Understanding the Role of Surface Modification of Randomized Trabecular Titanium Structures in Bone Tissue Regeneration: An Experimental Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design of the Study
- -
- Evaluation of a randomized trabecular titanium structure with the native surface versus a randomized trabecular titanium structure with the surface chemically treated, describing mechanical and morphological characteristics and cytocompatibility by means of an in vitro test and finally by a preclinical in vivo experiment in a sheep model.
- -
- Sample preparation;
- -
- Mechanical and morphological evaluation;
- -
- In vitro biological assay;
- -
- In vivo preclinical experiment.
2.2. Sample Preparation
2.2.1. Randomized Trabecular Titanium Structure with the Native Surface
2.2.2. Randomized Trabecular Titanium Structure with an Increased Micro-Roughness Surface
2.3. Mechanical and Morphological Evaluation
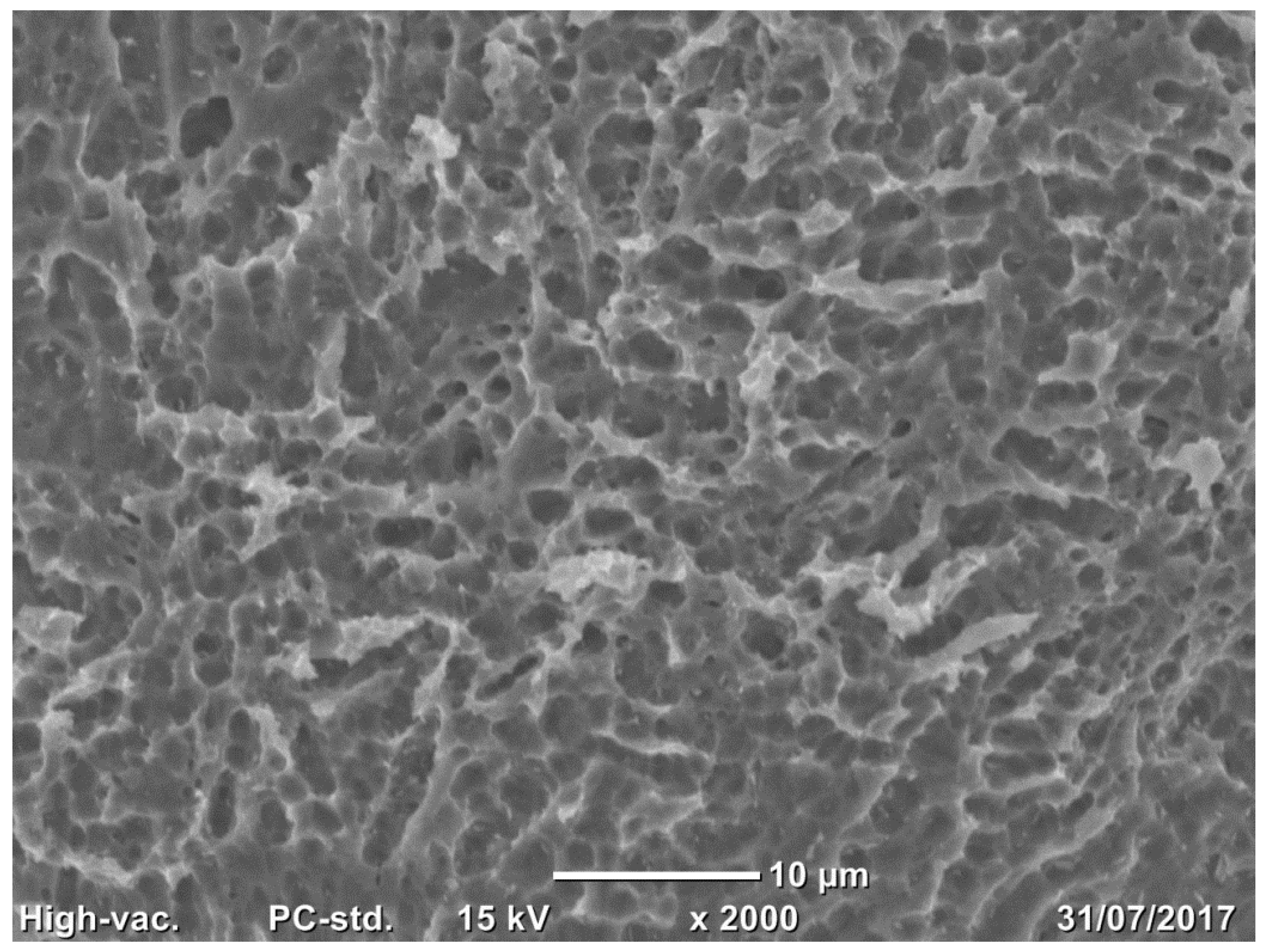
