A Novel Magnetic Resonance Imaging-Based Radiomics and Clinical Predictive Model for the Regrowth of Postoperative Residual Tumor in Non-Functioning Pituitary Neuroendocrine Tumor
Abstract
:1. Introduction
2. Materials and Methods
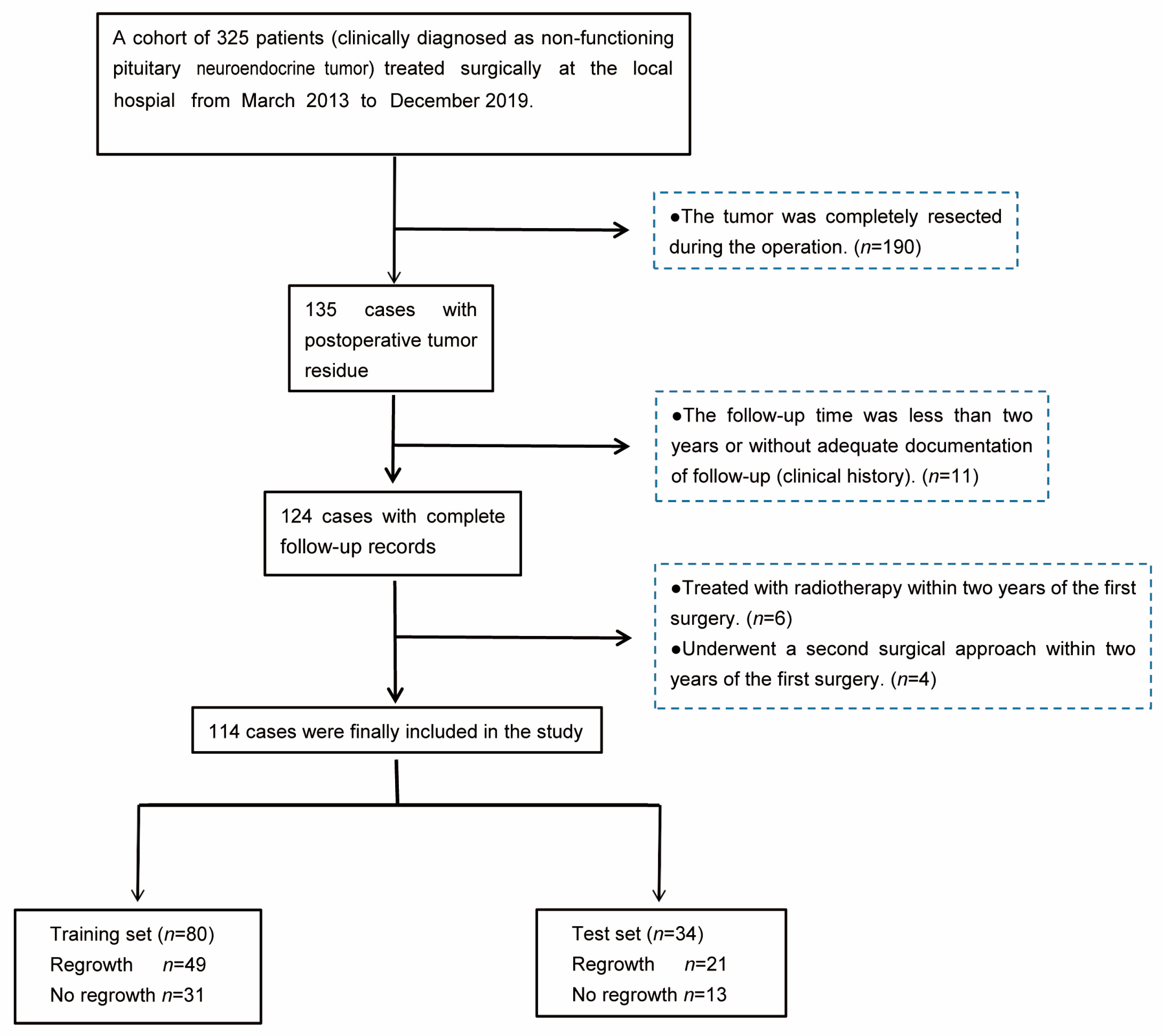
2.1. Patient Selection
2.2. Clinical Characteristics and Definitions
2.3. Image Acquisition
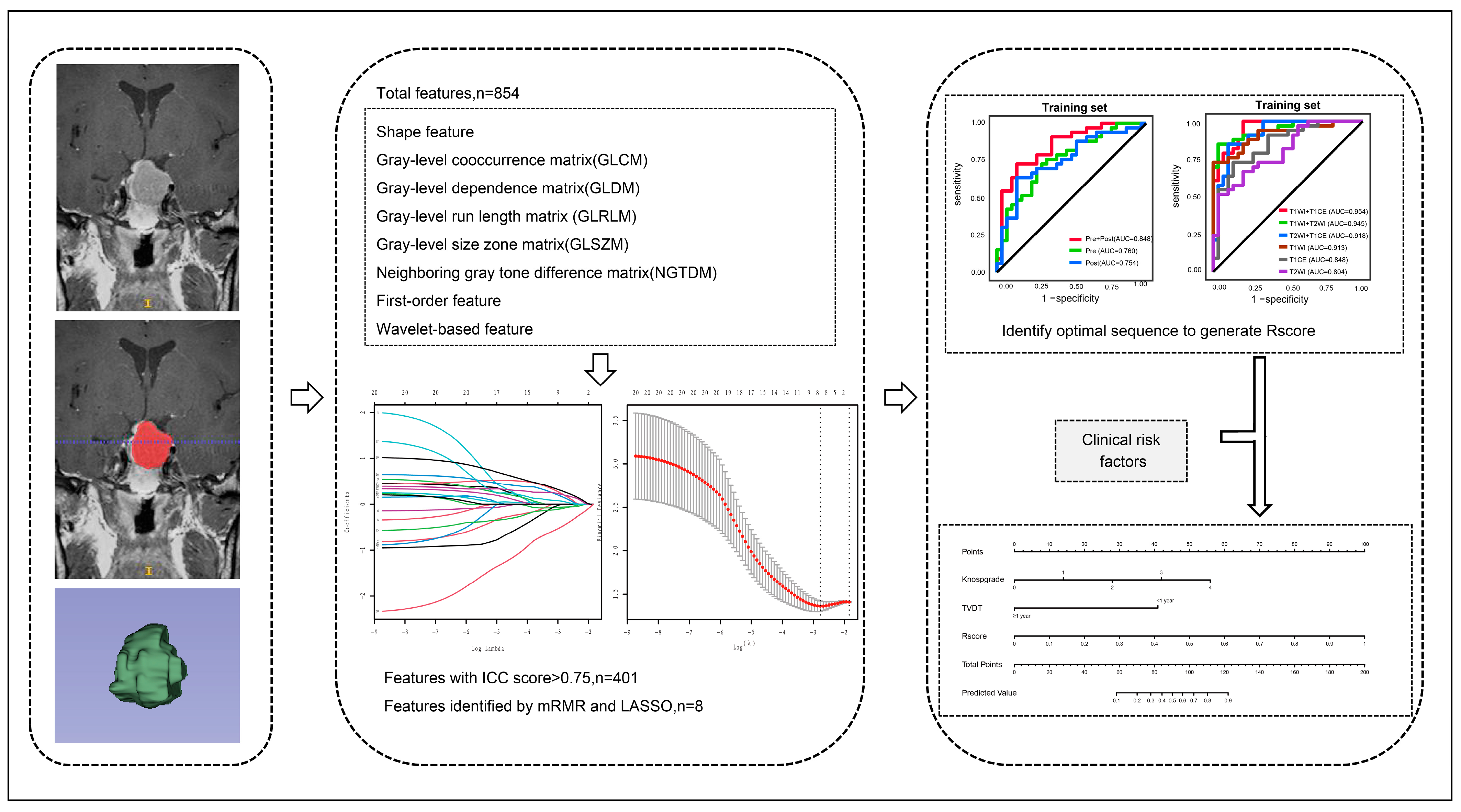
2.4. Image Segmentation and Feature Extraction
2.5. The Establishment and Validation of a Radiomics–Clinical Model
2.6. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
3.2. Radiomic Feature Selection
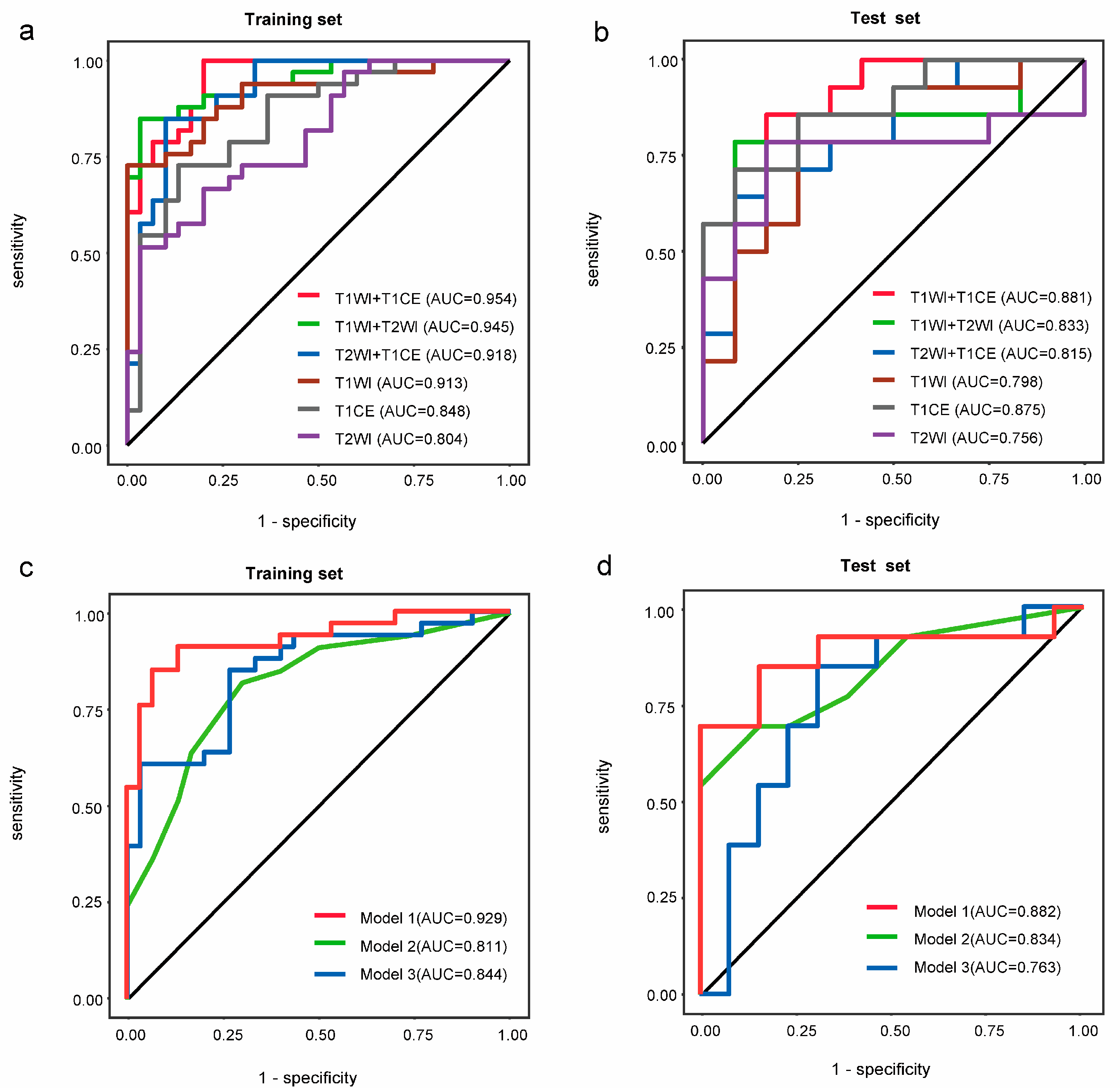
3.3. Construction of the Radiomics–Clinical Model
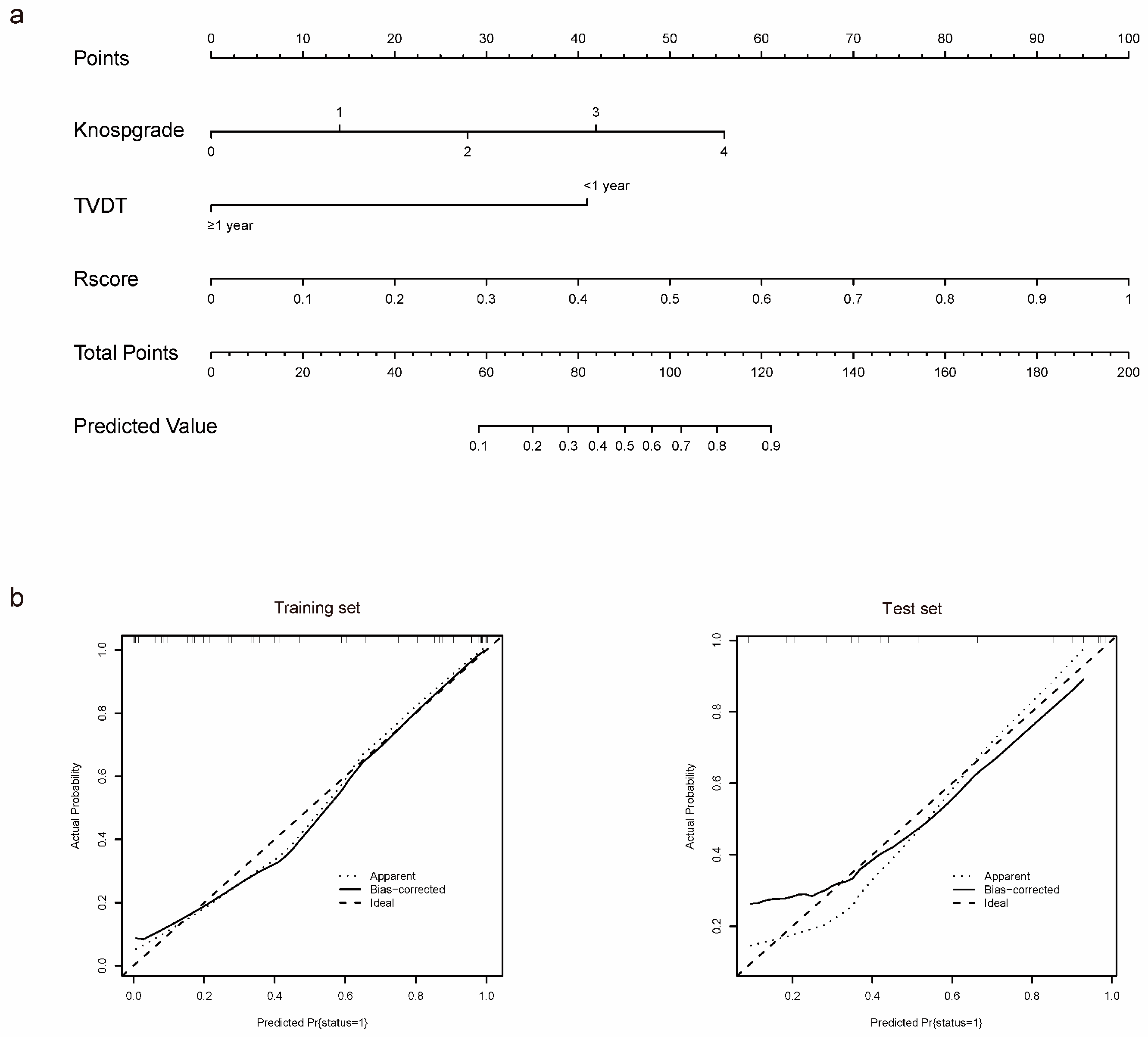
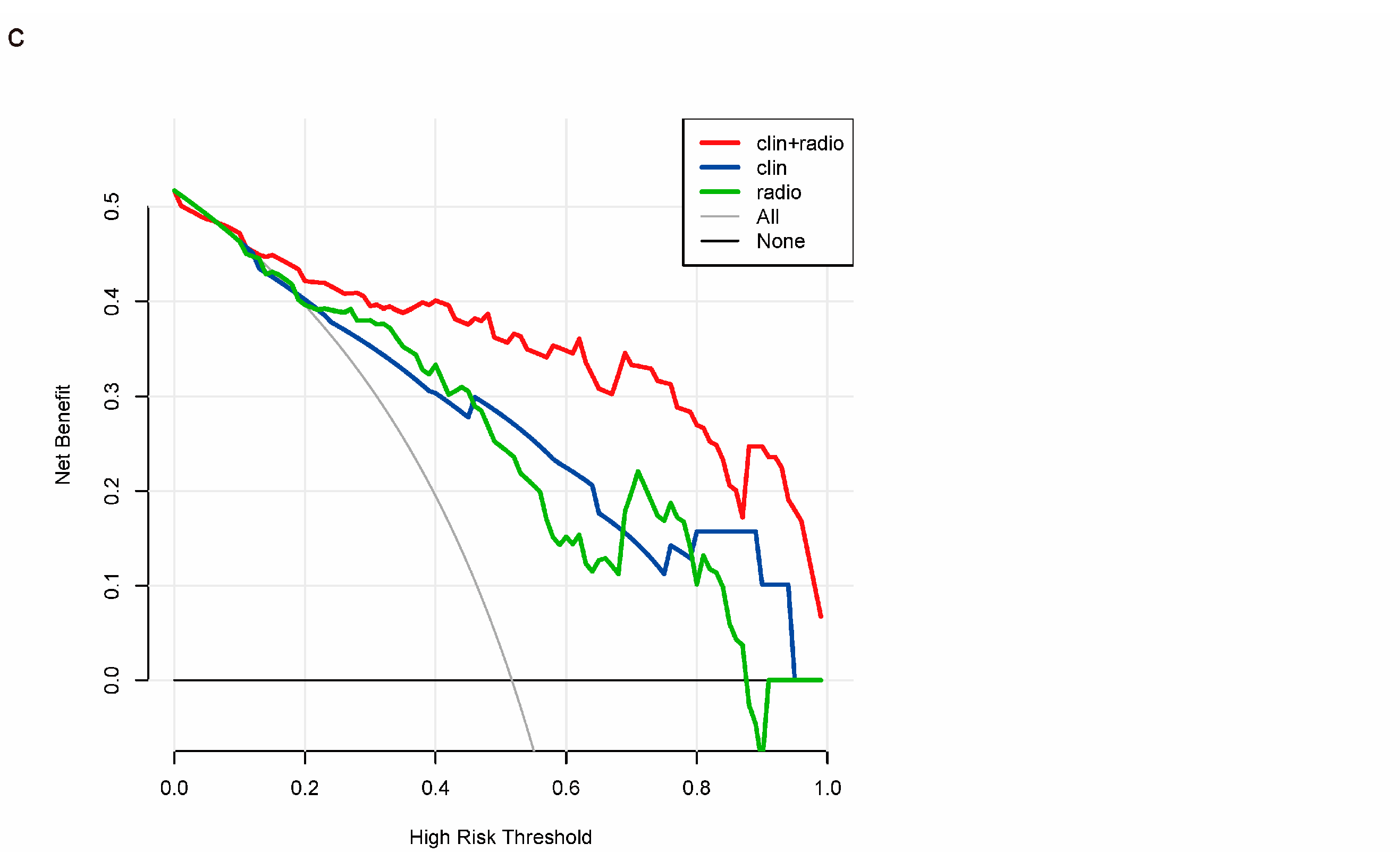
3.4. Individualized Nomogram Construction and Validation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Chen, Y.; Wang, C.D.; Su, Z.P.; Chen, Y.X.; Cai, L.; Zhuge, Q.C.; Wu, Z.B. Natural history of postoperative nonfunctioning pituitary adenomas: A systematic review and meta-analysis. Neuroendocrinology 2012, 96, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Price, M.; Neff, C.; Cioffi, G.; Waite, K.A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2015–2019. Neuro-Oncology 2022, 24 (Suppl. S5), v1–v95. [Google Scholar] [CrossRef]
- Ntali, G.; Wass, J.A. Epidemiology, clinical presentation and diagnosis of non-functioning pituitary adenomas. Pituitary 2018, 21, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Wu, J.; Li, C.; Li, Y.; Liu, C.; Li, G.; Li, W.; Hu, S.; Ying, X.; Zhang, Y. Predicting the regrowth of clinically non-functioning pituitary adenoma with a statistical model. J. Transl. Med. 2019, 17, 164. [Google Scholar] [CrossRef] [PubMed]
- Penn, D.L.; Burke, W.T.; Laws, E.R. Management of non-functioning pituitary adenomas: Surgery. Pituitary 2018, 21, 145–153. [Google Scholar] [CrossRef]
