast cancer treatment and survival. FASEB J. 2023, 37, e22720. [Google Scholar] [CrossRef]
- Zeng, H.; Irwin, M.L.; Lu, L.; Risch, H.; Mayne, S.; Mu, L.; Deng, Q.; Scarampi, L.; Mitidieri, M.; Katsaros, D.; et al. Physical activity and breast cancer survival: An epigenetic link through reduced methylation of a tumor suppressor gene L3MBTL1. Breast Cancer Res. Treat. 2012, 133, 127–135. [Google Scholar] [CrossRef]
- Barrès, R.; Yan, J.; Egan, B.; Treebak, J.T.; Rasmussen, M.; Fritz, T.; Caidahl, K.; Krook, A.; O’Gorman, D.J.; Zierath, J.R. Acute Exercise Remodels Promoter Methylation in Human Skeletal Muscle. Cell Metab. 2012, 15, 405–411. [Google Scholar] [CrossRef]
- Tsuji, K.; Matsuoka, Y.J.; Ochi, E. High-intensity interval training in breast cancer survivors: A systematic review. BMC Cancer 2021, 21, 184. [Google Scholar] [CrossRef]
- Moulton, C.; Lisi, V.; Silvestri, M.; Ceci, R.; Grazioli, E.; Sgrò, P.; Caporossi, D.; Dimauro, I. Impact of Physical Activity on DNA Methylation Signatures in Breast Cancer Patients: A Systematic Review with Bioinformatic Analysis. Cancers 2024, 16, 3067. [Google Scholar] [CrossRef]
- Voisin, S.; Eynon, N.; Yan, X.; Bishop, D.J. Exercise training and DNA methylation in humans. Acta Physiol. 2015, 213, 39–59. [Google Scholar] [CrossRef]
- Hickey, M.M.; Simon, M.C. Regulation of Angiogenesis by Hypoxia and Hypoxia-Inducible Factors. Curr. Top. Dev. Biol. 2006, 76, 217–257. [Google Scholar] [PubMed]
- Hanahan, D.; Folkman, J. Patterns and Emerging Mechanisms of the Angiogenic Switch during Tumorigenesis. Cell 1996, 86, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Bos, R.; Zhong, H.; Hanrahan, C.F.; Mommers, E.C.M.; Semenza, G.L.; Pinedo, H.M.; Abeloff, M.D.; Simons, J.W.; van Diest, P.J.; van der Wall, E. Levels of Hypoxia-Inducible Factor-1 During Breast Carcinogenesis. JNCI J. Natl. Cancer Inst. 2001, 93, 309–314. [Google Scholar] [CrossRef]
- Zhong, H.; De Marzo, A.M.; Laughner, E.; Lim, M.; Hilton, D.A.; Zagzag, D.; Buechler, P.; Isaacs, W.B.; Semenza, G.L.; Simons, J.W. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res. 1999, 59, 5830–5835. [Google Scholar] [PubMed]
- Schadler, K.L.; Thomas, N.J.; Galie, P.A.; Bhang, D.H.; Roby, K.C.; Addai, P.; Till, J.E.; Sturgeon, K.; Zaslavsky, A.; Chen, C.S.; et al. Tumor vessel normalization after aerobic exercise enhances chemotherapeutic efficacy. Oncotarget 2016, 7, 65429–65440. [Google Scholar] [CrossRef]
- McCullough, D.J.; Stabley, J.N.; Siemann, D.W.; Behnke, B.J. Modulation of Blood Flow, Hypoxia, and Vascular Function in Orthotopic Prostate Tumors During Exercise. JNCI J. Natl. Cancer Inst. 2014, 106, dju036. [Google Scholar] [CrossRef]
- Betof, A.S.; Lascola, C.D.; Weitzel, D.; Landon, C.; Scarbrough, P.M.; Devi, G.R.; Palmer, G.; Jones, L.W.; Dewhirst, M.W. Modulation of Murine Breast Tumor Vascularity, Hypoxia, and Chemotherapeutic Response by Exercise. JNCI J. Natl. Cancer Inst. 2015, 107, djv040. [Google Scholar] [CrossRef]
- Jones, L.W.; Viglianti, B.L.; Tashjian, J.A.; Kothadia, S.M.; Keir, S.T.; Freedland, S.J.; Potter, M.Q.; Moon, E.J.; Schroeder, T.; Herndon, J.E., 2nd; et al. Effect of aerobic exercise on tumor physiology in an animal model of human breast cancer. J. Appl. Physiol. 2010, 108, 343–348. [Google Scholar] [CrossRef]
- Ergun, M.; Eyigor, S.; Karaca, B.; Kisim, A.; Uslu, R. Effects of exercise on angiogenesis and apoptosis-related molecules, quality of life, fatigue and depression in breast cancer patients. Eur. J. Cancer Care 2013, 22, 626–637. [Google Scholar] [CrossRef] [PubMed]
- Schwappacher, R.; Schink, K.; Sologub, S.; Dieterich, W.; Reljic, D.; Friedrich, O.; Hans, J.H.; Markus, F.N.; Yurdagül, Z. Physical activity and advanced cancer: Evidence of exercise-sensitive genes regulating prostate cancer cell proliferation and apoptosis. J. Physiol. 2020, 598, 3871–3889. [Google Scholar] [CrossRef]
- Shaima, R. Banoon, Saade Abdalkareem Jasim, Abdolmajid Ghasemian. Effect of 12-week Aerobic Exercise on the Tumor Size and Expression of HIF-1α, BCL-2, Mir-15a, and VEGF Genes in BALB/C Female Mice with Breast Cancer. J. Chem. Health Risks 2023, 13, 283–290. [Google Scholar]
- Koohshoori, Y.S.; Marandi, S.M.; Kargarfard, M.; Vaseghi, G.; Moshtaghian, S.J. The Effect of 4 Weeks Aerobic Exercise Training with Detraining Courses in Various Prevention Phases on BCl-2 and BAX Genes Expression and Proteins. Int. J. Prev. Med. 2023, 14, 79. [Google Scholar] [CrossRef] [PubMed]
- Dias Reis, A.; João, B.; Garcia, S.; Rodrigues Diniz, R.; Silva-Filho, A.C.; Dias, C.J.; Leite, R.D.; Mostarda, C. Effect of exercise training and detraining in autonomic modulation and cardiorespiratory fitness in breast cancer survivors. J. Sports Med. Phys. Fit. 2017, 57, 1062–1068. [Google Scholar] [CrossRef]
- Whitham, M.; Parker, B.L.; Friedrichsen, M.; Hingst, J.R.; Hjorth, M.; Hughes, W.E.; Egan, C.L.; Cron, L.; Watt, K.I.; Kuchel, R.P.; et al. Extracellular Vesicles Provide a Means for Tissue Crosstalk during Exercise. Cell Metab. 2018, 27, 237–251.e4. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Casado, A.; Martín-Ruiz, A.; Pérez, L.M.; Provencio, M.; Fiuza-Luces, C.; Lucia, A. Exercise and the Hallmarks of Cancer. Trends Cancer 2017, 3, 423–441. [Google Scholar] [CrossRef]
