Periodontal Pathogen Adhesion, Cytotoxicity, and Surface Free Energy of Different Materials for an Implant Prosthesis Screw Access Hole
Abstract
:1. Introduction
2. Materials and Methods
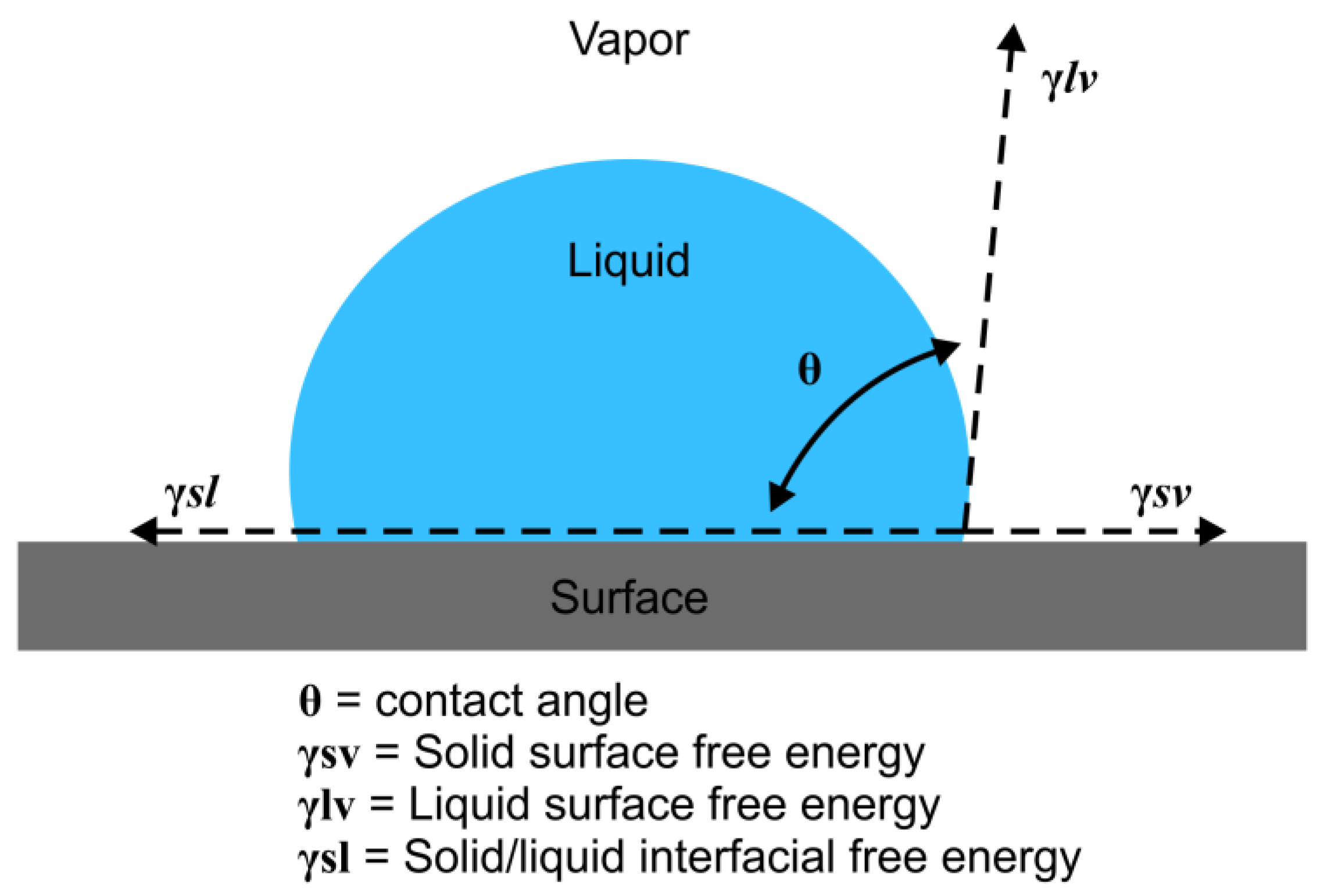
2.1. SFE Contact Angle Measurement
SFE Calculations
2.2. Preparation of Materials
2.3. Cell Culture Preparation
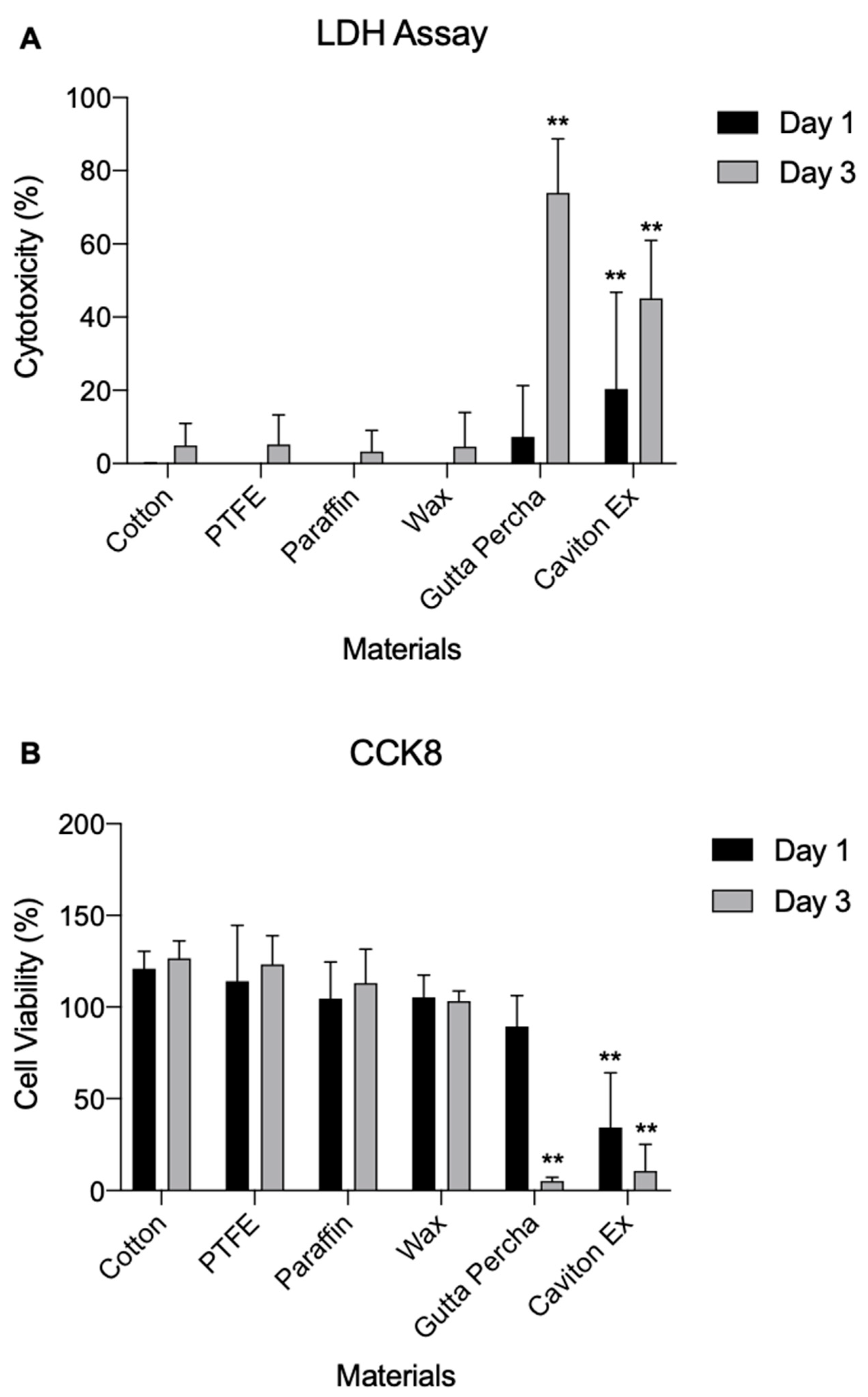
2.4. LDH Assay
2.5. Proliferation CCK8 Assay
2.6. Bacterial Adhesion Assay
2.7. Statistical Analyses
3. Results
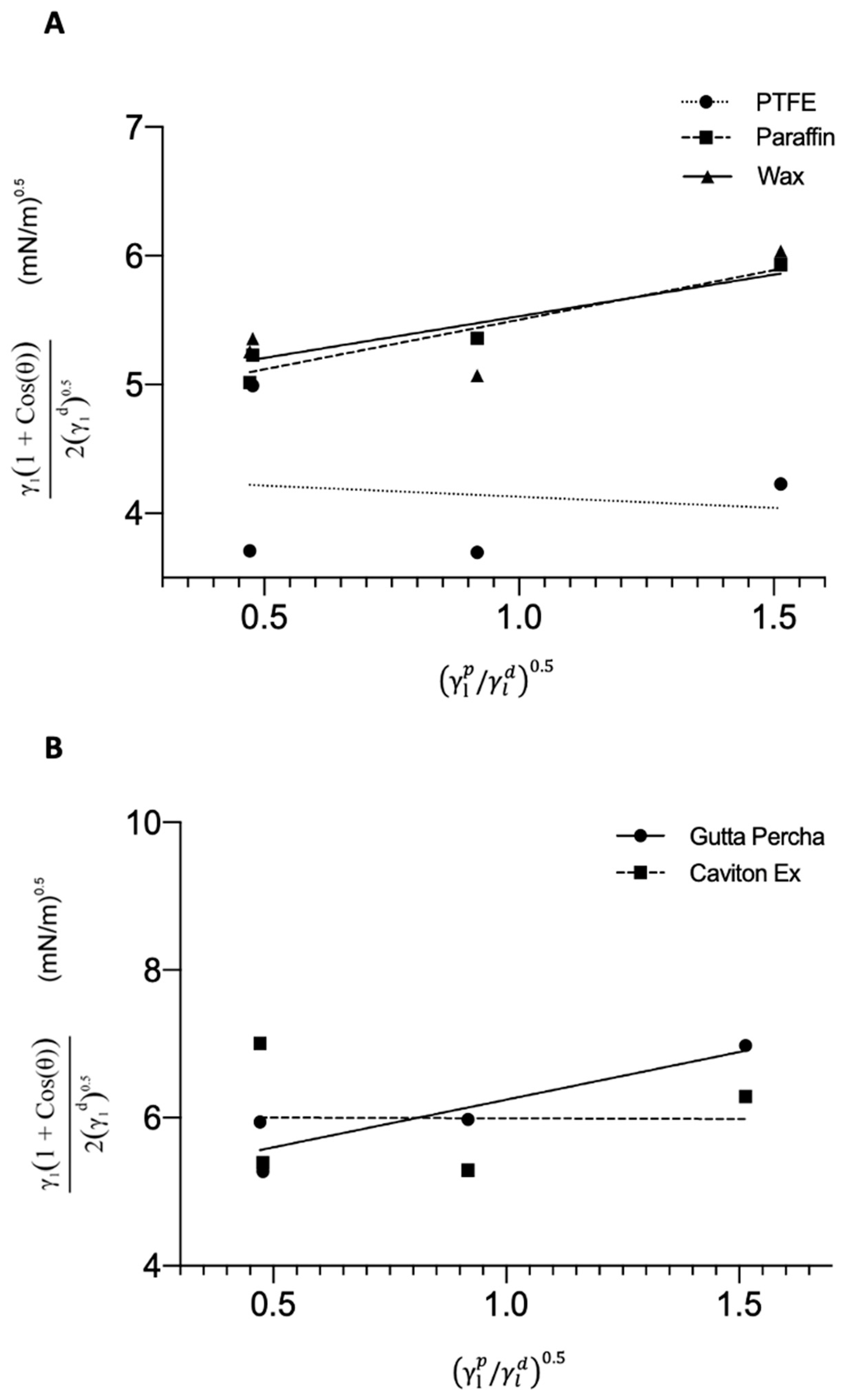
3.1. SFE
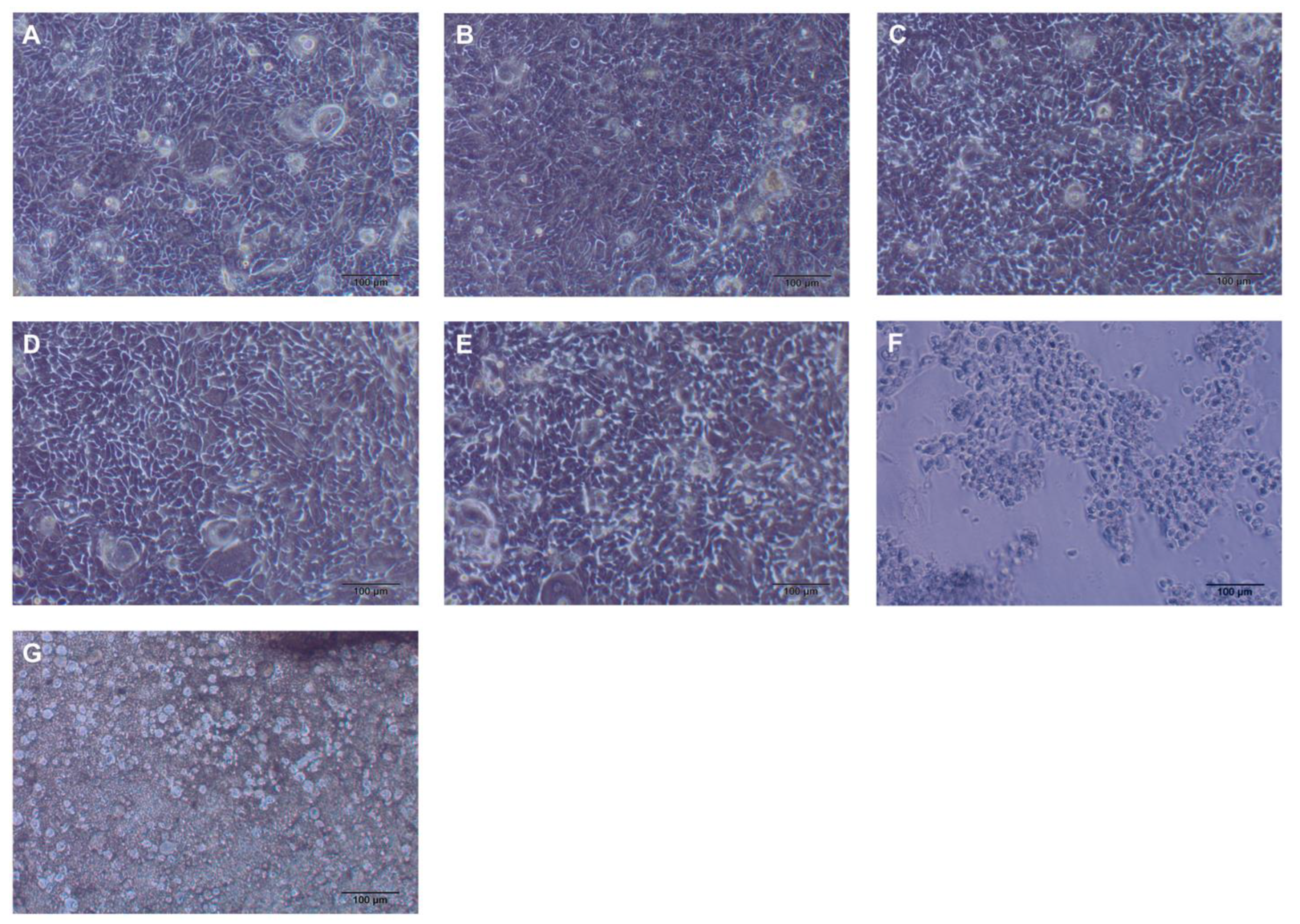
3.2. Cytotoxicity
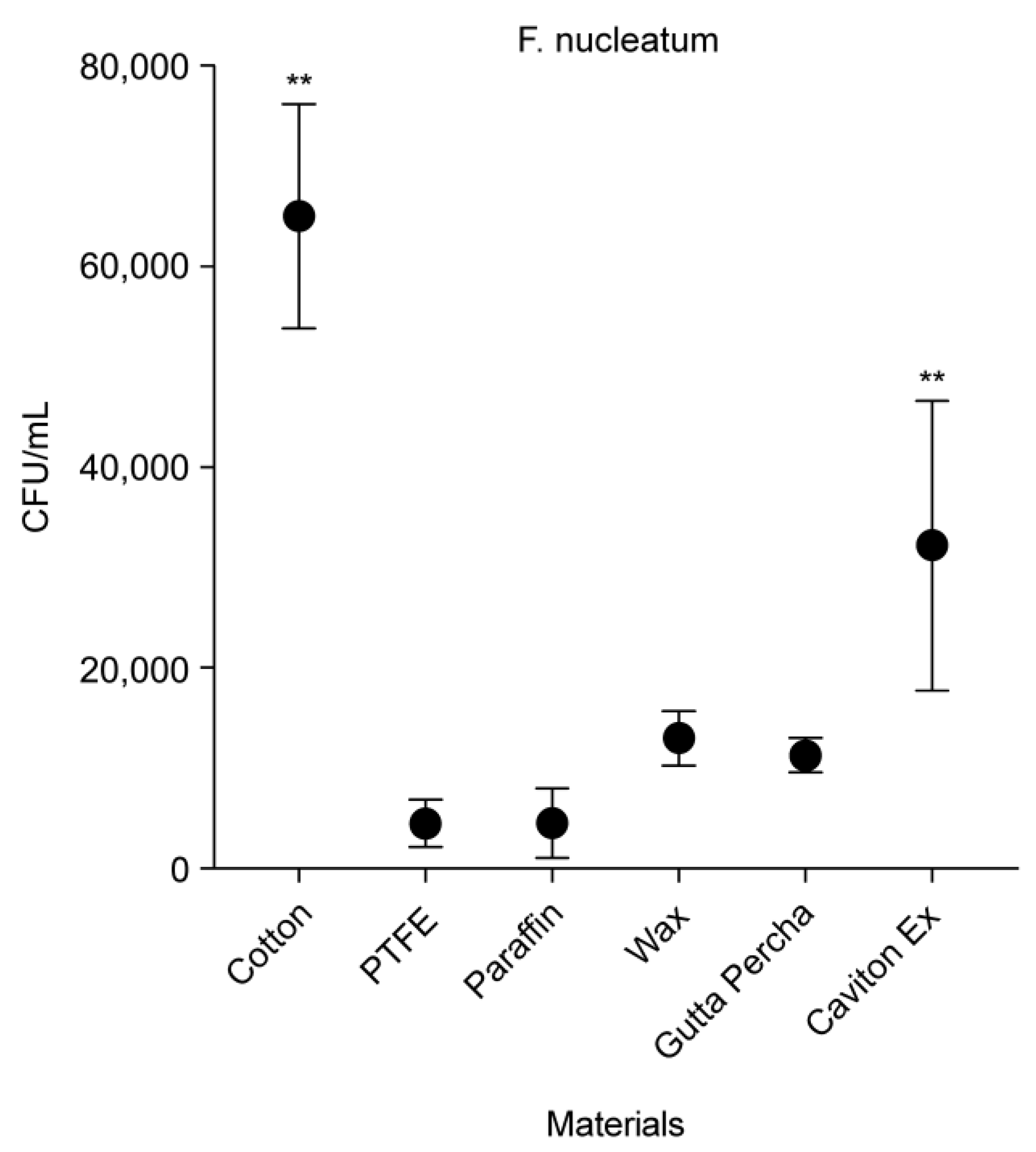
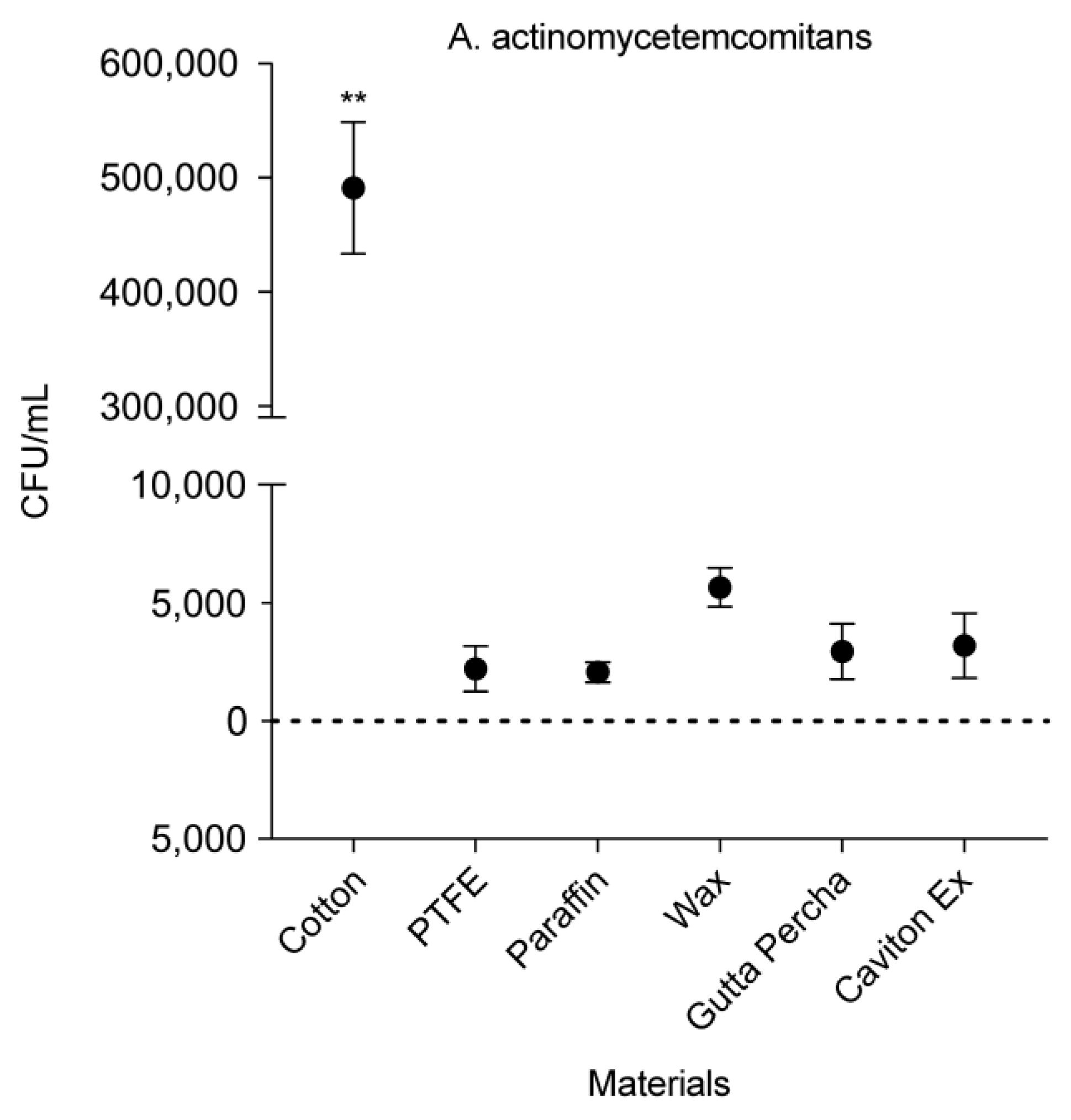
3.3. CFU Counts
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cortelli, S.C.; Cortelli, J.R.; Romeiro, R.L.; Costa, F.O.; Aquino, D.R.; Orzechowski, P.R.; Araújo, V.C.; Duarte, P.M. Frequency of periodontal pathogens in equivalent peri-implant and periodontal clinical statuses. Arch. Oral Biol. 2013, 58, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, M.; Albaqiah, H. Antimicrobial Efficacy of Materials Used for Sealing the Implant Abutment Screw Hole: An In Vitro Evaluation. Implant Dent. 2017, 26, 911–914. [Google Scholar] [CrossRef] [PubMed]
- Canullo, L.; Radovanović, S.; Delibasic, B.; Blaya, J.A.; Penarrocha, D.; Rakic, M. The predictive value of microbiological findings on teeth, internal and external implant portions in clinical decision making. Clin. Oral Implants Res. 2017, 28, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Teughels, W.; Van Assche, N.; Sliepen, I.; Quirynen, M. Effect of material characteristics and/or surface topography on biofilm development. Clin. Oral Implants Res. 2006, 17 (Suppl. 2), 68–81. [Google Scholar] [CrossRef] [PubMed]
- Fürst, M.M.; Salvi, G.E.; Lang, N.P.; Persson, G.R. Bacterial colonization immediately after installation on oral titanium implants. Clin. Oral Implants Res. 2007, 18, 501–508. [Google Scholar] [CrossRef] [PubMed]
- van Winkelhoff, A.J.; Goené, R.J.; Benschop, C.; Folmer, T. Early colonization of dental implants by putative periodontal pathogens in partially edentulous patients. Clin. Oral Implants Res. 2000, 11, 511–520. [Google Scholar] [CrossRef]
- Park, Y.; Simionato, M.R.; Sekiya, K.; Murakami, Y.; James, D.; Chen, W.; Hackett, M.; Yoshimura, F.; Demuth, D.R.; Lamont, R.J. Short fimbriae of Porphyromonas gingivalis and their role in coadhesion with Streptococcus gordonii. Infect. Immun. 2005, 73, 3983–3989. [Google Scholar] [CrossRef]
- Persson, G.R.; Renvert, S. Cluster of bacteria associated with peri-implantitis. Clin. Implant Dent. Relat. Res. 2014, 16, 783–793. [Google Scholar] [CrossRef]
- Leonhardt, A.; Dahlén, G.; Renvert, S. Five-year clinical, microbiological, and radiological outcome following treatment of peri-implantitis in man. J. Periodontol. 2003, 74, 1415–1422. [Google Scholar] [CrossRef]
- Macedo, J.P.; Pereira, J.; Vahey, B.R.; Henriques, B.; Benfatti, C.A.M.; Magini, R.S.; López-López, J.; Souza, J.C.M. Morse taper dental implants and platform switching: The new paradigm in oral implantology. Eur. J. Dent. 2016, 10, 148–154. [Google Scholar] [CrossRef]