- Brochier, S.; Galland, F.; Kujas, M.; Parker, F.; Gaillard, S.; Raftopoulos, C.; Young, J.; Alexopoulou, O.; Maiter, D.; Chanson, P. Factors predicting relapse of nonfunctioning pituitary macroadenomas after neurosurgery: A study of 142 patients. Eur. J. Endocrinol. 2010, 163, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Roelfsema, F.; Biermasz, N.R.; Pereira, A.M. Clinical factors involved in the recurrence of pituitary adenomas after surgical remission: A structured review and meta-analysis. Pituitary 2012, 15, 71–83. [Google Scholar] [CrossRef]
- Batista, R.L.; Trarbach, E.B.; Marques, M.D.; Cescato, V.A.; da Silva, G.O.; Herkenhoff, C.G.B.; Cunha-Neto, M.B.; Musolino, N.R. Nonfunctioning Pituitary Adenoma Recurrence and Its Relationship with Sex, Size, and Hormonal Immunohistochemical Profile. World Neurosurg. 2018, 120, e241–e246. [Google Scholar] [CrossRef]
- Wiedmann, M.K.H.; Brunborg, C.; Di Ieva, A.; Lindemann, K.; Johannesen, T.B.; Vatten, L.; Helseth, E.; Zwart, J.A. Overweight, obesity and height as risk factors for meningioma, glioma, pituitary adenoma and nerve sheath tumor: A large population-based prospective cohort study. Acta Oncol. 2017, 56, 1302–1309. [Google Scholar] [CrossRef]
- Tanaka, Y.; Hongo, K.; Tada, T.; Sakai, K.; Kakizawa, Y.; Kobayashi, S. Growth pattern and rate in residual nonfunctioning pituitary adenomas: Correlations among tumor volume doubling time, patient age, and MIB-1 index. J. Neurosurg. 2003, 98, 359–365. [Google Scholar] [CrossRef]
- Monsalves, E.; Larjani, S.; Loyola Godoy, B.; Juraschka, K.; Carvalho, F.; Kucharczyk, W.; Kulkarni, A.; Mete, O.; Gentili, F.; Ezzat, S.; et al. Growth patterns of pituitary adenomas and histopathological correlates. J. Clin. Endocrinol. Metab. 2014, 99, 1330–1338. [Google Scholar] [CrossRef] [PubMed]