- Vulczak, A.; Alberici, L.C. Physical Exercise and Tumor Energy Metabolism. Cancer Treat. Res. Commun. 2022, 32, 100600. [Google Scholar] [CrossRef]
- Sadovska, L.; Auders, J.; Keiša, L.; Romanchikova, N.; Silamiķele, L.; Kreišmane, M.; Zayakin, P.; Takahashi, S.; Kalniņa, Z.; Linē, A. Exercise-Induced Extracellular Vesicles Delay the Progression of Prostate Cancer. Front. Mol. Biosci. 2022, 8, 784080. [Google Scholar] [CrossRef]
- Koelwyn, G.J.; Quail, D.F.; Zhang, X.; White, R.M.; Jones, L.W. Exercise-dependent regulation of the tumour microenvironment. Nat. Rev. Cancer 2017, 17, 620–632. [Google Scholar] [CrossRef]
- Badouel, C.; McNeill, H. SnapShot: The Hippo Signaling Pathway. Cell 2011, 145, 484.e1. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.X.; Zhang, Y.; Park, H.W.; Jewell, J.L.; Chen, Q.; Deng, Y.; Pan, D.; Taylor, S.S.; Lai, Z.C.; Guan, K.L. Protein kinase A activates the Hippo pathway to modulate cell proliferation and differentiation. Genes Dev. 2013, 27, 1223–1232. [Google Scholar] [CrossRef]
- Du, T.; Wang, D.; Wan, X.; Xu, J.; Xiao, Q.; Liu, B. Regulatory effect of microRNA-223-3p on breast cancer cell processes via the Hippo/Yap signaling pathway. Oncol. Lett. 2021, 22, 516. [Google Scholar] [CrossRef]
- Dethlefsen, C.; Hansen, L.S.; Lillelund, C.; Andersen, C.; Gehl, J.; Christensen, J.F.; Pedersen, B.K.; Hojman, P. Exercise-Induced Catecholamines Activate the Hippo Tumor Suppressor Pathway to Reduce Risks of Breast Cancer Development. Cancer Res. 2017, 77, 4894–4904. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, I.R.; Batabyal, R.A.; Freishtat, R.; Cechinel, L.R. Potential involvement of circulating extracellular vesicles and particles on exercise effects in malignancies. Front. Endocrinol. 2023, 14, 1121390. [Google Scholar] [CrossRef]
- ElKhouly, A.M.; Youness, R.A.; Gad, M.Z. MicroRNA-486-5p and microRNA-486-3p: Multifaceted pleiotropic mediators in oncological and non-oncological conditions. Noncoding RNA Res. 2020, 5, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Lindholm, E.M.; Leivonen, S.K.; Undlien, E.; Nebdal, D.; Git, A.; Caldas, C.; Børresen-Dale, A.L.; Kleivi, K. miR-342-5p as a Potential Regulator of HER2 Breast Cancer Cell Growth. MicroRNA 2019, 8, 155–165. [Google Scholar] [CrossRef]
- Hou, Z.; Qin, X.; Hu, Y.; Zhang, X.; Li, G.; Wu, J.; Li, J.; Sha, J.; Chen, J.; Xia, J.; et al. Longterm Exercise-Derived Exosomal miR-342-5p. Circ. Res. 2019, 124, 1386–1400. [Google Scholar] [CrossRef]
- Llorente, A.; Brokāne, A.; Mlynska, A.; Puurand, M.; Sagini, K.; Folkmane, S.; Hjorth, M.; Martin-Gracia, B.; Romero, S.; Skorinkina, D.; et al. From sweat to hope: The role of exercise-induced extracellular vesicles in cancer prevention and treatment. J. Extracell. Vesicles 2024, 13, e12500. [Google Scholar] [CrossRef]
- Darkwah, S.; Park, E.J.; Myint, P.K.; Ito, A.; Appiah, M.G.; Obeng, G.; Kawamoto, E.; Shimaoka, M. Potential Roles of Muscle-Derived Extracellular Vesicles in Remodeling Cellular Microenvironment: Proposed Implications of the Exercise-Induced Myokine, Irisin. Front. Cell Dev. Biol. 2021, 9, 634853. [Google Scholar] [CrossRef]
- Gannon, N.P.; Vaughan, R.A.; Garcia-Smith, R.; Bisoffi, M.; Trujillo, K.A. Effects of the exercise-inducible myokine irisin on malignant and non-malignant breast epithelial cell behavior in vitro. Int. J. Cancer 2015, 136, E197–E202. [Google Scholar] [CrossRef]
- Mlynska, A.; Dobrovolskiene, N.; Suveizde, K.; Lukaseviciute, G.; Sagini, K.; Gracia, B.M.; Romero, S.; Llorente, A.; Line, A.; Butkute, A.; et al. Exercise-induced extracellular vesicles delay tumor development by igniting inflammation in an immunologically cold triple-negative breast cancer mouse model. J. Sport Health Sci. 2025, 101041. [Google Scholar] [CrossRef] [PubMed]
- Ficarra, S.; Thomas, E.; Bianco, A.; Gentile, A.; Thaller, P.; Grassadonio, F.; Papakonstantinou, S.; Schulz, T.; Olson, N.; Martin, A.; et al. Impact of exercise interventions on physical fitness in breast cancer patients and survivors: A systematic review. Breast Cancer 2022, 29, 402–418. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Chen, Y.; Liu, R.; Cao, B. Effect of exercise on rehabilitation of breast cancer surgery patients: A systematic review and meta-analysis of randomized controlled trials. Nurs. Open 2023, 10, 2030–2043. [Google Scholar] [CrossRef] [PubMed]
- Basha, M.A.; Aboelnour, N.H.; Alsharidah, A.S.; Kamel, F.H. Effect of exercise mode on physical function and quality of life in breast cancer–related lymphedema: A randomized trial. Support. Care Cancer 2022, 30, 2101–2110. [Google Scholar] [CrossRef]
- Park, Y.J.; Na, S.J.; Kim, M.K. Effect of progressive resistance exercise using Thera-band on edema volume, upper limb function, and quality of life in patients with breast cancer-related lymphedema. J. Exerc. Rehabil. 2023, 19, 105–113. [Google Scholar] [CrossRef]
- Pasyar, N.; Barshan Tashnizi, N.; Mansouri, P.; Tahmasebi, S. Effect of yoga exercise on the quality of life and upper extremity volume among women with breast cancer related lymphedema: A pilot study. Eur. J. Oncol. Nurs. 2019, 42, 103–109. [Google Scholar] [CrossRef]
- Northey, J.M.; Pumpa, K.L.; Quinlan, C.; Ikin, A.; Toohey, K.; Smee, D.J.; Rattray, B. Cognition in breast cancer survivors: A pilot study of interval and continuous exercise. J. Sci. Med. Sport. 2019, 22, 580–585. [Google Scholar] [CrossRef]