- Ranieri, R.; Ferreira, A.; Souza, E.; Arcoverde, J.; Dametto, F.; Gade-Neto, C.; Seabra, F.; Sarmento, C. The bacterial sealing capacity of morse taper implant-abutment systems in vitro. J. Periodontol. 2015, 86, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Lopes de Chaves, E.; Mello Dias, E.C.; Sperandio, M.; Napimoga, M.H. Association between Implant-Abutment Microgap and Implant Circularity to Bacterial Leakage: An In Vitro Study Using Tapered Connection Implants. Int. J. Oral Maxillofac. Implants 2018, 33, 505–511. [Google Scholar] [CrossRef]
- Tarica, D.Y.; Alvarado, V.M.; Truong, S.T. Survey of United States dental schools on cementation protocols for implant crown restorations. J. Prosthet. Dent. 2010, 103, 68–79. [Google Scholar] [CrossRef]
- Raab, P.; Alamanos, C.; Hahnel, S.; Papavasileiou, D.; Behr, M.; Rosentritt, M. Dental materials and their performance for the management of screw access channels in implant-supported restorations. Dent. Mater. J. 2017, 36, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Schoenbaum, T.R.; Wadhwani, C.; Stevenson, R.G. Covering the Implant Prosthesis Screw Access Hole: A Biological Approach to Material Selection and Technique. J. Oral Implantol. 2017, 43, 39–44. [Google Scholar] [CrossRef]
- Wang, Y.C.; Kan, J.Y.; Rungcharassaeng, K.; Roe, P.; Lozada, J.L. Marginal bone response of implants with platform switching and non-platform switching abutments in posterior healed sites: A 1-year prospective study. Clin. Oral Implants Res. 2015, 26, 220–227. [Google Scholar] [CrossRef]
- Vicente, C.M.S.; André, P.S.; Ferreira, R.A.S. Simple measurement of surface free energy using a web cam. Rev. Bras. Ensino Fis. 2012, 34, 1–5. [Google Scholar] [CrossRef]
- Di Giulio, M.; Traini, T.; Sinjari, B.; Nostro, A.; Caputi, S.; Cellini, L. Porphyromonas gingivalis biofilm formation in different titanium surfaces, an in vitro study. Clin. Oral Implants Res. 2016, 27, 918–925. [Google Scholar] [CrossRef]