- O′Reilly, M.W.; Reulen, R.C.; Gupta, S.; Thompson, C.A.; Dineen, R.; Goulden, E.L.; Bugg, G.; Pearce, H.; Toogood, A.A.; Gittoes, N.J.; et al. ACTH and gonadotropin deficiencies predict mortality in patients treated for nonfunctioning pituitary adenoma: Long-term follow-up of 519 patients in two large European centres. Clin. Endocrinol. 2016, 85, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Langlois, F.; Lim, D.S.T.; Yedinak, C.G.; Cetas, I.; McCartney, S.; Cetas, J.; Dogan, A.; Fleseriu, M. Predictors of silent corticotroph adenoma recurrence; a large retrospective single center study and systematic literature review. Pituitary 2018, 21, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Luo, Y.; Kong, X.; Wan, T.; Long, Y.; Ma, J. A Preoperative MRI-Based Radiomics-Clinicopathological Classifier to Predict the Recurrence of Pituitary Macroadenoma Within 5 Years. Front Neurol. 2021, 12, 780628. [Google Scholar] [CrossRef] [PubMed]
- Steno, A.; Bocko, J.; Rychly, B.; Chorvath, M.; Celec, P.; Fabian, M.; Belan, V.; Šteňo, J. Nonfunctioning pituitary adenomas: Association of Ki-67 and HMGA-1 labeling indices with residual tumor growth. Acta Neurochir. 2014, 156, 451–461. [Google Scholar] [CrossRef]
- Matousek, P.; Buzrla, P.; Reguli, S.; Krajca, J.; Dvorackova, J.; Lipina, R. Factors That Predict the Growth of Residual Nonfunctional Pituitary Adenomas: Correlations between Relapse and Cell Cycle Markers. BioMed Res. Int. 2018, 2018, 1876290. [Google Scholar] [CrossRef]
- Gillies, R.J.; Kinahan, P.E.; Hricak, H. Radiomics: Images Are More than Pictures, They Are Data. Radiology 2016, 278, 563–577. [Google Scholar] [CrossRef]