- Cešeiko, R.; Eglītis, J.; Srebnijs, A.; Timofejevs, M.; Purmalis, E.; Erts, R.; Vētra, A.; Tomsone, S. The impact of maximal strength training on quality of life among women with breast cancer undergoing treatment. Exp. Oncol. 2019, 41, 166–172. [Google Scholar] [CrossRef]
- Odynets, T.; Briskin, Y.; Todorova, V. Effects of Different Exercise Interventions on Quality of Life in Breast Cancer Patients: A Randomized Controlled Trial. Integr. Cancer Ther. 2019, 18, 1534735419880598. [Google Scholar] [CrossRef]
- Scott, J.M.; Thomas, S.M.; Peppercorn, J.M.; Herndon, J.E.; Douglas, P.S.; Khouri, M.G.; Dang, C.T.; Yu, A.F.; Catalina, D.; Ciolino, C.; et al. Effects of Exercise Therapy Dosing Schedule on Impaired Cardiorespiratory Fitness in Patients with Primary Breast Cancer. Circulation 2020, 141, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Cešeiko, R.; Thomsen, S.N.; Tomsone, S.; Eglītis, J.; Vētra, A.; Srebnijs, A.; Timofejevs, M.; Purmalis, E.; Wang, E. Heavy Resistance Training in Breast Cancer Patients Undergoing Adjuvant Therapy. Med. Sci. Sports Exerc. 2020, 52, 1239–1247. [Google Scholar] [CrossRef]
- Soriano-Maldonado, A.; Carrera-Ruiz, Á.; Díez-Fernández, D.M.; Esteban-Simón, A.; Maldonado-Quesada, M.; Moreno-Poza, N.; del Mar García-Martínez, M.; Alcaraz-García, C.; Vázquez-Sousa, R.; Moreno-Martos, H.; et al. Effects of a 12-week resistance and aerobic exercise program on muscular strength and quality of life in breast cancer survivors. Medicine 2019, 98, e17625. [Google Scholar] [CrossRef]
- Soriano-Maldonado, A.; Díez-Fernández, D.M.; Esteban-Simón, A.; Rodríguez-Pérez, M.A.; Artés-Rodríguez, E.; Casimiro-Artés, M.A.; Moreno-Martos, H.; Toro-de-Federico, A.; Hachem-Salas, N.; Bartholdy, C.; et al. Effects of a 12-week supervised resistance training program, combined with home-based physical activity, on physical fitness and quality of life in female breast cancer survivors: The EFICAN randomized controlled trial. J. Cancer Surviv. 2023, 17, 1371–1385. [Google Scholar] [CrossRef] [PubMed]
- Kiechle, M.; Engel, C.; Berling, A.; Hebestreit, K.; Bischoff, S.C.; Dukatz, R.; Siniatchkin, M.; Pfeifer, K.; Grill, S.; Yahiaoui-Doktor, M.; et al. Effects of lifestyle intervention in BRCA1/2 mutation carriers on nutrition, BMI, and physical fitness (LIBRE study): Study protocol for a randomized controlled trial. Trials 2016, 17, 368. [Google Scholar] [CrossRef]
- Neirich, L.; Yahiaoui-Doktor, M.; Lammert, J.; Basrai, M.; Seethaler, B.; Berling-Ernst, A.; Ramser, J.; Quante, A.S.; Schmidt, T.; Niederberger, U.; et al. Physical activity and Mediterranean diet as potential modulators of osteoprotegerin and soluble RANKL in gBRCA1/2 mutation carriers: Results of the lifestyle intervention pilot study LIBRE-1. Breast Cancer Res. Treat. 2021, 190, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Galasso, L.; Castelli, L.; Roveda, E.; Oliverio, A.; Baldassari, I.; Esposito, F.; Mulè, A.; Montaruli, A.; Pasanisi, P.; Bruno, E. Physical activity and sleep behaviour in women carrying BRCA1/2 mutations. Sci. Rep. 2022, 12, 12873. [Google Scholar] [CrossRef]
- Jones, L.W.; Eves, N.D.; Courneya, K.S.; Chiu, B.K.; Baracos, V.E.; Hanson, J.; Johnson, L.; Mackey, J.R. Effects of Exercise Training on Antitumor Efficacy of Doxorubicin in MDA-MB-231 Breast Cancer Xenografts. Clin. Cancer Res. 2005, 11, 6695–6698. [Google Scholar] [CrossRef]
- Khori, V.; Amani Shalamzari, S.; Isanejad, A.; Alizadeh, A.M.; Alizadeh, S.; Khodayari, S.; Shahbazi, S.; Zahedi, A.; Sohanaki, H. Effects of exercise training together with tamoxifen in reducing mammary tumor burden in mice: Possible underlying pathway of miR-21. Eur. J. Pharmacol. 2015, 765, 179–187. [Google Scholar] [CrossRef]
- Courneya, K.S.; Segal, R.J.; Mckenzie, D.C.; Dong, H.; Gelmon, K.; Friedenreich, C.M.; Yasui, Y.; Reid, R.D.; Crawford, J.J.; Mackey, J.R. Effects of Exercise during Adjuvant Chemotherapy on Breast Cancer Outcomes. Med. Sci. Sports Exerc. 2014, 46, 1744–1751. [Google Scholar] [CrossRef]
- Rao, R.; Cruz, V.; Peng, Y.; Harker-Murray, A.; Haley, B.B.; Zhao, H.; Xie, X.J.; Euhus, D. Bootcamp during Neoadjuvant Chemotherapy for Breast Cancer: A Randomized Pilot Trial. Breast Cancer 2012, 6, BCBCR.S9221. [Google Scholar] [CrossRef] [PubMed]
- Kirkham, A.A.; Gelmon, K.A.; Van Patten, C.L.; Bland, K.A.; Wollmann, H.; McKenzie, D.C.; Landry, T.; Campbell, K.L. Impact of Exercise on Chemotherapy Tolerance and Survival in Early-Stage Breast Cancer: A Nonrandomized Controlled Trial. J. Natl. Compr. Cancer Netw. 2020, 18, 1670–1677. [Google Scholar] [CrossRef]
- Pang, H.; Badehnoosh, B. Synergistic strength: Unleashing exercise and polyphenols against breast cancer. Cancer Cell Int. 2025, 25, 144. [Google Scholar] [CrossRef]
- Hashemi, S.A.; Karami, M.; Bathaie, S.Z. Saffron carotenoids change the superoxide dismutase activity in breast cancer: In vitro, in vivo and in silico studies. Int. J. Biol. Macromol. 2020, 158, 845–853. [Google Scholar] [CrossRef]
- Xu, Q.; Yu, J.; Jia, G.; Li, Z.; Xiong, H. Crocin attenuates NF-κB-mediated inflammation and proliferation in breast cancer cells by down-regulating PRKCQ. Cytokine 2022, 154, 155888. [Google Scholar] [CrossRef]