2.3.1. Scanning Electron Microscopy
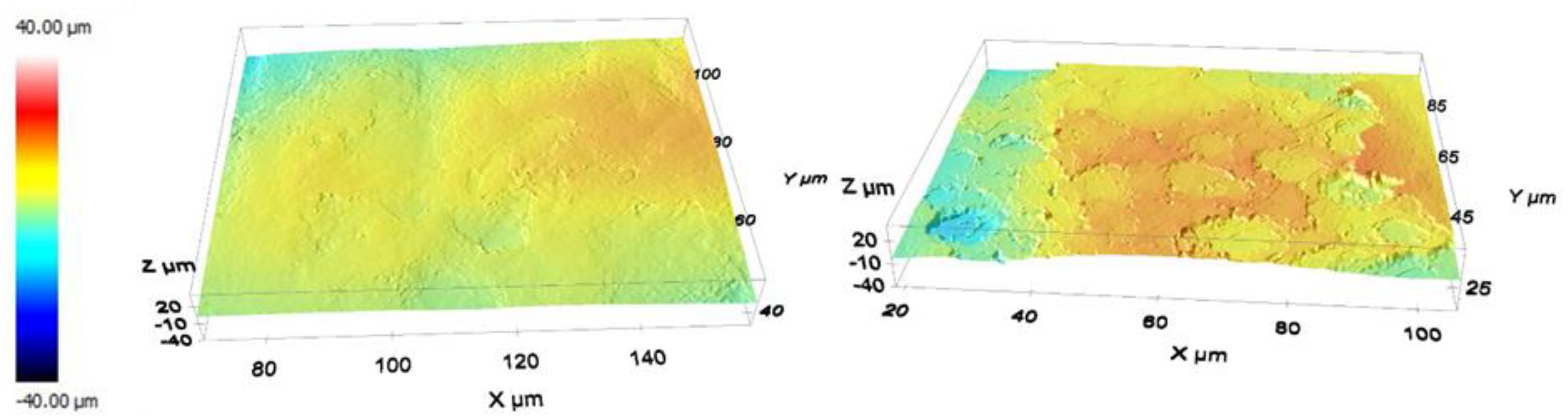
2.3.2. Surface Roughness Characterization
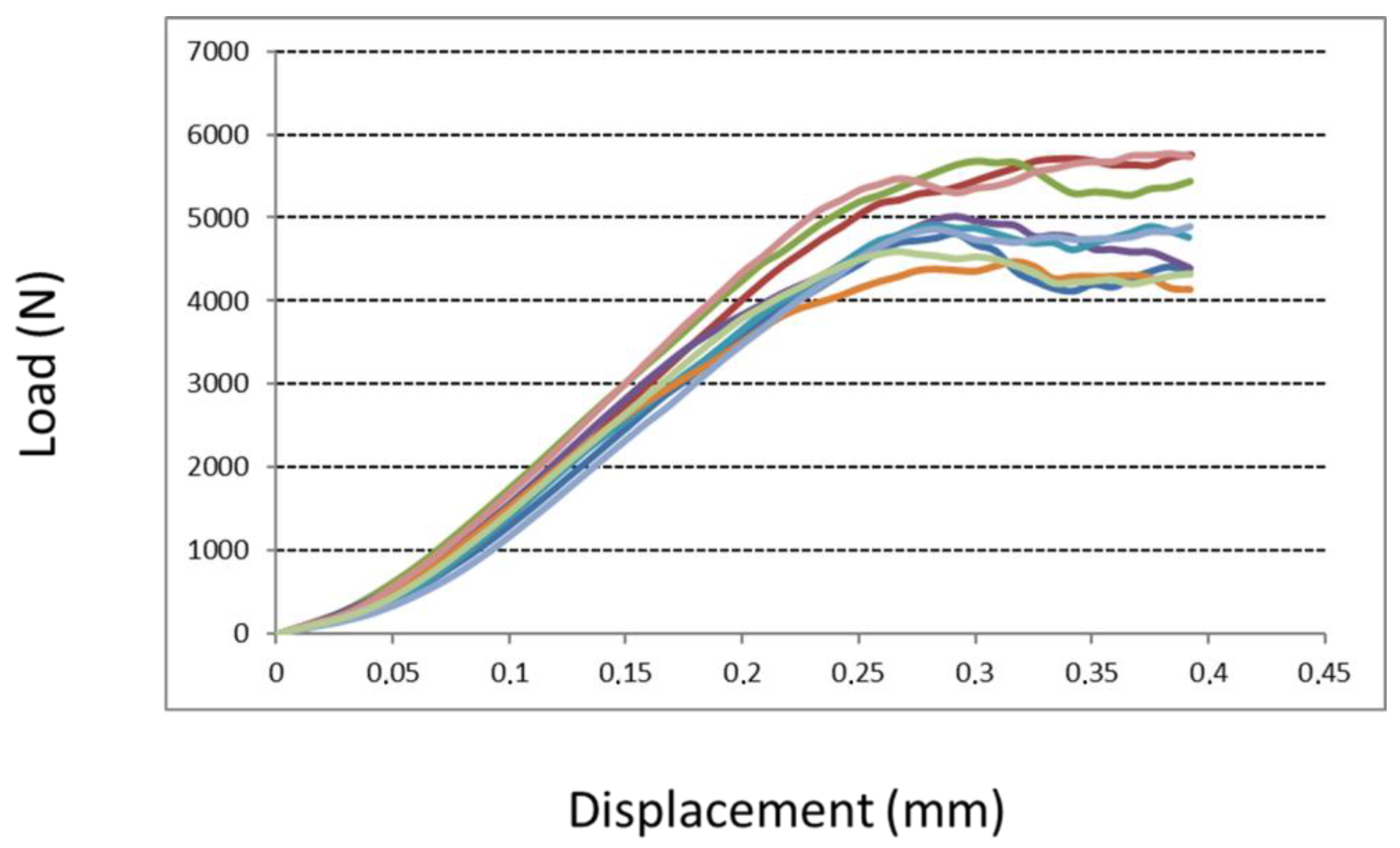
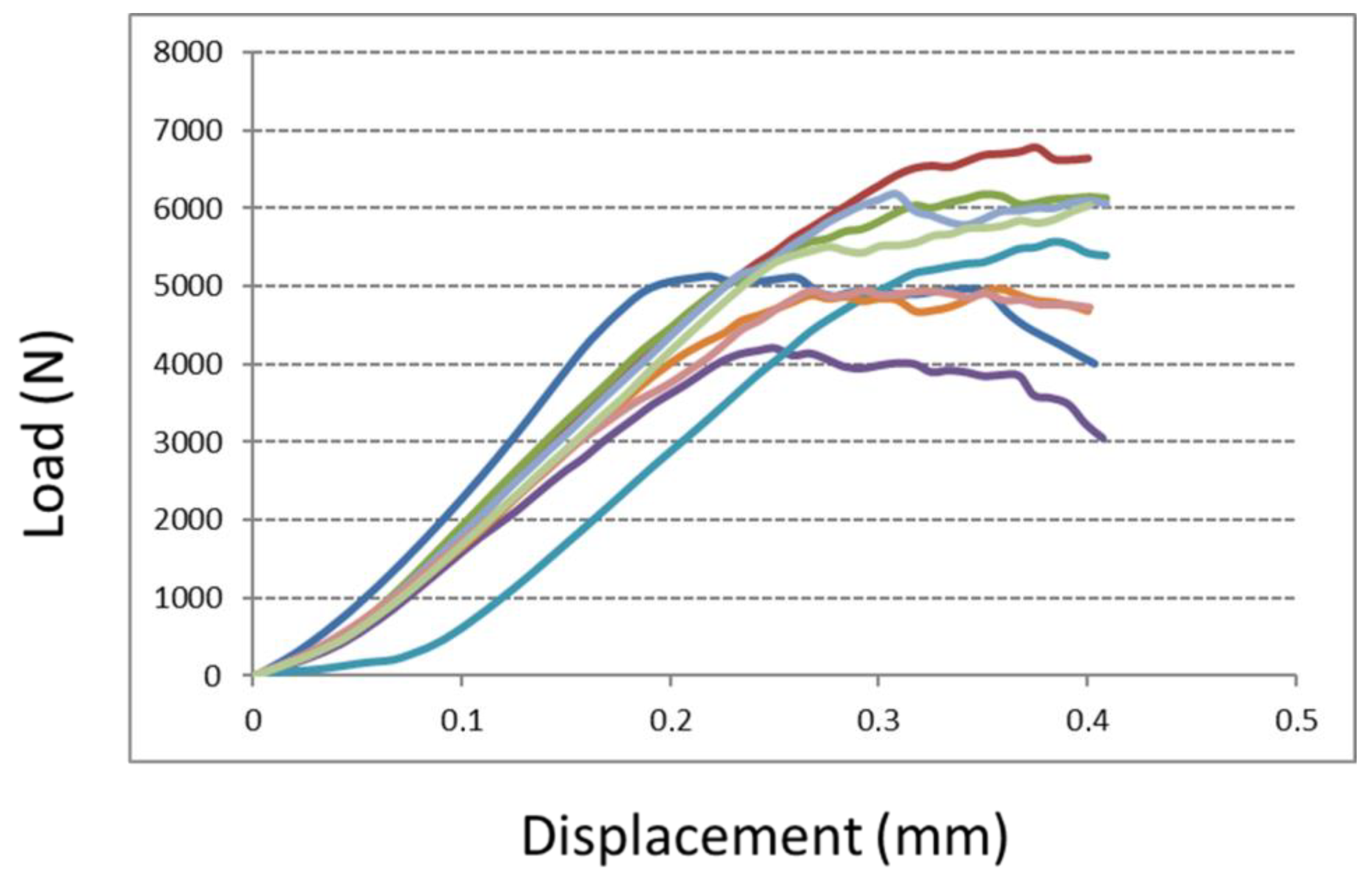
2.3.3. Compression Test
2.4. In Vitro Biological Assay
2.4.1. Cytotoxicity Test: In Vitro Evaluation
2.4.2. Cells Adhesion on the Implant Surface
2.5. In Vivo Preclinical Experiment
2.5.1. Surgical Procedure
2.5.2. Histological Process
2.5.3. Histological Assessment