- Stalder, A.F.; Melchior, T.; Müller, M.; Sage, D.; Blu, T.; Unser, M. Low-bond axisymmetric drop shape analysis for surface tension and contact angle measurements of sessile drops. Colloids Surf. A Physicochem. Eng. Asp. 2010, 364, 72–81. [Google Scholar] [CrossRef]
- Shen, J.; He, Y.; Wu, J.; Gao, C.; Keyshar, K.; Zhang, X. Liquid Phase Exfoliation of Two-Dimensional Materials. Nano Lett. 2015, 15, 5449–5454. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Hatakeyama, S.; Ohara-Nemoto, Y.; Yanai, N.; Obinata, M.; Hayashi, S.; Satoh, M. Establishment of gingival epithelial cell lines from transgenic mice harboring temperature sensitive simian virus 40 large T-antigen gene. J. Oral Pathol. Med. 2001, 30, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, W.; Ribeiro, R.F.; Sato, S.; Pedrazzi, V. Microleakage into and from two-stage implants: An in vitro comparative study. Int. J. Oral Maxillofac. Implants 2011, 26, 56–62. [Google Scholar] [PubMed]
- Berberi, A.; Maroun, D.; Kanj, W.; Amine, E.Z.; Philippe, A. Micromovement Evaluation of Original and Compatible Abutments at the Implant-abutment. Interface J. Contemp. Dent. Pract. 2016, 17, 907–913. [Google Scholar] [CrossRef]
- van Loosdrecht, M.C.; Lyklema, J.; Norde, W.; Schraa, G.; Zehnder, A.J. The role of bacterial cell wall hydrophobicity in adhesion. Appl. Environ. Microbiol. 1987, 53, 1893–1897. [Google Scholar] [CrossRef]
- Varshney, S.; Sain, A.; Gupta, D.; Sharma, S. Factors Affecting Bacterial Adhesion on Selected Textile Fibres. Indian J. Microbiol. 2021, 61, 31–37. [Google Scholar] [CrossRef]
- Hemmatian, T.; Lee, H.; Kim, J. Bacteria Adhesion of Textiles Influenced by Wettability and Pore Characteristics of Fibrous Substrates. Polymers 2021, 13, 223. [Google Scholar] [CrossRef]
- Categorizing Surface Energy. 3M. Access 2022. Available online: https://www.3m.co.id/3M/en_ID/bonding-and-assembly-id/resources/science-of-adhesion/categorizing-surface-energy/ (accessed on 28 December 2021).