- Arzi, L.; Riazi, G.; Sadeghizadeh, M.; Hoshyar, R.; Jafarzadeh, N. A Comparative Study on Anti-Invasion, Antimigration, and Antiadhesion Effects of the Bioactive Carotenoids of Saffron on 4T1 Breast Cancer Cells Through Their Effects on Wnt/β-Catenin Pathway Genes. DNA Cell Biol. 2018, 37, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Ghorbanzadeh, V.; Aljaf, K.A.H.; Wasman, H.M.; Dariushnejad, H. Crocin inhibit the metastasis of MDA-MB-231 cell line by suppressing epithelial to mesenchymal transition through WNT/β-catenin signalling pathway. Ann. Med. Surg. 2024, 86, 1401–1407. [Google Scholar] [CrossRef] [PubMed]
- Nezamdoost, Z.; Saghebjoo, M.; Hoshyar, R.; Hedayati, M.; Keska, A. High-Intensity Training and Saffron: Effects on Breast Cancer–related Gene Expression. Med. Sci. Sports Exerc. 2020, 52, 1470–1476. [Google Scholar] [CrossRef]
- Ahmadabadi, F.; Saghebjoo, M.; Huang, C.J.; Saffari, I.; Zardast, M. The effects of high-intensity interval training and saffron aqueous extract supplementation on alterations of body weight and apoptotic indices in skeletal muscle of 4T1 breast cancer-bearing mice with cachexia. Appl. Physiol. Nutr. Metab. 2020, 45, 555–563. [Google Scholar] [CrossRef]
- Ahmadabadi, F.; Saghebjoo, M.; Hedayati, M.; Hoshyar, R.; Huang, C.J. Treatment-induced tumor cell apoptosis following high-intensity interval training and saffron aqueous extract in mice with breast cancer. Physiol. Int. 2021, 108, 19–26. [Google Scholar] [CrossRef]
- Mirzaei, H.; Gharehgozlou, R.; Heydarirad, G.; Fahimi, S.; Ghafari, S.; Mosavat, S.H.; Moghani, M.M.; Hajian, P. Efficacy and Safety of Jollab (a Saffron-Based Beverage) on Cancer-Related Fatigue in Breast Cancer Patients: A Double-Blind Randomized Clinical Trial. Complement. Med. Res. 2022, 29, 437–445. [Google Scholar] [CrossRef]
- Mehta, K.; Pantazis, P.; McQueen, T.; Aggarwal, B.B. Antiproliferative effect of curcumin (diferuloylmethane) against human breast tumor cell lines. Anticancer. Drugs 1997, 8, 470–481. [Google Scholar] [CrossRef] [PubMed]
- Venkiteswaran, S.; Thomas, T.; Thomas, T.J. Curcumin inhibits the growth of HER-2 overexpressing human breast cancer cells by interference with the glutathione pathway. Cancer Res. 2007, 67 (Suppl. 9), 3467. [Google Scholar]
- Ashrafizadeh, M.; Zarrabi, A.; Hushmandi, K.; Zarrin, V.; Moghadam, E.R.; Hashemi, F.; Najafi, M.; Mirzaei, H. Toward Regulatory Effects of Curcumin on Transforming Growth Factor-Beta Across Different Diseases: A Review. Front Pharmacol. 2020, 11, 585413. [Google Scholar] [CrossRef]
- Rismanchi, H.; Malek Mohammadi, M.; Mafi, A.; Khalilzadeh, P.; Farahani, N.; Mirzaei, S.; Khorramdelazad, H.; Mahmoodieh, B.; Rahimzadeh, P.; Alimohammadi, M.; et al. The role of curcumin in modulating circular RNAs and long non-coding RNAs in cancer. Clin. Transl. Oncol. 2024, 27, 2416–2436. [Google Scholar] [CrossRef] [PubMed]
- Kouchaki Langroudi, F.; Peeri, M.; Delfan, M. The effect of endurance training in combination with curcumin on intratumoral gene expression of AngiomiR-126 and Angiopoietin-1 in female mice with breast cancer. J. Ardabil Univ. Med. Sci. 2020, 20, 410–420. [Google Scholar] [CrossRef]
- Delfan, M.; Ramzi, F. Efficient Synergistic Combination Effect of Endurance Exercise with Curcumin on Breast Cancer Progression Through Inflammatory Pathway Inhibition in BALB/C Mice. J. Shahid Sadoughi Univ. Med. Sci. 2021, 29. [Google Scholar] [CrossRef]
- Guo, Y.; Su, J.; Jiang, S.; Xu, Y.; Dou, B.; Li, T.; Zhu, J.; He, K. Transcriptomics and metabonomics study on the effect of exercise combined with curcumin supplementation on breast cancer in mice. Heliyon 2024, 10, e28807. [Google Scholar] [CrossRef]
- Sadeghian, S.; Kazemzadeh, Y.; Mohammadnejadpanah Kandi, Y.; Mirzayan Shanjani, S.; Sedaghati, S. Effect of Concomitant Use of Curcumin during Six Weeks of Aerobic Exercise on Antioxidant Indices of Liver Tissue in Mice with Induced Breast Cancer in the Doxorubicin Treatment Phase. Iran. J. Breast Dis. 2022, 15, 17–32. [Google Scholar] [CrossRef]
- Sadeghian, S.; Kazemzadeh, Y.; Mohammadnejadpanah Kandi, Y.; Mirzayan Shanjani, S.; Sedaghati, S. The effect of aerobic exercise with curcumin consumption on tissue apoptosis indices in the liver tissue of rats induced by breast cancer in the doxorubicin treatment phase: An experimental study. J. Rafsanjan Univ. Med. Sci. 2022, 21, 433–448. [Google Scholar] [CrossRef]
- Mirzayan Shanjani, S.; Benaifar, A.A. The Effect of Aerobic Exercise Training and Consumption of Curcumin Nano Micelles on the Expression Level of CASP3, CASP9, Bax and BCL2 Genes on Cardiac Tissues of Balb/C Mice with Induced Breast Cancer Treated with Doxorubicin. Iran. J. Breast Dis. 2023, 16, 67–83. [Google Scholar]
- Daryanoosh, F.; Zolfaghari, M.; Hashemi, S.M.; Jahromi, M.K.; Jalili, A.; Khazaei, H.; Ranjbar, K.; Amin, M.A.; Jahantigh, M.; Beluri, A. Synergistic effect of exercise training and curcumin supplementation on inflammation indices in overweight breast-cancer patients after adjuvant chemotherapy and/or radiation therapy: A randomized controlled trial study. Sport. Sci. Health 2025, 21, 251–259. [Google Scholar] [CrossRef]