- -
- Qualitative evaluation of cellular and tissue reaction
- -
- Histomorphometrical evaluation of osseointegration around the implant
- -
- Qualitative and semi-quantitative analysis of the regenerated area
- -
- Semi-quantitative analysis of the regenerated area
2.6. Statistical Analysis
3. Results
3.1. Mechanical and Morphological Evaluation
3.1.1. Implant Surface SEM
3.1.2. Profilometry
3.1.3. Compression Test
3.2. In Vitro Biological Assay
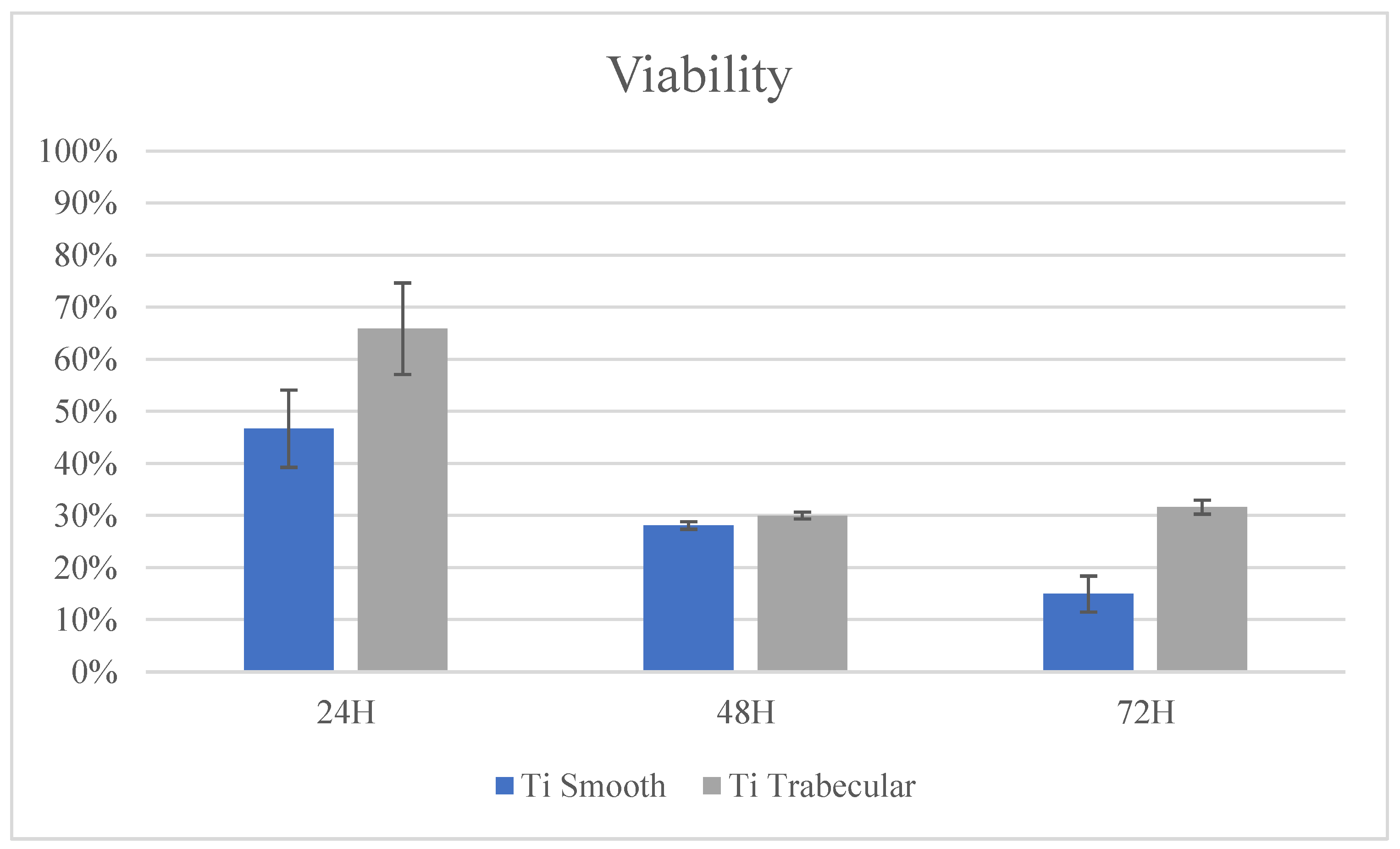
3.2.1. Cytotoxicity Test: In Vitro Evaluation
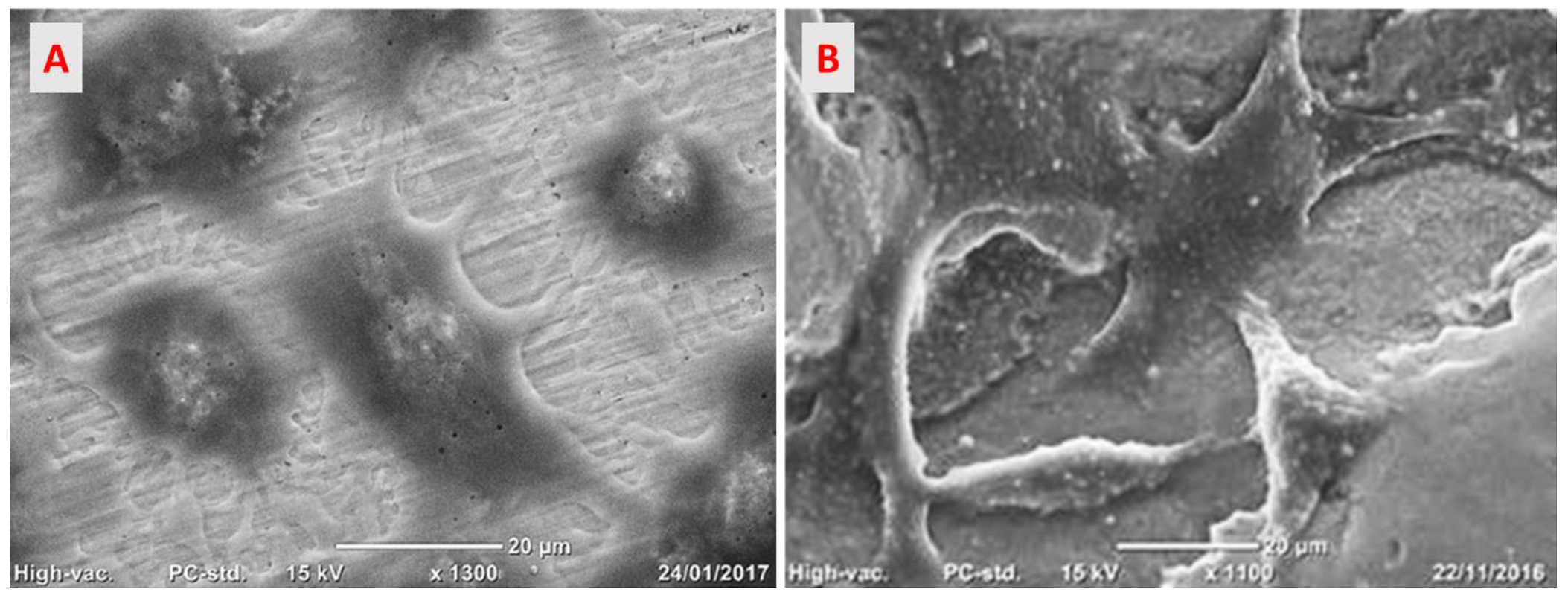
3.2.2. Cells Adhesion on the Implant Surface
3.3. In Vivo Preclinical Experiment
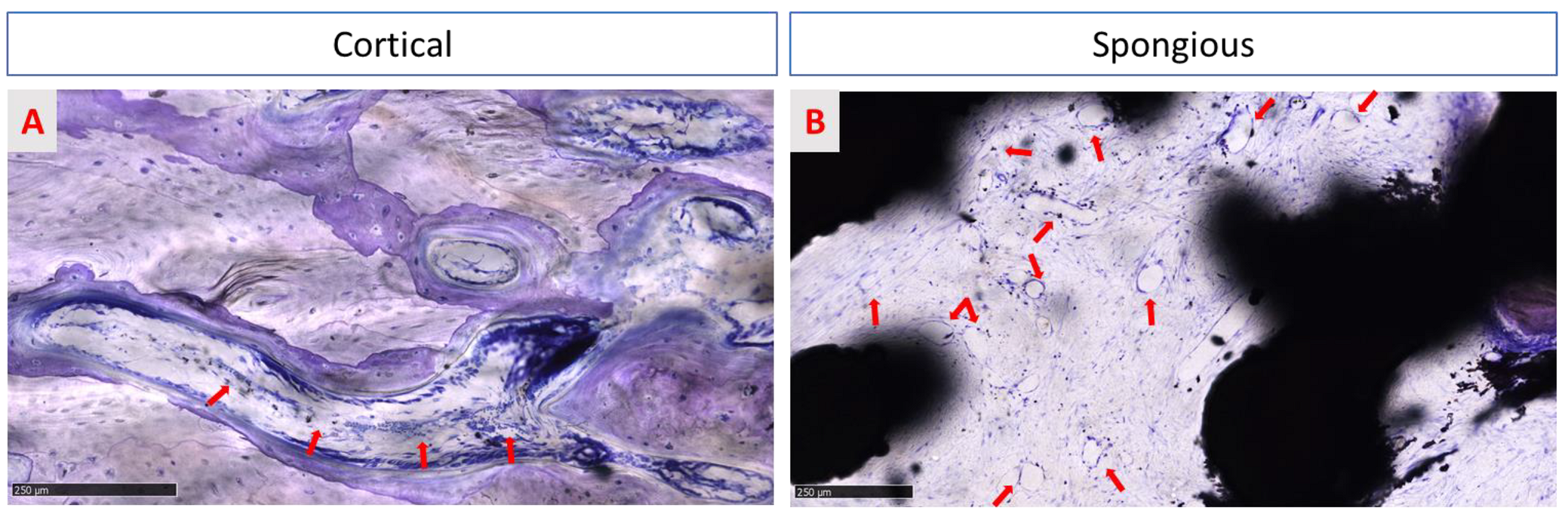
3.3.1. Histological Assessment
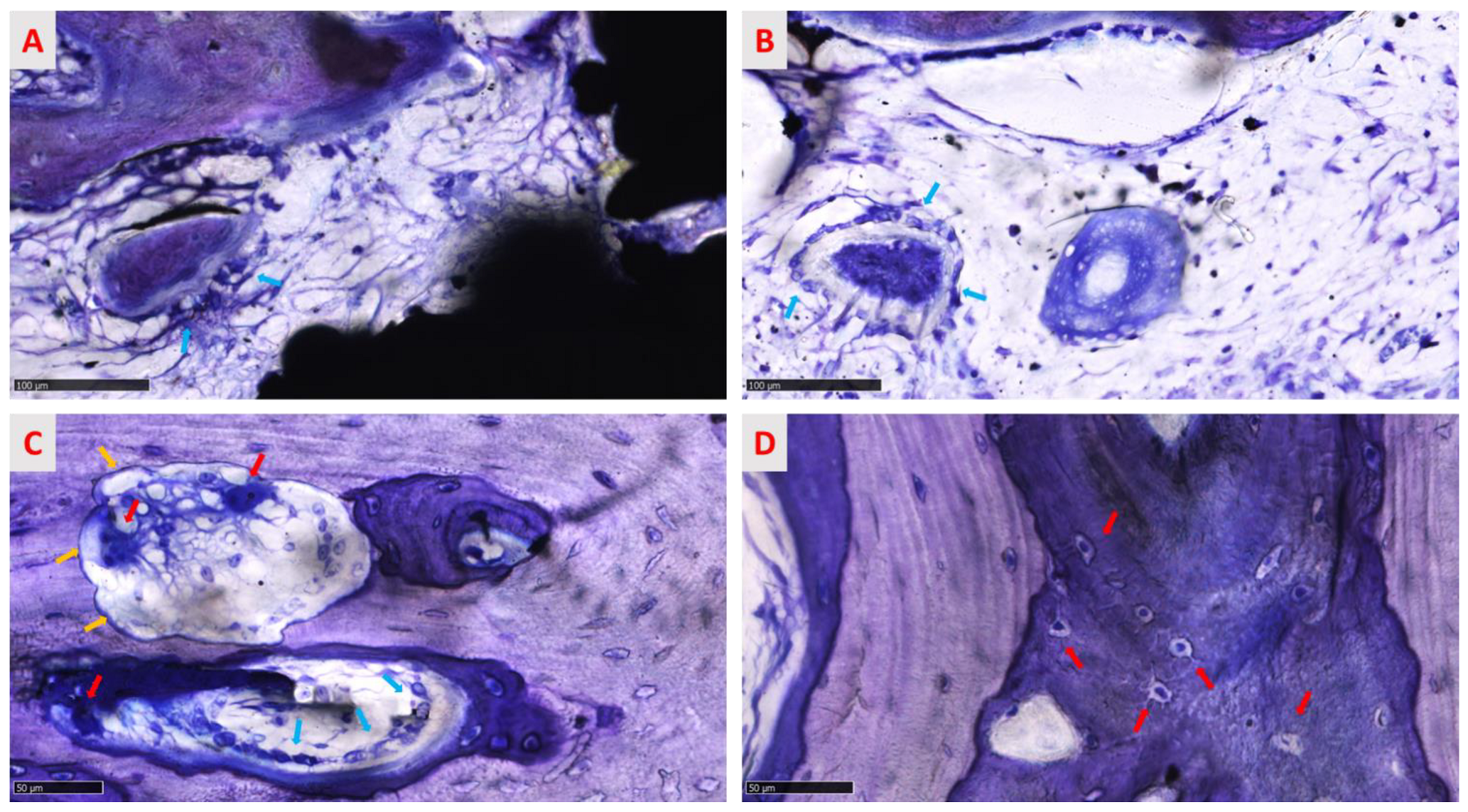
3.3.2. Qualitative Evaluation of Cellular and Tissue Reaction
3.3.3. Histomorphometric Evaluation of Osseointegration around the Implants
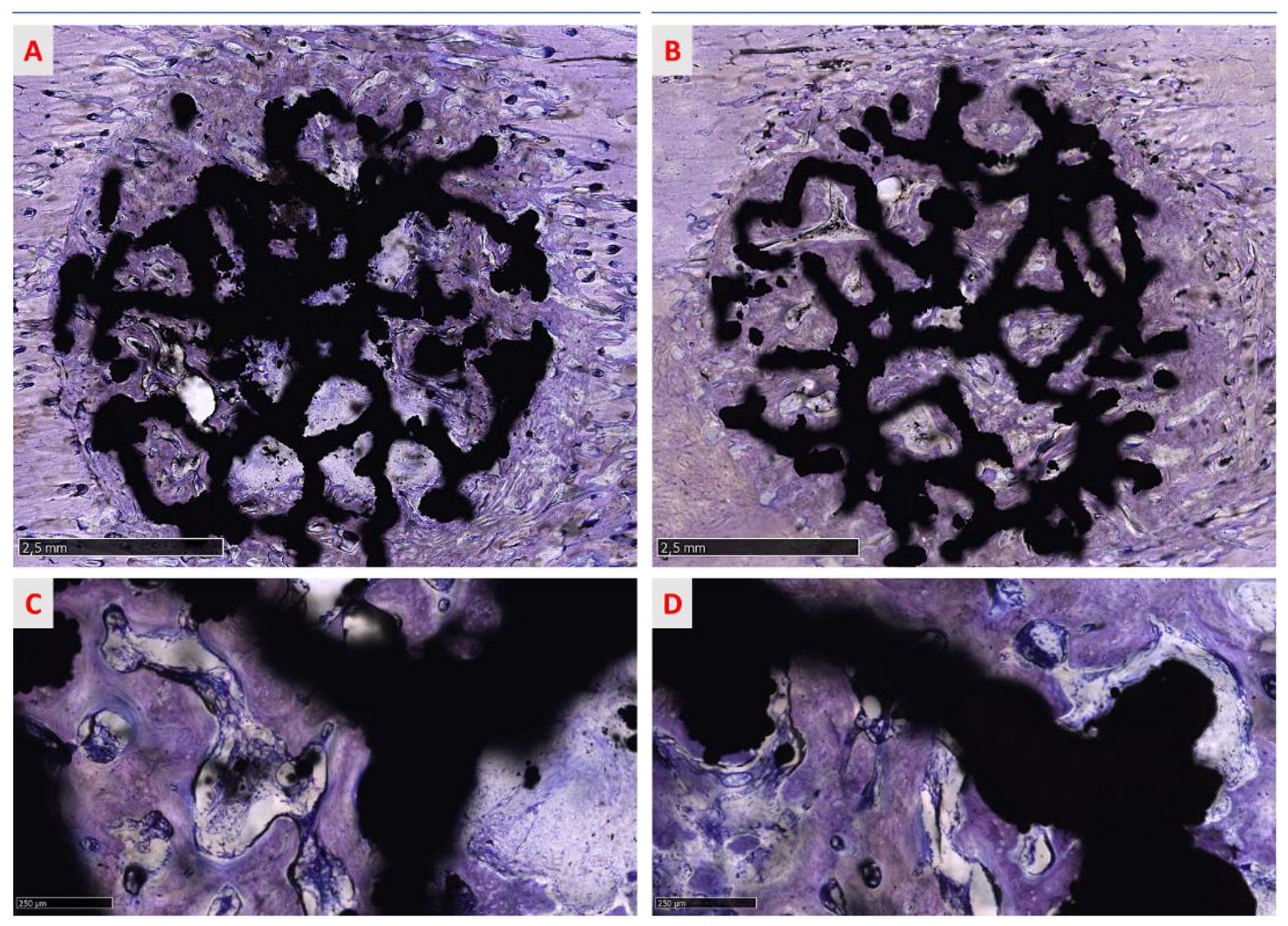