- Liu, Y.; Zhao, Q. Influence of surface energy of modified surfaces on bacterial adhesion. Biophys. Chem. 2005, 117, 39–45. [Google Scholar] [CrossRef]
- van Dijk, J.; Herkströter, F.; Busscher, H.; Weerkamp, A.; Jansen, H.; Arends, J. Surface-free energy and bacterial adhesion. An in vivo study in beagle dogs. J. Clin. Periodontol. 1987, 14, 300–304. [Google Scholar] [CrossRef]
- Pereni, C.I.; Zhao, Q.; Liu, Y.; Abel, E. Surface free energy effect on bacterial retention. Colloids Surf. B Biointerfaces 2006, 48, 143–147. [Google Scholar] [CrossRef]
- Szep, S.; Grumann, L.; Ronge, K.; Schriever, A.; Schultze, M.; Heidemann, D. In vitro cytotoxicity of medicated and nonmedicated gutta-percha points in cultures of gingival fibroblasts. J. Endod. 2003, 29, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Pascon, E.A.; Spngberg, L.S.W. In vitro cytotoxicity of root canal filling materials: 1. Gutta-percha. J. Endod. 1990, 16, 429–433. [Google Scholar] [CrossRef]
- Prabhakar, A.R.; Shantha Rani, N.; VNaik, S. Comparative Evaluation of Sealing Ability, Water Absorption, and Solubility of Three Temporary Restorative Materials: An in vitro Study. Int. J. Clin. Pediatr. Dent. 2017, 10, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Broggini, N.; McManus, L.M.; Hermann, J.S.; Medina, R.U.; Oates, T.W.; Schenk, R.K.; Buser, D.; Mellonig, J.T.; Cochran, D.L. Persistent acute inflammation at the implant-abutment interface. J. Dent. Res. 2003, 82, 232–237. [Google Scholar] [CrossRef]
- do Nascimento, C.; Barbosa, R.E.; Issa, J.P.; Watanabe, E.; Ito, I.Y.; Albuquerque, R.F., Jr. Bacterial leakage along the implant-abutment interface of premachined or cast components. Int. J. Oral Maxillofac. Surg. 2008, 37, 177–180. [Google Scholar] [CrossRef]
- Whittaker, C.J.; Klier, C.M.; Kolenbrander, P.E. Mechanisms of adhesion by oral bacteria. Annu. Rev. Microbiol. 1996, 50, 513–552. [Google Scholar] [CrossRef]



| Solvent | Surface Tension | ||
|---|---|---|---|
| Water | 72.75 | 22.10 | 50.65 |
| Ethanol | 23.70 | 19.30 | 4.40 |
| Glycerol | 63.40 | 29.00 | 34.40 |
| DMSO | 44.00 | 36.00 | 8.00 |
| Material | Liquid | |||
|---|---|---|---|---|
| Water | Ethanol | Glycerol | Dimethyl Sulfoxide | |
| PTFE | 116.986° ± 5.02 | 31.748° ± 4.87 | 111.827° ± 2.50 | 89.3586° ± 4.76 |
| Paraffin | 103.492° ± 3.71 | 20.327° ± 1.80 | 95.125° ± 2.04 | 68.403° ± 4.35 |
| Wax | 102.710° ± 3.93 | 9.550° ± 1.48 | 97.960° ± 2.90 | 64.328° ± 4.30 |
| Gutta Percha | 95.634° ± 3.20 | 16.793° ± 0.92 | 89.113° ± 2.30 | 51.608° ± 3.56 |
| Caviton Ex | 100.801° ± 3.10 | 0° ± 0.0 | 95.795° ± 4.34 | 24.340° ± 9.28 |
| PTFE | Paraffin | Wax | Gutta Percha | Caviton Ex | |
|---|---|---|---|---|---|
| SFE (mN.m−1) | 19.34 | 23.041 | 24.883 | 26.251 | 37.835 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, H.-Y.; Hou, J.; Takahashi, Y.; Tamura, Y.; Kasugai, S.; Kuroda, S.; Nakata, H. Periodontal Pathogen Adhesion, Cytotoxicity, and Surface Free Energy of Different Materials for an Implant Prosthesis Screw Access Hole. Medicina 2022, 58, 329. https://doi.org/10.3390/medicina58020329
Lu H-Y, Hou J, Takahashi Y, Tamura Y, Kasugai S, Kuroda S, Nakata H. Periodontal Pathogen Adhesion, Cytotoxicity, and Surface Free Energy of Different Materials for an Implant Prosthesis Screw Access Hole. Medicina. 2022; 58(2):329. https://doi.org/10.3390/medicina58020329
Chicago/Turabian StyleLu, Hsin-Ying, Jason Hou, Yuta Takahashi, Yukihiko Tamura, Shohei Kasugai, Shinji Kuroda, and Hidemi Nakata. 2022. "Periodontal Pathogen Adhesion, Cytotoxicity, and Surface Free Energy of Different Materials for an Implant Prosthesis Screw Access Hole" Medicina 58, no. 2: 329. https://doi.org/10.3390/medicina58020329
APA StyleLu, H.-Y., Hou, J., Takahashi, Y., Tamura, Y., Kasugai, S., Kuroda, S., & Nakata, H. (2022). Periodontal Pathogen Adhesion, Cytotoxicity, and Surface Free Energy of Different Materials for an Implant Prosthesis Screw Access Hole. Medicina, 58(2), 329. https://doi.org/10.3390/medicina58020329