- Hemati, S.; Mehrabinejad, F.; Elhaie, M.; Najafizade, N. Curcumin Supplementation as a Preventive Strategy Against Tamoxifen-Induced Nonalcoholic Fatty Liver Disease in ER+ Breast Cancer Patients: A Triple-Blind Randomized Placebo-Controlled Trial. J. Diet. Suppl. 2025, 22, 274–283. [Google Scholar] [CrossRef]
- Barrilleaux, T.L. Effects of Quercetin and Exercise on Tumor Progression in a Breast Cancer Mouse Model. Master’s Thesis, University of South Carolina, Columbia, SC, USA, 2009. [Google Scholar]
- Jalali, Z.; Shahidi, F. Interactive Effect of 6 Weeks of Aerobic Exercise and Quercetin Supplementation on TIE-2 and VEGF-A Expression in Tumor Tissue of Female Mice with Breast Cancer. Iran. J. Breast Dis. 2021, 14, 46–56. [Google Scholar] [CrossRef]
- Mudge, J. Immunological Effects of Berberine and Physical Activity in a Murine Breast Cancer Model. Master’s Thesis, University of Northern Colorado, Greeley, CO, USA, 2022. [Google Scholar]
- Ma, W.; Zhang, Y.; Yu, M.; Wang, B.; Xu, S.; Zhang, J.; Li, X.; Ye, X. In-vitro and in-vivo anti-breast cancer activity of synergistic effect of berberine and exercise through promoting the apoptosis and immunomodulatory effects. Int. Immunopharmacol. 2020, 87, 106787. [Google Scholar] [CrossRef]
- Wang, B.; Xu, H.; Hu, X.; Ma, W.; Zhang, J.; Li, Y.; Yu, M.; Zhang, Y.; Li, X.; Ye, X. Synergetic inhibition of daidzein and regular exercise on breast cancer in bearing-4T1 mice by regulating NK cells and apoptosis pathway. Life Sci. 2020, 245, 117387. [Google Scholar] [CrossRef] [PubMed]
- Malekpoor, Z.; Taghian, F.; Jalali Dehkordi, K. The effect of aerobic exercise combined with the combination of gallic acid and kaempferol on neurogenesis caused by the side effects of paclitaxel in mice with breast cancer. J. Isfahan Med. Sch. 2023, 41, 427–437. [Google Scholar]
- Lee, W.Y.; Hsu, K.F.; Chiang, T.A.; Chen, C.J. Phellinus Linteus Extract Induces Autophagy and Synergizes with 5-Fluorouracil to Inhibit Breast Cancer Cell Growth. Nutr. Cancer 2015, 67, 275–284. [Google Scholar] [CrossRef]
- Sliva, D.; Jedinak, A.; Kawasaki, J.; Harvey, K.; Slivova, V. Phellinus linteus suppresses growth, angiogenesis and invasive behaviour of breast cancer cells through the inhibition of AKT signalling. Br. J. Cancer 2008, 98, 1348–1356. [Google Scholar] [CrossRef]
- Syukriya, A.; Bankeeree, W.; Prasongsuk, S.; Yanatatsaneejit, P. In vitro antioxidant and anticancer activities of Smilax corbularia extract combined with Phellinus linteus extract against breast cancer cell lines. Biomed. Rep. 2023, 19, 63. [Google Scholar] [CrossRef]
- Li, S.; Lei, Y.; Jia, Y.; Li, N.; Wink, M.; Ma, Y. Piperine, a piperidine alkaloid from Piper nigrum re-sensitizes P-gp, MRP1 and BCRP dependent multidrug resistant cancer cells. Phytomedicine 2011, 19, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Grinevicius, V.M.A.S.; Andrade, K.S.; Mota, N.S.R.S.; Bretanha, L.C.; Felipe, K.B.; Ferreira, S.R.S.; Pedrosa, R.C. CDK2 and Bcl-xL inhibitory mechanisms by docking simulations and anti-tumor activity from piperine enriched supercritical extract. Food Chem. Toxicol. 2019, 132, 10644. [Google Scholar] [CrossRef] [PubMed]
- Greenshields, A.L.; Doucette, C.D.; Sutton, K.M.; Madera, L.; Annan, H.; Yaffe, P.B.; Knickle, A.F.; Dong, Z.; Hoskin, D.W. Piperine inhibits the growth and motility of triple-negative breast cancer cells. Cancer Lett. 2015, 357, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Do, M.T.; Kim, H.G.; Choi, J.H.; Khanal, T.; Park, B.H.; Tran, T.P.; Jeong, T.C.; Jeong, H.G. Antitumor efficacy of piperine in the treatment of human HER2-overexpressing breast cancer cells. Food Chem. 2013, 141, 2591–2599. [Google Scholar] [CrossRef]
- Tripathi, A.K.; Ray, A.K.; Mishra, S.K. Molecular and pharmacological aspects of piperine as a potential molecule for disease prevention and management: Evidence from clinical trials. Beni Suef Univ. J. Basic. Appl. Sci. 2022, 11, 16. [Google Scholar] [CrossRef]
- Yang, R.; Gao, N.; Chang, Q.; Meng, X.; Wang, W. The role of IDO, IL-10, and TGF-β in the HCV-associated chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma. J. Med. Virol. 2019, 91, 265–271. [Google Scholar] [CrossRef]
- Sadri Nahand, J.; Bokharaei-Salim, F.; Salmaninejad, A.; Nesaei, A.; Mohajeri, F.; Moshtzan, A.; Tabibzadeh, A.; Karimzadeh, M.; Moghoofei, M.; Marjani, A.; et al. microRNAs: Key players in virus-associated hepatocellular carcinoma. J. Cell Physiol. 2019, 234, 12188–12225. [Google Scholar] [CrossRef]
- Kakarala, M.; Brenner, D.E.; Korkaya, H.; Cheng, C.; Tazi, K.; Ginestier, C.; Liu, S.; Dontu, G.; Wicha, M.S. Targeting breast stem cells with the cancer preventive compounds curcumin and piperine. Breast Cancer Res. Treat. 2010, 122, 777–785. [Google Scholar] [CrossRef]
- Delecroix, B.; Abaïdia, A.E.; Leduc, C.; Dawson, B.; Dupont, G. Curcumin and Piperine Supplementation and Recovery Following Exercise Induced Muscle Damage: A Randomized Controlled Trial. J. Sports Sci. Med. 2017, 16, 147–153. [Google Scholar]
- Alves-Santos, A.M.; Sugizaki, C.S.A.; Lima, G.C.; Naves, M.M.V. Prebiotic effect of dietary polyphenols: A systematic review. J. Funct. Foods 2020, 74, 104169. [Google Scholar] [CrossRef]