- Zhang, S.; Song, G.; Zang, Y.; Jia, J.; Wang, C.; Li, C.; Tian, J.; Dong, D.; Zhang, Y. Non-invasive radiomics approach potentially predicts non-functioning pituitary adenomas subtypes before surgery. Eur. Radiol. 2018, 28, 3692–3701. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, Z.; Hou, B.; Li, L.; Liu, X.; Liu, Z.; Wang, R.; Lin, Y.; Feng, F.; Tian, J.; et al. Development and validation of an MRI-based radiomic signature for the preoperative prediction of treatment response in patients with invasive functional pituitary adenoma. Eur. J. Radiol. 2019, 121, 108647. [Google Scholar] [CrossRef]
- Zeynalova, A.; Kocak, B.; Durmaz, E.S.; Comunoglu, N.; Ozcan, K.; Ozcan, G.; Turk, O.; Tanriover, N.; Kocer, N.; Kizilkilic, O.; et al. Preoperative evaluation of tumour consistency in pituitary macroadenomas: A machine learning-based histogram analysis on conventional T2-weighted MRI. Neuroradiology 2019, 61, 767–774. [Google Scholar] [CrossRef]
- Zhang, Y.; Ko, C.C.; Chen, J.H.; Chang, K.T.; Chen, T.Y.; Lim, S.W.; Tsui, Y.-K.; Su, M.-Y. Radiomics Approach for Prediction of Recurrence in Non-Functioning Pituitary Macroadenomas. Front. Oncol. 2020, 10, 590083. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.W.; Eom, J.; Kim, S.; Kim, H.; Ahn, S.S.; Ku, C.R.; Kim, E.H.; Lee, E.J.; Kim, S.H.; Lee, S.-K. Radiomics With Ensemble Machine Learning Predicts Dopamine Agonist Response in Patients With Prolactinoma. J. Clin. Endocrinol. Metab. 2021, 106, e3069–e3077. [Google Scholar] [CrossRef] [PubMed]
- Wan, T.; Wu, C.; Meng, M.; Liu, T.; Li, C.; Ma, J.; Qin, Z. Radiomic Features on Multiparametric MRI for Preoperative Evaluation of Pituitary Macroadenomas Consistency: Preliminary Findings. J. Magn. Reason. Imaging 2022, 55, 1491–1503. [Google Scholar] [CrossRef] [PubMed]
- Briet, C.; Salenave, S.; Bonneville, J.F.; Laws, E.R.; Chanson, P. Pituitary Apoplexy. Endocr. Rev. 2015, 36, 622–645. [Google Scholar] [CrossRef]