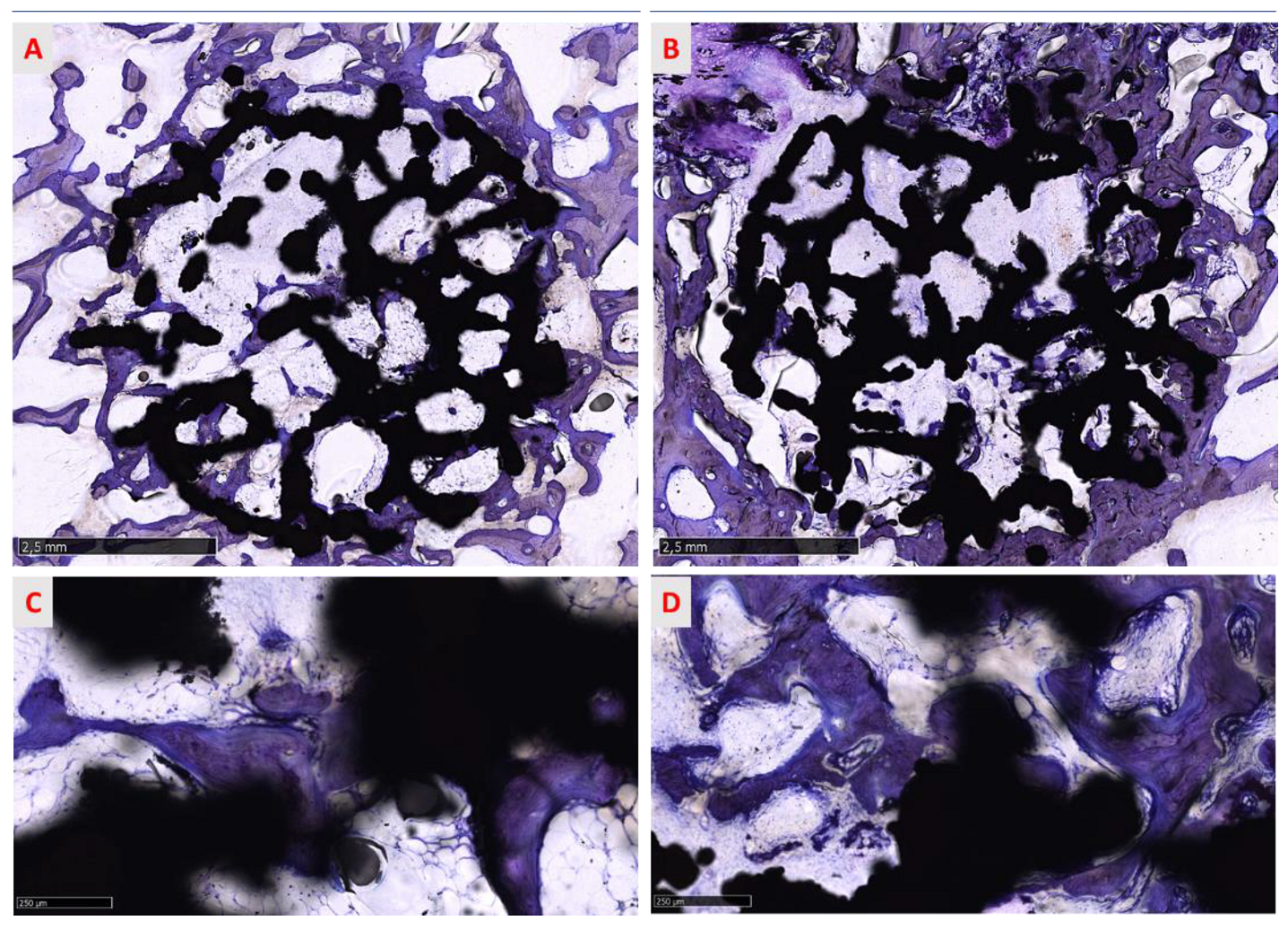
3.3.4. Qualitative Analysis of the Regenerated Area
3.3.5. Semi-Quantitative Analysis of the Regenerated Area
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hegedüs, T.; Kreuter, P.; Bányai, D.; Végh, A.; Hermann, P.; Végh, D. How Social Media Making an Impact on the Field of 3D Printing in Dentistry—Survey Study. Available online: https://www.sciencegate.app/document/10.2196/preprints.30046 (accessed on 11 January 2022).