- Luo, Q.; Cheng, D.; Huang, C.; Li, Y.; Lao, C.; Xia, Y.; Liu, W.; Gong, X.; Hu, D.; Li, B.; et al. Improvement of Colonic Immune Function with Soy Isoflavones in High-Fat Diet-Induced Obese Rats. Molecules 2019, 24, 1139. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Han, X.; Zhan, J.; You, Y.; Huang, W. Vanillin Alleviates High Fat Diet-Induced Obesity and Improves the Gut Microbiota Composition. Front. Microbiol. 2018, 9, 2733. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Chen, S.; Zang, D.; Sun, H.; Sun, Y.; Chen, J. Butyrate as a promising therapeutic target in cancer: From pathogenesis to clinic (Review). Int. J. Oncol. 2024, 64, 44. [Google Scholar] [CrossRef] [PubMed]
- Stoeva, M.K.; Garcia-So, J.; Justice, N.; Myers, J.; Tyagi, S.; Nemchek, M.; McMurdie, P.J.; Kolterman, O.; Eid, J. Butyrate-producing human gut symbiont, Clostridium butyricum, and its role in health and disease. Gut Microbes 2021, 13, 1–28. [Google Scholar] [CrossRef]
- Laborda-Illanes, A.; Sanchez-Alcoholado, L.; Dominguez-Recio, M.E.; Jimenez-Rodriguez, B.; Lavado, R.; Comino-Méndez, I.; Alba, E.; Queipo-Ortuño, M.I. Breast and Gut Microbiota Action Mechanisms in Breast Cancer Pathogenesis and Treatment. Cancers 2020, 12, 2465. [Google Scholar] [CrossRef]
- Evans, C.C.; LePard, K.J.; Kwak, J.W.; Stancukas, M.C.; Laskowski, S.; Dougherty, J.; Moulton, L.; Glawe, A.; Wang, Y.; Leone, V.; et al. Exercise Prevents Weight Gain and Alters the Gut Microbiota in a Mouse Model of High Fat Diet-Induced Obesity. PLoS ONE 2014, 9, e92193. [Google Scholar] [CrossRef]
- Estaki, M.; Pither, J.; Baumeister, P.; Little, J.P.; Gill, S.K.; Ghosh, S.; Ahmadi-Vand, Z.; Marsden, K.R.; Gibson, D.L. Cardiorespiratory fitness as a predictor of intestinal microbial diversity and distinct metagenomic functions. Microbiome 2016, 4, 42. [Google Scholar] [CrossRef]
- Dou, X.; Fu, P.; Zhang, Y.; Zhang, Y.; Ning, K.; Yang, B.; Niu, Y.; Wang, D.E.; Xu, H. Gut microbiota-derived butyrate enhances exercise-induced bone mineral density in humans. Mechanobiol. Med. 2025, 3, 100124. [Google Scholar] [CrossRef]
- Campbell, K.L.; Winters-Stone, K.M.; Wiskemann, J.; May, A.M.; Schwartz, A.L.; Courneya, K.S.; Zucker, D.S.; Matthews, C.E.; Ligibel, J.A.; Gerber, L.H.; et al. Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Med. Sci. Sports Exerc. 2019, 51, 2375–2390. [Google Scholar] [CrossRef]
- Hayes, S.C.; Newton, R.U.; Spence, R.R.; Galvão, D.A. The Exercise and Sports Science Australia position statement: Exercise medicine in cancer management. J. Sci. Med. Sport. 2019, 22, 1175–1199. [Google Scholar] [CrossRef]
- Friedenreich, C.M.; Neilson, H.K.; Farris, M.S.; Courneya, K.S. Physical Activity and Cancer Outcomes: A Precision Medicine Approach. Clin. Cancer Res. 2016, 22, 4766–4775. [Google Scholar] [CrossRef] [PubMed]
- Physical Activity Guidelines Advisory Committee. Physical Activity Guidelines Advisory Committee Scientific Report; US Public Health Service: Washington, DC, USA, 2018. [Google Scholar]

| Authors, Year | Design | Sample Size | Assessment of Physical Activity | Main Results |
|---|---|---|---|---|
| Kehm et al. [51], 2020 | Prospective cohort study | N = 15.550 women (6.503 families); 659 BRCA1, 526 BRCA2 carriers; 59% premenopausal | Physical activity questionnaire; METs estimation (hours/week). | Recreational physical activity in adulthood (highest 4 quintiles vs. lowest) associated with 20% lower BC risk (HR = 0.80). No association for adolescent activity. Effects not modified by familial risk or BRCA mutation status. |
| Lammert et al. [52], 2018 | Case–control study | N = 443 matched pairs of BRCA mutation carriers | Nurses’ Health Study II Physical Activity Questionnaire; METs estimation (hours/week): overall (ages 12–34), adolescence (ages 12–17), early adulthood (ages 18–34). | No association between total physical activity and BC risk overall. Moderate physical activity at ages 12–17 associated with 38% lower risk of premenopausal BC (OR = 0.62). No association with postmenopausal BC. |
| Grill et al. [53], 2017 | Randomized, prospective study | N = 68 mutation carriers; BRCA1 (61.8%) and BRCA2 (32.2%) | Interviews and questionnaires: type of physical activity, hours per week, and participation in sports. | Higher adolescent physical activity linked to lower cancer prevalence (p = 0.019); smoking status associated with cancer (p < 0.001); diseased carriers had lower BMI (p = 0.079) and lower physical activity than non-diseased carriers (p = 0.046). |
| Pijpe et al. [54], 2010 | Nationwide retrospective cohort study | Ν = 725, including 218 diagnosed with BC within the past 10 years | Lifetime sport type, weekly hours, age at practice (≥6 months, ≥1 h/wk.); METs assigned per Ainsworth et al. [55] for intensity analysis. | Sports activity linked to reduced BC risk in BRCA1/2 carriers. Medium lifetime intensity (11–22.7 MET-h/week) showed significant risk reduction (HR = 0.59). Strongest inverse association seen for activity before age 30 (HR = 0.58–0.60). No clear dose–response relationship; post-30 activity also protective (HR = 0.63). |
| Friedenreich et al. [56], 2009 | Prospective cohort study | Ν = 1.231 BC cases (1995–1997) followed ≥8.3 yrs for progression, recurrence, and new primaries | In-person baseline interviews; METs assigned per Ainsworth et al. [57] for intensity analysis. | Average total physical activity: 126 MET-h/week. Higher recreational physical activity was associated with lower BC death risk (HR = 0.54), especially at moderate (0.56) and vigorous intensity (0.74). Moderate-intensity recreational activity also reduced the risk of recurrence, progression, or new primary cancer (0.66). No association was observed between other physical activity types and BC survival. Recreational activity before diagnosis was particularly protective. |