- De Vries, F.; Lobatto, D.J.; Verstegen, M.J.T.; van Furth, W.R.; Pereira, A.M.; Biermasz, N.R. Postoperative diabetes insipidus: How to define and grade this complication? Pituitary 2021, 24, 284–291. [Google Scholar] [CrossRef]
- Miwa, Y.; Sugawara, T.; Kobayashi, D.; Maehara, T. Tumor doubling time as preoperative predictor of malignancy and recurrence in newly diagnosed meningioma. Neurosurg. Rev. 2022, 45, 3683–3687. [Google Scholar] [CrossRef]
- Cuocolo, R.; Ugga, L.; Solari, D.; Corvino, S.; D′Amico, A.; Russo, D.; Cappabianca, P.; Cavallo, L.M.; Elefante, A. Prediction of pituitary adenoma surgical consistency: Radiomic data mining and machine learning on T2-weighted MRI. Neuroradiology 2020, 62, 1649–1656. [Google Scholar] [CrossRef]
- Pappy, A.L., 2nd; Savinkina, A.; Bicknese, C.; Neill, S.; Oyesiku, N.M.; Ioachimescu, A.G. Predictive modeling for pituitary adenomas: Single center experience in 501 consecutive patients. Pituitary 2019, 22, 520–531. [Google Scholar] [CrossRef]
- Niu, J.; Zhang, S.; Ma, S.; Diao, J.; Zhou, W.; Tian, J.; Zang, Y.; Jia, W. Preoperative prediction of cavernous sinus invasion by pituitary adenomas using a radiomics method based on magnetic resonance images. Eur. Radiol. 2019, 29, 1625–1634. [Google Scholar] [CrossRef]
- Zhao, Z.; Xiao, D.; Nie, C.; Zhang, H.; Jiang, X.; Jecha, A.R.; Yan, P.; Zhao, H. Development of a Nomogram Based on Preoperative Bi-Parametric MRI and Blood Indices for the Differentiation Between Cystic-Solid Pituitary Adenoma and Craniopharyngioma. Front. Oncol. 2021, 11, 709321. [Google Scholar] [CrossRef]
- Losa, M.; Mortini, P.; Barzaghi, R.; Ribotto, P.; Terreni, M.R.; Marzoli, S.B.; Pieralli, S.; Giovanelli, M. Early results of surgery in patients with nonfunctioning pituitary adenoma and analysis of the risk of tumor recurrence. J. Neurosurg. 