- Yuan, L.; Ding, S.; Wen, C. Additive manufacturing technology for porous metal implant applications and triple minimal surface structures: A review. Bioact. Mater. 2018, 4, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Culmone, C.; Smit, G.; Breedveld, P. Additive manufacturing of medical instruments: A state-of-the-art review. Addit. Manuf. 2019, 27, 461–473. [Google Scholar] [CrossRef]
- Tofail, S.A.M.; Koumoulos, E.P.; Bandyopadhyay, A.; Bose, S.; O’Donoghue, L.; Charitidis, C. Additive manufacturing: Scientific and technological challenges, market uptake and opportunities. Mater. Today 2018, 21, 22–37. [Google Scholar] [CrossRef]
- Song, B.; Zhao, X.; Li, S.; Han, C.; Wei, O.; Wen, S.; Liu, J.; Shi, Y. Differences in microstructure and properties between selective laser melting and traditional manufacturing for fabrication of metal parts: A review. Front. Mech. Eng. 2015, 10, 111–125. [Google Scholar] [CrossRef]
- Sing, S.L.; An, J.; Yeong, W.Y.; Wiria, F.E. Laser and electron-beam powder-bed additive manufacturing of metallic implants: A review on processes, materials, and designs. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2016, 34, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Biffi, C.A.; Tuissi, A. Stato dell’arte sulle tecniche di produzione additiva per metalli. Metall. Ital. 2017, 1, 5–10. [Google Scholar]
- Demir, A.G.; Previtali, B. Additive manufacturing of cardiovascular CoCr stents by selective laser melting. Mater. Des. 2017, 119, 338–350. [Google Scholar] [CrossRef] [Green Version]
- Finazzi, V.; Demir, A.G.; Biffi, C.A.; Migliavacca, F.; Petrini, L.; Previtali, B. Design and functional testing of a novel balloon-expandable cardiovascular stent in CoCr alloy produced by selective laser melting. J. Manuf. Processes 2020, 55, 161–173. [Google Scholar] [CrossRef]
- Fiocchi, J.; Biffi, C.A.; Scaccabarozzi, D.; Saggin, B.; Tuissi, A. Enhancement of the Damping Behavior of Ti6Al4V Alloy through the Use of Trabecular Structure Produced by Selective Laser Melting. Adv. Eng. Mater. 2019, 22, 1900722. [Google Scholar] [CrossRef]
- Fuller, S.M.; Butz, D.R.; Vevang, C.B.; Makhlouf, M.V. Application of 3-dimensional printing in hand surgery for production of a novel bone reduction clamp. J. Hand Surg. Am. 2014, 39, 1840–1845. [Google Scholar] [CrossRef]
- Yamamoto, I.; Kishikawa, K.; Kondo, Y.; Lawn, M. Development of a Finger Like Multi-Joint Articulated Surgical Retractor for Use in Endoscopic Surgery. J. Vibroeng. 2018, 20, 1194–1201. [Google Scholar]
- Singh, R.; Suri, A.; Anand, S.; Baby, B. Validation of Reverse-Engineered and Additive-Manufactured Microsurgical Instrument Prototype. Surg. Innov. 2016, 23, 606–612. [Google Scholar] [CrossRef]
- Asa’ad, F.; Pelanyte, G.; Philip, J.; Dahlin, C.; Larsson, L. The Role of Epigenetic Functionalization of Implants and Biomaterials in Osseointegration and Bone Regeneration-A Review. Molecules 2020, 25, 5879. [Google Scholar] [CrossRef]
- Tsukanaka, M.; Yamamoto, K.; Fujibayashi, S.; Pattanayak, D.K.; Matsushita, T.; Kokubo, T.; Matsuda, S.; Akiyama, H. Evaluation of bioactivity of alkali- and heat-treated titanium using fluorescent mouse osteoblasts. J. Bone Miner. Metab. 2014, 32, 660–670. [Google Scholar] [CrossRef]
- Tsukanaka, M.; Fujibayashi, S.; Takemoto, M.; Matsushita, T.; Kokubo, T.; Nakamura, T.; Sasaki, K.; Matsuda, S. Bioactive treatment promotes osteoblast differentiation on titanium materials fabricated by selective laser melting technology. Dent. Mater. J. 2016, 35, 118–125. [Google Scholar] [CrossRef] [Green Version]
- de Wild, M.; Schumacher, R.; Mayer, K.; Schkommodau, E.; Thoma, D.; Bredell, M.; Kruse Gujer, A.; Grätz, K.W.; Weber, F.E. Bone regeneration by the osteoconductivity of porous titanium implants manufactured by selective laser melting: A histological and micro computed tomography study in the rabbit. Tissue Eng. Part A 2013, 19, 2645–2654. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.Y.; Chen, X.S.; Zhang, C.Y.; Liu, Y.; Wang, J.; Deng, F.L. Improved bioactivity of selective laser melting titanium: Surface modification with micro-/nano-textured hierarchical topography and bone regeneration performance evaluation. Mater. Sci. Eng. C 2016, 68, 229–240. [Google Scholar] [CrossRef]
- Járai-Szabó, F.; Néda, Z. On the size-distribution of Poisson Voronoi cells. Phys. A Stat. Mech. Its Appl. 2004, 385, 518–526. [Google Scholar]
- Ragone, V.; Canciani, E.; Arosio, M.; Olimpo, M.; Piras, L.A.; von Degerfeld, M.M.; Augusti, D.; D’Ambrosi, R.; Dellavia, C. In vivo osseointegration of a randomized trabecular titanium structure obtained by an additive manufacturing technique. Journal of materials science. Mater. Med. 2020, 31, 17. [Google Scholar] [CrossRef]
- Galliera, E.; Ragone, V.; Marazzi, M.G.; Selmin, F.; Banci, L.; Corsi Romanelli, M.M. Vitamin E-stabilized UHMWPE: Biological response on human osteoblasts to wear debris. Clin. Chim. Acta 2018, 486, 18–25. [Google Scholar] [CrossRef]