| Nkondjock et al. [58], 2006 | Case–control study | N = 80 French-Canadian families (250 members): 89 BRCA carriers with BC, 48 unaffected carriers | Interviews for physical activity assessment; METs assigned per Ainsworth et al. [57] for intensity analysis. FFQ to ascertain dietary intake. | Higher total energy intake linked to increased BRCA-related BC risk (OR = 2.76); no link found for other nutrients. Greater weight gain since age 18 (OR = 4.64) and 30 (OR = 4.11) and older age at max BMI (OR = 2.90) were also associated with higher risk. No association with BMI, smoking, or physical activity. |
| King et al. [59], 2003 | Cross-sectional study | N = 1008 index cases | Questionnaire: categorized as active vs. inactive during adolescence and young adulthood. | Lifetime BC risk among female mutation carriers was 82%; risk by age 50 increased from 24% (born < 1940) to 67% (born ≥ 1940). Ovarian cancer risk: 54% (BRCA1), 23% (BRCA2). Adolescent exercise and low obesity are linked to delayed onset. |
| Biomarker | Exercise Response | Clinical Utility |
|---|---|---|
| CRP | ↓ with aerobic | Monitor systemic inflammation |
| miR-133a | ↑ with resistance | Predict muscle strength adaptations |
| Estrogen | ↓ with aerobic (dose–response) | Modulate BC risk Target for prevention |
| SHBG | ↑ with aerobic (via improved insulin sensitivity) | Reduces bioavailable estrogens Protective role in menopause |
| Testosterone/Androgens | ↓ with aerobic and resistance training | Reduce pro-cancer signaling |
| Cortisol | Varied response ↓ with chronic exercise | Modulates HPA axis and inflammation |
| Insulin | ↓ with aerobic and resistance training (weight loss required) | Reduce mitogenic signaling and insulin resistance |
| IGF-1 | ↓ with aerobic and resistance training (reduced cancer signaling) | Downregulate growth-promoting pathways |
| miR-486-5p | ↑ with exercise | Enhances anti-tumor immunity Potential non-invasive market for BC prognosis |
| miR-342-5p | ↑ with exercise (miR-342-5p targets HER2, modulates tumor microenvironment) | Potential target for HER2 + BC therapy |
| Irisin | ↑ with exercise | Reduces BC cell viability, promotes apoptosis Potential therapeutic and chemosensitizer in BC |
| VEGF | ↓ with aerobic and interval training | Modulates angiogenesis Potential marker for tumor vascular response |
| SOD-2 | ↑ expression, ↓ promoter methylation with aerobic training | Enhances antioxidant defense Potential biomarker for oxidative stress response in BC |
| HIF-1α | ↓ with aerobic and interval training | Inhibits angiogenesis Marker of tumor hypoxia modulation |
| BCL-2 | ↓ with aerobic exercise | Pro-apoptotic shift Potential marker of exercise-induced tumor suppression |
| miR-206 | ↑ with combined exercise and hormone therapy | Tumor suppression miRNA Involved in proliferation and apoptosis regulation Anti-angiogenesis effects |
| let-7a | ↑ with exercise and hormone therapy | Tumor suppression miRNA Regulates oncogenes and inhibits cancer cell growth Anti-angiogenesis effects |
| miR-21 (oncomiR) | ↓ with exercise and hormone therapy | Ongogenic miRNA Reduction linked to decreased proliferation and metastasis |
| Authors, Year | Design | Sample Size | Exercise Intervention (Type, Duration, Frequency and Intensity) | Main Results |
|---|---|---|---|---|
| Northey et al. [161], 2019 | Pilot RCT | 17 BC survivors randomized to HIIT (n = 6), MOD (n = 5), or control (n = 6). | HIIT and MOD groups trained on a cycle ergometer for 3/week for 12 weeks. MOD: 55–65% peak power; HIIT: 105% peak power (90% HRmax) with self-selected active recovery. | All 17 completed follow-up. Adherence was similar (HIIT: 78.7%, MOD: 79.4%). No significant cognitive/cerebrovascular differences, but HIIT showed moderate–large positive effects on memory, executive function, and cerebral blood flow. VO2peak ↑ 19.3% (HIIT, d = 1.28); MOD ↑ 5.6% (d = 0.72); control ↓ 2.6%. |
| Cešeiko et al. [162], 2019 | RCT | 55 BC patients (stage I–III) randomized to training or control group | Training group performed maximal strength training at 85–90% 1RM, 2×/week for 3 months; control group received standard care without strength training. | Training group ↑ 1RM by 20.4 kg (20%, p = 0.001, d = 0.9); control ↓ 8.9 kg (9%, p = 0.001, d = 0.5). QoL ↑ 13% in training (p = 0.002, d = 0.6), no change in control. Fatigue ↓ 24% in training (p = 0.03, d = 0.6); ↑ 25% in control (p = 0.02, d = 0.4). Significant between-group differences (p ≤ 0.01, d = 0.6–0.9). |
| Odynets et al. [163], 2019 | RCT | 115 BC patients were randomly allocated to water exercise (Group A, n = 45), Pilates exercise (Group B, n = 40), and yoga exercise (Group C, n = 30) interventions | All 3 groups attended 144 rehab sessions over 1 year (3×/week). Pilates intensity: 45–60% HRR | QoL improved in all groups. Group A showed higher emotional well-being vs. Group B (+1.40, p < 0.05) and Group C (+1.69, p < 0.01), and higher BC subscale scores vs. Group B (+2.15, p < 0.05). Group C scored higher in social/family well-being vs. Group A (+2.80, p < 0.01). |