2008, 108, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Raverot, G.; Vasiljevic, A.; Jouanneau, E. Prognostic factors of regrowth in nonfunctioning pituitary tumors. Pituitary 2018, 21, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Watts, A.K.; Easwaran, A.; McNeill, P.; Wang, Y.Y.; Inder, W.J.; Caputo, C. Younger age is a risk factor for regrowth and recurrence of nonfunctioning pituitary macroadenomas: Results from a single Australian centre. Clin. Endocrinol. 2017, 87, 264–271. [Google Scholar] [CrossRef] [PubMed]
| Variables (N = 114) | Training Set (N = 80) | Test Set (N = 34) | ||||
|---|---|---|---|---|---|---|
| Residual | No Residual Regrowth (N = 31) | p-Value | Residual | No Residual Regrowth | p-Value | |
| Regrowth (N = 49) | Regrowth (N = 21) | (N = 13) | ||||
| Gender, n (%) | 0.935 | 0.481 | ||||
| Male | 28 (57.1%) | 18 (58.1%) | 12 (57.1%) | 9 (69.2%) | ||
| Female | 21 (42.9%) | 13 (41.9%) | 9 (42.9%) | 4 (30.8%) | ||
| Age (years) | 54 (41.5–66) | 51 (41–66) | 0.771 | 51 (44–70) | 46 (36–61) | 0.103 |
| Weight (kg) | 64.0 (59.0–71.5) | 64.0 (58.0–69.0) | 0.909 | 60.0 (53.0–65.0) | 62.0 (58.0–72.0) | 0.166 |
| Height (m) | 1.65 ± 0.07 | 1.62 ± 0.06 | 0.103 | 1.63 ± 0.08 | 1.65 ± 0.07 | 0.363 |
| BMI | 23.66 (21.08–26.34) | 23.95 (22.04–26.57) | 0.295 | 22.04 (21.22–23.95) | 22.17 (20.00–25.96) | 0.818 |
| Headache, n (%) | 0.009 * | 0.004 * | ||||
| Yes | 29 (59.2%) | 9 (29.0%) | 14 (66.7%) | 2 (15.4%) | ||
| No | 20 (40.8%) | 22 (71.0%) | 7 (33.3%) | 11 (84.6%) | ||
| Vision changes, n (%) | 0.444 | 0.727 | ||||
| Yes | 28 (56.2%) | 15 (48.4%) | 13 (61.9%) | 9 (69.2%) | ||
| No | 21 (43.8%) | 16 (51.6%) | 8 (38.1%) | 4 (30.8%) | ||
| Pituitary apoplexy, n (%) | 0.931 | 0.498 | ||||
| Yes | 6 (12.2%) | 4 (12.9%) | 2 (9.5%) | 2 (15.4%) | ||
| No | 43 (87.8%) | 27 (87.1%) | 19 (90.5%) | 11 (84.6%) | ||
| Knosp grade, n (%) | <0.001 * | 0.005 * | ||||
| 0 | 2 (4.1%) | 12 (38.7%) | 1 (4.8%) | 7 (53.8%) | ||