- Canciani, E.; Dellavia, C.; Ferreira, L.M.; Giannasi, C.; Carmagnola, D.; Carrassi, A.; Brini, A.T. Human Adipose-Derived Stem Cells on Rapid Prototyped Three-Dimensional Hydroxyapatite/Beta-Tricalcium Phosphate Scaffold. J. Craniofacial Surg. 2016, 27, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Dellavia, C.; Canciani, E.; Pellegrini, G.; Tommasato, G.; Graziano, D.; Chiapasco, M. Histological assessment of mandibular bone tissue after guided bone regeneration with customized computer-aided design/computer-assisted manufacture titanium mesh in humans: A cohort study. Clin. Implant Dent. Relat. Res. 2021, 23, 600–611. [Google Scholar] [CrossRef]
- Varoni, E.; Canciani, E.; Palazzo, B.; Varasano, V.; Chevallier, P.; Petrizzi, L.; Dellavia, C.; Mantovani, D.; Rimondini, L. Effect of Poly-L-Lysine coating on titanium osseointegration: From characterization to in vivo studies. J. Oral Implantol. 2015, 41, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Humayun, A.; Cohen, D.J.; Boyan, B.D.; Schwartz, Z. Additively manufactured 3D porous Ti-6Al-4V constructs mimic trabecular bone structure and regulate osteoblast proliferation, differentiation and local factor production in a porosity and surface roughness dependent manner. Biofabrication 2014, 6, 045007. [Google Scholar] [CrossRef] [PubMed]
- Gómez, S.; Vlad, M.D.; López, J.; Fernández, E. Design and properties of 3D scaffolds for bone tissue engineering. Acta Biomater. 2016, 42, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Warnke, P.H.; Douglas, T.; Wollny, P.; Sherry, E.; Steiner, M.; Galonska, S.; Becker, S.T.; Springer, I.N.; Wiltfang, J.; Sivananthan, S. Rapid prototyping: Porous titanium alloy scaffolds produced by selective laser melting for bone tissue engineering. Tissue Eng. Part C Methods 2009, 15, 115–124. [Google Scholar] [CrossRef] [Green Version]
- Horn, T.J.; Harrysson, O.L. Overview of current additive manufacturing technologies and selected applications. Sci. Prog. 2012, 95, 255–282. [Google Scholar] [CrossRef]
- Basalah, A.; Shanjani, Y.; Esmaeili, S.; Toyserkani, E. Characterizations of additive manufactured porous titanium implants. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 1970–1979. [Google Scholar] [CrossRef]
- Rong, M.; Lu, H.; Wan, L.; Zhang, X.; Lin, X.; Li, S.; Zhou, L.; Lv, Y.; Su, Y. Comparison of early osseointegration between laser-treated/acid-etched and sand-blasted/acid-etched titanium implant surfaces. J. Mater. Sci. Mater. Med. 2018, 29, 43. [Google Scholar] [CrossRef]
- Carmagnola, D.; Botticelli, D.; Canciani, E.; Rossi, F.; Milani, S.; Dellavia, C. Histologic and immunohistochemical description of early healing at marginal defects around implants. Int. J. Periodontics Restor. Dent. 2014, 34, e50–e57. [Google Scholar] [CrossRef] [Green Version]
- Kienapfel, H.; Sprey, C.; Wilke, A.; Griss, P. Implant fixation by bone ingrowth. J. Arthroplast. 1999, 14, 355–368. [Google Scholar] [CrossRef]
- Bonsignore, L.A.; Goldberg, V.M.; Greenfield, E.M. Machine oil inhibits the osseointegration of orthopaedic implants by impairing osteoblast attachment and spreading. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2015, 33, 979–987. [Google Scholar] [CrossRef]


| 6 Weeks | ||||
|---|---|---|---|---|
| Bone | BIC | BIn | ||
| Mean | SD | Mean | SD | |
| Cortical Bone | ||||
| Treated | 74.79% | 16.92% | 73.72% | 11.47% |
| Native | 77.10% | 16.21% | 81.58% | 9.87% |
| Spongious Bone | ||||
| Treated | 27.20% | 3.84% | 29.25% | 3.88% |
| Native | 30.43% | 9.80% | 33.53% | 20.05% |
| Lamellar Bone | Woven Bone | Osteoid | Soft Tissue | |
|---|---|---|---|---|
| Cortical Bone | ||||
| Treated | 3.63 | 1.5 | 1.13 | 1.38 |
| Native | 3.83 | 1.5 | 1,00 | 1.13 |
| Spongious Bone | ||||
| Treated | 1.13 | 1.25 | 2.38 | 3.00 |
| Native | 1.63 | 1.5 | 1.63 | 3.38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canciani, E.; Ragone, V.; Biffi, C.A.; Valenza, F.; D’Ambrosi, R.; Olimpo, M.; Cristofalo, A.; Galliera, E.; Dellavia, C. Understanding the Role of Surface Modification of Randomized Trabecular Titanium Structures in Bone Tissue Regeneration: An Experimental Study. Medicina 2022, 58, 315. https://doi.org/10.3390/medicina58020315
Canciani E, Ragone V, Biffi CA, Valenza F, D’Ambrosi R, Olimpo M, Cristofalo A, Galliera E, Dellavia C. Understanding the Role of Surface Modification of Randomized Trabecular Titanium Structures in Bone Tissue Regeneration: An Experimental Study. Medicina. 2022; 58(2):315. https://doi.org/10.3390/medicina58020315
Chicago/Turabian StyleCanciani, Elena, Vincenza Ragone, Carlo Alberto Biffi, Fabrizio Valenza, Riccardo D’Ambrosi, Matteo Olimpo, Aurora Cristofalo, Emanuela Galliera, and Claudia Dellavia. 2022. "Understanding the Role of Surface Modification of Randomized Trabecular Titanium Structures in Bone Tissue Regeneration: An Experimental Study" Medicina 58, no. 2: 315. https://doi.org/10.3390/medicina58020315
APA StyleCanciani, E., Ragone, V., Biffi, C. A., Valenza, F., D’Ambrosi, R., Olimpo, M., Cristofalo, A., Galliera, E., & Dellavia, C. (2022). Understanding the Role of Surface Modification of Randomized Trabecular Titanium Structures in Bone Tissue Regeneration: An Experimental Study. Medicina, 58(2), 315. https://doi.org/10.3390/medicina58020315