| Pasyar et al. [160], 2019 | RCT | 40 women with BC-related lymphedema were randomized to intervention or control group | Intervention: 8-week yoga program (2×/week instructor-led, 1×/week home practice) | Post-intervention, QoL improved in the yoga group: role functioning (4 weeks, p = 0.03), physical and emotional functioning (8 weeks, p < 0.05); trends showed increased cognitive, role, physical, and emotional functioning, and reduced fatigue, pain, insomnia, and financial difficulty. No significant change in edema volume. |
| Scott et al. [164], 2020 | RCT | 117 BC survivors | Exercise interventions followed linear (LET: 70% VO2peak, fixed intensity, 160 min/week) or nonlinear (NLET: 55–95% VO2peak, variable intensity, ~120 min/week) dosing, 3–4×/week for 16 weeks, vs. a matched stretching control. | No serious adverse events. Attendance: 64% (control), 75% (LET), 80% (NLET). VO2peak ↑ 0.6 ± 1.7 (p = 0.05, LET) and 0.8 ± 1.8 (p = 0.07, NLET) vs. control. Range: −2.7 to 4.1 (LET), −3.6 to 5.1 (NLET). ~40% were VO2peak responders (Δ ≥ 1.32). NLET improved all patient-reported outcomes vs. control |
| Cešeiko et al. [165], 2020 | RCT | 55 stage I–III BC patients, scheduled for adjuvant therapy, were randomized to maximal strength training or control. | Maximal strength training group: 4 × 4 leg press at 85–90% 1RM, 2×/week for 12 weeks | Maximal strength training group improved 1RM (+20 ± 8%), walking economy (+9 ± 8%), time to exhaustion (+9 ± 8%), 6MWD (+10 ± 7%), chair rise (+30 ± 20%), and stair climb (+12 ± 7%; all p < 0.001); control declined in all. They also maintained quadriceps mass (−7 ± 10% in control, p < 0.001). Changes in 1RM strongly correlated with functional gains (r = 0.75–0.81, p < 0.001) |
| Basha et al. [158], 2022 | RCT | 60 patients with BC-related lymphedema were randomly divided into two groups: the Xbox Kinect group received VR Kinect-based games (n = 30) and resistance exercise group received resistance training (n = 30). | Intervention: 5 sessions/week for 8 weeks | Xbox Kinect group showed greater improvements in pain intensity (VAS), upper limb disability (DASH), shoulder range of motion (p < 0.001), bodily pain (p = 0.002), general health (p < 0.001), and vitality (p = 0.006). Resistance exercise group showed greater gains in shoulder flexion (p = 0.002), external rotation (p = 0.004), abduction, and handgrip strength (p < 0.001) |
| Park et al. [159], 2023 | RCT | 20 patients with BC-related lymphedema were randomized to progressive resistance exercise group or self-home resistance exercise group | Both groups performed Thera-band resistance exercises. Sessions were 50 min, 3×/week for 6 weeks. Same investigator rated pre/post outcomes. | No significant intergroup difference in edema volume, but significant intragroup change. Both groups showed significant inter- and intragroup improvements in handgrip strength and upper extremity function. QoL improved significantly in progressive resistance exercise group (global health, physical, role, cognitive function, dyspnea); role function and global QoL differed significantly between groups. No QoL changes in self-home resistance exercise group. |
| Soriano-Maldonado et al. [166,167], 2023 | RCT | 60 BC survivors were randomized to intervention or control group | Experimental group: 24 resistance training sessions over 12 weeks (2 weeks individual + 10 weeks micro-group) at 50%–65% HRR + 10,000 steps/day. Control group: 10,000 steps/day only. | 32 participants in resistance training group (29 ≥ 75% attendance), 28 in control (all completed). Resistance training showed significantly greater gains in full-body strength vs. control (Δ = 0.718; p < 0.001, d = 1.04), consistent across upper- (Δ = 0.727) and lower-body (Δ = 0.709) strength. No significant effects on cardiorespiratory fitness, shoulder flexion, fatigue, depression, QoL, or life satisfaction. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michou, V.; Zervoudis, S.; Eskitzis, P.; Tsamos, G.; Vasdeki, D.; Vouxinou, A.; Markja, A.; Iatrakis, G. Exercise Interventions in Breast Cancer: Molecular Mechanisms, Physical Benefits, and Practical Recommendations. Medicina 2025, 61, 1167. https://doi.org/10.3390/medicina61071167
Michou V, Zervoudis S, Eskitzis P, Tsamos G, Vasdeki D, Vouxinou A, Markja A, Iatrakis G. Exercise Interventions in Breast Cancer: Molecular Mechanisms, Physical Benefits, and Practical Recommendations. Medicina. 2025; 61(7):1167. https://doi.org/10.3390/medicina61071167
Chicago/Turabian StyleMichou, Vasiliki, Stefanos Zervoudis, Panagiotis Eskitzis, Georgios Tsamos, Dimitra Vasdeki, Andriani Vouxinou, Anisa Markja, and Georgios Iatrakis. 2025. "Exercise Interventions in Breast Cancer: Molecular Mechanisms, Physical Benefits, and Practical Recommendations" Medicina 61, no. 7: 1167. https://doi.org/10.3390/medicina61071167
APA StyleMichou, V., Zervoudis, S., Eskitzis, P., Tsamos, G., Vasdeki, D., Vouxinou, A., Markja, A., & Iatrakis, G. (2025). Exercise Interventions in Breast Cancer: Molecular Mechanisms, Physical Benefits, and Practical Recommendations. Medicina, 61(7), 1167. https://doi.org/10.3390/medicina61071167