| 1 | 6 (12.2%) | 8 (25.8%) | 3 (14.3%) | 2 (15.4%) | ||
| 2 | 11 (22.4%) | 5 (16.1%) | 2 (9.5%) | 2 (15.4%) | ||
| 3 | 11 (22.4%) | 4 (12.9%) | 5 (23.8%) | 1 (7.7%) | ||
| 4 | 19 (38.9%) | 2 (6.5%) | 10 (47.6%) | 1 (7.7%) | ||
| Cystic, n (%) | 0.433 | 0.427 | ||||
| Yes | 15 (30.6%) | 7 (22.6%) | 7 (33.3%) | 2 (15.4%) | ||
| No | 34 (69.4%) | 24 (77.4%) | 14 (66.7%) | 11 (84.6%) | ||
| Hardy grade, n (%) | 0.54 | 0.191 | ||||
| 0 | 16 (32.6%) | 6 (19.4%) | 4 (19.0%) | 1 (7.7%) | ||
| I | 19 (38.8%) | 14 (45.2%) | 8 (38.1%) | 4 (30.8%) | ||
| II | 10 (20.4%) | 9 (29.0%) | 4 (19.0%) | 6 (46.2%) | ||
| III | 2 (4.1%) | 0 (0%) | 4 (19.0%) | 0 (0%) | ||
| IV | 2 (4.1%) | 2 (6.4%) | 1 (4.9%) | 2 (15.3%) | ||
| TVDT, n (%) | 0.002 * | <0.001 * | ||||
| <1 year | 27 (55.1%) | 6 (19.4%) | 15 (71.4%) | 1 (7.7%) | ||
| ≥1 year | 22 (44.9%) | 25 (80.6%) | 6 (28.6%) | 12 (92.3%) | ||
| Surgical resection ratio (%) | 67.34 (16.25–98.58) | 69.68 (21.69–98.60) | 0.933 | 73.64 (37.10–98.01) | 80.81 (22.54–95.59) | 0.326 |
| Consistency, n (%) | 0.083 | 0.574 | ||||
| Soft | 28 (57.1%) | 21 (67.7%) | 14 (66.7%) | 11 (84.6%) | ||
| Medium | 7 (14.3%) | 0 (0%) | 2 (9.5%) | 0 (0%) | ||
| Hard | 14 (28.6%) | 10 (32.3%) | 5 (23.8%) | 2 (15.4%) | ||
| Residual position, n (%) | 0.349 | 0.894 | ||||
| Intrasellar | 20 (40.8%) | 15 (48.4%) | 10 (47.6%) | 5 (38.5%) | ||
| Suprasellar | 16 (32.7%) | 12 (38.7%) | 8 (38.1%) | 5 (38.5%) | ||
| Both | 13 (26.5%) | 4 (12.9%) | 3 (14.3%) | 3 (23.0%) | ||
| Postoperative T1 enhancement, n (%) | 0.029 | 0.21 | ||||
| Yes | 34 (69.4%) | 28 (90.3%) | 15 (71.4%) | 12 (92.3%) | ||
| No | 15 (30.6%) | 3 (9.7%) | 6 (28.6%) | 1 (7.7%) | ||
| Ki-67, n (%) | 0.132 | 0.21 | ||||
| <3% | 30 (61.2%) | 24 (77.4%) | 15 (71.4%) | 12 (92.3%) | ||
| ≥3% | 19 (38.8%) | 7 (22.6%) | 6 (28.6%) | 1 (7.7%) |
| Training Set (n = 80) | Test Set (n = 34) | |||||||
|---|---|---|---|---|---|---|---|---|
| AUC (95% CI) | ACC | SEN | SPE | AUC (95% CI) | ACC | SEN | SPE | |
| Model 1 | 0.929 (0.865, 0.993) | 0.888 | 0.848 | 0.933 | 0.882 (0.735, 1.000) | 0.823 | 0.818 | 0.833 |
| Model 2 | 0.811 (0.704, 0.918) | 0.762 | 0.818 | 0.700 | 0.834 (0.676, 0.992) | 0.794 | 0.800 | 0.785 |
| Model 3 | 0.844 (0.748, 0.941) | 0.787 | 0.848 | 0.733 | 0.763 (0.569, 0.958) | 0.735 | 0.764 | 0.705 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, C.; Liu, X.; Jin, J.; Han, C.; Wu, L.; Wu, Z.; Su, Z.; Chen, X. A Novel Magnetic Resonance Imaging-Based Radiomics and Clinical Predictive Model for the Regrowth of Postoperative Residual Tumor in Non-Functioning Pituitary Neuroendocrine Tumor. Medicina 2023, 59, 1525. https://doi.org/10.3390/medicina59091525
Shen C, Liu X, Jin J, Han C, Wu L, Wu Z, Su Z, Chen X. A Novel Magnetic Resonance Imaging-Based Radiomics and Clinical Predictive Model for the Regrowth of Postoperative Residual Tumor in Non-Functioning Pituitary Neuroendocrine Tumor. Medicina. 2023; 59(9):1525. https://doi.org/10.3390/medicina59091525
Chicago/Turabian StyleShen, Chaodong, Xiaoyan Liu, Jinghao Jin, Cheng Han, Lihao Wu, Zerui Wu, Zhipeng Su, and Xiaofang Chen. 2023. "A Novel Magnetic Resonance Imaging-Based Radiomics and Clinical Predictive Model for the Regrowth of Postoperative Residual Tumor in Non-Functioning Pituitary Neuroendocrine Tumor" Medicina 59, no. 9: 1525. https://doi.org/10.3390/medicina59091525
APA StyleShen, C., Liu, X., Jin, J., Han, C., Wu, L., Wu, Z., Su, Z., & Chen, X. (2023). A Novel Magnetic Resonance Imaging-Based Radiomics and Clinical Predictive Model for the Regrowth of Postoperative Residual Tumor in Non-Functioning Pituitary Neuroendocrine Tumor. Medicina, 59(9), 1525. https://doi.org/10.3390/medicina59091525







